Abstract
Exposure of DNA to chemicals can result in the formation of DNA adducts, a molecular initiating event in genotoxin-induced carcinogenesis. O6-Methylguanine (O6-MeG) is a highly mutagenic DNA adduct that forms in human genomic DNA upon reaction with methylating agents of dietary, environmental, or endogenous origin. In this work, we report the design and synthesis of novel non-natural nucleoside analogues 1’-β-[1-naphtho[2,3-d]imidazol-2(3H)-one)]-2’-deoxy-D-ribofuranose and (1’-β-[1-naphtho[2,3-d]imidazole]-2’-deoxy-D-ribofuranose and their use for quantifying O6-MeG within mutational hotspots of the human KRAS gene. The novel nucleoside analogues were incorporated into oligonucleotides conjugated to gold nanoparticles to comprise a DNA hybridization probe system for detecting O6-MeG in a sequence-specific manner on the basis of colorimetric readout of the nanoparticles. The concept described herein is unique in utilizing new nucleoside analogues with elongated hydrophobic surfaces to successfully measure in-gene abundance of O6-MeG in mixtures with competing unmodified DNA.
Introduction
DNA is exposed to chemical and environmental insults such as ultraviolet radiation, reactive oxygen species, and alkylating agents. Endogenous and exogenous methylating agents react with and modify DNA nucleobases, producing primarily N7-methylguanine (N7-MeG) and O6-methylguanine (O6-MeG). These adducts are present in DNA isolated from blood cells and various human tissues.1 O6-MeG is formed in DNA by SN1-type methylating agents,2 represented by N-nitroso compounds including known human carcinogens present in the environment, diet, and tobacco.3 Endogenous sources of methylation include S-adenosylmethionine4 and endogenous nitrosation products.5,6
The formation of O6-MeG is a molecular initiating event leading to carcinogenesis and cytotoxicity. Upon encountering O6-MeG, DNA polymerases often preferentially incorporate thymidine.7–9 The resulting mismatch is a substrate for repair; however, the new strand can persist and normal cells harboring O6-MeG display a high rate of G→A mutations.10 Additionally, when cancer cells are treated with methylating agents, futile cycling of mismatch repair is exploited to induce cytotoxicity.11 G→A mutations have been causally related with the development of tumors in experimental animals treated with methylating carcinogens.12 The observation of frequent G→A transition mutations in KRAS or p53 genes of human colon and lung tumors lacking O6-MeG–DNA methyltransferase, an enzyme that repairs O6-MeG in DNA,13 further supports the etiologic involvement of O6-MeG in colon and lung carcinogenesis, and underscores the value of detecting its presence in tumor-suppressor or tumor-promoter gene sequences as a potential prognostic marker for cancer risk.
Assessing the accumulation of adducts such as O6-MeG in DNA is a considerable challenge owing to their extremely low occurrence, which is on the order of only tens to thousands per genome.14–17 O6-MeG has been quantified by 32P-postlabeling,15,17 immunoassay14,18,19 and mass spectrometry,20 wherein the DNA must be completely digested prior to analysis. Therefore it is not possible to assess O6-MeG abundance in particular genomic loci of interest, such as oncogenes. Furthermore, these methods provide little information regarding the distribution of alkylation events in the genome, needed to characterize causal relationships between adduct occurrence and genetic mutations observed in cancer. Therefore, a major standing goal for DNA adduct detection is direct quantitative sequencing of genes containing DNA adducts.
Several strategies are emerging for determining the location of DNA adducts within the genome. First, ligation-mediated polymerase chain reaction in combination with PAGE was used to map the distribution of DNA lesions within gene sequences.21 This methodology can be used to examine the distribution of single strand breaks22 and alkali labile adducts (e.g. N7-MeG),23,24 but not to map the distribution of O6-MeG. Sequencing of DNA with adducts was also achieved by single-molecule real-time sequencing,25 which enables the detection of an adduct on the basis of the slower rate of polymerase-mediated nucleotide incorporation opposite an adduct. More recently, a method based on the enzymatic removal of oxidation adducts followed by the introduction of marker nucleotides at the gapped sites and subsequent PCR amplification and nanopore sequencing was reported.26 Another related approach was based on the combination of DNA glycosylase-mediated excision of specific DNA oxidation adducts with ligase-mediated formation of deletion mutations at the sites of the lesions, which could then be detected by DNA sequencing.27 These strategies represent major conceptual and practical advances, but quantification of adduct abundance remains to be addressed.
An approach that provides a plausible chemical basis for quantitative in-gene detection of DNA alkylation adducts is based on DNA adduct-directed nucleoside probes containing artificial nucleobase surrogates that complement DNA adducts. For example, a method for site-specifically labeling O6-MeG with fluorescent tags transferred from complementary artificial nucleosides was reported.28 It is not known whether this approach is viable for sensing O6-MeG in mixtures of targets. With the goal of expanding alkylation adduct recognition, we have devised a class of synthetic nucleosides with mixed aromatic and H-bonding moieties.29–35 The first example was a perimidinone-derived nucleoside (Per, Figure S1) that formed more stable DNA duplexes when paired with O6-benzylguanine (O6-BnG) than with G.29 In a recent proof-of-principle study, we demonstrated that coupling hybridization probes containing Per with gold nanoparticles (AuNPs) allowed for the quantification of the model O6-alkylG adduct O6-BnG within a defined, albeit non-biologically relevant, DNA sequence in the presence of excess unmodified DNA strands.36
When hydrophobic perimidinone- and benzimidazole-derived nucleoside analogues (Benzi, BIM, and Peri, Figure S1) were incorporated into oligonucleotides, more stable DNA duplexes were formed when the synthetic bases were placed opposite adducts vs. unmodified bases.30 Ultimately, the larger nucleosides Peri and Per were found to better stabilize O6-BnG than did smaller analogues.30 Nucleobases with large π surface areas stack more strongly with neighboring bases than their smaller counterparts since they have more area of overlap with neighboring bases, as demonstrated for benzene, naphthalene, and pyrene nucleosides37,38 and size-expanded natural nucleobases.39–42 Structural characterization studies with short duplexes indicated that Per adopts a syn conformation and intercalates into the duplex when placed opposite O6-BnG, whereas opposite G it adopts the anti conformation and forms a less stable wobble pair.43 We proposed, therefore, to re-orient the aromatic rings in Per and Peri in order to probe the potential for their elongated surface to improve hybridization performance.
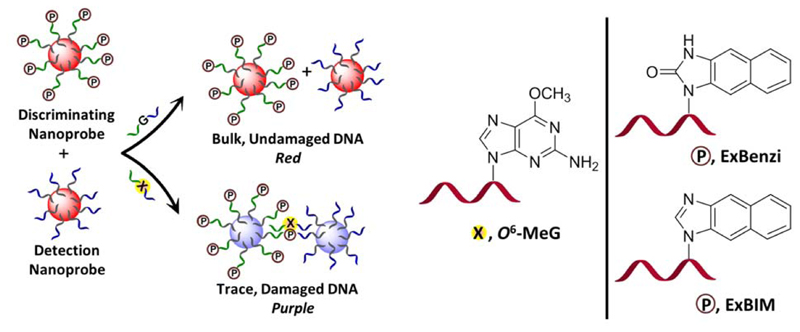
New hydrophobic, elongated adduct-directed nucleoside analogues 1’-β-[1-naphtho[2,3-d]imidazol-2(3H)-one)]-2’-deoxy-D-ribofuranose (ExBenzi) and (1’-β-[1-naphtho[2,3-d]imidazole]-2’-deoxy-D-ribofuranose (ExBIM) (Figure 1) were synthesized and incorporated into oligonucleotides. The modified oligonucleotides formed more stable DNA duplexes when the artificial nucleosides were placed opposite O6-MeG vs. G. Furthermore, oligonucleotides with ExBenzi and ExBIM were conjugated to AuNPs to create nanoprobes effective for colorimetric detection of O6-MeG in cancer-related hotspots of the KRAS oncogene. The colorimetric detection of O6-MeG is based on the distance-dependent color change caused by AuNP aggregation upon recognition of a target nucleic acid by the nanoprobes (Figure 1).
Figure 1.
Schematic representation of the nanoprobe-based detection of the highly mutagenic O6-MeG. The novel synthetic nucleosides ExBenzi and ExBIM lead to the formation of more stable DNA duplexes when they are paired with O6-MeG than with G. Gold nanoparticles (AuNPs) functionalized with ordinary oligonucleotides serve as detection nanoprobes while AuNPs functionalized with oligonucleotides containing a synthetic nucleoside (indicated by terminal P) serve as discriminating nanoprobes. In the presence of O6-MeG target DNA strands, the detection nanoprobes can align in a tail-to-tail fashion with the discriminating nanoprobes. The hybridization of the target leads to aggregation of AuNPs accompanied by a change in the color of the solution from red to purple.
Results and Discussion
Synthesis of elongated nucleoside analogues and oligonucleotides
ExBenzi and ExBIM were designed to explore the influence of elongated nucleobase shape on adduct recognition. ExBenzi is structurally similar to Benzi but is extended by addition of a benzene ring (Figure 1). Likewise, ExBIM is an extended version of BIM.
Phosphoramidites of ExBenzi and ExBIM nucleosides were newly synthesized as substrates for solid phase DNA synthesis (Scheme S1). ExBenzi (1) was prepared by heating neat 2,3-diaminonaphthalene and urea,44 while ExBIM (2) was prepared by heating 2,3-diaminonaphthalene in formic acid.45,46 The nucleobases 1 and 2 were glycosylated by nucleophilic displacement of chloride from 1-(α)-chloro-3,5-di-O-p-toluoyl-2-deoxyribose to yield O-toluoylated nucleosides, which were deprotected with NaOMe to yield nucleosides 3 and 4.29,30,34 Protection of the 5'-OH of 3 and 4 was initially attempted using DMTCl, but very low yields of product were obtained. Thus, DMT groups were installed with the more reactive reagent DMT·BF4 to obtain 5 and 6.47–49 Finally, phosphoramidites 7 and 8 were formed by alkylating the 3'-OH of 5 and 6 with chlorinated phosphotidylating reagent.29 These phosphoramidites were incorporated into oligonucleotides by solid-phase DNA synthesis with an automated DNA synthesizer.
Thermal stability of DNA duplexes containing ExBenzi or ExBIM opposite O6-MeG
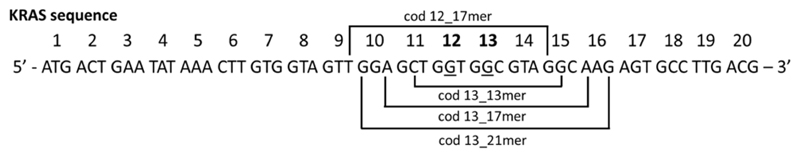
The capability of ExBenzi and ExBIM to stabilize DNA duplexes containing a site-specific O6-MeG modification relative to those containing G was evaluated in DNA duplexes having the sequence of the KRAS gene surrounding codon 12 and codon 13 (Figure 2). The KRAS oncogene is a gene frequently mutated in patients with colorectal cancer and nearly 97% of all KRAS mutations are localized to codons 12 and 13.50 The evaluation studies were performed with 13-mer target oligonucleotides for which the middle base of the target was the middle base of KRAS codon 13. These targets contained at the middle base position either O6-MeG or G (O6-MeG_13mer target and G_13mer target respectively, Table S1). Respective complementary probe oligonucleotides contained ExBenzi, ExBIM, or C at the middle base position (ExBenzi_Probe_1, ExBIM_probe_1, C_Probe_1, Table S1).
Figure 2.
KRAS gene sequence studied herein. The target oligonucleotides were designed such that their middle base was the middle base of either codon 12 or codon 13 (underlined bases). The length of the targets was varied for the evaluation studies performed with targets centered on codon 13 (13-, 17-, 21-mer). The target was a 17-mer for the codon 12-based studies.
The stability of DNA duplexes was investigated by measuring their melting temperature (Tm). The duplex containing G:C at the middle position had a Tm of 66.0 °C (Table 1). When O6-MeG was paired with C, the Tm decreased to 55.2 °C. The diminished stability of this duplex is consistent with previous studies suggesting that O6-MeG:C adopts a wobble configuration at physiological pH and thus diminished stability could be attributed to the possibility for two instead of three hydrogen bonds.51 Additionally, the higher degree of lipophilicity of O6-MeG in comparison to G appears to disrupt stacking interactions within both the modified and complementary strands.52 In contrast, when the duplex contained ExBenzi opposite O6-MeG, the Tm of the duplex was slightly higher than when the duplex contained ExBenzi opposite G (55.0 °C vs 52.0°C, ΔTm=3.0 °C, Table 1). Similarly, when the probe strand contained ExBIM, the duplex containing O6-MeG in the target strand had a higher Tm compared to when there was a G in the target strand (Tm=54.0 °C for ExBIM: O6-MeG vs 51.7 °C for ExBIM:G, Table 1). This preference may be rationalized on the basis of more favorable hydrophobic interactions of the nucleobase analogues with the alkylated base over G, consistent with previous observations.53–58 Thus, the synthesized probes seem to discriminate between G and O6-MeG by destabilizing the duplexes containing G.
Table 1.
Melting temperatures of 13-mer DNA duplexes centered on KRAS codon 13 in which the target strand contains in the middle position either G or O6-MeG and the base in the middle position of the complementary strand is C, ExBenzi, or ExBIM.
| Paired bases | Tm(°C) | ΔTm(°C)a |
|---|---|---|
| C:G C:O6-MeG |
66.0±0.6 55.2±0.2 |
-10.8±0.6 |
| ExBenzi:G ExBenzi: O6-MeG |
52.0±0.7 55.0±0.7 |
+3.0±1.0 |
| ExBIM:G ExBIM: O6-MeG |
51.7±0.6 54.0±1.0 |
+2.3±1.2 |
ΔTm= Tm(O6-MeG duplex)- Tm(G duplex)
Data are mean ± SD from three independent experiments
Colorimetric discrimination of O6-MeG in KRAS
Encouraged by the oligonucleotide duplex thermal stability data, we used oligonucleotides containing ExBenzi and ExBIM to construct AuNP-based nanoprobes targeting O6-MeG within the KRAS sequence context. The nanoprobes were constructed by functionalizing AuNPs (d=20 nm) with thiol-modified oligonucleotides. Two types of nanoprobes were prepared: a detection nanoprobe and discriminating nanoprobes (Figure 1). The detection nanoprobe was functionalized with a 5’-thiol-modified oligonucleotide (5’-thiol_1, Table S1) with a 16-mer sequence comprised of a (T)10 spacer and a 6-mer recognition sequence. The discriminating nanoprobes were functionalized with 17- mer oligonucleotides that were 3’-thiol-modified and consisted of a (A)10 spacer and a 7-mer recognition sequence ending with a 5’-ExBenzi or ExBIM (3’-thiol_ExBenzi_1, 3’-thiol_ExBIM_1, respectively, Table S1). The nanoprobes were designed such that the discriminating nanoprobe (ExBenzi nanoprobe or ExBIM nanoprobe) could hybridize to the target with its 5’-tail and the detection nanoprobe could hybridize to the target with its 3’-tail; therefore the nanoprobes were created with the capability to align in a tail-to-tail fashion in order to form a sequence complementary to the target sequences (Figure 1).
The functionalized AuNPs exhibited a characteristic surface plasmon resonance (SPR) band at 530 nm (Figure S16). In the absence of a matched target, the AuNPs remained dispersed, and the solution had a red color. When a matched target was added, it hybridized to the covalently attached probe oligonucleotides, bringing detection and discriminating nanoprobes in close proximity. Thus, a AuNP-DNA network aggregate was formed causing a change in the dielectric environment of the solution. The close proximity of the AuNPs caused a coupling of their individual localized plasmon fields and induced a red-to-purple color change that could be quantified by comparing the absorbance spectra of the solutions. Upon AuNP aggregation, the 530-nm SPR peak was red-shifted and the spectrum broadened, indicating a decrease in inter-particle distance and an increase of aggregate size (Figure S16). As a result, the absorbance at 530 nm (A530) decreased while absorbance in the 700-nm (A700) region increased (Figure S16). Thus, the absorbance ratio at 700 nm vs. 530 nm (A700/A530) is indicative of the aggregation state.59
Evaluation of thermal stability of AuNP probe:DNA aggregates
To assess the capability of the nanoprobes to distinguish between adducted and non-adducted target strands, the detection nanoprobes (1 nM) were mixed with discriminating nanoprobes containing either ExBenzi or ExBIM (1 nM). (The mixture of the detection nanoprobes with the discriminating nanoprobes containing ExBenzi or ExBIM will hereafter be referred to as ExBenzi nanoprobes and ExBIM nanoprobes, respectively). ExBenzi and ExBIM nanoprobes were supplemented with the same amount of either the O6-MeG or G target (20 nM final concentration). Upon aggregation, the thermal stability of the aggregates was evaluated; the Tm values of the AuNP probe:target aggregates were determined by monitoring the absorbance of solutions at 530 nm (A530) as a function of temperature. Increased absorbance reflects denaturation of the hybridized strands within the aggregates. The aggregates exhibited exceptionally sharp melting transitions characteristic of AuNP-DNA conjugates.60 Aggregates of the ExBenzi nanoprobes and G target had a Tm of 29.0 °C (Table 2), while those from the O6-MeG target had a Tm of 32.0 °C (Table 2). In the case of the ExBIM nanoprobes, aggregates formed in the presence of modified target also had a higher Tm than the aggregates formed in the presence of the natural target (32.7 °C vs 29.4 °C, Table 2).
Table 2.
Melting temperatures of AuNP aggregates formed by either the ExBenzi or ExBIM nanoprobes and the 13-mer KRAS codon 13 target (containing either O6-MeG or G in the middle position).
| Paired bases | Tm(°C) | ΔTm(°C)a |
|---|---|---|
| ExBenzi:G ExBenzi: O6-MeG |
29.0±0.0 32.0±0.0 |
+3.0±0.0 |
| ExBIM:G ExBIM: O6-MeG |
29.4±0.6 32.7±0.6 |
+3.3±0.8 |
ΔTm= Tm(O6-MeG aggregates)- Tm(G aggregates)
Data are mean ± SD from three independent experiments
The increased discrimination achieved with the nanoprobe system is noteworthy considering that the concentration of target oligonucleotide was 110-fold lower for the nanoprobe system in comparison to the free DNA duplexes (20 nM vs 2.2 uM). The degree of discrimination (ΔTm = Tm probe:O6-MeG - Tm probe:G) for ExBIM nanoprobes was 3.3 °C vs. 2.3 °C measured for the corresponding free duplexes. The sensitivity gained by incorporating the AuNPs relates to their sharp melting transitions. These are due to a combination of cooperativity of nanoparticle dissociation60 (the network of interconnected AuNPs gets progressively weaker as multiple DNA linkers dissociate) and the extremely high molar extinction coefficient of AuNPs (9 × 108 L cm-1 mol-1 for AuNPs with d= 20 nm).61 Moreover, in the nanoprobe system, the detection and discriminating nanoprobes bind to adjacent positions on the target forming a nicked DNA duplex. This design allows better mismatch discrimination from the standard DNA duplex format through cooperative hybridization of the AuNP-conjugated DNA strands to the target.
O6-MeG recognition in mixed targets
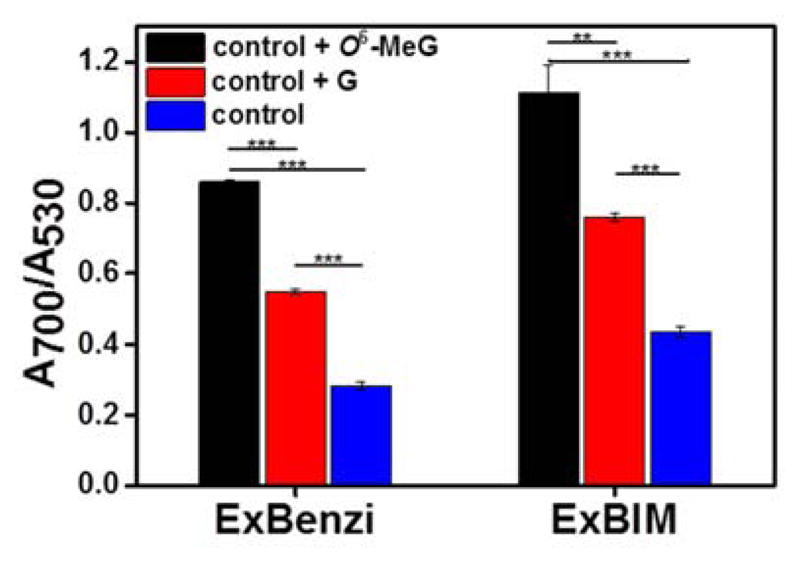
In the aforementioned studies either methylated or undamaged DNA was targeted individually. To test whether ExBenzi and ExBIM nanoprobes could sense O6-MeG in mixtures containing excess undamaged DNA strands we investigated the magnitude of aggregation of the nanoprobes when they were supplemented with a mixture of different targets. Solutions containing either ExBenzi (1 nM) or ExBIM nanoprobes (1 nM) were supplemented with a mixture of G target and a non-complementary 13-mer target (20 nM each, final concentration). The non-complementary target (Table S1) was included to mimic the presence of non-specific targets in the mixture. Each mixture of nanoprobes and targets was further supplemented with either 2 pmol of O6-MeG target (20 nM final concentration) or 2 pmol of G target (40 nM final concentration). Therefore the total DNA concentration for the two supplemented solutions was equal (60 nM) but the amounts of the two competitive DNA strands were different (20 nM O6-MeG and 20 nM G target vs 40 nM G target). Absorbance ratios (A700/A530) were determined in order to assess the magnitude of aggregation (Figure 3). For ExBenzi nanoprobes, aggregates from solutions supplemented with the O6-MeG target exhibited higher A700/A530 values than the solutions supplemented with the G target (Δ(A700/A530) = 0.31, Figure 3). Similarly, for the ExBIM nanoprobes, aggregates from solutions supplemented with the O6-MeG target exhibited higher A700/A530 values than the solutions supplemented with the G target (Δ(A700/A530)= 0.36, Figure 3). These results indicated that the selective recognition of O6-MeG by ExBenzi or ExBIM lead to larger aggregates of the corresponding ExBenzi and ExBIM nanoprobes when mixed with the O6-MeG target even in the presence of the competing G target.
Figure 3.
Absorbance ratios of aggregates formed from ExBenzi or ExBIM nanoprobes and target mixtures (20 nM of each G and non-complementary target, control) supplemented with either the O6-MeG (control + O6-MeG, 20 nM final concentration) or G target (control + G, 40 nM final concentration). Aggregates formed from the initial mixture (control) served as the control. Data are mean±SD from three independent experiments. Significant differences indicated as ** for P<0.01 and *** for P<0.001.
Sensitivity of nanoprobes for detecting O6-MeG in KRAS sequence: ExBenzi vs ExBIM nanoprobes
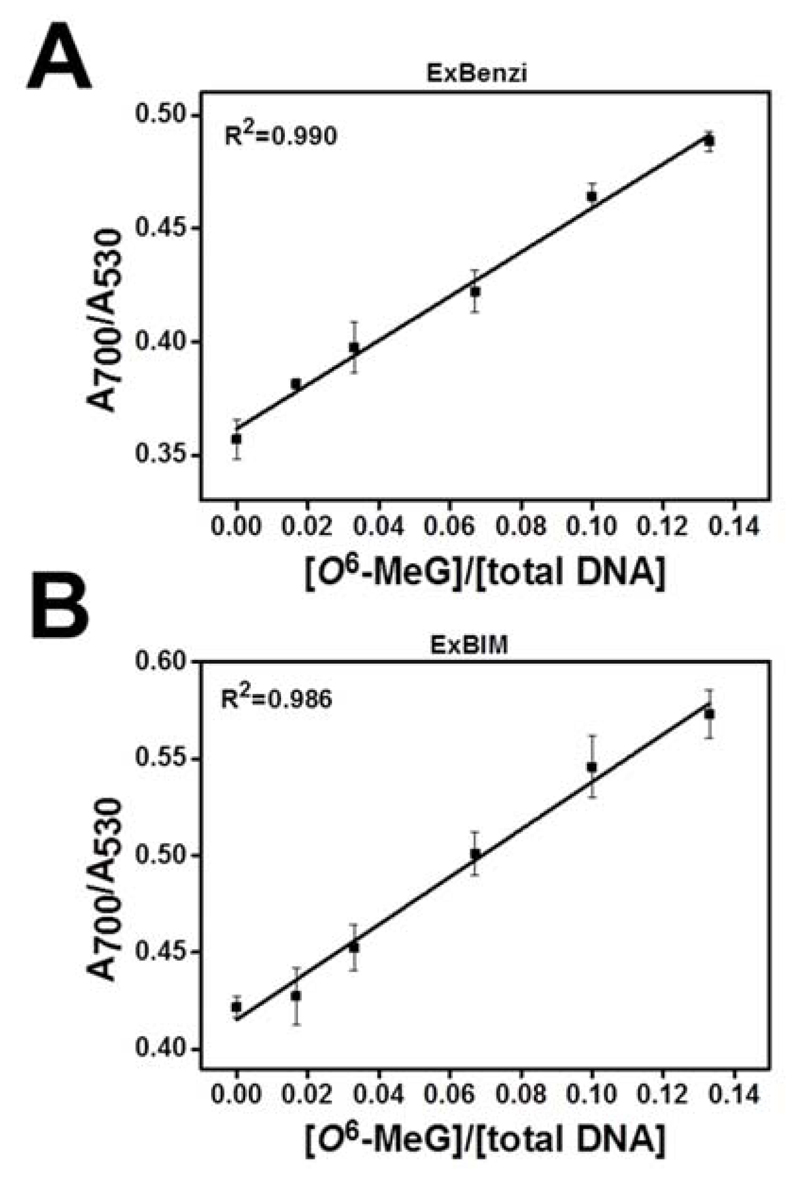
The sensitivity of the nanoprobes for detecting O6-MeG in the KRAS sequence was evaluated by measuring aggregation in samples containing decreasing concentrations of the O6-MeG target in the presence of competitive strands. Aggregates containing either the ExBenzi or ExBIM nanoprobes (1 nM each) were mixed with the G and non-complementary targets (20 nM each) and supplemented with O6-MeG and G targets at different ratios (6 pmol in total) so that the final relative O6-MeG concentration ([O6-MeG]/[total DNA]) ranged from 0-13.3 % (Table S2). Upon aggregation, absorbance ratios (A700/A530) were measured. For both ExBenzi (Figure 4A) and ExBIM nanoprobes (Figure 4B) A700/A530 values increased linearly as a function of the relative amount of O6-MeG target, indicating a corresponding increase in aggregate size.
Figure 4.
Absorbance ratios (A700/A530) as a function of relative O6-MeG target concentrations ([O6-MeG] / [total target DNA] for aggregates formed from (A) ExBenzi or (B) ExBIM nanoprobes. Data points indicate mean ± SD from three independent experiments.
In order to compare the capability of the ExBenzi and ExBIM nanoprobes to detect O6-MeG, we determined the limit of detection (LOD) for O6-MeG target DNA strands (Figure 4). For the ExBenzi nanoprobes (Figure 4A) the LOD was 2.3 % O6-MeG target or 138 fmol O6-MeG target in the presence of 6 pmol DNA. For the ExBIM nanoprobes (Figure 4B) the LOD was lower, namely 1.6 % O6-MeG target or 96 fmol O6-MeG target in the presence of 6 pmol DNA. Absorbance ratios (A700/A530) were also plotted as a function of the relative concentration [O6-MeG]/[G] and corresponding standard curves appear in the Supporting Information (Figure S17). These results demonstrate that both ExBenzi and ExBIM nanoprobes, can be used for the competitive quantitative detection of O6-MeG, with ExBIM being approximately 1.4-fold more sensitive in the tested sequence context, and that they are effective in the presence of G target DNA strands even when O6-MeG target is a minor component of the mixture.
It is notable that the nanoprobes distinguish the presence of a single methyl group in one of the 13 bases of a target strand, and that the selection is achieved without heating and under non-stringent salt conditions, which are often needed for mismatch discrimination for single nucleotide polymorphism (SNP) detection. A feature of these nanoprobes that suggests their further potential in bioanalytical applications is their compatibility with AuNP-based techniques currently used for SNP detection in unamplified genomic samples.62–66 In this study, the increase in AuNP aggregation associated with the presence of the O6-MeG target was monitored by UV-Vis spectroscopy. It can be anticipated that with further engineering, the adduct-sensitive AuNP aggregation could be monitored with analytical readouts that are significantly more sensitive than absorbance, such as light-scattering,65,66 scanometric62,64 or electrical detection63 currently applied for SNP detection in unamplified genomic samples.
Data from melting studies of the DNA duplexes, as well as AuNP-DNA aggregates, together with data from the colorimetric ratiometric detection studies suggest that both ExBenzi and ExBIM have a higher affinity for O6-MeG than for G. The relative performance of the two novel nucleoside analogues is compared in Table S3. In duplex DNA, there appears to be a slight increase in discrimination in the case of ExBenzi, whereas the detection capacity of the nanoprobe system is slightly improved in the case of ExBIM. The similarity between the two analogues suggest a lack of relevant hydrogen bonding interactions between the adduct and probes since the analogues differ only in H-bonding capacity. From a synthetic perspective, however, ExBIM can be produced with consistently higher yields primarily due to better solubility characteristics during work-up and purification.
Detection of O6-MeG oligonucleotide targets in the presence of human genomic DNA
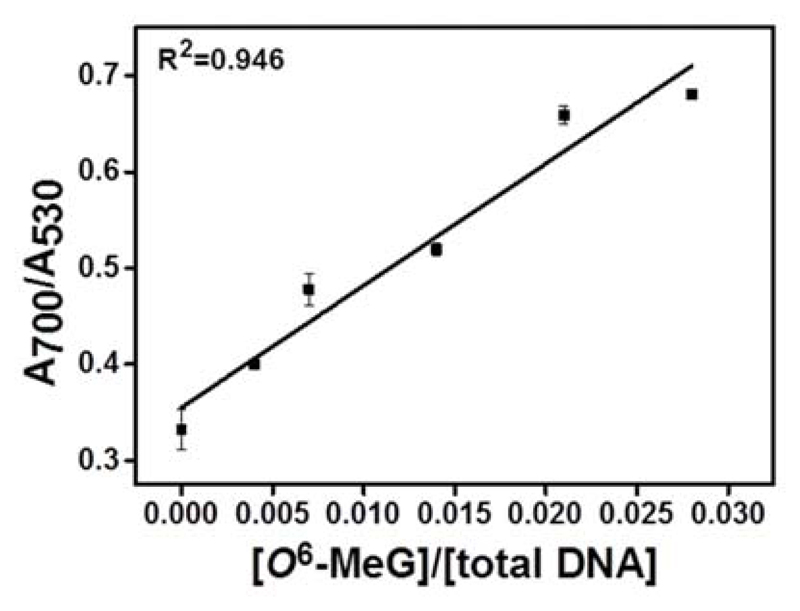
We investigated the performance of the ExBIM nanoprobes for detecting O6-MeG oligonucleotide targets in the presence of human genomic DNA. Genomic DNA extracted from SW480 colon carcinoma cells was fragmented by ultrasonication (Figure S18). ExBIM nanoprobes (1 nM) were combined with fragmented genomic DNA (100 ng) and O6-MeG oligonucleotides. The same target sequence with G at the modification position was also added as a direct competitor. Ratios of added O6-MeG to G targets where the same as when the sensitivity of the nanoprobes was assessed with non-complementary oligonucleotides as background. The final relative concentration of O6-MeG in the sample ([O6-MeG]/[total DNA]) ranged from 0-2.8 % (Table S4). Upon target-induced aggregation, absorbance ratios (A700/A530) were measured. Ratiometric absorbance values A700/A530 increased linearly as a function of O6-MeG target concentration (Figure 5), indicating a corresponding increase in aggregation. The LOD for O6-MeG DNA strands with genomic DNA as background (Figure 5) was 0.24 %, indicating that the presence of a large background of fragmented genomic DNA did not interfere with the sensing response of the nanoprobes and quantification of O6-MeG.
Figure 5.
Absorbance ratios (A700/A530) as a function of relative O6-MeG target concentrations ([O6-MeG] / [total target DNA] for aggregates formed from ExBIM nanoprobes in the presence of fragmented human genomic DNA. Data points indicate mean±SD from three independent experiments.
Specificity of ExBIM nanoprobes
The specificity of ExBIM towards O6-MeG as opposed to adducts containing other alkyl groups on the O6-position of guanine was also examined. ExBIM nanoprobes (1 nM) were mixed with the same amount (20 nM, final concentration) of a 13-mer KRAS sequence target that contained either O6-MeG or O6-BnG at the middle position (O6-BnG_13mer target, Table S1). The color of the O6-MeG-supplemented suspension turned purple, whereas the color of the O6-BnG-supplemented suspension remained red (Figure S19). This observation suggested that the O6-BnG target did not induce any aggregation. To further confirm the accuracy of this simple visual observation, the solutions were gradually heated and A530 was monitored as a function of temperature. Characteristic sharp melting transitions were observed for O6-MeG whereas the A530 did not change for O6-BnG, further indicating the absence of aggregates and confirming the naked-eye inspection (Figure S20). These results indicate a lack of affinity of ExBIM for O6-BnG. Furthermore, the convenience of visually judging aggregation due to color changes associated with the SPR phenomenon suggests a future utility of the assay for screening diverse analogues with the goal of optimizing base surrogates for selection toward different DNA adducts.
Effect of target length
Hypothesizing that longer target sequences would result in lower discrimination, we investigated thermal stability differences for longer DNA duplexes containing ExBIM placed opposite either O6-MeG or G. Therefore, we synthesized longer target oligonucleotides (17 and 21-mer) for which the middle base of the target was the middle base of KRAS codon 13 (Figure 2) and contained in the middle position either O6-MeG or G (Table S1). The corresponding complementary probe oligonucleotides containing ExBIM in the middle position were also synthesized (Table S1) and the Tm of the DNA duplexes formed from the probe and target strands was determined. For the 17-mer DNA duplex the ΔTm was 1.6 °C (Table S5). For the 21-mer duplex the ΔTm was 1.3 °C (Table S5). It should be noted that the melting analysis experiments for the longer targets (17 and 21-mer) were performed with a lower DNA concentration (1 uM) than for the 13-mer targets (2.2 uM) to ensure that the A260 measurements were in the linear response range of the UV/Vis spectrophotometer.
To test the capability of ExBIM to distinguish between O6-MeG and G in longer DNA strands using nanoprobes, we prepared detection and discriminating ExBIM nanoprobes designed to hybridize with either the 17-mer or 21-mer KRAS codon 13 targets. Thus, the same type of AuNPs (d=20 nm) were functionalized with longer 5’-thiolated oligonucleotides (5’-thiol_2 and 5’-thiol_3, Table S1) to yield detection nanoprobes targeting the 17- and 21-mer target, respectively. Similarly, discriminating ExBIM nanoprobes were produced by functionalizing AuNPs with longer 3’-thiolated oligonucleotides (3’-thiol_ExBIM_2 and 3’-thiol_ExBIM_3, Table S1). The detection and discriminating ExBIM nanoprobes (1 nM each) were mixed with the same amount (20 nM final concentration) of either O6-MeG or G target, and the Tm values for the resulting aggregates were determined. For the 17-mer target, aggregates formed in the presence of O6-MeG target exhibited a higher Tm than the aggregates formed in the presence of G target (47.7 °C for O6-MeG vs 44.1 °C for G, ΔTm= 3.6 °C, Table S6). Similarly, for the 21- mer target, aggregates formed in the presence of O6-MeG exhibited a higher Tm than aggregates formed from the ExBIM nanoprobes and G target (62.0 °C for O6-MeG vs 60.2 °C for G, ΔTm= 1.8 °C, Table S6). The thermal stability of AuNP-DNA aggregates is influenced by the interparticle distance, which is modulated by a combination of the length of the interconnecting oligonucleotides and efficiency of hybridization. Hybridization efficiency, in turn, is influenced by the ratio of mismatched to matched bases. Thus, as hypothesized, discrimination diminished for the longest target. However, balancing the combination of interparticle distance and hybridization efficiency influences, discrimination for the 17-mer target was no worse than for the 13-mer. The 17-mer target was therefore preferred for further studies because there was no loss in discrimination.
Detection of O6-MeG in KRAS cod12
To address the potential influence of sequence context, ExBIM nanoprobes targeting KRAS codon 12 were also developed. Thus, 17-mer target oligonucleotides in which the middle base (either O6-MeG or G) was the middle base of KRAS codon 12 were synthesized (Figure 2, Table S7). Aggregates formed between KRAS codon 12-targeting nanoprobes in the presence of the O6-MeG target (20 nM final concentration) exhibited a higher Tm than those formed in the presence of the G target (ΔTm = 2.0 °C). These results indicate that ExBIM can also discriminate between O6-MeG and G in targets with different sequence context.
In conclusion, the design, synthesis, and incorporation of the novel nucleoside analogues ExBenzi and ExBIM into DNA hybridization probes are reported in this study. Both of the novel probes have a higher affinity for O6-MeG than for G in DNA duplexes containing sequences of cancer-related mutational hotspots. Hybridization probes containing ExBenzi and ExBIM were coupled to AuNPs. These were the basis of a method for the sequence-specific quantification of DNA strands containing O6-MeG in the presence of different competitor strands and human genomic DNA. To the best of our knowledge, this is the first sensing strategy for in-gene quantification of the highly mutagenic DNA adduct O6-MeG in a complex mixture. Studies to elucidate the molecular origin of discrimination of O6-MeG from G by ExBIM and ExBenzi on the basis of their orientation in DNA and stacking capacity are anticipated. Finally, integration of the nanoprobes developed in this study with hybrid capture techniques regularly used for targeted enrichment of nucleic acids prior to next generation sequencing and with well-established ultrasensitive AuNP-based DNA detection technologies used for SNP detection in unamplified genomic samples may enable monitoring the in-gene abundance of O6-MeG in biological samples.
Supplementary Material
Supporting Information. Detailed experimental procedures, synthesis, chemical characterization, additional figures and tables. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This work was supported ba the European Research Council (260341 and 680920) and the Swiss national Science Foundation (31003A_156280). We thank Larissa Roth for synthesizing oligonucleotides and for performing DNA duplex melting analyses. We thank Dr. Laura Wyss for helping with the synthesis of phosphoramidites and writing.
Funding Sources
This work was supported by the European Research Council (260341) and the Swiss National Science Foundation (31003A_156280).
Notes
The authors declare no competing financial interest.
References
- (1).Kyrtopoulos SA, Ampatzi P, Davaris P, Haritopoulos N, Golematis B. Carcinogenesis. 1990;11:431. doi: 10.1093/carcin/11.3.431. [DOI] [PubMed] [Google Scholar]
- (2).Kyrtopoulos SA. Mutat Res Fundam Mol Mech Mutagen. 1998;405:135. doi: 10.1016/s0027-5107(98)00130-4. [DOI] [PubMed] [Google Scholar]
- (3).Mirvish SS. Cancer Lett. 1995;93:17. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- (4).Rydberg B, Lindahl T. EMBO J. 1982;1:211. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Harrison KL, Jukes R, Cooper DP, Shuker DEG. Chem Res Toxicol. 1999;12:106. doi: 10.1021/tx980057n. [DOI] [PubMed] [Google Scholar]
- (6).Sedgwick B. Carcinogenesis. 1997;18:1561. doi: 10.1093/carcin/18.8.1561. [DOI] [PubMed] [Google Scholar]
- (7).Abbott PJ, Saffhill R. Biochim Biophys Acta Nucleic Acids Protein Synth. 1979;562:51. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- (8).Saffhill R, Hall JA. Chem-Biol Interact. 1985;56:363. doi: 10.1016/0009-2797(85)90017-1. [DOI] [PubMed] [Google Scholar]
- (9).Saffhill R, Margison GP, O'Connor PJ. Biochim Biophys Acta Rev Cancer. 1985;823:111. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- (10).Rossi SC, Conrad M, Voigt JM, Topal MD. Carcinogenesis. 1989;10:373. doi: 10.1093/carcin/10.2.373. [DOI] [PubMed] [Google Scholar]
- (11).O'Reilly SM, Newlands ES, Brampton M, Glaser MG, Rice-Edwards JM, Illingworth RD, Richards PG, Kennard C, Colquhoun IR, Lewis P, Stevens MFG. Eur J Cancer. 1993;29:940. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- (12).Mirsalis JC, Monforte JA, Winegar RA. Crit Rev Toxicol. 1994;24:255. doi: 10.3109/10408449409021608. [DOI] [PubMed] [Google Scholar]
- (13).Pegg AE. Cancer Res. 1990;50:6119. [PubMed] [Google Scholar]
- (14).Foiles PG, Miglietta LM, Akerkar SA, Everson RB, Hecht SS. Cancer Res. 1988;48:4184. [PubMed] [Google Scholar]
- (15).Kang H-i, Konishi C, Kuroki T, Huh N-h. Carcinogenesis. 1995;16:1277. doi: 10.1093/carcin/16.6.1277. [DOI] [PubMed] [Google Scholar]
- (16).Kang H, Konishi C, Kuroki T, Huh N. Environ Health Perspect. 1993;99:269. doi: 10.1289/ehp.9399269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kang H, Konishi C, Eberle G, Rajewsky MF, Kuroki T, Huh N. Cancer Res. 1992;52:5307. [PubMed] [Google Scholar]
- (18).Georgiadis P, Kaila S, Makedonopoulou P, Fthenou E, Chatzi L, Pletsa V, Kyrtopoulos SA. Cancer Epidemiol Biomarkers Prev. 2011;20:82. doi: 10.1158/1055-9965.EPI-10-0788. [DOI] [PubMed] [Google Scholar]
- (19).vanDelft JHM, Steenwinkel M, deGroot AJL, vanZeeland AA, EberleAdamkiewicz G, Rajewsky MF, Thomale J, Baan RA. Fundam Appl Toxicol. 1997;35:131. doi: 10.1006/faat.1996.2272. [DOI] [PubMed] [Google Scholar]
- (20).Brink A, Lutz U, Volkel W, Lutz WK. J Chromatogr B. 2006;830:255. doi: 10.1016/j.jchromb.2005.10.046. [DOI] [PubMed] [Google Scholar]
- (21).Pfeifer GP, Drouin R, Holmquist GP. Mutat Res Fundam Mol Mech Mutagen. 1993;288:39. doi: 10.1016/0027-5107(93)90206-u. [DOI] [PubMed] [Google Scholar]
- (22).Tornaletti S, Pfeifer G. In: Technol Detect DNA Damage Mutat. Pfeifer G, editor. Springer; US: 1996. p. 199. [Google Scholar]
- (23).Cloutier J-F, Drouin R, Castonguay A. Chem Res Toxicol. 1999;12:840. doi: 10.1021/tx990025f. [DOI] [PubMed] [Google Scholar]
- (24).Cloutier J-F, Drouin R, Weinfeld M, O’Connor TR, Castonguay A. J Mol Biol. 2001;313:539. doi: 10.1006/jmbi.2001.4997. [DOI] [PubMed] [Google Scholar]
- (25).Clark T, Spittle K, Turner S, Korlach J. Genome Integr. 2011;2:10. doi: 10.1186/2041-9414-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Riedl J, Ding Y, Fleming AM, Burrows CJ. Nat Commun. 2015:6. doi: 10.1038/ncomms9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Riedl J, Fleming AM, Burrows CJ. J Am Chem Soc. 2016;138:491. doi: 10.1021/jacs.5b11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Onizuka K, Nishioka T, Li Z, Jitsuzaki D, Taniguchi Y, Sasaki S. Chem Commun. 2012;48:3969. doi: 10.1039/c2cc17621a. [DOI] [PubMed] [Google Scholar]
- (29).Gong J, Sturla SJ. J Am Chem Soc. 2007;129:4882. doi: 10.1021/ja070688g. [DOI] [PubMed] [Google Scholar]
- (30).Gahlon HL, Sturla SJ. Chem Eur J. 2013;19:11062. doi: 10.1002/chem.201204593. [DOI] [PubMed] [Google Scholar]
- (31).Angelov T, Dahlmann HA, Sturla SJ. Bioorg Med Chem. 2013;21:6212. doi: 10.1016/j.bmc.2013.07.036. [DOI] [PubMed] [Google Scholar]
- (32).Wyss LA, Nilforoushan A, Eichenseher F, Suter U, Blatter N, Marx A, Sturla SJ. J Am Chem Soc. 2015;137:30. doi: 10.1021/ja5100542. [DOI] [PubMed] [Google Scholar]
- (33).Nilforoushan A, Furrer A, Wyss LA, van Loon B, Sturla SJ. J Am Chem Soc. 2015;137:4728. doi: 10.1021/ja512547g. [DOI] [PubMed] [Google Scholar]
- (34).Gahlon HL, Schweizer WB, Sturla SJ. J Am Chem Soc. 2013;135:6384. doi: 10.1021/ja311434s. [DOI] [PubMed] [Google Scholar]
- (35).Sturla SJ. Curr Opin Chem Biol. 2007;11:293. doi: 10.1016/j.cbpa.2007.05.021. [DOI] [PubMed] [Google Scholar]
- (36).Trantakis IA, Sturla SJ. Chem Commun. 2014;50:15517. doi: 10.1039/c4cc07184k. [DOI] [PubMed] [Google Scholar]
- (37).Guckian KM, Schweitzer BA, Ren RX, Sheils CJ, Tahmassebi DC, Kool ET. J Am Chem Soc. 2000;122:2213. doi: 10.1021/ja9934854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Guckian KM, Schweitzer BA, Ren RXF, Sheils CJ, Paris PL, Tahmassebi DC, Kool ET. J Am Chem Soc. 1996;118:8182. doi: 10.1021/ja961733f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lu H, He K, Kool ET. Angew Chem Int Ed. 2004;43:5834. doi: 10.1002/anie.200461036. [DOI] [PubMed] [Google Scholar]
- (40).Gao J, Liu H, Kool ET. J Am Chem Soc. 2004;126:11826. doi: 10.1021/ja048499a. [DOI] [PubMed] [Google Scholar]
- (41).Liu H, Gao J, Kool ET. J Org Chem. 2005;70:639. doi: 10.1021/jo048357z. [DOI] [PubMed] [Google Scholar]
- (42).Lee AHF, Kool ET. J Org Chem. 2005;70:132. doi: 10.1021/jo0483973. [DOI] [PubMed] [Google Scholar]
- (43).Kowal EA, Lad RR, Pallan PS, Dhummakupt E, Wawrzak Z, Egli M, Sturla SJ, Stone MP. Nucleic Acids Res. 2013;41(15):7568. doi: 10.1093/nar/gkt488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kidwai M, Saxena S, Mohan R. J Heterocycl Chem. 2005;42:703. [Google Scholar]
- (45).Herbert JM, Woodgate PD, Denny WA. J Med Chem. 1987;30:2081. doi: 10.1021/jm00394a025. [DOI] [PubMed] [Google Scholar]
- (46).Sachs F. Liebigs Ann Chem. 1909;365:53. [Google Scholar]
- (47).Hansen AS, Thalhammer A, El-Sagheer AH, Brown T, Schofield CJ. Bioorg Med Chem Lett. 2011;21:1181. doi: 10.1016/j.bmcl.2010.12.098. [DOI] [PubMed] [Google Scholar]
- (48).Lakshman MK, Zajc B. Nucleosides Nucleotides. 1996;15:1029. [Google Scholar]
- (49).Bleasdale C, Ellwood SB, Golding BT. J Chem Soc Perk T 1. 1990:803. [Google Scholar]
- (50).Arrington AK, Heinrich EL, Lee W, Duldulao M, Patel S, Sanchez J, Garcia-Aguilar J, Kim J. Int J Mol Sci. 2012;13:12153. doi: 10.3390/ijms131012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Leonard GA, Thomson J, Watson WP, Brown T. Proc Natl Acad Sci U S A. 1990;87:9573. doi: 10.1073/pnas.87.24.9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wong C-W, Tan N-W, Li BFL. J Mol Biol. 1992;228:1137. doi: 10.1016/0022-2836(92)90321-a. [DOI] [PubMed] [Google Scholar]
- (53).Ogawa AK, Wu Y, McMinn DL, Liu J, Schultz PG, Romesberg FE. J Am Chem Soc. 2000;122:3274. [Google Scholar]
- (54).Wu Y, Ogawa AK, Berger M, McMinn DL, Schultz PG, Romesberg FE. J Am Chem Soc. 2000;122:7621. doi: 10.1002/1521-3773(20000818)39:16<2940::aid-anie2940>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- (55).Brotschi C, Häberli A, Leumann CJ. Angew Chem Int Ed. 2001;40:3012. doi: 10.1002/1521-3773(20010817)40:16<3012::AID-ANIE3012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- (56).Kool ET, Morales JC, Guckian KM. Angew Chem Int Ed. 2000;39:990. doi: 10.1002/(sici)1521-3773(20000317)39:6<990::aid-anie990>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- (57).O'Neill BM, Ratto JE, Good KL, Tahmassebi DC, Helquist SA, Morales JC, Kool ET. J Org Chem. 2002;67:5869. doi: 10.1021/jo025884e. [DOI] [PubMed] [Google Scholar]
- (58).Guckian KM, Morales JC, Kool ET. J Org Chem. 1998;63:9652. doi: 10.1021/jo9805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Trantakis IA, Bolisetty S, Mezzenga R, Sturla SJ. Langmuir. 2013;29:10824. doi: 10.1021/la401211u. [DOI] [PubMed] [Google Scholar]
- (60).Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL. J Am Chem Soc. 1998;120:1959. [Google Scholar]
- (61).Liu X, Atwater M, Wang J, Huo Q. Colloids Surf B. 2007;58:3. doi: 10.1016/j.colsurfb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- (62).Bao YP, Huber M, Wei TF, Marla SS, Storhoff JJ, Muller UR. Nucleic Acids Res. 2005:33. doi: 10.1093/nar/gni017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Park S-J, Taton TA, Mirkin CA. Science. 2002;295:1503. doi: 10.1126/science.1067003. [DOI] [PubMed] [Google Scholar]
- (64).Taton TA, Mirkin CA, Letsinger RL. Science. 2000;289:1757. doi: 10.1126/science.289.5485.1757. [DOI] [PubMed] [Google Scholar]
- (65).Storhoff JJ, Lucas AD, Garimella V, Bao YP, Müller UR. Nat Biotechnol. 2004;22:883. doi: 10.1038/nbt977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Storhoff JJ, Marla SS, Bao P, Hagenow S, Mehta H, Lucas A, Garimella V, Patno T, Buckingham W, Cork W, Müller UR. Biosens Bioelectron. 2004;19:875. doi: 10.1016/j.bios.2003.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.