Abstract
Background
Autologous fat transplantation is used after breast reconstruction to improve the breast profile. There are a variety of different methods used for fat harvesting, preparation, and reinjection. This study describes the specific techniques we used in this series of autologous fat transplantations in breast reconstruction patients and reports their outcomes compared with other studies in the literature.
Patients and methods
At the University Hospital of Parma between May 2012 and December 2016, we performed 53 autologous fat transplantations for secondary breast reconstruction patients with an average age of 49 years (range: 34–65 y). A tumescent fluid (NaCl, epinephrine, and a local anaesthetic) was injected, and the lipoaspirate was harvested using a closed aspiration–injection system connected to a 50 ml syringe, a 4 mm infiltration cannula, and a −650 mmHg vacuum. The average amount of lipoaspirate obtained was 100 ml (range: 50–200 ml). Centrifugation of the lipoaspirate (3000 rpm for 3 min) was performed to isolate the adipose tissue (average amount obtained, 80 ml; range: 30–180 ml). Under local anaesthesia, the retrograde injection of thin layers of fat graft in multiple tunnels was performed in the subcutaneous and/or subglandular planes.
Results
Average follow-up was six months. Comparable to other studies, our complication rate was 7.4% (n = 4/53) and included cyst formation at the injection site (n = 1/53) and hematoma at the donor site (n = 3/53). Repeat fat grafting was performed in 28.3% of patients (n = 15/53) due to fat graft resorption.
Conclusions
Autologous fat transplantation is a useful procedure for correcting irregularities in the breast contour in secondary breast reconstruction.
Keywords: Autologous fat transplantation, Secondary breast reconstruction, Harvesting, Processing, Outcomes
Introduction
Breast cancer is the leading cause of cancer in females across Europe, is estimated to affect more than one in 10 women, and accounts for 28.8% of cancer cases in females (1). Treatment typically includes surgery in the form of mastectomy or breast-conserving surgery (BCS). This is often combined with radiotherapy (RT), chemotherapy, and/or hormonal therapy (2). Autologous fat transplantation (AFT) represents a simple solution to restore the profile of the breast after reconstruction (3). AFT was initially performed by Neuber in 1893 to fill in depressed scars. Its current use dates back to 1987, when Bircoll (4) described a method that coupled liposuction with autologous transplantation of harvested fat into the breast. The major advantage cited for this new technique was the presence of virtually limitless donor tissue that was soft and malleable (4). In breast reconstruction, unlike elsewhere in the body, adipocytes are implanted in a loose, poorly vascularized space (5). Therefore, for adipocyte survival, the fat needs greater contact with the host tissue to ensure adequate nutrition and immobilization during the first few days as the adipocytes become incorporated (5). These anatomical characteristics in the breast explain the high resorption rate found in literature. In 1987, Coleman developed the concept of structural fat grafting which describes the insertion of small amounts of fat in multiple tunnels and in many layers and directions. This results in the largest possible number of adipocytes being in contact with host tissue and, thus, receiving adequate nutrition for survival (6). Coleman’s technique consisted of three steps: manual lipoaspiration under low pressure, centrifugation for 3 min at 3000 rpm, and reinjection in three dimensions (3, 6, 7). This technique is superior to conventional liposuction for harvesting and processing fat grafts because the fat grafts contain a greater number of viable adipocytes and sustain more optimal cellular functioning than fat grafts harvested with conventional liposuction performed by an experienced surgeon (3, 6, 7). Numerous modifications to the Coleman technique have been attempted to improve the survival of the injected fat, including atraumatic fat harvesting, fat washing to eliminate inflammatory mediators, centrifugation, and incubation of fat grafts with different bioactive agents (3, 6, 7).
Plastic surgeons and patients seeking breast reconstruction may have drastically different images in mind of what constitutes an attractive, natural, and ideal breast shape (8). The indications for fat grafting in post-mastectomy reconstruction are numerous, but all essentially involve improving the contour, shape, and volume (9). There are important landmarks in the female breast, for example, the creation of a well-defined inframammary fold is a fundamental element in obtaining a good aesthetic result after breast reconstruction (9). Losken et al. (10) reported a study of 107 patients with a history of breast cancer who had undergone autologous fat grafting for secondary breast reconstruction. They found locations needing contour improvement and additional volume in all quadrants; however, in implant-based reconstructions, the upper pole was a more common site of fat grafting to provide a more natural transition between the implant and the upper chest wall. In thin patients with implant rippling, additional coverage should be achieved with care taken to not violate the implant pocket (10). In fact, AFT can be used after reconstruction with implants or muscle flaps with or without tissue expansion. Appropriate tissue expansion allows the use of autologous flaps or the insertion of definitive prosthetic implants for breast reconstruction. This could be carried out with the aid of a computer program to help the surgeon select the proper tissue expander while planning breast reconstruction (11, 12). Fat grafts are preferred over other graft types for the correction of volume and contour defects because fat is autologous, abundant, and easily harvested (3).
AFT is now commonly used, and many variations in the techniques for fat harvesting, preparation, and reinjection have been reported (7). In breast reconstruction surgery, fat transplantation can be used not only to fill atrophic scars, but also to reduce scar contracture as a regenerative alternative to other surgical techniques (13). Indeed, fat is an active and dynamic tissue composed of several different cell types, including adipocytes, fibroblasts, smooth muscle cells, endothelial cells, and adipogenic progenitor cells called pre-adipocytes (14, 15). Stem cells isolated from lipoaspirates have demonstrated a broad in vitro adipogenic, chondrogenic, osteogenic, and myogenic lineage commitment (16–18) as well as the ability to differentiate into pancreatic cells, hepatocytes, and neurogenic cells (16–18). Cytometric analysis of adipose-derived stem cells (ASCs) has shown that these cells do not express CD31 and CD45, but do express CD34, CD73, CD105, and the mesenchymal stem cell marker, CD90 (19,20). ASCs have a differentiation potential similar to that of other mesenchymal stem cells as well as a higher yield upon isolation, and a greater proliferative rate in culture than bone marrow-derived stem cells (21). Owing to these properties, and because they can be easily harvested in great amounts with minimal donor-site morbidity, ASCs are particularly promising for regenerative therapies (22). Mojallal et al. (23) showed that fat tissue grafting stimulates the neosynthesis of collagen fibres at the recipient site and makes the dermis thicker, thereby improving skin quality. Rigotti et al. (24) reported that the transplantation of lipoaspirates containing adult ASCs is a highly effective therapeutic approach for the treatment of chronic degenerative lesions that are late effects of oncologic radiation treatments. In the 1980s, concerns over the development of fat necrosis and consequent calcification, which could compromise the early detection of breast cancer, led to widespread scepticism about the application of the technique to breast deformities (25). In 2009, a task force of the American Society of Plastic Surgeons made recommendations for the safe and efficacious use of fat grafting to the breast (26).
Patients and methods
From May 2012 to December 2016 at the University Hospital of Parma, we performed 53 autologous fat transplantations for secondary breast reconstruction after simple mastectomy or BCS. The average patient age was 49 years (range: 34–65). The type of primary reconstruction included latissimus dorsi with implants (6%, n = 3), simple mastectomy with implant reconstruction (43%, n = 23), and BCS (51%, n = 27). Fat injection was performed in 48% of the patients (n = 24) in the left breast, in 35% (n = 19) in the right breast, and in 17% (n = 10) bilaterally. In this study, we described the harvest and processing technique, the amount of fat injected, and the outcomes through a comparison with data reported in the literature.
Harvesting
It is widely accepted that less traumatic methods result in increased viability of adipocytes and graft survival (27). Several techniques have been proposed for fat harvesting, and there is ongoing debate in the literature as to which method produces more viable and functional adipocytes (3). The most frequently used methods for fat harvesting are vacuum aspiration or syringe aspiration with or without the infiltration of tumescent fluid (3). No difference in cell viability, cell metabolic activity, or adipogenic response was found after harvesting fat by syringe liposuction compared with pump-assisted liposuction (28). The tumescent technique causes hydrodissection and enlarges the target fat layer, facilitating the subsequent aspiration and decreasing pain and ecchymosis (27).
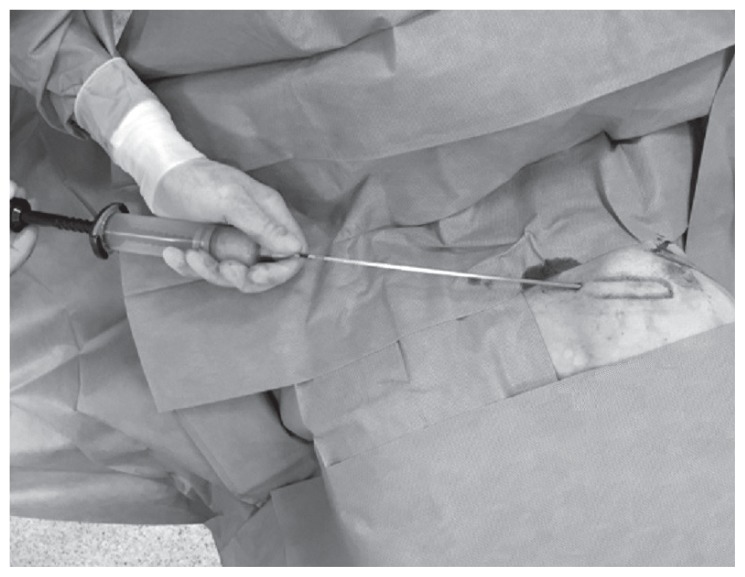
We performed autologous fat transplantation for secondary breast reconstruction under local anesthesia and sedation as day surgery. The operating room had a centrifuge (Lipokit; Medikhan, Korea) equipped with a closed aspiration–injection system connected to a 50 ml syringe (Figure 1) that was used to introduce the Klein solution and subsequently to harvest the fat graft. First of all, the various adipose areas of the body were examined to identify the natural fat deposits. No differences in cell viability have been found among fat removed from the abdomen, flank, thigh, and medial knee (29). However, increased adipose stem cell levels have been observed in fat from the lower abdomen compared with those in the other anatomical locations (29). Once the area from which the fat graft was to be harvested was identified, a local anaesthetic was administered, and a stab incision was made into the fat tissue. Then, through a 4 mm infiltration cannula and a 50 ml syringe connected to the closed aspiration–injection system, Klein solution (30), which is a tumescent fluid consisting of NaCl, epinephrine, and a local anaesthetic drug, was injected (Figure 2). It has been observed that the shear stress exerted on harvested fat affects adipocyte viability, with lower shear stresses associated with improved graft survival (31). Therefore, the lipoaspirate was harvested using a 4 mm suction cannula and a 50 ml syringe connected to a closed aspiration–injection system with a −650 mmHg vacuum (Figure 3). If it was necessary to process more lipoaspirate, we used additional 50 ml syringes. The average amount of lipoaspirate harvested was 100 ml (range: 50–200 ml).
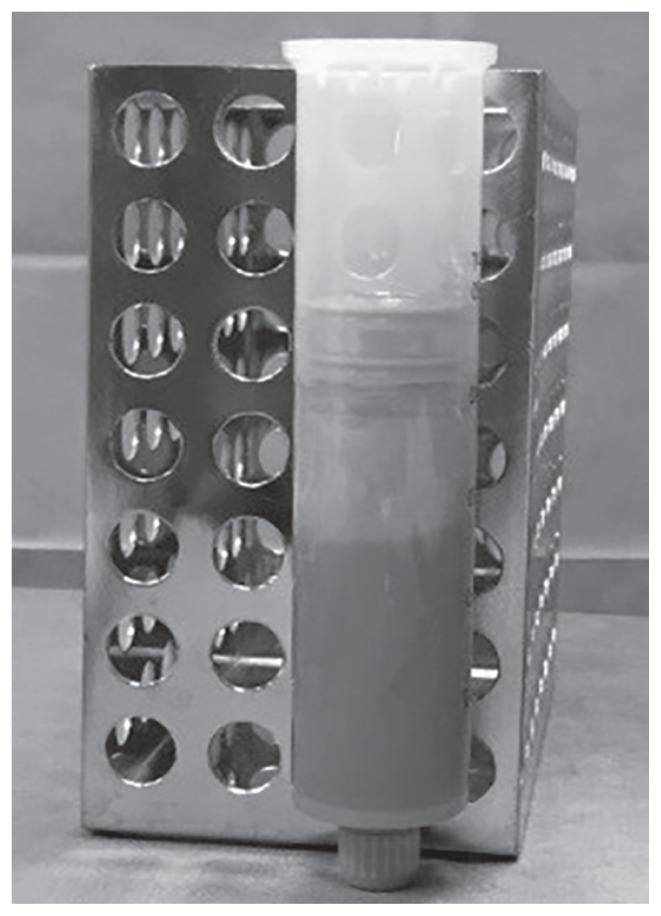
Figure 1.
Centrifuge equipped with a closed aspiration–injection system (Lipokit; Medikhan, Korea).
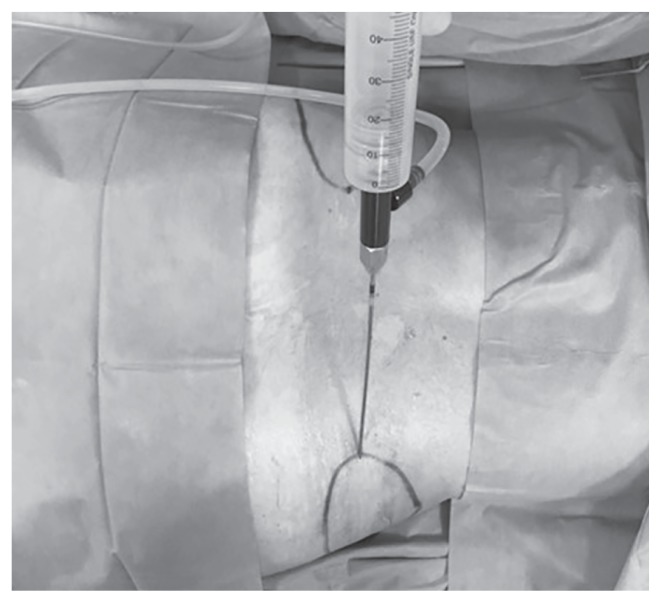
Figure 2.
Klein’s Solution introduced at the the donor site by means of a 50 ml syringe connected to a closed aspiration–injection system.
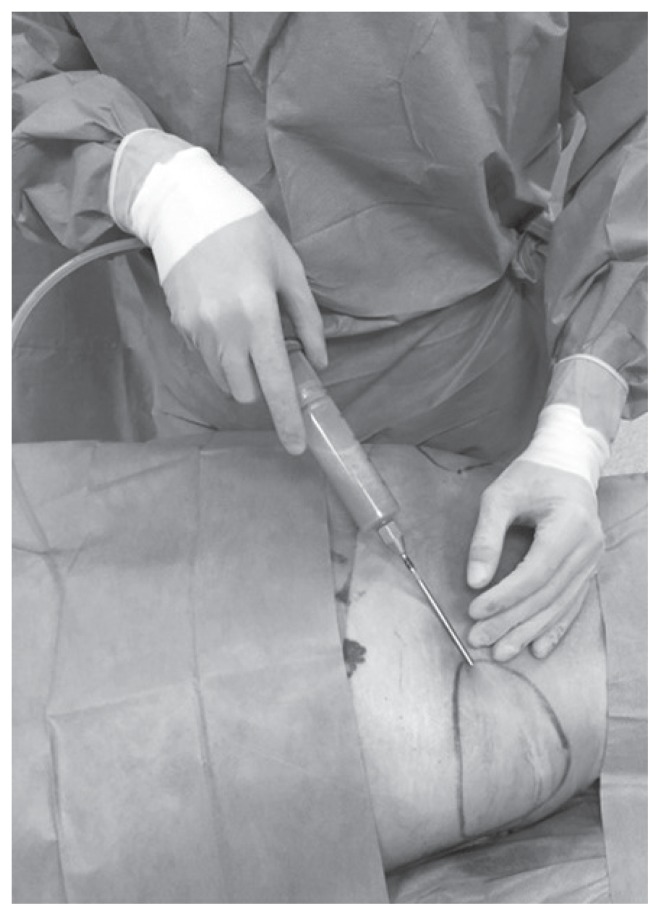
Figure 3.
Lipoaspirate harvested using a 4 mm suction cannula and a 50 ml syringe connected to a closed aspiration–injection system, with a −650 mmHg vacuum.
Cell damage of greater than 10 percent has been demonstrated to occur with the use of a −700 mmHg vacuum (32). An inverse relationship exists between cellular damage and the diameter of the instrument used to extract the fat (32). In their prospective study, Ozsoy et al. (33) demonstrated a greater number of viable adipocytes with a 4 mm diameter cannula than with 2 or 3 mm cannulas. Erdim et al. (34) reported increased graft viability in fat harvested by liposuction using a 6 mm cannula compared with grafts obtained by 4 mm and 2 mm cannulas.
Processing
The principal fat-processing techniques described in the literature include centrifugation, filtering, decantation, and washing. The decision which technique to use often is dependent on the volume of fat required for transplantation, the availability of equipment (e.g., centrifuge), and the surgeon’s own personal preference (35). Fat processing is necessary because the lipoaspirate contains not only adipocytes but also collagen fibres, blood, and debris. These elements can cause inflammation at the recipient site, which can be detrimental for the fat graft (36). Blood must be extracted because blood accelerates the degradation of the transplanted fat (37). Centrifugation based processing resulted in higher ADSC numbers but decreased cell viability counts than decantation (38). When centrifugation is used, several of the articles suggested that forces greater than 3000 rpm (1200 g) cause more cellular damage (7).
We separated the purified fat from cell debris by centrifugation, as described in the widely-used protocol by Coleman (39). Once the fat was harvested, the fat syringes were centrifuged at 3000 rpm for 3 min in the Lipokit. After centrifugation, three layers are observed: the first layer includes lipids, which are removed using absorbent material; the second layer is composed of the fatty tissue; and the third layer, containing blood, tissue fluid, and the local anesthetic, is ejected from the base of syringe (Figures 4, 5). The middle layer is routinely used for adipose tissue grafting (3, 39). The average amount of adipose tissue obtained by processing of the lipoaspirate was 80 ml (range: 30–180 ml).
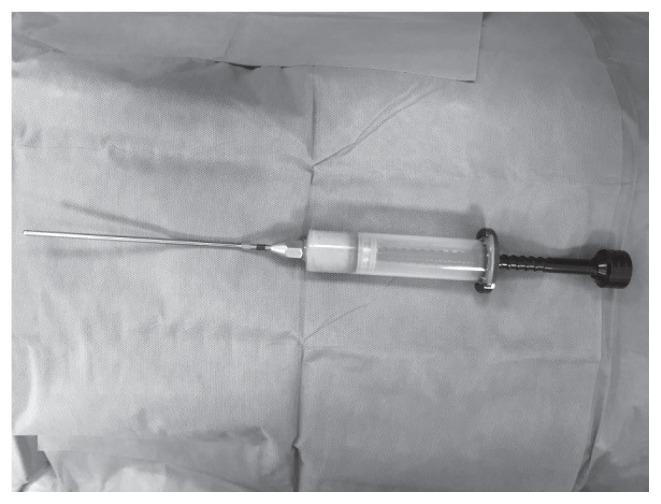
Figure 4.
Lipoaspirate after centrifugation at 3000 rpm for 3 min. It is possible to observe three layers: the first consists of lipids; the second is composed of fatty tissue; and the third contains blood, tissue fluid, and local anaesthetic.
Figure 5.
Adipose tissue obtained after the ejection of the third layer from the base of the syringe.
Injection
In our patients, under local anaesthesia and through a small skin incision in the breast, a 4 mm cannula was introduced to release dermofascial adhesions and scar tissue. Then, the same cannula was used to inject the fat graft into the subcutaneous and subglandular plane after breast-conserving surgery, or into the subcutaneous plane after mastectomy (Figure 6). Retrograde injection of thin layers of fat in multiple tunnels was performed. We avoided excessively large deposits of fat since they may result in liponecrosis and graft loss.
Figure 6.
The injection of fat graft to restore the profile of the left breast in a patient who previously underwent mastectomy and reconstruction with an implant.
Most surgeons practice Coleman’s method, in which small amounts of fat are injected in thin strips at different levels of the subcutis (39). Graft survival is dependent on the diffusion of nutrients from neighbouring capillaries and, hence, fat parcels must be kept small to maximize survival. Carpaneda et al. (40) assessed graft viability as a function of injected volume. They postulated that nutrition was obtained by imbibition from plasma across a distance of 1.5 mm and showed that in grafts with diameters greater than 3 mm, graft viability was inversely proportional to graft diameter (40).
Outcomes
The average follow-up period for our patients was 6 months (range: 3–9 months). The rate of complications was 7.4% (n = 4/53). Complications at the fat injection site (cyst formation) occurred in 1.8% of the patients (n = 1/53). A hematoma occurred at the donor site in 5.6% of patients (n = 3/53). Fat injection was repeated in 28.3% of patients (n = 15/53); it was repeated two times in nine patients and three times in six patients. Repeat treatment was required due to fat graft resorption. The average time between the first and second treatments was 5 months (range: 3–9 months). Our complication rate and the need to repeat the treatment after fat graft resorption was consistent with data reported by others in the literature.
In a review of 33 studies with a total of 5502 patients, Groen et al. (41) reported 461 complications, for a total complication rate of 8.4% (95% CI: 7.6–9.1). Complications included nodules/masses in 11.5%, cyst formation in 6.9%, hematoma in 6.3%, calcifications in 5.2%, breast striae in 4.4%, fat/liponecrosis in 4%, granulomas in 3.6%, infections/cellulitis in 0.8%, seroma in 0.8%, donor site infection in 0.7%, abscess in 0.6%, pneumothorax in 0.2%, and delayed wound healing in 0.1%. Beck et al. (42) reported an objective computed tomography analysis that showed low fat resorption rates during the first 3 months (0–9.54%), followed by an increase in the average fat resorption rate to 51.72% by the ninth month. The rate of fat resorption remained stable after the ninth month, and even seemed to be lower (44.02%) after 3 years. In a review of 24 studies, Agha et al. (43) reported 207 complications, occurring in 7.3% of 2832 treated breasts.
Discussion
Autologous fat transplantation is a promising technique for improving aesthetic results after BCS and breast reconstructions with prostheses or autologous tissues following mastectomy (44). It is a simple procedure that can help obviate the need for more extensive surgery such as that using myocutaneous flaps, and it is often used to correct small defects after breast cancer reconstruction (44). Often, however, patients find the improvement after this procedure insufficient, and the procedure should be repeated to obtain a satisfactory result. We performed 53 autologous fat transplantations by using a closed aspiration–injection system with a −650 mmHg vacuum, 4 mm cannulas, and processing by centrifugation. Recent studies have demonstrated that fat harvested using a blunt cannula and refined by centrifugation as described above has a greater than 90 percent ASC survival rate in histologic studies (45) and near-normal adipose cellular enzyme activity (45, 46). Concerning the effects of cannula sizes on fat graft outcomes, Shiffman and Mirrafati used cannulas ranging from 2.5–3.7 mm in diameter, various suction pressures, and injection needles to discern if there was an effect on cellular viability levels. The only significant result was that cellular damage greater than 10 percent occurred when a vacuum pressure of −700 mmHg was exceeded (31). The pool of ASCs in the grafted fat plays an important role in enhancing angiogenesis, minimizing the local inflammatory response, and improving integration of the transplanted fat and the overall long-term results (45). As reported by Klinger et al., the regenerative effects of ASCs in the fat graft reduce the scar contracture rate, improve the skin texture, and decrease neuropathic pain (47). The chances of survival are higher the less the fat graft is manipulated and the more quickly it is re-injected (48). Choi et al. (49) analysed volumetric data obtained using 3D imaging over a certain time course, and found that breast tissue has a resolution similar to that of soft tissue edema, and that approximately 40–50% of the injected fat volume was retained at 5 months after the procedure (49). In our series, we performed the secondary fat grafting in 15 patients at an average of the fifth postoperative month. The low rate of very minor complications (7.4%), which is compatible with other data in the literature, highlights the safety of this procedure. Similarly, in 2005, Spear et al. (50) reported an 8.5% complication rate for the correction of contour deformities in 37 patients.
Conclusion
In secondary breast reconstruction, autologous fat transplantation is an acceptable procedure for correcting irregularity and deformities that does not compromise oncological outcomes and minimizes patient discomfort.
Footnotes
Disclosure
The authors declares that there is no conflict of interest regarding the publication of this paper.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.NHS Information Centre. Fourth Annual Report - 2011-Datasets DGU. 2011. National mastectomy and brest reconstruction audit. [Google Scholar]
- 3.Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Autologous fat transplantation for breast reconstruction: A literature review. Ann Med Surg (Lond) 2016;12:94–100. doi: 10.1016/j.amsu.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bircoll M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast Reconstr Surg. 1987;79:267–271. doi: 10.1097/00006534-198702000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Claro F, Jr, Figueiredo JC, Zampar AG, Pinto-Neto AM. Applicability and safety of autologous fat for reconstruction of the breast. Br J Surg. 2012;99:768–780. doi: 10.1002/bjs.8722. [DOI] [PubMed] [Google Scholar]
- 6.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesth Plast Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 7.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 8.Raposio E, Belgrano V, Santi P, Chiorri C. Which is the Ideal Breast Size?: Some Social Clues for Plastic Surgeons. Ann Plast Surg. 2016;76:340–345. doi: 10.1097/SAP.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Raposio E, Wang J, Nordström RE. Development of the inframammary fold and ptosis in breast reconstruction with textured tissue expanders. Aesthetic Plast Surg. 2002;26:219–222. doi: 10.1007/s00266-002-1477-0. [DOI] [PubMed] [Google Scholar]
- 10.Losken A, Pinell XA, Sikoro K, Yezhelyev MV, Anderson E, Carlson GW. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg. 66:518–522. doi: 10.1097/SAP.0b013e3181fe9334. 201. [DOI] [PubMed] [Google Scholar]
- 11.Raposio E, Cicchetti S, Adami M, Ciliberti RG, Santi PL. Computer planning for breast reconstruction by tissue expansion: an update. Plast Reconstr Surg. 2004;113:2095–2097. doi: 10.1097/01.prs.0000121189.51406.12. [DOI] [PubMed] [Google Scholar]
- 12.Raposio E, Caregnato P, Barabino P, Gualdi A, Orefice A, Spagnolo A, Capello C, Santi PL. Computer-based preoperative planning for breast reconstruction in the woman with unilateral breast hypoplasia. Minerva Chir. 2002;57:711–714. [PubMed] [Google Scholar]
- 13.Khouri RK, Smit JM, Cardoso E, Pallua N, Lantieri L, Mathijssen IM, Khouri RK, Jr, Rigotti G. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280–1290. doi: 10.1097/PRS.0b013e3182a4c3a9. [DOI] [PubMed] [Google Scholar]
- 14.Raposio E, Guida C, Baldelli I, Benvenuto F, Curto M, Paleari L, Filippi F, Fiocca R, Robello G, Santi PL. Characterization and induction of human pre-adipocytes. Toxicol In Vitro. 2007;21:330–334. doi: 10.1016/j.tiv.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Raposio E, Guida C, Coradeghini R, Scanarotti C, Parodi A, Baldelli I, Fiocca R, Santi PL. In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008;41:739–754. doi: 10.1111/j.1365-2184.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coradeghini R, Guida C, Scanarotti C, Sanguineti R, Bassi AM, Parodi A, Santi PL, Raposio E. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010;191:466–477. doi: 10.1159/000273266. [DOI] [PubMed] [Google Scholar]
- 17.Aluigi MG, Coradeghini R, Guida C, Scanarotti C, Bassi AM, Falugi C, Santi P, Raposio E. Pre-adipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic molecules. Cell Biol Int. 2009;33:594–601. doi: 10.1016/j.cellbi.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Scanarotti C, Bassi AM, Catalano M, Guida C, Coradeghini R, Falugi C, Aluigi M, Santi P, Raposio E. Neurogenic-committed human pre-adipocytes express CYP1A isoforms. Chem Biol Interact. 2010;184:474–483. doi: 10.1016/j.cbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Raposio E, Caruana G, Petrella M, Bonomini S, Grieco MP. A Standardized Method of Isolating Adipose-Derived Stem Cells for Clinical Applications. Ann Plast Surg. 2016;76:124–126. doi: 10.1097/SAP.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 20.Raposio E, Caruana G, Bonomini S, Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133:1406–1409. doi: 10.1097/PRS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 21.Raposio E, Bertozzi N, Bonomini S, Bernuzzi G, Formentini A, Grignaffini E, Pio Grieco M. Adipose-derived Stem Cells Added to Platelet-rich Plasma for Chronic Skin Ulcer Therapy. Wounds. 2016;28:126–131. [PubMed] [Google Scholar]
- 22.Caruana G, Bertozzi N, Boschi E, Pio Grieco M, Grignaffini E, Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Ann Ital Chir. 2015;86:1–4. [PubMed] [Google Scholar]
- 23.Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier JL, Braye F, Damour O. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg. 2009;124:765–774. doi: 10.1097/PRS.0b013e3181b17b8f. [DOI] [PubMed] [Google Scholar]
- 24.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 25.Pierrefeu-Lagrange AC, Delay E, Guerin N, Chekaroua K, Delaporte T. Radiological evaluation of breasts reconstructed with lipomodeling. Ann Chir Plast Esthet. 2006;51:18–28. doi: 10.1016/j.anplas.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Gutowski KA ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124:272–280. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 27.Kakagia D, Pallua N. Autologous fat grafting: in search of the optimal technique. Surg Innov. 2014;21:327–336. doi: 10.1177/1553350613518846. [DOI] [PubMed] [Google Scholar]
- 28.Leong DT, Hutmacher DW, Chew FT, Lim TC. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J Dermatol Sci. 2005;37:169–176. doi: 10.1016/j.jdermsci.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Padoin AV, Braga-Silva J, Martins P, Rezende K, Rezende AR, Grechi B, Gehlen D, Machado DC. Sources of processed lipoaspirate cells: Influence of donor site on cell concentration. Plast Reconstr Surg. 2008;122:614–618. doi: 10.1097/PRS.0b013e31817d5476. [DOI] [PubMed] [Google Scholar]
- 30.Klein JA. Tumescent technique for local anesthesia improves safety in large-volume liposuction. Plast Reconstr Surg. 1993;92:1085–98. [PubMed] [Google Scholar]
- 31.Shiffman MA, Mirrafati S. Fat transfer techniques: The effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001;27:819–826. doi: 10.1046/j.1524-4725.2001.01062.x. [DOI] [PubMed] [Google Scholar]
- 32.Campbell G-L, Laudenslager N, Newman J. The effect of mechanical stress on adipocyte morphology and metabolism. Am J Cosmet Surg. 1987;4:89–94. [Google Scholar]
- 33.Ozsoy Z, Kul Z, Bilir A. The role of cannula diameter inimproved adipocyte viability: A quantitative analysis. Aesthetic Surg J. 2006;26:287–289. doi: 10.1016/j.asj.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Erdim M, Tezel E, Numanoglu A, Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthet Surg. 2009;62:1210–1214. doi: 10.1016/j.bjps.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff M, Wu W, Zellner E, Steinbacher DM. Processing technique for lipofilling influences adipose-derived stem cell concentration and cell viability in lipoaspirate. Aesthetic Plast Surg. 2014;38:224–229. doi: 10.1007/s00266-013-0261-7. [DOI] [PubMed] [Google Scholar]
- 36.Mojallal A, Foyatier JL. The effect of different factors on the survival of transplanted adipocytes. Ann Chir Plast Esthet. 2004;49:426–436. doi: 10.1016/j.anplas.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159–1566. [PubMed] [Google Scholar]
- 38.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381. doi: 10.1016/j.bjps.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Coleman SR. Structural fat grafting. Aesthet Surg J. 1998;18:386–388. doi: 10.1016/S1090-820X(98)70098-6. [DOI] [PubMed] [Google Scholar]
- 40.Carpaneda CA, Ribeiro MT. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast Surg. 1994;18:17–19. doi: 10.1007/BF00444242. [DOI] [PubMed] [Google Scholar]
- 41.Groen JW, Negenborn VL, Twisk DJ, Rizopoulos D, Ket JC, Smit JM, Mullender MG. Autologous fat grafting in onco-plastic breast reconstruction: A systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J Plast Reconstr Aesthet Surg. 2016;69:742–764. doi: 10.1016/j.bjps.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Beck M, Amar O, Bodin F, Lutz JC, Lehmann S, Bruant-Rodier C. Evaluation of breast lipofilling after sequelae of conservative treatment for cancer. European Journal of Plastic Surgery. 2012;35:221–228. [Google Scholar]
- 43.Agha RA, Fowler AJ, Herlin C, Goodacre TE, Orgill DP. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J Plast Reconstr Aesthet Surg. 2015;68:143–161. doi: 10.1016/j.bjps.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 44.Rietjens M, De Lorenzi F, Rossetto F, Brenelli F, Manconi A, Martella S, Intra M, Venturino M, Lohsiriwat V, Ahmed Y, Petit JY. Safety of fat grafting in secondary breast reconstruction after cancer. J Plast Reconstr Aesthet Surg. 2011;64:477–483. doi: 10.1016/j.bjps.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Von Heimburg D, Hemmrich K, Haydarlioglu S, Staiger H, Pallua N. Comparison of viable cell yield from excised versus aspirated adipose tissue. Cells Tissues Organs. 2004;178:87–92. doi: 10.1159/000081719. [DOI] [PubMed] [Google Scholar]
- 46.Pu LL, Cui X, Fink BF, Cibull ML, Gao D. The viability of fatty tissues within adipose aspirates after conventional liposuction: A comprehensive study. Ann Plast Surg. 2005;54:288–292. [PubMed] [Google Scholar]
- 47.Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci V. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–1615. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 48.Smith P, Adams WP, Jr, Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg. 2006;117:1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 49.Choi M, Small K, Levovitz C, Lee C, Fadl A, Karp NS. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 50.Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg. 2005;5:1300–1305. doi: 10.1097/01.prs.0000181509.67319.cf. [DOI] [PubMed] [Google Scholar]