Abstract
Background:
Synthetic oxytocin, the primary tool for labor augmentation, is less effective among obese women, leading to more unplanned cesarean deliveries for slow labor progress. It is not known if obese women require higher doses of oxytocin due to maternal, fetal, or labor factors related to maternal obesity.
Objectives:
This study had two main objectives: (1) examine the influence of maternal body mass index (BMI) on hourly doses of oxytocin from augmentation initiation until vaginal delivery in obese women; and (2) examine the influence of other maternal, fetal, and labor factors on hourly doses of oxytocin in obese women.
Study Design:
Longitudinal study of a cohort (N = 136) of healthy, nulliparous, spontaneously laboring obese women (BMI ≥ 30 kg/m2) who received oxytocin augmentation and achieved vaginal delivery. We performed iterative multilevel analyses to examine the influence of maternal BMI and other factors on hourly oxytocin doses.
Results:
Maternal BMI explained 16.56% (95% confidence interval [CI] = [13.7, 20.04], p < .001) of the variance in hourly oxytocin doses received in a multilevel model controlling for influence of maternal, fetal, and labor characteristics. Maternal age, gestational age, status of amniotic membranes at hospital admission, and admission cervical dilation examination were not significant; however, neonatal birthweight and cervical dilation at oxytocin initiation were significant predictors of hourly oxytocin dose in these women (p < .001).
Conclusions:
Even when parturition preparation has progressed adequately for spontaneous labor initiation, there still may be some obesity-related blunting of myometrial contractility and response to oxytocin used for augmentation.
Keywords: obesity, pregnancy, labor, oxytocin, augmentation, dose
Obesity (body mass index [BMI] ≥ 30 kg/m2) complicates the health of nearly 40% of women 20−39 years of age in the United States (Flegal, Kruszon-Moran, Carroll, Fryar, & Ogden, 2016). Maternal obesity results in altered parturition physiology with multiple effects on the intrapartum period, including delayed labor onset (Arrowsmith, Wray, & Quenby, 2012), slow labor progress (Kominiarek et al., 2011), and decreased labor endurance (Carlson, Hernandez, & Hurt, 2015). Intrapartum care of obese women is characterized by more frequent use of interventions such as early hospital admission, artificial rupture of membranes (AROM), epidural anesthesia, and oxytocin augmentation compared to care provided to normal weight women (Carlson & Lowe, 2014). Despite these efforts to correct labor abnormalities, obese women are more likely than normal weight women to end labor with unplanned cesarean delivery (Chu et al., 2007), with subsequent increased risk for serious postoperative complications (Stamilio & Scifres, 2014). Careful maternal care of obese pregnant women, with resources (bariatric equipment, specialized fetal heart rate monitoring, etc.) and staff training on the unique aspects of obese women’s labor, is necessary to avoid or lessen the extent of poor labor outcomes in this population.
Oxytocin infusions in labor are carefully titrated to the milliunit, with infusion rates often being increased every 20−30 min until women’s contraction patterns become stronger and more regular and cervical changes occur. During particular times in labor, for example, the late active phase when a woman’s myometrial cells may be more responsive to synthetic oxytocin and she produces increased amounts of her own oxytocin, lower (or steady) hourly doses of synthetic oxytocin are often sufficient to effect continued cervical progression. Thus, each woman’s hourly synthetic oxytocin dose is a result of her unique infusion pattern during that time, which in turn is influenced by a mix of factors ranging from her labor phase to her baby’s birth weight and myometrial responsiveness. Higher maternal BMI is linked to decreased myometrial efficiency (Moynihan, Hehir, Glavey, Smith, & Morrison, 2006; Smith, Babiychuk, Noble, Draeger, & Wray, 2005; Zhang, Kendrick, Quenby, & Wray, 2007) and higher neonatal birth weights (Scott-Pillai, Spence, Cardwell, Hunter, & Holmes, 2013). However, protocols for oxytocin dosing are not individualized by maternal BMI or other maternal or neonatal factors. Therefore, obese women are more likely to “fail” oxytocin augmentation (i.e., not exhibit stronger contractions or progressive cervical change in response to medication), leading to increased risk of unplanned cesarean delivery for the indication of slow labor progress (Carlson & Lowe, 2014; Chu et al., 2007; Walsh, Foley, & O’Herlihy, 2011). It is unknown how or to what extent maternal obesity or other maternal or neonatal factors influence effective oxytocin augmentation dosage, and no research to date has examined the effects of infusing higher doses (larger hourly dose, longer duration of therapy, or some combination of both) in obese women on labor outcomes.
Among women having their labors induced, higher maternal BMI has been associated with larger median oxytocin dose and longer oxytocin infusion durations (Hill, Reed, & Cohen, 2014; Pevzner, Powers, Rayburn, Rumney, & Wing, 2009; Roloff, Peng, Sanchez-Ramos, & Valenzuela, 2015). By contrast, investigators found no difference in the total dose of oxytocin augmentation by maternal BMI among women with spontaneous labor onset who achieved vaginal delivery (Roloff et al., 2015). However, in another study including women with spontaneous labor onset who had either cesarean or vaginal deliveries, oxytocin augmentation was less effective among obese compared to normal weight women, more often failing to prevent unplanned cesarean delivery for slow labor progress (Walsh et al., 2011).
Although research has not found that maternal BMI predicts the total dose of oxytocin augmentation in spontaneously laboring women, it remains unknown whether maternal obesity predicts the hourly doses of oxytocin during augmentation. If BMI predicts hourly oxytocin dose, this information might be used to inform new strategies of oxytocin administration in obese women to more frequently avoid cesarean delivery for slow labor progress. Furthermore, if it were found that obese women do require higher hourly doses of oxytocin during labor augmentation, it would be important to know whether this difference reflected larger body size requirements or if other maternal, fetal, or labor factors related to maternal obesity were influencing hourly oxytocin dose. For example, could larger birth weights (Ovesen, Rasmussen, & Kesmodel, 2011) or more advanced gestational ages (Arrowsmith et al., 2012), both known to increase with maternal BMI, influence oxytocin augmentation dose requirements in obese women?
In the present study, we had two main objectives: (1) to examine the influence of maternal BMI on the hourly doses of oxytocin from augmentation initiation until vaginal delivery in obese women and (2) to examine the influence of other maternal, fetal, and labor factors on hourly doses of oxytocin in obese women.
Material and Method
Sample
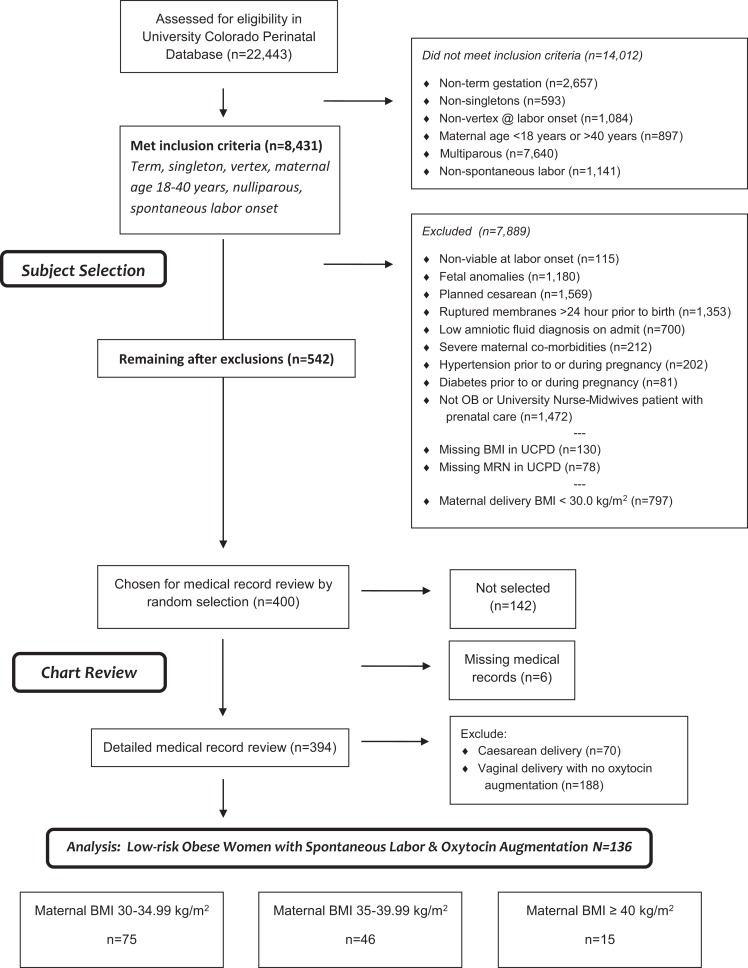
The present study is a secondary analysis of data from an original cohort study comparing intrapartum care and outcomes between obese women cared for by obstetricians (OBs) and those cared for by certified nurse-midwives (CNMs). We identified subjects who delivered at the University of Colorado Hospital (UCH) between October 1, 2005, and December 31, 2012, inclusive (n = 22,443) from the University of Colorado Perinatal Database (UCPD), a comprehensive database maintained by the University of Colorado School of Medicine Department of Obstetrics and Gynecology containing information on maternal history, prenatal course, intrapartum processes, and pregnancy outcomes collected by research personnel on a continuing basis for all births occurring at the University of Colorado Hospital over inclusion years (Figure 1). Of the total identified, 14,012 did not meet the inclusion criteria of nulliparous, singleton birth, vertex presentation, term delivery (37 0/7 to 41 6/7 weeks based on certain first day of last menstrual period and/or first trimester ultrasound), maternal age between 18 and 40 years, spontaneous onset of painful contractions with progressive cervical change prior to hospital admission, and intrapartum and prenatal care with either an OB or CNM. We excluded an additional 7,889 because UCPD admission data indicated one of the following: major fetal anomaly (chromosomal or ultrasound evidence of anomaly), intrauterine fetal growth restriction, intrauterine fetal death, planned cesarean delivery, prelabor rupture of membranes (>24 hr prior to delivery), low amniotic fluid (anhydramnios or oligohydramnios), severe maternal comorbidities (cancer, major cardiac disease), chronic or gestational hypertension, preeclampsia, pregestational or gestational diabetes, intrapartum care with a private UCH midwife group serving patients who self-select for midwifery care, missing maternal height or medical record number, or mother was not obese at delivery (<30 kg/m2). A total of 542 women met the inclusion–exclusion criteria. From these, we randomly selected two cohorts of 200 women each who differed by provider type (OB or CNM) but were otherwise similar in age and other variables to assess whether oxytocin dosing differed by provider status. The lead author, a clinician with expertise in the area of intrapartum nursing, then performed detailed medical record reviews on these 400 labors using REDCap (NIH/NCRR Colorado CTSI Grant UL1TR001082), a secure Health Insurance Portability and Accountability Act (HIPAA)-compliant, web-based application designed to support data capture for research studies (Harris et al., 2009). We tested the performance of the data collection instrument used for medical record abstraction on 10 patients excluded for these analyses prior to use in the present study. Then, to confirm the accuracy of data taken from medical records, we randomly selected 20 women included in this sample for repeat medical record review, which represented 5% of the reviews we performed in this study, as recommended in protocols for chart-abstraction reliability testing (To, Estrabillo, Wang, & Cicutto, 2008). Record reviews showed 95.14% of data were accurate, meeting accuracy standards of >95% (Mi, Collins, Lerner, Losina, & Katz, 2013). After reviewing the medical records, we excluded labors that did not include oxytocin augmentation and vaginal delivery, resulting in a final sample of N = 136 women split among three groups by maternal BMI. The Colorado multiple institutional review board approved this study as exempt (Protocol #14-0557).
Figure 1.
Subject-selection flow diagram. Process for applying inclusion and exclusion criteria to select final sample for study. BMI = body mass index; MRN = medical record number; OB = obstetrician; UCPD = University of Colorado Perinatal Database.
Data Collection
We calculated maternal BMI using the measured height and weight of subjects upon hospital admission for labor (maternal delivery BMI) and classified them using World Health Organization (WHO) BMI criteria: obese I (30.00−34.99 kg/m2), obese II (35.00−39.99 kg/m2), and obese III (≥40kg/m2; WHO, 2000). We used maternal delivery BMI rather than prepregnancy BMI because it better reflects maternal physiology during labor, the timing of interest for this study. In a previous study of the relationship between maternal obesity and labor performance, investigators found that, regardless of prepregnancy BMI, women who gained enough weight during pregnancy to cross into a higher BMI category by WHO criteria showed a 30% increased risk for unplanned cesarean delivery (Kominiarek et al., 2010). Therefore, it did not appear that there was any chronic physiologic changes in myometrial functioning caused by maternal obesity that existed prior to pregnancy. Instead, women’s BMI at the time of labor onset, which incorporates both prepregnancy BMI and gestational weight gain, best predicted their risk for poor labor outcome. Moreover, other investigators examining the relationship between maternal obesity and intrapartum processes or outcomes used delivery BMI rather than prepregnancy BMI, including all others who published investigations examining the relationship between oxytocin dose and maternal obesity (Hill et al., 2014; Kominiarek et al., 2010, 2011; Norman et al., 2012; Pevzner et al., 2009; Roloff et al., 2015).
From each medical record, we collected information on maternal characteristics, including self-reported race, ethnicity, married/partnered status, comorbid conditions, and alcohol/drug/smoking during pregnancy. We calculated maternal age from maternal date of birth and gestational age at the time of hospital admission for labor and delivery using either a certain last menstrual period or estimated date of delivery from a first trimester ultrasound. We collected information on each woman’s cervical examination and whether she had leaking amniotic fluid at hospital admission. We also collected information from nurses’ and providers’ notes on labor interventions, including AROM and anesthesia in labor (epidural or combined spinal–epidural), duration of first- and second-stage labor (first-stage labor defined as period between 4- and 10-cm cervical dilation, second-stage labor defined as period between 10-cm cervical dilation and vaginal delivery), and labor outcomes including rate of operative vaginal delivery (forceps or vacuum), shoulder dystocia, third- or fourth-degree perineal lacerations, postpartum hemorrhage (≥500 ml estimated blood loss for vaginal delivery), maternal fever in labor (>38.0°C), Apgar score less than 7 at 5 min of age, and neonatal intensive care (NICU) admission in first 24 hr of life. Finally, for each subject, we collected detailed information on the timing and results of every cervical examination and oxytocin infusion titration change (time from admission until oxytocin initiation, cervical dilation at time of augmentation initiation, total time and total dose of oxytocin received, rate and dose of oxytocin infusion during each hour of augmentation, and rate of cervical change in labor). Although the dose of oxytocin infusion for labor augmentation is standardized in the United States, we reported rate as well as hourly dose because healthcare providers use rate when infusing oxytocin, and it is therefore more familiar for clinicians.
Statistical Analyses
Our final sample size of N = 136 nulliparous, obese women who received oxytocin augmentation and achieved vaginal delivery (Figure 1) met a priori sample size calculations for a minimum total sample of 100 subjects needed to run our planned multilevel analysis of hourly oxytocin doses by maternal integer BMI (power = .80, α = .05, moderate effect size = .30, integer BMI divisions [e.g., Division 1 = BMI 30.00−30.99, Division 2 = BMI 31.00−31.99…] = 18, average anticipated number of hours of oxytocin dose per woman = 7, minimal clinically important variation of hourly oxytocin dose by maternal BMI division = 10%). We used Optimal Design software (Version 3.01) for our sample size calculations (Spybrook et al., 2011).
We compared data on maternal characteristics, labor characteristics, oxytocin infusion details, and labor outcomes by maternal delivery BMI WHO obesity category using χ2 analysis for categorical variables or one-way analysis of variance for continuous variables. Next, we constructed multilevel (hierarchical) linear models to examine the effect of maternal delivery BMI on hourly oxytocin doses over the course of each labor augmentation. Multilevel modeling provided the ability to test the influence of maternal integer BMI while controlling for other variables that we theorized might also change the hourly dose of oxytocin (gestational age, neonatal birthweight, intrapartum provider type, and maternal age). Our primary variables of interest were the hourly dose of oxytocin (dependent) and maternal delivery BMI (independent) in this longitudinal study.
We ran successive iterations of multilevel models to find one with the best fit for hourly oxytocin data in the present sample. We described two levels for this model: Level 1 was the hourly dose of oxytocin received by each subject throughout her augmentation. Level 2 predictors were maternal delivery integer BMI score of the woman and hours of oxytocin infusion (time trend fixed effect). Once we described an initial model that included our primary Level 2 variables, we tested the fit of more complex models after adding additional variables as fixed effects (neonatal birthweight, cervical dilation at onset of oxytocin infusion, maternal age, gestational age, type of intrapartum provider [OB or CNM], status of amniotic membranes at hospital admission, and the admission cervical dilation examination) using Akaike’s information criterion, Bayesian information criterion, and −2 log-likelihood statistics. We chose these covariates based on existing evidence showing their association with changes in the pace of labor progression, myometrial inefficiency (Hiersch et al., 2015; Siggelkow et al., 2008; Zaki, Hibbard, & Kominiarek, 2013; Zhang et al., 2010), or oxytocin dosing (Jackson, 2003). We estimated all univariate analyses using SPSS, V. 23.0.0.0, 2015, and all multilevel models for this analysis in R 2.14.2 Statistical Software, R Development Team, 2013, using the lme4 package (Bates, 2009).
Results
Results by Maternal BMI WHO Categories
Maternal and pregnancy characteristics were similar across maternal BMI WHO categories at the time of hospital admission (Table 1). Labor characteristics also showed no significant differences by maternal delivery BMI except for cervical dilation at time of AROM, which was significantly smaller among morbidly obese women (BMI ≥ 40 kg/m2) compared to women with BMI 30−34.99 kg/m2, p = .028 (Table 2). Although we excluded women leaking amniotic fluid for longer than 24 hr prior to delivery from this study, we tracked the number of women with spontaneously ruptured membranes at the time of hospital admission and found this proportion to be similar across maternal BMI categories (Table 2). We also considered women’s amniotic membrane status in our multivariate model predicting hourly oxytocin doses. We found no significant differences by maternal BMI category on the rate of cervical dilation change per hour of labor, the total duration of labor, or the duration of either first- or second-stage labor (Table 2). Regarding labor outcomes in this sample, there were no maternal or neonatal deaths, and only three neonates (2.2% of total sample) spent time in the NICU in the first 24 hr of life. We found no differences by maternal BMI category in neonatal birthweight or poor maternal/neonatal outcomes (maternal fever in labor, operative vaginal delivery, third- or fourth-degree perineal laceration, shoulder dystocia, or postpartum hemorrhage; Table 2).
Table 1.
Maternal and Pregnancy Characteristics by Maternal Delivery Body Mass Index (BMI) Category.
| Maternal Variable | Obese I (30−34.99 kg/m2; n = 75) | Obese II (35−39.99 kg/m2; n = 46) | Obese III (≥40 kg/m2; n = 15) | p Valuea |
|---|---|---|---|---|
| Race/ethnicity, n (%) | ||||
| Caucasian | 22 (45.8) | 19 (39.6) | 7 (14.6) | 0.25 |
| Black | 12 (60.0) | 6 (30.0) | 2 (10.0) | 0.89 |
| Asian | 9 (81.8) | 2 (18.2) | 0 | 0.16 |
| Hispanic | 28 (56.0) | 16 (32.0) | 6 (12.0) | 0.93 |
| Missing | 4 (57.2) | 2 (28.6) | 1 (14.3) | |
| Other maternal factors | ||||
| Married/partnered, n (%) | 46 (55.4) | 28 (33.7) | 9 (10.8) | 0.90 |
| Maternal height (m), mean (SD) | 1.62 (0.07) | 1.64 (0.08) | 1.64 (0.06) | 0.13 |
| Maternal age (years), mean (SD) | 25.16 (5.02) | 23.43 (4.09) | 22.87 (3.85) | 0.06 |
| Gestational age at labor onset (weeks/days), mean (SD days) | 39 5/7 (6 days) | 39 5/7 (7 days) | 39 2/7 (7 days) | 0.26 |
| Gestational weight gain (lb), mean (SD) | 37.97 (13.99) | 41.75 (19.65) | 33.07 (19.54) | 0.20 |
| Type of intrapartum provider, n (%) | 0.45 | |||
| Obstetrician | 42 (53.2) | 26 (32.9) | 11 (13.9) | |
| Nurse-midwife | 33 (57.9) | 20 (35.1) | 4 (7.0) | |
a p Value for difference by BMI category calculated using χ2 (categorical) or one-way analysis of variance (continuous).
Table 2.
Labor Characteristics by Maternal Delivery Body Mass Index (BMI) Category.
| Labor Variable | Obese I (30−34.99 kg/m2; n = 75) | Obese II (35−39.99 kg/m2; n = 46) | Obese III (≥40 kg/m2; n = 15) | p Valuea |
|---|---|---|---|---|
| Hospital admission | ||||
| Cervical dilation on hospital admission (cm), mean (SD) | 3.69 (1.29) | 3.85 (1.44) | 3.47 (0.83) | 0.61 |
| Spontaneously ruptured membranes on hospital admit, n (%) | 18 (52.9) | 13 (38.2) | 3 (8.8) | 0.78 |
| Artificial rupture of membranes (AROM) | ||||
| Had AROM in labor, n (%) | 34 (51.5) | 24 (36.4) | 8 (12.1) | 0.71 |
| Cervical dilation at time of AROM (cm), mean (SD) | 6.66 (1.96) | 6.60 (2.00) | 4.80 (1.30) | 0.03 |
| Anesthesia and analgesia use in labor | ||||
| Had epidural analgesia in labor, n (%) | 71 (55.9) | 44 (34.6) | 12 (9.4) | 0.09 |
| Cervical dilation at time of epidural (cm), mean (SD) | 4.39 (1.55) | 4.64 (1.63) | 3.80 (0.45) | 0.11 |
| Systemic analgesic use in labor, n (%) | 20 (26.7) | 13 (28.3) | 4 (26.7) | 0.98 |
| Duration and pace of labor | ||||
| Total duration of first-stage labor (hr), bmean (SD) | 8.99 (4.86) | 9.23 (4.96) | 10.28 (6.49) | 0.67 |
| Total duration of second-stage labor (min), cmean (SD) | 112.00 (81.77) | 99.89 (109.91) | 141.33 (227.90) | 0.48 |
| Rate of cervical change per hr (cm dilation/hr), dmean (SD) | 0.69 (1.15) | 0.69 (1.56) | 0.75 (1.32) | 0.93 |
| Labor outcomes | ||||
| Neonatal birthweight (g), mean (SD) | 3,238.88 (390.68) | 3,326.70 (448.83) | 3,263.13 (408.87) | 0.40 |
| Maternal fever in labor > 38.0ºC), n (%) | 11 (40.7) | 13 (48.1) | 3 (11.1) | 0.19 |
| Operative vaginal delivery, n (%) | 10 (66.7) | 2 (13.3) | 3 (20.0) | 0.16 |
| Third- or fourth-degree perineal laceration, n (%) | 8 (53.3) | 6 (40.0) | 1 (6.7) | 0.78 |
| Postpartum hemorrhage ≥ 500 ml, n (%) | 11 (44.0) | 11 (44.0) | 3 (12.0) | 0.44 |
Note. Additional labor outcomes: No neonates had Apgar score <7 at 5 min of age. Only three neonates (2.2% of total) were admitted to the neonatal intensive care unit (NICU) in the first 24 hr of life, all born to Obese I mothers. No neonatal or maternal deaths following birth in this sample.
a p Value for difference by BMI category calculated using χ2 (categorical) or one-way analysis of variance (continuous). bFirst-stage labor = 4- to 10-cm dilation. cSecond-stage labor = 10-cm dilation to vaginal delivery. dMean (SD) calculated on all cervical exams, n = 432 among Obese I women, n = 270 among Obese II women, and n = 81 among Obese III women.
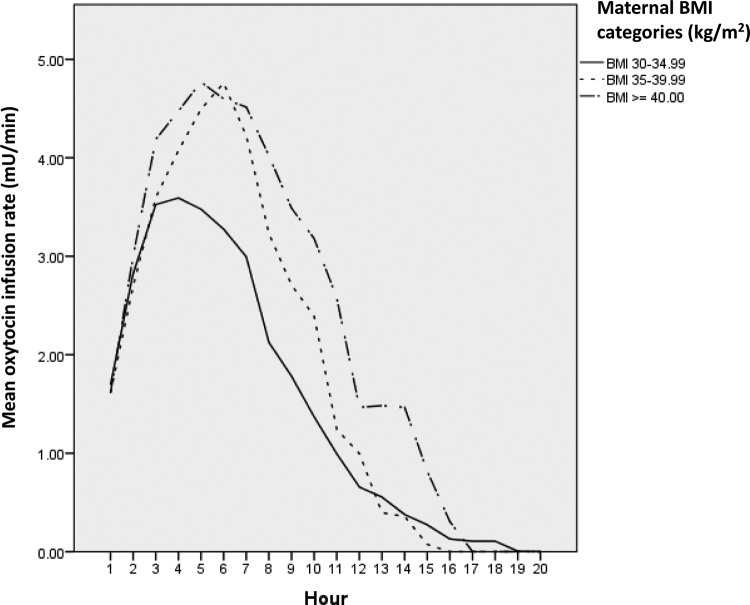
Oxytocin infusions were initiated after a similar time interval following hospital admission and at similar cervical dilation across maternal BMI categories (Table 3). There was a higher mean rate of oxytocin infusion in women in the Obese II category (1.84 mU/min) compared to women in the Obese I category (1.49 mU/min, p = .047) and in the Obese III category (2.29 mU/min) compared to the Obese I category (p = .001; Table 3). Although not statistically significant, both total dose and total duration of oxytocin in labor increased in a stepwise manner with more advanced maternal obesity, as did the time interval from oxytocin initiation until vaginal delivery. To more clearly examine the relationship between maternal BMI and oxytocin infusion rates, we plotted the average oxytocin infusion rates (mU/min) for each hour of augmentation by maternal BMI category (Figure 2), demonstrating that obese women with higher BMIs received higher infusion rates than obese women with lower BMIs.
Table 3.
Oxytocin Infusion Details by Maternal Delivery Body Mass Index (BMI) Category.
| Oxytocin Infusion Variable | Obese I (30−34.99 kg/m2; n = 75) | Obese II (35−39.99 kg/m2; n = 46) | Obese III (≥40 kg/m2; n = 15) | p Valuea |
|---|---|---|---|---|
| Time intervals describing oxytocin infusion | ||||
| Hospital admission to oxytocin augmentation initiation (hr) | 7.49 (3.83) | 6.75 (3.53) | 6.65 (3.30) | .49 |
| Oxytocin augmentation initiation to vaginal delivery (hr) | 6.2 (3.87) | 6.51 (3.76) | 8.48 (4.25) | 0.13 |
| Total duration of oxytocin augmentation in labor (hr) | 4.81 (3.49) | 4.86 (3.28) | 6.22 (4.27) | .35 |
| Cervical dilation at time of oxytocin augmentation initiation (cm) | 6.27 (2.62) | 5.91 (2.45) | 5.00 (2.01) | .20 |
| Rate of oxytocin infusion across entire augmentation (mU/min) | 1.49 (2.96) | 1.84 (3.97) | 2.29 (3.96) | Ob1 − Ob2 p = .047 Ob1 − Ob3 p = .001 Ob2 − Ob3 p = .14 |
| Total dose of oxytocin in labor (mU) | 1,791.67 (1,961.42) | 2,210.85 (2,668.53) | 2,757.60 (3,037.27) | .29 |
Note. Cells display mean (SD). Mean (SD) oxytocin rates calculated based on hourly measures during infusion (n = 1,500 measurements in Obese I women, n = 920 in Obese II women, and n = 300 in Obese III women.
a p Value for difference by BMI category calculated using one-way analysis of variance.
Figure 2.
Plot of mean infusion rate (mU/min) over each hour of oxytocin augmentation during spontaneous labor in obese, nulliparous women achieving vaginal delivery. Solid and dotted lines designate the average rate of oxytocin infusion for each hour of labor augmentation by maternal delivery body mass index (BMI) categories defined by World Health Organization criteria.
Multilevel Modeling of Hourly Oxytocin Dose by Maternal Delivery BMI Integer Categories
We calculated interclass correlation (ICC) and the 95th-percentile intervals of hourly oxytocin doses for each of 18 BMI integer divisions to verify that multilevel modeling was an appropriate choice for the hourly oxytocin data. We found that over 50% of the hourly oxytocin dose intervals within a single integer BMI division did not cross the median hourly dose for the entire sample and that the ICC = .067, which together indicated that multilevel modeling was a better choice than a flat multivariate model using BMI as a continuous variable for these data. Although we tested each of maternal age, gestational age, type of intrapartum provider (OB or CNM), status of amniotic membranes at hospital admission, and the admission cervical dilation examination in the model, none contributed to a better fit for hourly oxytocin dose and were therefore discarded from later models. Thus, none of these variables predicted hourly oxytocin doses.
In the multilevel model that best fit data (p < .001 over other models), significant predictors of hourly oxytocin dose were maternal integer BMI, hours since oxytocin initiation, cervical dilation at oxytocin initiation, and neonatal birthweight (p < .001). Variation in the maternal BMI explained 16.56% (95% CI [13.7, 20.04], p < .001) of the total variance in the hourly oxytocin doses received during labor augmentation by obese women (ICC = .1656, Table 4). By contrast, the hours since infusion onset, reflecting the duration of oxytocin infusion, predicted only 0.0044% of the variance in hourly oxytocin doses.
Table 4.
Statistics for Multilevel Model Predicting Hourly Oxytocin Dosage by Body Mass Index (BMI) Grouping, Hours of Oxytocin Infusion, Cervical Dilation at Infusion Onset, and Neonatal Birthweight.
| Variable | Multilevel Model |
|---|---|
| Hourly oxytocin dose (intercept): Average hourly dose of oxytocin infused for labor augmentation across all maternal BMI groups | 182.66 mU |
| Group predictor (Level 2) of hourly oxytocin dose | |
| BMI grouping (ICC)a: Percent of variance in hourly oxytocin doses explained by maternal delivery BMI group when controlling for hour of infusion, cervical dilation at infusion onset, and neonatal birthweight | 16.56% 95% CI [13.7, 20.04] |
| Individual-level predictors (Level 1) of hourly oxytocin dose | |
| Hour of oxytocin infusion (beta coefficient): Average change in hourly oxytocin dose with each additional hour of infusion | −12.79 mU |
| Cervical dilation at start of oxytocin (beta coefficient): Average change in hourly oxytocin dose with each 1 cm increase in maternal cervical dilation at onset of oxytocin infusion | −20.17 mU |
| Neonatal birthweight (beta coefficient): Average change in hourly oxytocin dose with each increase of 1 kg in neonatal birthweight | 53 mU |
aInterclass correlation coefficient (ICC) calculation for percent of variance in hourly oxytocin dose explained by maternal BMI grouping: BMI group variance/residual + BMI group variance + hour group variance = 6,216.10/(31,299.23 + 6,216.10 + 10.66) = 0.1656 = 16.56% of variance in hourly oxytocin dose explained by maternal delivery BMI.
According to the best-fitting multilevel model (Table 4), women tended to require higher hourly doses of oxytocin when their babies were heavier or when their cervices were less dilated at the time of oxytocin onset. The average hourly oxytocin dose decreased by 20.17 mU each time a woman moved up by 1 cm in cervical dilation at the time of oxytocin augmentation initiation. Also, according to this model, if two women of equivalent BMIs had the same starting cervical dilations and were in the same hour of oxytocin infusion, the woman with a baby weighing 3-kg baby would receive an average of 53 mU less oxytocin in an hour (i.e., her infusion rate would be about 0.88 mU/min slower if not changed during that hour) than the matched woman with a baby weighing 4 kg.
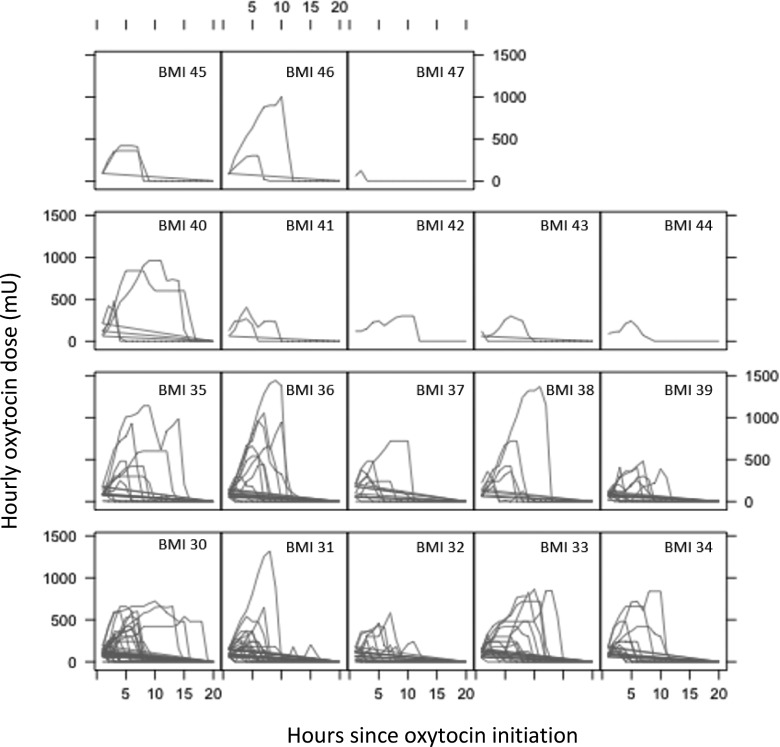
To more clearly demonstrate this complex relationship, we created a plot of hourly oxytocin dose across time for each integer BMI division (Figure 3). Each line in this figure traces the hourly oxytocin doses for a woman from the time of oxytocin initiation until the end of infusion. Women are grouped in boxes by their delivery integer BMI division. In general, hourly oxytocin dose moved upward in a sharp slope upon initiation of infusion to a high point, followed by maintenance at a high level for a period of time (creating a plateau in the slope) or by a rapid decrease in dose when oxytocin infusion was ceased for that woman. With higher maternal BMI (35−40 kg/m2), more women reached higher hourly oxytocin doses (higher peaks) and continued over more hours of oxytocin exposure (longer infusion lines) compared to women in the lower BMI groups (BMI 30−34 kg/m2). Groups for BMI 41−47 kg/m2 each had only a few subjects, resulting in graphs that have too little information to identify clear patterns. Figure 3 demonstrates results at the individual level that are similar to those illustrated by Figure 2, showing that women in higher BMI categories received higher oxytocin infusion rates continued over more hours during labor augmentation.
Figure 3.
Plot of hourly oxytocin dose trajectories by maternal body mass index (BMI) grouping, hour of infusion for groups defined by maternal delivery BMI (kg/m2). For each group of women separated into boxes by their integer BMI values at delivery, lines designate the hourly oxytocin dose for each hour of labor augmentation. The graphs demonstrate longer periods of augmentation (longer lines) and higher hourly oxytocin doses (higher peaks in the lines) as maternal delivery BMI value increases.
Discussion
As Roloff et al. (2015) found in a previous investigation, the total dose of oxytocin used to augment spontaneous labors among obese women in the present sample was not statistically significantly associated with maternal delivery BMI WHO category. However, we found that the mean rate of oxytocin infusion across women’s labor augmentation was significantly higher among Obese II and Obese III women compared to Obese I women in the present study (Table 3). In addition, 16.56% of the variance in the hourly dose of oxytocin received by obese women in the present sample was explained by integer BMI in multilevel analysis when controlling for cervical dilation at infusion onset, the elapsed time of infusion, and neonatal birthweight. Despite higher hourly doses of oxytocin and higher overall mean rates of oxytocin infusion with increased maternal BMI, we found no significant difference in the rate of cervical change or in the total duration of labor by maternal BMI WHO obesity category. However, morbidly obese women were at a significantly smaller average cervical dilation than obese women with BMI 30.00−34.99 kg/m2 when their providers artificially ruptured their membranes, a labor intervention typically intended to speed labor progress.
Prior to this study, investigators showed that labor augmentation with oxytocin occurred more frequently among women with higher BMI (Carlson & Lowe, 2014; Walsh et al., 2011), and obese women undergoing labor induction required higher rates of oxytocin infusion during the first stage and greater total dosages of oxytocin than normal weight women to achieve vaginal delivery (Roloff et al., 2015). These clinical findings are corroborated by tissue studies revealing that cholesterol, leptin, and apelin, all found at higher levels in obese pregnant women, blunt the effect of oxytocin of stimulating myometrial contractility (Carlson et al., 2015; Hehir & Morrison, 2012; Moynihan et al., 2006; Zhang et al., 2007). In the present study, we showed for the first time that even in term, spontaneously laboring, healthy, nulliparous women with no prelabor rupture of membranes, obese women with higher BMIs had significantly increased mean oxytocin infusion rates compared to obese women with lower BMIs and less advanced cervical dilation at the time of AROM. Further, increased maternal BMI independently predicted higher hourly oxytocin doses. Thus, it appears that even when parturition preparation has progressed adequately for spontaneous labor initiation, there still may be some obesity-related blunting of myometrial contractility and response to oxytocin.
Research has linked higher maternal BMI to decreased myometrial efficiency (Moynihan et al., 2006; Smith et al., 2005; Zhang et al., 2007) and slower labor progress, especially during early active-phase labor (Kominiarek et al., 2011). However, two women of different BMIs and with theoretically different myometrial efficiencies might still have the same total oxytocin dose if, for example, the woman with a higher BMI had a shorter pushing phase of labor (as has been shown with morbidly obese women compared to women with BMI 30−35 kg/m2; Kominiarek et al., 2011) or if the women with the lower BMI also had a heavier baby. Our analysis adds to the existing literature by controlling for neonatal birthweight and starting cervical dilation in multivariate models of hourly oxytocin dose to allow for a clearer view of maternal BMI’s influence on oxytocin doses.
Other labor factors that could change oxytocin dosing besides maternal BMI-related myometrial inefficiency include the use of other systemic medications during labor and the accuracy of uterine-contraction monitoring to guide oxytocin dosing. We collected data on the use of systemic opioids during labor and found that women of different BMI groups used this intervention with approximately the same frequency (p = .98, Table 2). Therefore, any change caused by systemic opioids on uterine oxytocin response was theoretically experienced equally across BMI groups. Another possible explanation for the increased rates of oxytocin infusion among women with higher BMIs is that nurses dosed oxytocin titrations higher in more obese women simply because they had more difficulty monitoring uterine contractions accurately in women with more abdominal adipose tissue. Increased maternal BMI is known to decrease the accuracy of external uterine-contraction monitoring (Cohen & Hayes-Gill, 2014). Whatever the reasons for the observation that women with increasing levels of obesity received higher doses of oxytocin, it is important to note that higher rates of oxytocin administration and higher hourly doses of oxytocin among more obese women did not result in a faster pace of labor progression or a shorter labor duration than occurred among less obese women. Therefore, increased exposure to oxytocin among more obese women did not result in more efficient contractions.
We conducted post hoc power analysis for the multilevel analysis of hourly oxytocin doses, finding that a minimum sample size of 123 women was needed. We also conducted post hoc power analysis on the outcome of total oxytocin dose by maternal BMI WHO category (Obese I, Obese II, and Obese III), and found that we needed 550 subjects to identify significance at the 5% level on observed differences in the total dose of oxytocin by maternal BMI ( δ = .24, 80% power). Although this study was therefore adequately powered for our multilevel analysis, future analyses including larger samples of women, especially those with morbid obesity, could establish more definitively the influence of maternal delivery BMI on hourly and total oxytocin dose. Although the average rates of oxytocin infusion were significantly higher in Obese II and Obese III women compared to Obese I women, absolute differences between these mean rates were small (mean difference of 0.35 mU/min in average infusion rate between Obese I and II women, 0.80 mU/min mean difference between Obese III and Obese I women). In a larger sample of women, however, these small differences in infusion rates might result in significant changes in overall oxytocin dose or risk for postpartum hemorrhage.
There are several important limitations of this study. First, conclusions are limited by its retrospective design. Further investigation using an experimental prospective design of labor management among obese, nulliparous women would be necessary to establish causality. However, through careful sample selection, we were able to focus these analyses on a group of women with similar pregnancy features (obese, healthy, nulliparous women with term labor and singleton, vertex fetus), similar labor onset (spontaneous labor with no prelabor rupture of membranes), and exposure to similar oxytocin administration protocols and clinicians (UCH). Therefore, the differences we notice here in hourly oxytocin dose and oxytocin rates are less likely to be related to sampling bias. A second limitation of this study is generalizability to other populations. Our sample was taken from a single institution with low rates of cesarean delivery and only included healthy subjects who had comprehensive prenatal care. Labor outcomes and interventions by maternal BMI would likely vary from our results in other studies if the hospital had high overall rates of cesarean delivery or subjects were included who had comorbidities or absent or substandard prenatal care.
To conclude, the present study demonstrated that even in spontaneously laboring obese women who achieved vaginal delivery, higher BMI predicted higher hourly oxytocin doses and higher average rates of oxytocin infusion. Hourly oxytocin dose in obese women was also related to neonatal birthweight and cervical dilation at oxytocin initiation but not to other maternal or labor characteristics. Further research among larger groups of morbidly obese women is needed to establish if the higher hourly oxytocin doses required by obese women during labor augmentation are simply related to larger body size or are also influenced by obesity-related decreases in myometrial response and contractility. Finally, our study was limited by the distribution of BMI in the final sample, with the majority of subjects (62.5%) having BMIs between 30 and 34.99 kg/m2. BMI effects on labor performance and oxytocin response are known to be dose dependent (Carlson & Lowe, 2014; Walsh et al., 2011). Therefore, the clustering of our sample around low levels of maternal obesity limits our conclusions. Future studies should include samples with greater ranges of maternal BMI and less clustering of subjects around one area of that range.
Our study has several potentially important implications for intrapartum nursing and nurse-midwifery care of obese women. Despite the fact that current oxytocin protocols are not individualized by the degree of maternal obesity, maternal delivery BMI appears to predict nearly 17% of women’s hourly oxytocin doses during labor augmentation. Unfortunately, currently available gold-standard techniques for monitoring contraction intensity (intrauterine pressure catheters) cannot discriminate between obese and normal weight women’s contractions in late first-stage (Chin, Henry, Holmgren, Varner, & Branch, 2012) or second-stage labor (Buhimschi, Buhimschi, Malinow, & Weiner, 2004) and thus are of questionable use for nurses wishing to determine oxytocin dose in obese women by myometrial response. Our findings suggest the need for further research upon which to base revised protocols for dosing oxytocin in obese women, including the possibilities of dosing oxytocin over longer periods of time to correct for obesity-related myometrial inefficiency, dosing oxytocin at higher levels, or some combination of these strategies.
Acknowledgments
The authors would like to thank Oliwier Dziadkowiec, PhD, University of Colorado Denver, and Sudeshna Paul, PhD, Emory University School of Nursing, for statistical consultation.
Footnotes
Author Contribution: Nicole S. Carlson contributed to conception, design, and acquisition; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Elizabeth J. Corwin contributed to conception and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Nancy K. Lowe contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received Financial support from National Institutes of Health (5F31NR014061-02), March of Dimes.
References
- Arrowsmith S., Wray S., Quenby S. (2012). Maternal obesity and labor complications after induction of labor in prolonged pregnancy. Obstetric Anesthesia Digest, 32, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. (2009). lme vs. lmer. Retrieved from https://stat.ethz.ch/pipermail/r-sig-mixed-models/2009q3/002912.html
- Buhimschi C. S., Buhimschi I. A., Malinow A. M., Weiner C. P. (2004). Intrauterine pressure during the second stage of labor in obese women. Obstetrics & Gynecology, 103, 225–230. (Erratum published 2004, Obstetrics & Gynecolgy, 103, p. 1019) [DOI] [PubMed] [Google Scholar]
- Carlson N. S., Hernandez T. L., Hurt K. J. (2015). Parturition dysfunction in obesity: Time to target the pathobiology. Reproductive Biology and Endocrinology, 13, 135 doi:10.1186/s12958-015-0129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson N. S., Lowe N. K. (2014). Intrapartum management associated with obesity in nulliparous women. Journal of Midwifery & Women’s Health, 59, 43–53. doi:10.1111/jmwh.12073 [DOI] [PubMed] [Google Scholar]
- Chin J. R., Henry E., Holmgren C. M., Varner M. W., Branch D. W. (2012). Maternal obesity and contraction strength in the first stage of labor. American Journal of Obstetrics and Gynecology, 207, 129e121–129.e126. doi:10.1016/j.ajog.2012.06.044 [DOI] [PubMed] [Google Scholar]
- Chu S. Y., Kim S. Y., Schmid C. H., Dietz P. M., Callaghan W. M., Lau J., Curtis K. M. (2007). Maternal obesity and risk of cesarean delivery: A meta-analysis. Obesity Reviews, 8, 385–394. doi:10.1111/j.1467-789X.2007.00397.x [DOI] [PubMed] [Google Scholar]
- Cohen W. R., Hayes-Gill B. (2014). Influence of maternal body mass index on accuracy and reliability of external fetal monitoring techniques. Acta Obstetricia et Gynecologica Scandinavica, 93, 590–595. doi:10.1111/aogs.12387 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Kruszon-Moran D., Carroll M. D., Fryar C. D., Ogden C. L. (2016). Trends in obesity among adults in the United States, 2005 to 2014. Journal of the American Medical Association, 315, 2284–2291. doi:10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. doi:10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehir M. P., Morrison J. J. (2012). The adipokine apelin and human uterine contractility. American Journal of Obstetrics & Gynecology, 206, 359e351–355. doi:10.1016/j.ajog.2012.01.032 [DOI] [PubMed] [Google Scholar]
- Hiersch L., Rosen H., Salzer L., Aviram A., Ben-Haroush A., Yogev Y. (2015). Does artificial rupturing of membranes in the active phase of labor enhance myometrial electrical activity? Journal of Maternal-Fetal & Neonatal Medicine, 28, 515–518. doi:10.3109/14767058.2014.927431 [DOI] [PubMed] [Google Scholar]
- Hill M., Reed K. L., Cohen W. R. (2014). Oxytocin utilization for labor induction in obese and lean women. Journal of Perinatal Medicine, 43, 703–706. doi:10.1515/jpm-2014-0134 [DOI] [PubMed] [Google Scholar]
- Jackson D. J. (2003). Impact of collaborative management and early admission in labor on method of delivery. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 32, 147–157. [DOI] [PubMed] [Google Scholar]
- Kominiarek M., Vanveldhuisen P., Hibbard J., Landy H., Haberman S., Learman L.…Zhang J. (2010). The maternal body mass index: A strong association with delivery route. American Journal of Obstetrics & Gynecology, 203, 264 e261–267. doi:10.1016/j.ajog.2010.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominiarek M., Zhang J., VanVeldhuisen P., Troendle J., Beaver J., Hibbard J. U. (2011). Contemporary labor patterns: The impact of maternal body mass index. American Journal of Obstetrics and Gynecology, 205, 244.e241–244.e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi M. Y., Collins J. E., Lerner V., Losina E., Katz J. N. (2013). Reliability of medical record abstraction by non-physicians for orthopedic research. BMC Musculoskeletal Disorders, 14, 181 doi:10.1186/1471-2474-14-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan A. T., Hehir M. P., Glavey S. V., Smith T. J., Morrison J. J. (2006). Inhibitory effect of leptin on human uterine contractility in vitro. American Journal of Obstetrics and Gynecology, 195, 504–509. [DOI] [PubMed] [Google Scholar]
- Norman S. M., Tuuli M. G., Odibo A. O., Caughey A. B., Roehl K. A., Cahill A. G. (2012). The effects of obesity on the first stage of labor. Obstetrics & Gynecology, 120, 130–135. doi:10.1097/AOG.0b013e318259589c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovesen P., Rasmussen S., Kesmodel U. (2011). Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstetrics & Gynecology, 118, 305–312. doi:10.1097/AOG.0b013e3182245d49 [DOI] [PubMed] [Google Scholar]
- Pevzner L., Powers B. L., Rayburn W. F., Rumney P., Wing D. A. (2009). Effects of maternal obesity on duration and outcomes of prostaglandin cervical ripening and labor induction. Obstetrics & Gynecology, 114, 1315–1321. [DOI] [PubMed] [Google Scholar]
- Roloff K., Peng S., Sanchez-Ramos L., Valenzuela G. J. (2015). Cumulative oxytocin dose during induction of labor according to maternal body mass index. International Journal of Gynaecology & Obstetrics, 131, 54–58. doi:10.1016/j.ijgo.2015.04.038 [DOI] [PubMed] [Google Scholar]
- Scott-Pillai R., Spence D., Cardwell C. R., Hunter A., Holmes V. A. (2013). The impact of body mass index on maternal and neonatal outcomes: A retrospective study in a UK obstetric population, 2004-2011. British Journal of Obstetrics and Gynaecology, 120, 932–939. doi:10.1111/1471-0528.12193 [DOI] [PubMed] [Google Scholar]
- Siggelkow W., Boehm D., Skala C., Grosslercher M., Schmidt M., Koelbl H. (2008). The influence of macrosomia on the duration of labor, the mode of delivery and intrapartum complications. Archives of Gynecology & Obstetrics, 278, 547–553. doi:10.1007/s00404-008-0630-7 [DOI] [PubMed] [Google Scholar]
- Smith R. D., Babiychuk E. B., Noble K., Draeger A., Wray S. (2005). Increased cholesterol decreases uterine activity: Functional effects of cholesterol alteration in pregnant rat myometrium. American Journal of Physiology—Cell Physiology, 288, C982–C988. [DOI] [PubMed] [Google Scholar]
- Spybrook J., Bloom H., Congdon R., Hill C., Martinez A., Raudenbush S. (2011). Optimal Design plus Empirical Evidence: Documentation for the “Optimal Design” software, 216 Retrieved from http://hlmsoft.net/od/od-manual-20111016-v300.pdf [Google Scholar]
- Stamilio D. M., Scifres C. M. (2014). Extreme obesity and postcesarean maternal complications. Obstetrics & Gynecology, 124, 227–232. doi:10.1097/AOG.0000000000000384 [DOI] [PubMed] [Google Scholar]
- To T., Estrabillo E., Wang C., Cicutto L. (2008). Examining intra-rater and inter-rater response agreement: A medical chart abstraction study of a community-based asthma care program. BMC Medical Research Methodology, 8, 29 doi:10.1186/1471-2288-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J., Foley M., O’Herlihy C. (2011). Dystocia correlates with body mass index in both spontaneous and induced nulliparous labors. Journal of Maternal-Fetal & Neonatal Medicine, 24, 817–821. doi:10.3109/14767058.2010.531313 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2000). Obesity: Preventing and managing the global epidemic. Retrieved from http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed]
- Zaki M. N., Hibbard J. U., Kominiarek M. A. (2013). Contemporary labor patterns and maternal age. Obstetrics & Gynecology, 122, 1018–1024. doi:10.1097/AOG.0b013e3182a9c92c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kendrick A., Quenby S., Wray S. (2007). Contractility and calcium signaling of human myometrium are profoundly affected by cholesterol manipulation: Implications for labor? Reproductive Sciences, 14, 456–466. doi:10.1177/1933719107306229 [DOI] [PubMed] [Google Scholar]
- Zhang J., Landy H. J., Branch D. W., Burkman R., Haberman S., Gregory K. D., Reddy U. M. (2010). Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstetrics & Gynecology, 116, 1281–1287. doi:10.1097/AOG.0b013e3181fdef6e [DOI] [PMC free article] [PubMed] [Google Scholar]