Abstract
Question
What is the impact of simulated historical tree litter removal on understorey plants and soil properties in a temperate deciduous forest? What is the role of seasonal timing of tree litter removal on understorey plants?
Location
Podyjí National Park, Czech Republic.
Methods
We conducted an experiment in a randomized complete block design of 45 plots (5 × 5 m). Each block (N = 15) consisted of one plot for each of the three treatments. Treatments consisted of (i) tree litter removal during spring, (ii) tree litter removal during autumn, or (iii) no litter removal as control treatment. These treatments were repeated for a duration of four years. In each plot we recorded the understorey plant species composition and collected soil samples prior to treatment (year 0) and in each subsequent year (years 1–4). Temporal trends in species richness were analysed using repeated measures ANOVAs. The impact of the treatments on vegetation composition over time was analysed using Principal Response Curves.
Results
Total species richness per plot significantly changed over time, but this was not related to treatment. Annual species richness increased significantly, but only for the autumn treatment. Annual species also showed the highest inter-annual variation. Endangered species were not affected. When compared to the control treatment, the effect of autumn raking on species composition was stronger than the effect of spring raking. Although the amount of removed nutrients substantially exceeded ambient nitrogen input, no changes in soil conditions were detected.
Conclusions
The season in which tree litter removal took place had a small but significant impact on the understorey vegetation, in particular affecting the germination and establishment of annual species. The large inter-annual variation in species richness calls for a long-term field experiment. The removal of nutrients via litter raking greatly exceeds atmospheric nutrient deposition, warranting a further investigation of litter raking as a potential tool for forest conservation.
Keywords: Central Europe, Conservation management, Litter raking, Permanent plots, Species diversity, Temperate woodland, Traditional management, Vascular plants
Introduction
Litter raking is a form of land use whereby large quantities of dead leaf biomass are collected from the forest ground using rakes, resulting in the removal of large quantities of nutrients from the forest and a mechanical disturbance of the top soil (Glatzel 1991; Gimmi et al. 2013). Subsistence litter raking was once a widespread activity in the woodlands of central Europe (Ebermayer 1876; Bürgi 1999). Leaf litter was primarily applied as bedding for farm animals and, after mixing with animal excrements, used as a fertiliser on arable land (Glatzel 1991). Already by the 1850s environmental consequences of litter raking such as severe nutrient depletion of forest soil and adverse impacts on soil conditions were widely known (Ebermayer 1876). At the time these impacts were deemed undesired as negatively affecting the production of main forest commodities such as fuel and timber. The practice of litter raking gradually diminished during the 19th century until it was largely abandoned by the beginning of the 20th century (Bürgi 1999; Glatzel 1999). To this day, litter raking persists in a few areas of southeast Europe, but is gradually disappearing (Čarni et al. 2007; Šilc et al. 2008). Since subsistence litter raking was largely abandoned more than a century ago, little is known about the ecological impact of this type of historical land use on the forest understorey vegetation.
The litter layer has many functions in a woodland ecosystem (Facelli & Pickett 1991). For example, it forms a mechanical barrier buffering temperature and humidity fluctuations between the outer environment and the soil. In addition, the litter layer maintains a microclimate favouring the decomposition and mineralisation of dead organic matter (Xu et al. 2013). Due to litter removal, fewer nutrients enter the decomposition cycle. Indeed, long-term studies show a strong reduction of, amongst others, nitrogen, phosphorus and potassium (Sayer 2005). Therefore the soil environment gradually becomes less favourable for decomposition processes (Facelli & Pickett 1991). Mechanical soil disturbance increases the nitrogen mineralisation rate (Tamm 1991). Litter raking disturbs the underlying soil, albeit unintentionally, and could therefore result in an increase of the nitrogen mineralisation rate. In addition, erosion and compaction increase due to the direct impact of raindrops on soil (Benkobi et al. 1993; Li et al. 2014). As a result, abiotic conditions and biotic linkages between the soil and understorey may change considerably.
Vascular plants are a neglected subject of litter removal experiments. The most-detailed long-term field experiment (16 years), conducted in a mixed oak-pine woodland in Poland, showed that a decrease in soil nutrient availability resulted in a change in species composition (Dzwonko & Gawroński 2002a; Dzwonko & Gawroński 2002b). In this experiment litter removal prevented a further spread of the competitive sedge species Carex brizoides, while conserving threatened acidophilous woodland species (Dzwonko & Gawroński 2002a). Litter removal hampered natural succession: plots subjected to litter removal remained unchanged while control plots became more eutrophic. Increased nitrogen deposition has become one of the major threats to global biodiversity (Bobbink et al. 1998; Bobbink et al. 2010). The potential of litter removal to counteract this effect gave rise to ideas to use litter raking as a tool for forest ecosystem restoration and conservation of declining ecosystems and species (Prietzel & Kaiser 2005; Bürgi & Gimmi 2007). Litter raking also increases the colonization rate of vascular plants and bryophytes. For example, a 3-year experiment on litter removal from treefall pits in a deciduous forest in central New York showed that species richness increased due to increased germination and seedling establishment (Beatty & Sholes 1988). In addition, a 6-month experiment in a Swedish deciduous forest fragment reported an inhibitory effect of litter on seedling recruitment (Eriksson 1995). However, Dzwonko & Gawroński (2002b) observed large inter-annual variability in seedling recruitment. The authors attributed this variability to inter-annual differences in climatic conditions, especially in late winter and early spring. These results suggest that the seasonality of litter raking, whether this takes place before or after the winter season, is likely to have an effect on vascular plants.
Timing of litter raking has the potential to affect soil chemistry and species composition via multiple pathways. Autumn raking removes litter in an early decomposition phase and, thus, removes carbon, nitrogen and other nutrients from the system that would otherwise gradually become available. On the other hand, if not removed until spring, organic material is available for decomposition during winter. Mainly polyphenols and soluble carbohydrates are used by decomposers in the first months, unlike other litter constituents like lignin and holocellulose (Bocock 1964). However, another mechanism involves enrichment of the forest floor by nutrients from animal excrements during winter. If litter is removed in autumn, these nutrients are added directly to the soil and are readily available during the growing season. Under a regime of spring raking, however, animal excrements — mixed in with litter during the winter period — are removed from the system (Bocock 1964; Osono & Takeda 2001). Therefore it is not clear if more nutrients are removed when litter is removed in autumn or in spring, and during which season leaf litter removal has the strongest impact on soil conditions. Due to the ability of soil to buffer chemistry fluctuations, plants may fail to respond to initial changes in nutrient conditions (Sayer 2005). Mechanical soil disturbance and physical absence of the litter layer, on the other hand, are likely to have an immediate effect on species composition.
At the start of the growing season, a litter-free soil will warm up faster and receive more light, stimulating seed germination and advancing the start of the growing season. Easily dispersing annual species are well adapted to rapidly colonise such disturbed soils (Grime 2001; Wilson & Tilman 2002). The short life cycle of annuals enables the colonisation of regularly disturbed plots by litter raking. However, this can make seedlings vulnerable to early-spring frost spells (Facelli & Pickett 1991). It is therefore likely that some species will perform better when litter is removed in autumn while others will thrive if the forest floor remains covered until spring.
The response of a forest ecosystem to litter raking timing can have significant implications for the interpretation of historical practice. Although historical sources fail to mention during which season litter raking occurred, it is widely assumed that litter raking primarily took place during autumn, because bedding material for cattle was needed in stables during winter (Gimmi et al. 2007). Moreover, in areas where tree litter is still used, litter is collected during the autumn season (U. Šilc, Institute of Biology ZRC SAZU, Slovenia, pers. comm.). Therefore, field litter removal experiments generally study the impact of litter raking in autumn only (e.g., Dzwonko and Gawrónski 2002a, b).
In this study we focus on the impact of repeated litter raking in a temperate forest in central Europe. We were specifically interested if the seasonal timing of litter raking affected vegetation diversity and composition and if it changed soil conditions. We used a field experiment simulating the historical removal of leaf litter biomass during autumn and spring. We tested the hypotheses that: (1) repeated litter removal has an impact on understorey species richness and composition, specifically annual species, perennial species and endangered species, (2) the season in which litter removal takes place matters: litter removal in autumn has a different impact on the ecosystem than litter removal in spring. In addition, we aimed to establish a soil chemistry baseline in anticipation for long-term research on the impact of repeated litter removal. We do not expect any short-term effects of litter removal on soil conditions. Finally, we discuss our results in the context of litter raking as a potential conservation tool.
Methods
Study area
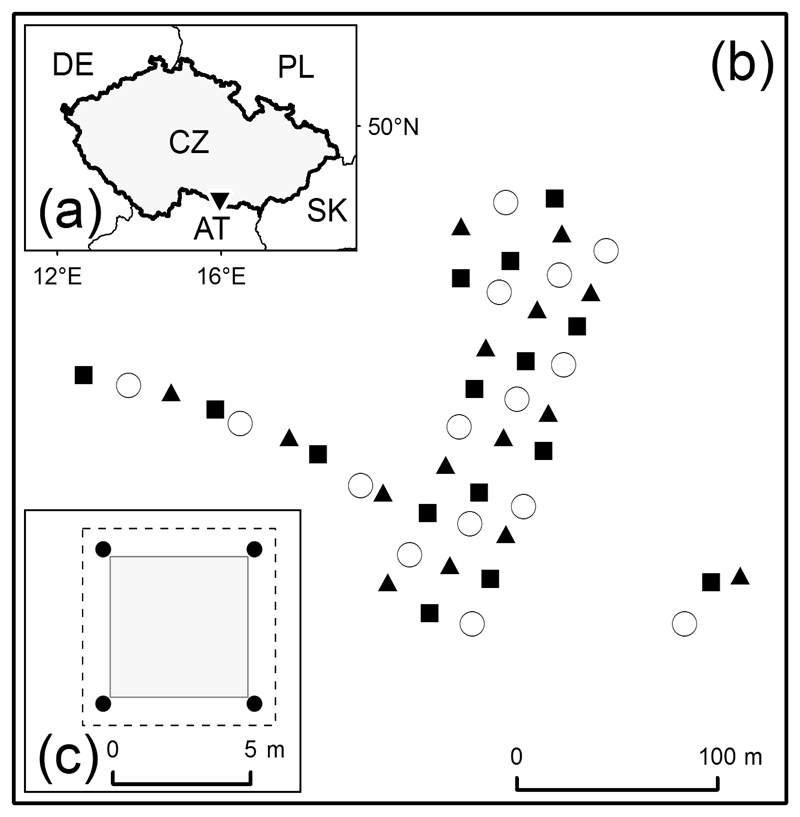
We selected a 4-ha forest stand in the Podyjí National Park, Czech Republic (Fig. 1a; 48°48' N, 15°57' E). Climate in this region is subcontinental, with an average temperature of -1.5 °C in January and 18.5 °C in July. Average precipitation is 313 mm during the growing season (April–September), and 163 mm outside the growing season (October–March). Average annual snow cover duration is 45 days (Tolasz et al. 2007). The selected forest stand was relatively homogeneous in terms of environmental conditions, vegetation structure and composition. Soil type was oligotrophic cambisol with a pH (H2O) of 4.0–5.5. The dominating bedrock was granite. The forest stand relief was homogenous against an insignificant slope with an elevation range of 365–375 m a.s.l. This area was used as pasture until the 19th century, after which it was converted to woodland. At present, this forest stand is dominated by 10–12 m high Quercus petraea agg. with occasional Pinus sylvestris and Carpinus betulus.
Fig. 1.
(a) Location of the study area in the south of the Czech Republic (▼), (b) Overview of the distribution of plots in a randomized complete block design with symbols depicting the respective treatments: litter removal during autumn (■), during spring (▲), and no litter removal as control (○). Each block (N = 15) consisted of one plot per treatment, and (c) schematic overview of the treatment and sampling protocol of each 5 × 5 m plot (grey square). Litter removal treatment also took place in a 1-m buffer around plots (dashed outline). Soil samples were taken from four places within the buffer zone (●).
Experimental design
A total of 45 plots of 5 × 5 m were selected in a randomized complete block design. Each block (N = 15) consisted of one plot for each of the three treatments. To ensure that an experimental treatment affected the respective plot only, a minimum distance between plots was set at 6.0 m (Fig. 1b). Experimental treatment was applied as follows: (i) autumn litter raking annually in November, (ii) spring litter raking annually in March, and (iii) no litter raking as control. Our observations indicated that the area was not subjected to strong winds, suggesting that fencing or other means to prevent post-treatment litter accumulation was unnecessary. Litter raking consisted of collecting and weighing all leaf litter from a plot using a standard leaf rake (Fiskars, Large Leaf Rake, Helsinki, Finland), including the removal of litter from the adjacent 1.0-m buffer zone. A sample of the collected biomass from each plot was air-dried at 60 °C, weighted and the water ratio subsequently used to calculate the total amount of dry litter removed per plot.
Data collection
To determine the pre-treatment state of vegetation and soil, all plots were sampled in July or August 2010 (year 0). To quantify how treatment affected species composition and diversity over time, abundance of all vascular plant species was assessed for each plot in each of the four consecutive growing seasons of 2011–2014 (year 1–4). All herb layer species were recorded and their cover-abundance estimated using the nine-level Braun-Blanquet scale (Dengler et al. 2008).
Soil samples were taken at the same time as the vegetation recording. In each plot we collected four subsamples with a 4-cm diameter core and to a maximum depth of 15 cm (Fig. 1c). The organic layer was removed from the core samples. Organic-mineral (horizon A) and mineral (horizon B) layers were separated while sampling and analysed separately. Subsamples were compiled within plot and dried at room temperature. Dry samples were sieved through a 2-mm mesh prior analysis. For analysis of total C and N, the 2-mm fraction was sieved to a 0.1-mm fraction and measured using a CHN Carlo Erba NC 2500 analyzer. Available phosphorus was determined colorimetrically using a Unicam UV-400 spectrometer at 630 nm in mixed samples prepared with a 1 M solution of sodium bicarbonate with a pH of 8.5 (Olsen 1982). Contents of cations were determined in Mehlich II extraction of soil prepared by shaking and filtering. Ca2+ and Mg2+ contents were determined with atomic absorption spectroscopy, K+ content with emission absorption spectroscopy. An AAS 9200X Unicam spectrometer was used for both types of analyses. Sulphuric acid and lanthanum chloride were added to the extraction to eliminate potential influence of sulphides and metals. Soil acidity (pH) was measured using a glass electrode from a suspension of 20 g dried soil and 50 ml distilled water. Soil analyses were conducted prior the litter raking treatment (year 0) and for the first two years of the experiment (year 1 and 2) to establish a baseline of soil nutrient composition.
Data analysis
To test if species richness was affected by the season in which litter removal took place, repeated measures ANOVAs were fitted using time, i.e. the year in which plots were sampled, as the within-subject variable and treatment as the between-subjects factor. For all models we initially included block of plots as random variable. This random variable was subsequently omitted from further analysis because it never contributed significantly to overall variation. Four categories of response variable were analysed: (i) all vascular plant species, (ii) annual species only, (iii) perennial species only, and (iv) red-listed species as endangered plants (Klotz & Kühn 2002; Grulich 2012). Annual species were not a characteristic group of species for this forest type (Chytrý & Tichý 2003), but this life form sporadically occurs here nevertheless. The number of Red List plant species was used to evaluate the conservation potential of litter raking. Each response variable displayed a normal distribution of residuals., We adopted a Huynh-Feldt correction for ε > 0.75 and a Greenhouse-Geisser correction for ε < 0.75 where Mauchly's test of sphericity showed that the assumption of sphericity was not met (Quinn & Keough 2002). We used Tukey HSD post-hoc tests to assess the effect of each particular treatment. The impact of litter raking on soil properties was analysed using the same method. All repeated measures ANOVAs were fitted using SPSS 20.0 (SPSS, Chicago, IL, USA). Since species richness can vary considerably between years due to variation in climatic conditions, we also analysed the relationship between species richness and regional weather conditions. We calculated generalized least squares models (GLS) of the relationship between mean species richness per plot as response variable against the environmental variables precipitation and temperature for each of the three treatments (autumn litter raking, spring litter raking, and control). These models are based on a restricted maximum likelihood estimation using a first-order autoregressive correlation structure to adjust for temporal autocorrelation (Piepho et al. 2004). We acquired regional precipitation and temperature measurements from the Czech Hydrometeorological Institute (CHMI 2014). Cumulative precipitation and mean monthly temperature were used from the period December–March prior to plot sampling.
To assess the impact of litter raking over time on species composition and response of selected species, we used Principal Response Curves (PRC; Van den Brink & Ter Braak 1999). This method is a special case of a Redundancy Analysis in which treatments are compared against a control while time is used as a covariate. Therefore, variability between years caused by an external variable such as weather fluctuations is accounted for. Species cover data were standardised using Wisconsin double standardisation. The significance of treatments over time was compared using a permutation test for the first constrained eigenvalue with 9999 runs. The PRCs cover standardisation and statistical tests were calculated using the vegan package (Oksanen et al. 2014; functions prc and permutest) in an R environment (version 2.14.1; R Development Core Team, 2014).
Results
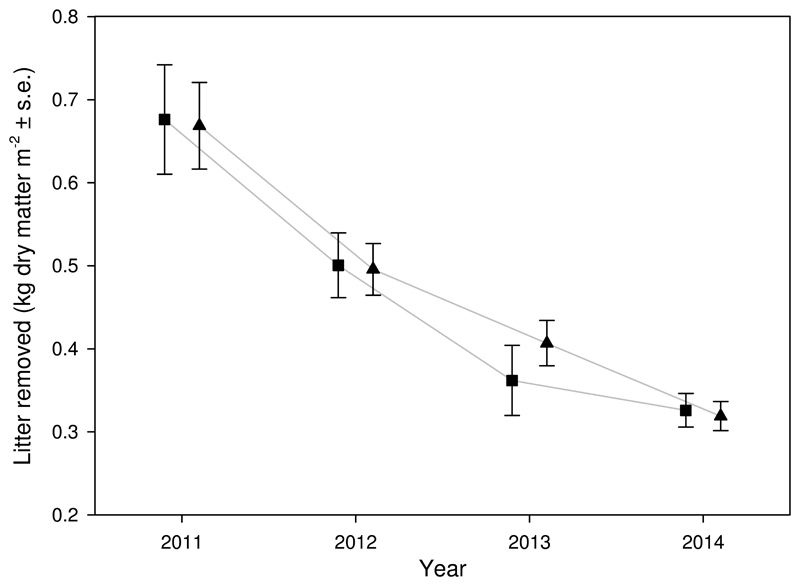
The biomass removed during the experimental treatments followed a negative exponential relationship over time (Fig. 2). Removed amounts of litter were similar for spring and autumn treatment.
Fig. 2.
Error plot illustrating the average amount of litter removed (kg dry matter m-2 ± s.e.) per year. Litter removal treatment consisted of annually repeated litter removal during autumn (■) or spring (▲) season prior to the vegetation recording.
Species richness
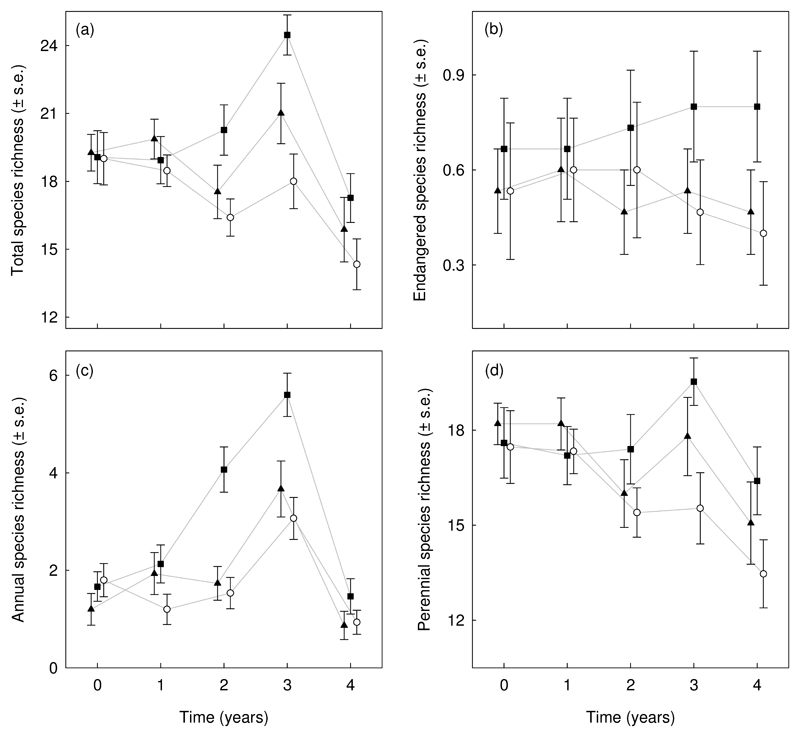
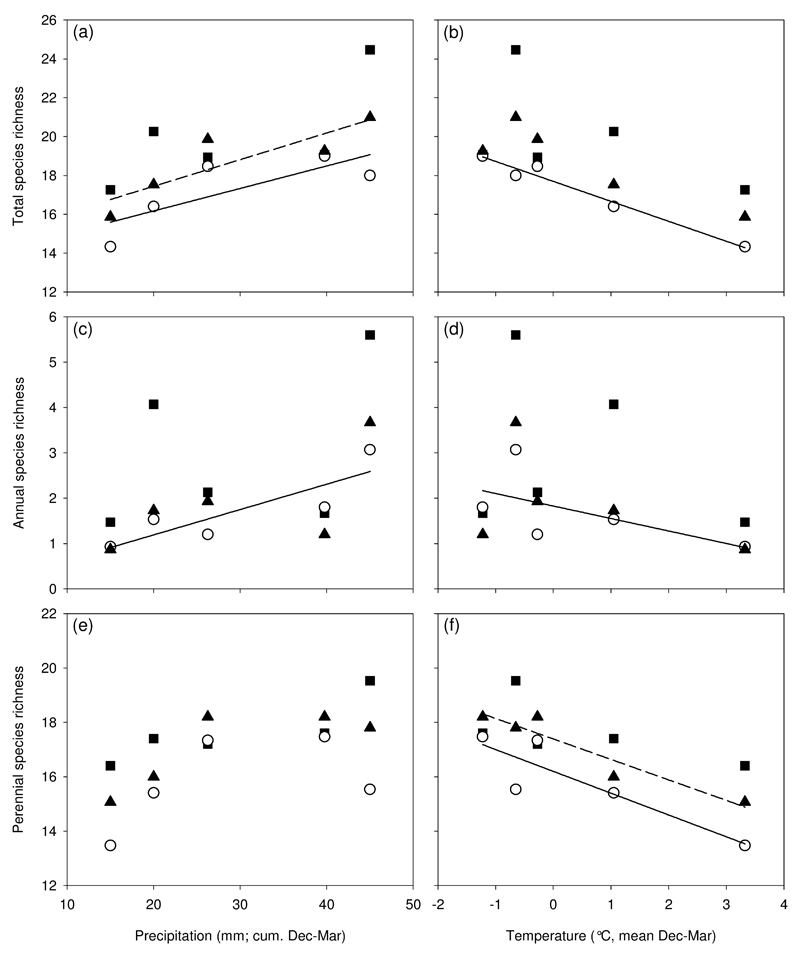
Plot species richness varied considerably between years but was also influenced by the season in which litter raking treatment took place (Fig. 3). The effects of year and the season in which litter raking treatment took place were significant for all examined species groups, except for the endangered species (Table 1). Autumn litter raking resulted in higher species richness for annual species only. Richness of annual species varied strongly between years, with a peak in year 3. Richness of perennial species showed a similar pattern, but had considerably lower variation. Endangered species showed stable species richness and were least prone to inter-annual changes. We recorded higher species richness in years characterised by high precipitation and low temperatures. Litter raking treatment resulted in higher variability among years with different climatic conditions, while the control treatment showed significant relationships between species richness and the two weather variables (Table 2; Fig. 4).
Fig. 3.
Error plots illustrating how species richness (mean ± s.e.) in 5 × 5 m plots responded to the various repeated litter removal treatments in time for total species richness (a), endangered species (b), annual species (c) and perennial species (d). Litter removal treatments consisted of annually repeated litter removal during autumn (■), spring (▲) and without litter removal as control (○).
Table 1.
Results of repeated measures ANOVAs for plot-level total species richness, number annual species, number of perennial species and number of endangered species. The effect of time and the interaction between treatment and time were tested (for all models: df = 4). P values of Tukey’s post-hoc test are shown for spring and autumn treatments, respectively, against control.
| Parameter | Variable | F | P | Spring | Autumn |
|---|---|---|---|---|---|
| All species | Time | 30.038 | <0.001 | ||
| Time*Treatment | 5.046 | <0.001 | 0.51 | 0.102 | |
| Annual species | Time | 54.548 | <0.001 | ||
| Time*Treatment | 5.803 | <0.001 | 0.906 | 0.009 | |
| Perennial species | Time | 14.368 | <0.001 | ||
| Time*Treatment | 2.912 | 0.005 | 0.598 | 0.334 | |
| Endangered species | Time | 0.291 | 0.884 | ||
| Time*Treatment | 0.995 | 0.442 |
Table 2.
Generalized least squares model details of the relationship between mean species richness per plot as response variable against the environmental variables precipitation and temperature for each of the three treatments (autumn litter raking, spring litter raking, and control). The response variable was modelled for three species groups: total species richness, number of annual species only, and number of perennial species only. Models with a P-value < 0.05 are indicated in bold (see also Fig. 4).
| Species group | Treatment | Precipitation | Temperature | ||
|---|---|---|---|---|---|
| t | P | t | P | ||
| All species | Autumn | 2.795 | 0.068 | -2.366 | 0.099 |
| Control | 7.806 | 0.004 | -11.431 | 0.001 | |
| Spring | 3.778 | 0.032 | -1.215 | 0.311 | |
| Annuals | Autumn | 1.522 | 0.225 | -1.741 | 0.18 |
| Control | 5.238 | 0.014 | -3.456 | 0.041 | |
| Spring | 2.25 | 0.11 | -3.03 | 0.056 | |
| Perennials | Autumn | 2.943 | 0.06 | -1.82 | 0.166 |
| Control | 2.853 | 0.065 | -4.701 | 0.018 | |
| Spring | 2.769 | 0.07 | -3.72 | 0.034 | |
Fig. 4.
Scatterplots depicting the relationship between mean species richness per plot and per treatment (autumn litter raking (■), spring litter raking (▲), and control (○)) against the environmental variables precipitation (a, c, e) and temperature (b, d, f). Mean plot species richness was categorized into three groups: total species richness (a, b), number of annual species only (c, d), and number of perennial species only (e, f). To illustrate the general relationship between species richness and the environment variables, generalized least squares models with a P-value < 0.05 are indicated with a solid line (see also Table 2). Solid lines indicate significant models for control treatment, whereas dashed lines indicate significant models for the spring treatments.
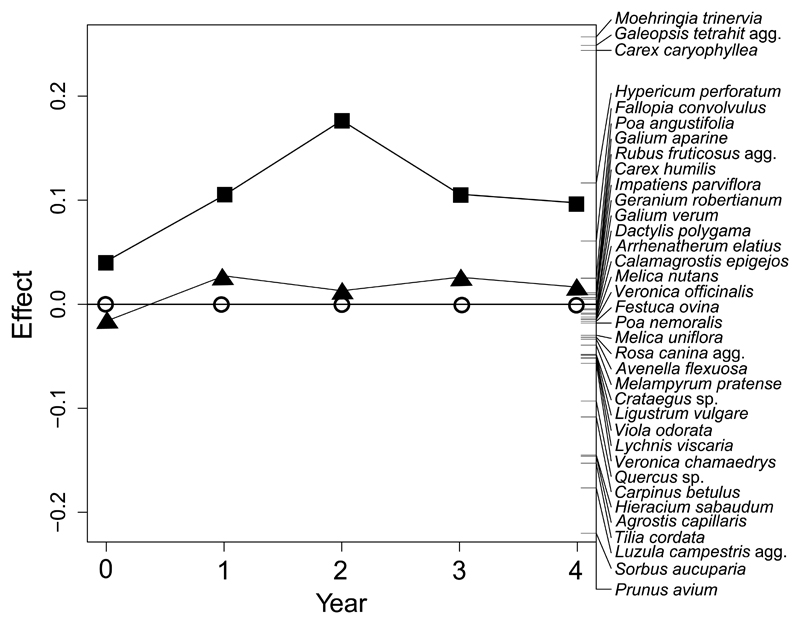
Species composition
The season in which litter raking took place did not affect species composition (Fig. 5; permutation test; pseudo-F = 4.34, P = 0.294). Variation in species composition was high for both treatments, especially for autumn litter raking, but neither treatment resulted in a change in species composition. Many species were positively associated with litter raking, such as annual species indicative of disturbed grounds (Moehringia trinervia, Galeopsis tetrahit, Fallopia convolvulus) or of clearings (Hypericum perforatum, Poa angustifolia). Species associated with the control treatment were mostly graminoids (Agrostis capillaris, Melica nutans) or woodland perennials (Hieracium sabaudum, Viola odorata).
Fig. 5.
Principal Response Curves (PRC) showing treatment effect on species composition across time with year 0 indicating the pre-treatment situation. Spring litter raking treatment (▲) and autumn litter raking treatment (■) are compared against control (○). Species with the highest increase or decrease in response to treatment are displayed at the top and bottom of the right-hand side axis.
Soil analysis
Most soil elements (Ca, Mg, N, P, K, Na) including pH showed a high variability among years. However, none of these soil element concentrations changed over time, nor were they affected by the season in which litter raking took place (all P-values > 0.05; Appendix S1)
Discussion
Our field experiment showed that repeated litter raking and the seasonal timing of raking both affected species richness and overall species composition, while there was no short-term effect on soil conditions. A delay in the response in soil nutrient composition is in agreement with most litter removal experiments (Sayer 2005). The exponential decrease in removed litter was likely the result of a proportion of the dead organic matter being removal in concurrence with litter removal. In the initial four years of the litter raking experiment, the impact on species richness and composition could not be attributed to changed content of chemical elements. Other mechanisms therefore determine these changes.
Species performance
Autumn litter raking, unlike spring litter raking, resulted in an increase of annual species richness. The life strategy of annual species likely played a key role in their response to litter raking. Annuals are mainly ruderals — which have a short life cycle and invest a large amount of energy in seed production (Grime 2001) — whereas most indigenous oak forest species are perennials. Litter raking created conditions suitable for the seed germination of ruderal species, notably by increased fluctuation of temperature, and increased nitrogen and light availability to seeds in the organic matter layer (Vincent & Roberts 1977). Moreover, annuals were able to take advantage of the absence of competition on a litter-free soil surface (Monk & Gabrielson 1985). Seed germination of some species (e.g., Moehringia trinervia, Geranium robertianum or Fallopia convolvulus) can be initiated by mechanical disturbances (Baskin & Baskin 2014). Since soil was rich in coarse-grained mineral particles, it is possible that seeds were scarified during raking; breaking the outer seed coat, initiating water intake and starting germination (Baskin & Baskin 2014). In contrast to our results, such increase in annual species richness was not observed in a comparable experiment conducted in Poland (Dzwonko & Gawroński 2002a). Many ruderal species are nutrient demanding (Grime 2001). In our study area, the nitrogen content was more than three times higher and the phosphorus content more than five times higher than the values observed by Dzwonko & Gawroński (2002a). Therefore, a difference in soil nutrient composition between the study areas could be responsible for this discrepancy. The strong inter-annual variation in annual species richness indicates that other factors are involved too, such as variation in temperature and precipitation (Dzwonko & Gawroński 2002b). Year 3, for example, was characterized by high precipitation, low temperature and a high species richness, while in the year 4 the opposite was observed. If winter temperatures are high, and seeds are not cold stratified, the probability of spring germination decreases (Baskin & Baskin 2014). Indeed, we found significant relationships between species richness and precipitation, and temperatures in late winter and early spring (Fig. 4; Table 2; Appendix S2). We are, however, cautious to draw conclusions from these relationships due to the short time-span over which these climate data were collected.
Our results indicate that seasonality of litter raking matters. These results are in accordance with a review on the effect of litter on plants by Xiong & Nilsson (1999). The authors concluded that litter removal has a stronger effect on seedling germination than on establishment. Therefore mechanical disturbance during the early onset of the growing season has a stronger impact than disturbance after the growing season. This finding is further supported by experiments carried out in grasslands and crop fields reporting higher establishment rates when the soil was mechanically disturbed during autumn (Calado et al. 2008; Hellström et al. 2009). Moreover, if litter is removed by the end of the growing season, weather extremes affecting the topsoil during winter are more likely to break seed dormancy and stimulate germination (Baskin & Baskin 2014). This mechanism could explain our observation of increased abundance of Moehringia trinervia, Galeopsis tetrahit, Hypericum perforatum and Poa angustifolia (Fig. 5). Lower richness of annual plants in spring raking plots can also be attributed to leaching of seedling emergence inhibiting chemicals from leaves during the winter period (Koorem et al. 2011).
The impact of litter raking on endangered species is particularly important if litter raking were to be considered as a conservation measure. Endangered species richness was not affected by litter raking and showed little inter-annual variation. However, the small contribution of this species group to overall species composition calls for a careful interpretation of results (Appendix S3). If nutrients are consistently removed from the system, nutrient demanding species will decrease, providing space for species such as endangered species of nutrient-poor soils (Gabrielová et al. 2013). Therefore, we cannot rule out the possibility that litter raking may have a long-term effect on endangered species, but this needs to be studied over a longer period of time.
Soil properties
Soil buffer capacity is crucial for an ecosystem’s ability to maintain nutrient and cation availability. The impact of litter raking therefore strongly depends on bedrock type and soil conditions. Basic substrates have a higher buffer of basic cations, as compared to acidic substrates, and are less susceptible to cation loss. Similarly, nutrient-poor soils are rapidly depleted from nitrogen and phosphorus when subjected to litter raking (Hofmeister et al. 2008; Leff et al. 2012; Ito et al. 2014). However, most of previous studies showed no immediate impact of litter raking on soil chemistry (Sayer 2005). Our baseline data, on the first years of soil nutrient composition following litter raking, confirmed no short-term effect on soil conditions. This was expected as cation and nutrient content in the topsoil were relatively high. However, once the storage buffer is depleted, a fast decrease can be expected (Sayer 2005). Nitrogen depletion can therefore be expected in the long term, but the timing thereof depends on the soil’s nutrient reserves and amount of decomposed organic matter.
Conservation implications
For centuries, tree litter removal was a wide-spread type of forest land use, affecting soil nutrients and tree species composition (Hüttl & Schaaf 1995; Sayer 2005; Gimmi & Wohlgemuth 2010). Historical litter raking was predominantly practiced in the pasture woodlands of mountainous regions (Gimmi & Bürgi 2007; Gimmi et al. 2013). These woodlands have, therefore, been impoverished of nutrients for centuries (Bergmeier et al. 2010). However, with the cessation of litter raking in the 19th century and increasing atmospheric nitrogen deposition in the 20th century, many oligotrophic species became endangered, especially in high-pollution areas such as the Czech Republic (Kopáček et al. 2001; Hédl et al. 2010).
The amount of nutrients that we removed during the first years of treatment exceeded annual atmospheric nutrient deposition by more than 17-fold. This is a rough estimate based on nitrogen content in litter of Quercus petraea (0.94 %, Carlisle et al. 1967) and nitrogen deposition in the Czech Republic (CHMI 2010). A regime of sporadic litter removal would therefore already compensate for the impact of atmospheric nutrient deposition, as documented from an experimental study in a mixed pine-oak wood (Dzwonko & Gawroński 2002a). Such practice might be particularly beneficial to biodiversity in oligotrophic oak woodlands, if it were implemented in a present-day situation. Due to high labour costs, however, it would be very expensive to implement as a practical conservation means. An economic use of this litter removal may at least partially compensate for such costs. For example, if an adequate logistic management system is in place, removed litter could serve as a source of biofuel or landscaping mulch (Loqué et al. 2011; Dickens et al. 2012). The potential of litter raking for conservation purposes needs to be investigated further.
Conclusion
Repeated litter raking had a significant effect on species richness of herbaceous vegetation. Seasonality of litter raking affected which species benefitted most from altered environmental conditions: especially annual species increased under a regime of autumn litter raking. Soil nutrient composition, on the other hand, remained unaffected, suggesting the presence of a significant soil buffer. A continuation of this litter raking experiment is warranted to establish the long-term response of plants to persistent soil impoverishment in more detail.
Supplementary Material
Soil properties and the degree of change in time.
Winter precipitation and temperature for each of the sampling years.
List of annual and perennial species with the indication of endangered species.
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013) / ERC Grant agreement no 278065, research grant IAA600050812 and long-term research project RVO 67985939, both from the Czech Academy of Sciences. We are grateful to Borja Jiménez-Alfaro, Jan Roleček and Kateřina Šumberová for fruitful discussions on seed ecology, and to Tom R. Bishop for stylistic suggestions.
References
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press; San Diego, CA: 2014. [Google Scholar]
- Beatty SW, Sholes ODV. Leaf litter effect on plant species composition of deciduous forest treefall pits. Canadian Journal of Forest Research. 1988;18:553–559. [Google Scholar]
- Benkobi L, Trlica MJ, Smith JL. Soil loss as affected by different combinations of surface litter and rock. Journal of Environmental Quality. 1993;22:657–661. [Google Scholar]
- Bergmeier E, Petermann J, Schröder E. Geobotanical survey of wood-pasture habitats in Europe: diversity, threats and conservation. Biodiversity and Conservation. 2010;19:2995–3014. [Google Scholar]
- Bobbink R, Hicks K, Galloway JN, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hornung M, Roelofs JGM. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. Journal of Ecology. 1998;86:717–738. [Google Scholar]
- Bocock K. Changes in the amounts of dry matter, nitrogen, carbon and energy in decomposing woodland leaf litter in relation to the activities of the soil fauna. Journal of Ecology. 1964;52:273–284. [Google Scholar]
- Bürgi M. A case study of forest change in the Swiss lowlands. Landscape Ecology. 1999;14:567–575. [Google Scholar]
- Bürgi M, Gimmi U. Three objectives of historical ecology: the case of litter collecting in Central European forests. Landscape Ecology. 2007;22:77–87. [Google Scholar]
- Calado J, Basch G, de Carvalho M. Appearance of spontaneous plants from disturbed and undisturbed soil under mediterranean conditions. Revista de Ciências Agrárias. 2008;31:68–78. [Google Scholar]
- Carlisle A, Brown A, White E. The nutrient content of tree stem flow and ground flora litter and leachates in a sessile oak (Quercus petraea) woodland. Journal of Ecology. 1967;55:615–627. [Google Scholar]
- CHMI. Odhad celkové roční depozice uvedených složek na plochu České republiky (78 841 km2) v tunách, 2010. [accessed 29 December 2014];2010 URL: http://portal.chmi.cz/
- CHMI. ČHMÚ Historická data – meteorologie a klimatologie. [accessed 14 October 2014];2014 URL: http://www.chmi.cz/
- Chytrý M, Tichý L. Diagnostic, constant and dominant species of vegetation classes and alliances of the Czech Republic: a statistical revision. Folia facultatis scientiarum naturalium universitatis Masarykianae Brunensis. 2003;108:1–231. [Google Scholar]
- Čarni A, Košir P, Marinšek A, Šilc U, Zelnik I. Changes in structure, floristic composition and chemical soil properties in a succession of birch forests. Periodicum Biologorum. 2007;109:13–20. [Google Scholar]
- Dengler J, Chytrý M, Ewald J. Phytosociology. In: Jørgensen SE, Fath BD, editors. Encyclopedia of Ecology. Elsevier; Oxford, UK: 2008. pp. 2767–2779. [Google Scholar]
- Dickens ED, Moorhead DJ, Morris LA. Pine straw – an economically important forest product in Georgia. University of Georgia; Athens, GA USA: 2012. [Google Scholar]
- Dzwonko Z, Gawroński S. Effect of litter removal on species richness and acidification of a mixed oak-pine woodland. Biological Conservation. 2002a;106:389–398. [Google Scholar]
- Dzwonko Z, Gawroński S. Influence of litter and weather on seedling recruitment in a mixed oak-pine woodland. Annals of Botany. 2002b;90:245–251. doi: 10.1093/aob/mcf178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebermayer E. Die gesammte Lehre der Waldstreu mit Rücksicht auf die chemische Statik des Waldbaues. Springer; Berlin, Germany: 1876. [Google Scholar]
- Eriksson O. Seedling recruitment in deciduous forest herbs: the effects of litter, soil chemistry and seed bank. Flora. 1995;190:65–70. [Google Scholar]
- Facelli JM, Pickett S. Plant litter: its dynamics and effects on plant community structure. Botanical Review. 1991;57:1–32. [Google Scholar]
- Gabrielová J, Münzbergová Z, Tackenberg O, Chrtek J. Can we distinguish plant species that are rare and endangered from other plants using their biological traits? Folia Geobotanica. 2013;48:449–466. [Google Scholar]
- Gimmi U, Bürgi M. Using oral history and forest management plans to reconstruct traditional non-timber forest uses in the Swiss Rhone Valley (Valais) since the late nineteenth century. Environment and History. 2007;13:211–246. [Google Scholar]
- Gimmi U, Bürgi M, Stuber M. Reconstructing anthropogenic disturbance regimes in forest ecosystems: a case study from the Swiss Rhone valley. Ecosystems. 2007;11:113–124. [Google Scholar]
- Gimmi U, Poulter B, Wolf A, Portner H, Weber P, Bürgi M. Soil carbon pools in Swiss forests show legacy effects from historic forest litter raking. Landscape Ecology. 2013;28:835–846. [Google Scholar]
- Gimmi U, Wohlgemuth T. Land-use and climate change effects in forest compositional trajectories in a dry Central-Alpine valley. Annals of Forest Science. 2010;67:701. [Google Scholar]
- Glatzel G. Causes and consequences of forest growth trends in Europe: Results of the recognition project. In: Karjalainen T, Spieker H, Laroussinie O, editors. Causes and consequences of accelerated tree growth in Europe. European Forest Institute; Joensuu, Finland: 1999. pp. 65–74. [Google Scholar]
- Glatzel G. The impact of historic land use and modern forestry on nutrient relations of Central European forest ecosystems. Fertilizer Research. 1991;27:1–8. [Google Scholar]
- Grime JP. Plant strategies, vegetation processes, and ecosystem properties. John Wiley and Sons; Chichester, UK: 2001. [Google Scholar]
- Grulich V. Red List of vascular plants of the Czech Republic: 3rd edition. Preslia. 2012;84:631–645. [Google Scholar]
- Hellström K, Huhta A-P, Rautio P, Tuomi J. Seed introduction and gap creation facilitate restoration of meadow species richness. Journal for Nature Conservation. 2009;17:236–244. [Google Scholar]
- Hofmeister J, Oulehle F, Krám P, Hruška J. Loss of nutrients due to litter raking compared to the effect of acidic deposition in two spruce stands, Czech Republic. Biogeochemistry. 2008;88:139–151. [Google Scholar]
- Hüttl RF, Schaaf W. Nutrient supply of forest soils in relation to management and site history. Plant and Soil. 1995;168-169:31–41. [Google Scholar]
- Ito E, Toriyama J, Araki M, Kiyono Y, Kanzaki M, Tith B, Keth S, Chandararity L, Chann S. Physicochemical surface-soil properties after litter-removal manipulation in a Cambodian lowland dry evergreen forest. Japan Agricultural Research Quarterly. 2014;48:195–211. [Google Scholar]
- Klotz S, Kühn I. BIOLFLOR – Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. Bundesamt für Naturschutz; Bonn, Germany: 2002. [Google Scholar]
- Koorem K, Price JN, Moora M. Species-specific effects of woody litter on seedling emergence and growth of herbaceous plants. PLoS ONE. 2011;6:e26505. doi: 10.1371/journal.pone.0026505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopáček J, Veselý J, Stuchlík E. Sulphur and nitrogen fluxes and budgets in the Bohemian Forest and Tatra Mountains during the Industrial Revolution (1850–2000) Hydrology and Earth System Sciences. 2001;5:391–405. [Google Scholar]
- Kubát K, Hrouda L, Chrtek J Jr, Kaplan Z, Kirschner J, Štěpánek J, editors. Klíč ke květeně České republiky. Academia, Praha, Czech Republic; 2002. [Google Scholar]
- Leff JW, Wieder WR, Taylor PG, Townsend AR, Nemergut DR, Grandy A, Cleveland CC. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Global Change Biology. 2012;18:2969–79. doi: 10.1111/j.1365-2486.2012.02749.x. [DOI] [PubMed] [Google Scholar]
- Li X, Niu J, Xie B. The effect of leaf litter cover on surface runoff and soil erosion in Northern China. PLoS ONE. 2014;9:e107789. doi: 10.1371/journal.pone.0107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loqué D, Eudes A, Yang F. Biomass availability and sustainability for biofuels. In: Simmons B, editor. Chemical and biochemical catalysis for next generation biofuels. Royal Society of Chemistry; Cambridge, UK: 2011. [Google Scholar]
- Monk CD, Gabrielson F. Effects of shade, litter and root competition on old-field vegetation in South Carolina. Bulletin of the Torrey Botanical Club. 1985;112:383–392. [Google Scholar]
- Olsen RS. Phosphorus. In: Page AL, editor. Methods in Soil Analysis. Madison, Wisconsin, USA: 1982. [Google Scholar]
- Oksanen J, Blanchet GF, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson G, Solymos P, Stevens MHH, Wagner HH. Vegan: Community Ecology Package. R package version 2.0-10. 2014. [Google Scholar]
- Osono T, Takeda H. Organic chemical and nutrient dynamics in decomposing beech leaf litter in relation to fungal ingrowth and succession during 3-year decomposition processes in a cool temperate deciduous forest in Japan. Ecological Research. 2001;16:649–670. [Google Scholar]
- Piepho H, Büchse A, Richter C. A mixed modelling approach for randomized experiments with repeated measures. Journal of Agronomy & Crop Science. 2004;190:230–247. [Google Scholar]
- Prietzel J, Kaiser KO. De-eutrophication of a nitrogen-saturated Scots pine forest by prescribed litter-raking. Journal of Plant Nutrition and Soil Science. 2005;168:461–471. [Google Scholar]
- Quinn G, Keough M. Experimental design and data analysis for biologists. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Sayer EJ. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biological Reviews. 2005;81:1–31. doi: 10.1017/S1464793105006846. [DOI] [PubMed] [Google Scholar]
- Šilc U, Čarni A, Košir P, Marinšek A, Zelnik I. Litter-raking forests in SE Slovenia and in Croatia. Hacquetia. 2008;7:71–88. [Google Scholar]
- Tamm CO. Nitrogen in terrestrial ecosystems: Questions of productivity, vegetational changes, and ecosystem stability. Springer; Berlin, Germany: 1991. [Google Scholar]
- Tolasz R, Míková T, Valeriánová T, Voženílek V. Climate atlas of Czechia. Czech Hydrometeorological Institute and Palacký University; Olomouc, Czech Republic: 2007. [Google Scholar]
- Van den Brink P, Ter Braak C. Principal response curves: analysis of time-dependent multivariate responses of biological community to stress. Environmental Toxicology and Chemistry. 1999;18:138–148. [Google Scholar]
- Vincent EM, Roberts EH. The interaction of light, nitrate and alternating temperature in promoting the germination of dormant seeds of common weed species. Seed Science and Technology. 1977;5:659–670. [Google Scholar]
- Wilson S, Tilman D. Quadratic variation in old-field species richness along gradients of disturbance and nitrogen. Ecology. 2002;83:492–504. [Google Scholar]
- Xiong S, Nilsson C. The effects of plant litter on vegetation: a meta-analysis. Journal of Ecology. 1999;87:984–994. [Google Scholar]
- Xu S, Liu LL, Sayer EJ. Variability of above-ground litter inputs alters soil physicochemical and biological processes: a meta-analysis of litterfall-manipulation experiments. Biogeosciences. 2013;10:7423–7433. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Soil properties and the degree of change in time.
Winter precipitation and temperature for each of the sampling years.
List of annual and perennial species with the indication of endangered species.