Abstract
Background and Purpose
Women have worse post-stroke outcomes than men. We evaluated sex-specific clinical and neuroimaging characteristics of white matter in association with functional recovery after acute ischemic stroke (AIS).
Methods
We performed a retrospective analysis of AIS patients with admission brain MRI and 3 to 6 month modified Rankin scale score (mRS). White matter hyperintensity (WMH) and acute infarct volume were quantified on fluid attenuated inversion recovery (FLAIR) and diffusion-tensor imaging MRI, respectively. Diffusivity anisotropy metrics were calculated in normal appearing white matter (NAWM) contralateral to the acute ischemia.
Results
Among 319 AIS patients, women were older (68.0 vs. 62.7 years; p=0.004), had increased incidence of atrial fibrillation (21.4% vs. 12.2%; p=0.04), and lower rate of tobacco use (21.1% vs. 35.9%; p=0.03). There was no sex-specific difference in WMH volume, acute infarct volume, NIHSS, pre-stroke mRS, or NAWM diffusivity anisotropy metrics. However, women were less likely to have an excellent outcome (mRS <2: 49.6% vs. 67.0%; p=0.005). In logistic regression analysis, female sex and the interaction of sex with FA, RD, and AD were independent predictors of functional outcome.
Conclusions
Female sex is associated with decreased likelihood of excellent outcome following AIS. The correlation between markers of white matter integrity and functional outcomes in women, but not men, suggests a potential sex-specific mechanism.
Keywords: Leukoaraiosis, brain ischemia, diffusion-weighted imaging
INTRODUCTION
Following acute ischemic stroke (AIS), women have worse functional outcomes and increased likelihood of death compared to men.1, 2 Identifying and understanding the factors that mediate the disparity in sex-specific outcomes after AIS therefore offers a critical opportunity to improve patient care. White matter hyperintensity (WMH) burden represents one factor that has been associated with poor post-stroke functional outcomes.3–6 Moreover, in patients with AIS, normal appearing white matter (NAWM) structural integrity has also been shown to influence post-stroke functional outcomes.7 In this analysis, we characterized sex-specific differences in clinical and neuroimaging variables of post-stroke functional recovery.
METHODS
The details of the study design have been described previously by Etherton et al.7 For additional details of the methods please refer to the online supplement.
Patients greater than 18 years old presenting to our hospital with signs and symptoms of AIS and a brain MRI with a confirmed acute lesion on diffusion-weighted imaging (DWI) and available fluid attenuated inversion recovery (FLAIR) and diffusion tensor imaging (DTI) sequences were eligible for this study. Clinical variables were obtained on admission. Modified Rankin scale (mRS) scores were obtained at 3–6 months post-stroke. Excellent functional outcome was defined as mRS < 2.
Total WMH volume (WMHv) and DWI volume (DWIv) were determined using a semi-automated approach and normalized for differences in head size (nWMHv and nDWIv).8, 9 Using the constructed contralesional NAWM mask, median voxel values were calculated for fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD), and radial diffusivity (RD).7, 10
Logistic regression was performed with a model of age, sex, WMHv, diffusivity metrics, and excellent functional outcome. Paired t-test, Wilcoxon rank sum, and correlation analysis using Spearman or Pearson correlation were performed when appropriate (R version 3.3.2). Statistical significance was set at p-value < 0.05 in all analyses. The authors agree to make available to any researcher the data, methods used in the analysis, and materials used to conduct the research for the express purposes of reproducing the results and with the explicit permission for data sharing by the local IRB.
RESULTS
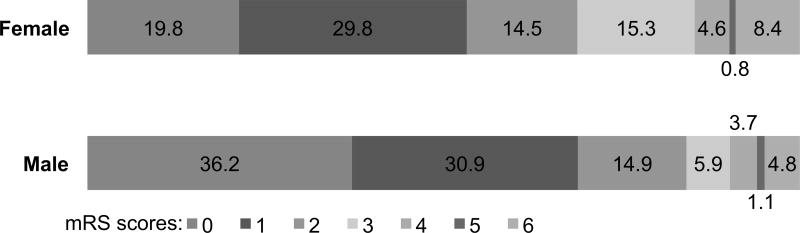
Of an initial 480 participants, 319 participants met criteria for this analysis. Men comprised 58.9% (n=188 subjects) of the cohort. Women were older (68.0 vs. 62.7 years, p=0.004), reported lower rates of tobacco use (21.1% vs. 37.0%, p=0.03), had lower admission diastolic blood pressure (78.6 vs. 83.2 mmHg, p=0.02), and had increased rates of antecedent atrial fibrillation (21.4% vs. 12.2%, p=0.04; Supplementary Table I). There was no significant difference between sexes in pre-stroke mRS, admission NIHSS, DWIv, WMH burden, or rates of administration of intravenous tPA (Supplementary Table I). At three to six months after AIS, there were significantly fewer women with an excellent outcome compared to men (49.6% vs. 67.0%, p=0.005; Figure 1).
Figure 1.
Follow up mRS at 3 to 6 months post AIS according to sex. Horizontal bar chart depicts follow up mRS as a percentage of total patients per sex.
We observed no difference between men and women in contralesional NAWM diffusivity anisotropy metrics (Supplementary Table II). MD, AD, and RD correlated with follow up mRS in both sexes; however, FA was inversely correlated with mRS in women but not men (ρ=−0.182, p=0.048 vs. ρ=−0.096, p=0.201; Supplementary Table III). We observed a statistically significant negative correlation between FA and age in women but not men (women: ρ=−0.259, p= 0.003; men: ρ=−0.087, p= 0.25). NWMHv was positively correlated with mRS in both sexes (women: ρ= 0.221, p=0.015; men: ρ= 0.160, p=0.030). In a logistic regression model of excellent functional outcome (mRS < 2), sex and the interaction of sex with FA, AD, and RD were independently associated with excellent functional outcome (Table 1).
Table 1.
Determinants of excellent functional outcome (mRS<2).
| Variable | Estimate | p-value |
|---|---|---|
| Age | −0.02 | 0.217 |
| Female sex | −39.07 | 0.002 |
| nWMHv | 0.02 | 0.151 |
| FA | −22.07 | 0.166 |
| MD | −9.11 | 0.584 |
| AD | 12.34 | 0.230 |
| RD | −22.08 | 0.265 |
| Age*sex | −0.01 | 0.616 |
| nWMHv*sex | 0.02 | 0.440 |
| FA*sex | 97.39 | 0.002 |
| MD*sex | −2.72 | 0.884 |
| AD*sex | −52.23 | 0.003 |
| RD*sex | 101.82 | 0.003 |
DISCUSSION
In this retrospective analysis of AIS patients, we found that despite no observed differences in stroke severity, WMH burden, or treatment with intravenous tPA, fewer women achieved an excellent functional outcome (mRS < 2) after stroke than men. Whereas the underlying explanation for this observation is unclear, several findings from our study suggest potential associated factors.
In our population, women were older and more likely to have premorbid atrial fibrillation. The advanced age, as compared to men, could contribute to the disparity in functional outcomes and agrees with prior studies demonstrating worse outcomes in women after AIS.1, 2, 11, 12, We also demonstrated that, despite similar WMH burden and contralesional NAWM diffusivity anisotropy metrics, contralesional FA correlated with functional outcomes in women but not men. This observed inverse relationship between FA and mRS suggests that reduced FA may be associated with worse functional outcomes after stroke. These findings are further supported by our logistic regression model demonstrating that female sex was an independent determinant of worse functional outcomes, and that the interaction between sex and multiple diffusivity metrics was independently associated with functional outcomes. We previously demonstrated that the median FA values of contralesional NAWM are predictive of functional outcomes after AIS.7 Moreover, an inverse correlation between FA and age in women, but not men, suggests that there may be sex-specific differences in the loss of white matter microstructural integrity with age. While the tissue integrity of NAWM is negatively impacted by age and hypertension, no sex-specific differences have been reported to date.13 Thus, our data suggest that white matter structural integrity may be a contributing variable on AIS functional outcomes in women.
There are several limitations of the current study that merit consideration in the overall interpretation and applicability of our findings including: exclusion of patients with infratentorial or bilateral strokes; the relatively low stroke severity of our population (median NIHSS 3); lack of data on discharge destination and intensity of rehabilitation; and a potential bias from partial volume averaging occurring because of non-isotropic voxel sizes for DTI analysis. Going forward, a large-scale study looking at clinical and neuroimaging variables in relation to sex-specific functional outcomes is warranted.
Supplementary Material
Acknowledgments
None
Sources of funding: This study was supported in part by NIH-National Institute of Neurological Disorders and Stroke K23NS064052, R01NS082285, P50NS051343 and R01NS086905; National Institute of Biomedical Imaging and Bioengineering (P41EB015896); American Heart Association 17CPOST33680102, American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N); and Deane Institute for Integrative Study of Atrial Fibrillation and Stroke.
Footnotes
Disclosures:
Lisa Cloonan is employed by Decision Resources Group. All other authors report no disclosures.
References
- 1.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: A systematic review. Stroke; a journal of cerebral circulation. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 2.Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac D, Sturm J, et al. Sex differences in long-term mortality after stroke in the instruct (international stroke outcomes study): A meta-analysis of individual participant data. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003436. [DOI] [PubMed] [Google Scholar]
- 3.Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis. 2012;33:525–531. doi: 10.1159/000337335. [DOI] [PubMed] [Google Scholar]
- 5.Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Clinical prediction of functional outcome after ischemic stroke: The surprising importance of periventricular white matter disease and race. Stroke; a journal of cerebral circulation. 2009;40:530–536. doi: 10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YH, Xia ZX, Wei W, Gao GR, Gong JJ, Li Y, et al. The impact of leucoaraiosis on neurological function recovery in elderly patients with acute cerebral infarction: Clinical study involving 279 chinese patients. J Int Med Res. 2014;42:857–862. doi: 10.1177/0300060513507386. [DOI] [PubMed] [Google Scholar]
- 7.Etherton MR, Wu O, Cougo P, Giese AK, Cloonan L, Fitzpatrick KM, et al. Integrity of normal-appearing white matter and functional outcomes after acute ischemic stroke. Neurology. 2017;88:1701–1708. doi: 10.1212/WNL.0000000000003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rost NS, Rahman RM, Biffi A, Smith EE, Kanakis A, Fitzpatrick K, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75:1670–1677. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: A validated method for estimating intracranial volume. J Neuroimaging. 2005;15:76–78. doi: 10.1177/1051228404270243. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, et al. Human acute cerebral ischemia: Detection of changes in water diffusion anisotropy by using mr imaging. Radiology. 1999;212:785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 11.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A International Stroke Trial Collaborative G. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 12.Gall SL, Donnan G, Dewey HM, Macdonell R, Sturm J, Gilligan A, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology. 2010;74:975–981. doi: 10.1212/WNL.0b013e3181d5a48f. [DOI] [PubMed] [Google Scholar]
- 13.Munoz Maniega S, Chappell FM, Valdes Hernandez MC, Armitage PA, Makin SD, Heye AK, et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37:644–656. doi: 10.1177/0271678X16635657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.