Abstract
Objectives
To evaluate the relation between voided volume and void trial “success” to create an algorithm that minimizes the need for postvoid residual volume (PVR) assessment in backfill-assisted void trials.
Methods
This article is an ancillary analysis of deidentified data from a randomized trial evaluating prophylactic antibiotics after urogynecologic surgery. Void trials were routinely performed after surgery; voided volumes, PVR, and void trial outcomes were collected. The void trial regimen was as follows: the bladder was backfilled with 300 mL of normal saline or until the patient reported the urgency to void, the catheter was removed, and the participant was prompted to void immediately. PVR volume was measured either by sonographic bladder scan or catheterization. Voided volumes were categorized in 25-mL increments from 50 to 225 mL. For each voided volume range, the PVR and void trial outcome data were incorporated to calculate sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) in terms of ability of voided volume alone to predict a passing void trial result. An algorithm was created using the voided volumes that optimize PPV and NPV.
Results
The study population included 255 participants. Voided volumes <100 mL and ≥200 mL were identified as optimal thresholds to predict failure and passage of backfill-assisted void trials, respectively. When patients voided <100 mL, 3% passed their void trial (NPV odds ratio 96.7, 95% confidence interval 88.6–99.5). When patients voided ≥200 mL, 97% passed (PPV odds ratio 97.4, 95% confidence interval 93.5–99.3).
Conclusions
We propose an algorithm for void trials after urogynecologic surgery. After backfilling the bladder if voided volume is ≥200 mL, the void trial is successful and no PVR is needed; if voided volume is between 100 and 199 mL, the void trial is indeterminate and PVR is recommended; and if voided volume is <100 mL, the void trial is unsuccessful and catheterization is needed. Applying this algorithm to our study population would have eliminated the need for PVR in 85% of patients. Calculated PPVs and NPVs depend on the prevalence of voiding dysfunction in the population being studied, and therefore may be unique to our institution.
Keywords: postoperative care algorithm, postoperative voiding dysfunction, postvoid residual, urogynecologic surgery, void trial
An increasing number of women undergo surgery for pelvic organ prolapse, stress urinary incontinence, or both each year.1,2 Postoperative voiding dysfunction is common following these procedures. Historically, after open colposuspenion and bladder neck sling surgeries, suprapubic catheters or indwelling transurethral Foley catheters were routinely placed for several days to weeks until normal voiding function was ensured.3–7 More recently, with the widespread adoption of minimally invasive surgery and the advent of midurethral slings, the rate and the duration of postoperative catheterization have been reduced.8–10 Postoperative voiding dysfunction continues to be a significant concern, however, with a reported incidence after pelvic reconstructive surgery ranging from 11% to 84% in some studies.8,11–18
Void trials are still routinely performed after reconstructive pelvic surgery and usually involve either spontaneous bladder filling or bladder backfill, followed by an attempted void and measurement of the postvoid residual volume (PVR).19 Studies have shown that the backfill-assisted void trial is faster and preferred by patients.13,16,20 The backfill-assisted method, however, requires a PVR measurement, which is a time-consuming step that increases nurse workload, patient anxiety, and if obtained via catheterization, potential for patient discomfort. The PVR values used to define adequate voiding have been variably defined. In some studies a predetermined PVR is used as demonstration of return to normal voiding; other studies use a percentage of total bladder volume, with no guideline as to what is acceptable.21,22 Studies have examined ways to avoid PVR measurement, but these studies require additional nursing and patient education and have been conducted only in patients undergoing midurethral sling procedures.23,24
We hypothesized that it would be possible to develop a protocol that greatly minimizes the need for PVR assessment and that can be used in the majority of patients undergoing surgery for prolapse as well as stress incontinence, without the need for additional patient or nurse education. When patients void a small amount of the backfilled volume, there is little chance that they have voided adequately; conversely, when patients void the majority of the backfilled volume, there is a high probability that their bladder function has recovered. Our primary aim was to examine outcomes of backfill-assisted void trials in patients who underwent pelvic reconstructive surgery to determine whether certain voided volumes predict the final void trial result and, if so, to define the upper and lower thresholds of voided volume at which the PVR assessment is not necessary.
Methods
This study was initiated after the Duke University Medical Center institutional review board granted exempt status. All of the subjects underwent urogynecologic surgery between August 2011 and February 2013. This study was an ancillary analysis of data collected during a previously performed randomized controlled trial (RCT) of antibiotics compared with placebo to prevent urinary tract infection (UTI) after urogynecologic surgery.25 All of the women included in the parent RCT were eligible. Inclusion criteria for the RCT stipulated that women were undergoing surgery for pelvic organ prolapse, stress urinary incontinence, or both. Exclusion criteria for the RCT were age younger than 21 years, pregnancy, allergy to nitrofurantoin, dependence on catheterization to accomplish voiding preoperatively, or no facility with the English language. Patients undergoing fistula repair, sacral neuromodulation surgery, vaginal mesh excision, or diverticulum repair also were excluded. In addition, women being treated for UTI at the time of surgery or women with a preoperative creatinine clearance of <60 mL/min were excluded from the parent RCT. For this ancillary analysis, patients who did not have recorded void trial data also were excluded.
We retrospectively reviewed deidentified research records to gather data on participant demographics; past obstetric, gynecologic, medical and surgical histories; body mass index; pelvic organ prolapse quantification scores; preoperative baseline PVR; surgical procedure(s) performed; operative characteristics such as estimated blood loss, operative time, and type of anesthesia; and postoperative catheterization data. Additional information was collected, including voided volumes, PVRs, and void trial outcomes (ie, pass/fail) from the last void trial before discharge. Per protocol, void trials from the parent RCT were performed via the backfill-assisted method. The bladder was backfilled with 300 mL of normal saline or until the patient reported the urgency to void, the catheter was removed, and the participant was prompted to void immediately. PVR volume was measured after voiding either by sonographic bladder scan or catheterization. A priori criteria for passing the void trial were a PVR <100 mL or a PVR <50% of the total bladder volume (PVR + voided volume), with a voided volume ≥200 mL. This was consistent with the standard of care at the time of the RCT.
For analysis, the voided volumes were categorized into increments of 25 mL from 50 to 225 mL. For each voided volume range (eg, voided volume <50 mL or >50 mL), we assessed the percentage of participants who passed and failed the void trial. The sensitivity, specificity, and positive predictive values (PPVs) and negative predictive values (NPVs) were assessed for a passing result for each incremental volume range. Because the PPV and NPV are the most applicable predictors when assessing the clinical utility of a test, these two values were used to create the algorithm. The optimal PPV and NPV combinations were identified to create two thresholds: a lower voided volume threshold at which PVR assessment would be unnecessary because the chance of passing the void trial was minimal to none, and an upper voided volume threshold at which PVR would be unnecessary because the chance of passing the void trial was extremely high. Using these lower and upper volume thresholds, the algorithm was created for a PVR-minimizing, backfill-assisted void trial protocol. To validate the upper voided volume threshold, a receiver operating characteristic (ROC) curve was created to examine the voided volume that produced the maximum sensitivity in predicting void trial success. Using these thresholds, the proportions of participants from the parent RCT in which a PVR could have been avoided were calculated.
Categorical variables were analyzed using the χ2 test and continuous variables were analyzed using the Student t test to assess whether patient or surgical factors were associated with the void trial outcome. Significance was determined at P ≤ 0.05. Statistical analysis was performed using SPSS version 20.0 (IBM SPSS Statistics, Armonk, NY) and ROC analysis was performed using JMP Pro 11 (SAS Institute, Cary, NC).
Results
Of the 264 women in the parent randomized trial, 255 had the necessary void trial data and were included. The preoperative, intraoperative, and postoperative characteristics of the study population are presented in Table 1.
Table 1.
Participant characteristics (N = 255)
| Age, y | 56 (±12) |
| BMI, kg/m2 | 29 (±6) |
| Diabetes mellitus (%) | 31 (12) |
| Tobacco use (%) | 47 (18) |
| POP-Q stage | 2 (2–3) |
| Preoperative PVR, mL | 74.23 (±108) |
| Postoperative void trial, mL | |
| Postoperative PVR | 136 (±177) |
| Voided volume | 221 (±203) |
| Total surgical time, min | 152 (±97) |
| Estimated blood loss, mL | 85 (±122) |
| Surgical procedures (%) | |
| Midurethral sling | 144 (54) |
| Hysterectomy | 90 (34) |
| Anterior repair | 75 (28) |
| Posterior repair | 72 (27) |
| Vault suspension | 119 (45) |
| Obliterative prolapse repair | 10 (4) |
| Anesthesia administered (%) | |
| General | 231 (88) |
| MAC | 24 (9) |
| Neuraxial | 11 (4) |
Data reported as means ± standard deviations, n (%) or median (interquartile range 25th–75th percentile). BMI, body mass index; MAC, monitored anesthesia care; POP-Q, pelvic organ prolapse quantification; PVR, postvoid residual volume.
Overall, 69% of patients passed their void trial and were discharged home without catheterization. Table 2 shows the participants with voided volume at or above the voided volume threshold; the percentage of participants who passed and failed the void trial; and the sensitivity, specificity, PPV, and NPV for correctly identifying a passing void trial result. We identified a lower voided volume threshold of 100 mL and an upper voided volume threshold of 200 mL as the optimal thresholds that would minimize the need for PVR assessment. When patients in the study population voided <100 mL, the NPV was 96.7%, meaning that a patient who voided <100 mL had a 96.7% chance of failing the void trial. When patients voided ≥200 mL, the PPV was 97.0%, meaning that a patient who voided ≥200 mL had a 97.0% chance of passing the void trial after calculating the voiding efficiency. As such, we propose the following backfill-assisted void trial algorithm (Fig. 1):
Table 2.
Voided volume range; void trial pass and fail rates; and sensitivity, specificity, PPV, and NPV for chance of successful void trial
| Voided vol, mL | Subjects with voided vol at or above threshold (N = 255) | Void trial result | Successful void trial outcome | ||||
|---|---|---|---|---|---|---|---|
| Pass | Fail | Sensitivity | Specificity | PPV | NPV | ||
| ≥ 50 | 213 | 176 (82.6) | 37 (17.4) | 100 (97.9–100) | 53.2 (41.6–64.5) | 82.6 (76.9–87.5) | 100 (91.5–100) |
| ≥ 75 | 200 | 174 (87.0) | 26 (13.0) | 98.9 (96.0–99.8) | 67.1 (55.6–77.3) | 87.0 (81.5–91.3) | 96.4 (87.5–99.5) |
| ≥ 100 | 194 | 174 (89.7) | 20 (10.3) | 98.9 (96.0–99.8) | 74.7 (63.6–83.8) | 89.7 (84.5–93.6) | 96.7 (88.6–99.5) |
| ≥ 125 | 181 | 168 (92.8) | 13 (7.2) | 95.5 (91.2–98.0) | 83.5 (73.5–90.9) | 92.8 (88.0–96.1) | 89.2 (79.8–95.2) |
| ≥ 150 | 179 | 168 (93.9) | 11 (6.1) | 95.5 (91.2–98.0) | 86.1 (76.5–92.8) | 93.9 (89.3–96.9) | 89.5 (80.3–95.3) |
| ≥ 175 | 171 | 164 (95.9) | 7 (4.1) | 93.2 (88.4–96.4) | 91.1 (82.6–96.4) | 95.9 (91.7–98.3) | 85.7 (76.4–92.4) |
| ≥ 200 | 155 | 151 (97.4) | 4 (2.6) | 85.8 (79.8–90.6) | 94.9 (87.5–98.6) | 97.4 (93.5–99.3) | 75.0 (65.3–83.1) |
| ≥ 225 | 118 | 116 (98.3) | 2 (1.7) | 65.9 (58.4–72.9) | 97.5 (91.1–99.6) | 98.3 (94.0–99.8) | 56.2 (47.5–64.7) |
Pass and fail results are shown as N (percentage), with percentages calculated from the total N for that row (ie, total number of patients who voided at or above the specified voided volume). Sensitivity, specificity, PPV, and NPV are calculated for the ability of voided volume to predict a successful void trial (ie, passing result) and are shown as % (95% confidence interval). NPV, negative predictive value; PPV, positive predictive value.
Fig. 1.
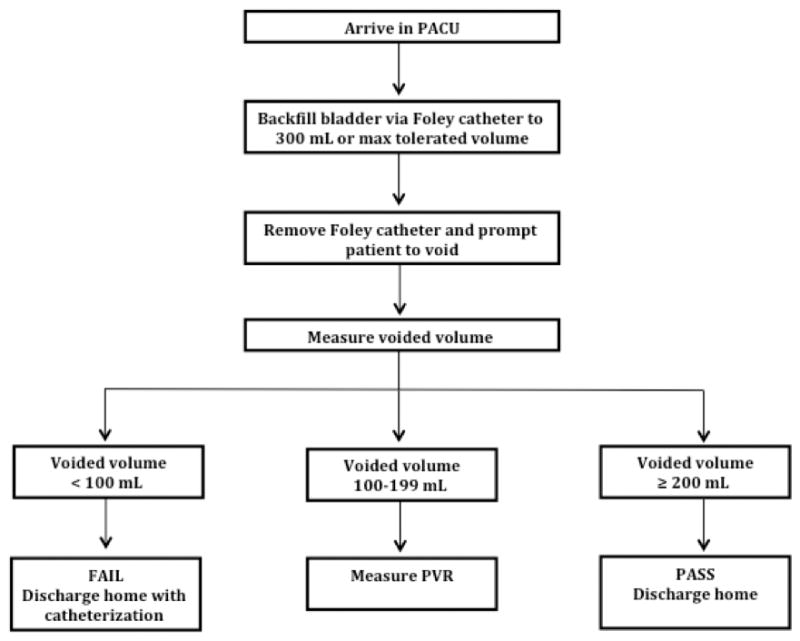
Flowchart of proposed void trial algorithm. PACU, postanesthesia care unit.
If the patient voids ≥200 mL, the void trial is successful and a PVR is unnecessary.
If the patient voids between 100 and 199 mL, the void trial is indeterminate and PVR assessment is recommended.
If the patient voids <100 mL, the void trial is unsuccessful, PVR is unnecessary, and catheterization is indicated.
If the proposed algorithm were used in our study population, it would have eliminated the need for PVR measurement in the majority (85%, 216 of 255) of patients.
The calculated sensitivity and specificity for voided volume to predict void trial success is illustrated in the ROC curve in Figure 2. The area under the curve (AUC) was 0.97. Because an AUC of 1 indicates that a test can perfectly predict an outcome, the data indicate that voided volume alone performed exceedingly well as a predictor of final void trial results. Based on the ROC curve, the maximal AUC, indicating the voided volume that best predicted void trial success, was 180 mL. This cutoff had a sensitivity of 92.0% and a specificity of 94.0% in predicting void trial results. The proposed upper threshold of 200 mL in the algorithm uses a slightly more conservative (higher) voided volume to determine void trial success.
Fig. 2.
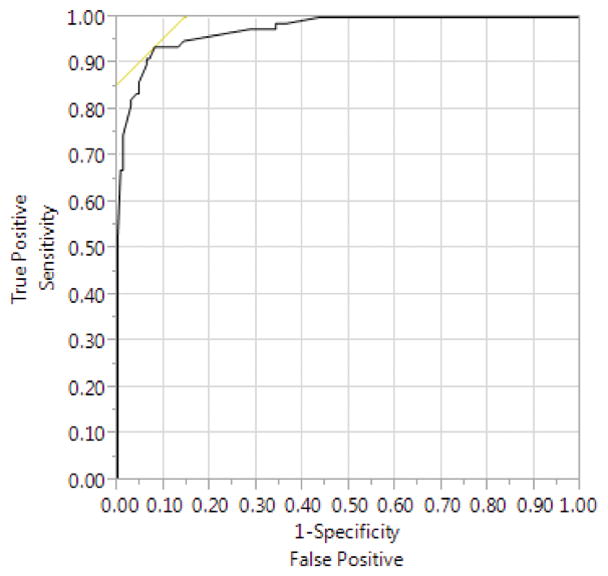
Receiver operating characteristic curve depicting test characteristics (sensitivity and specificity) when using postoperative voided volume to predict void trial success.
After evaluating all of the variables collected, midurethral sling surgery was the only factor that achieved statistical significance, such that patients with a sling procedure had a higher void trial pass rate (78.8% passed with sling vs 60.6% passed without sling, P = 0.002). We repeated our original analyses in the subpopulations of patients who did and did not undergo midurethral sling surgery and the results were unchanged.
Of note, 2 (0.8%) women voided <100 mL and subsequently passed their void trial after calculating voiding efficiency, whereas 4 (1.6%) women voided ≥200 mL and failed the void trial. Of the 2 who voided <100 mL and passed, the actual backfill volume was not recorded for one and for the other the voided volume was 50 mL with a PVR of 27 mL. Both of these instances suggest that nonstandard procedures were applied for those backfills. Of the 4 patients who voided ≥200 mL, all 4 were backfilled to 300 mL and 3 of the 4 had PVR measurements greater than their voided volume (263–700 mL). Two of these patients were discharged home with indwelling Foley catheters and two with intermittent self-catheterization, and the duration of catheterization ranged from 1 to 4 days.
Discussion
Based on the results, we propose a new algorithm to expedite postoperative void trials in a urogynecologic surgical population. With the use of this proposed algorithm, we estimate that the majority of patients (>80%) would no longer require a PVR assessment. The ability to avoid PVR can reduce total time in the postanesthesia care unit without necessitating any additional work or training for nursing staff and in fact would reduce nursing workload by avoiding the additional step of a PVR measurement. A further benefit to this proposed voiding trial algorithm may be a reduction in patient anxiety.
Two other studies specifically have examined ways to avoid PVR assessment and streamline postoperative void trials. Ingber and colleagues prospectively investigated a patient’s subjective force of urinary stream after a backfill-assisted void trial to predict the need for catheterization following a midurethral sling procedure.23 This study had a 92% void trial pass rate, defined as the postoperative force of stream being ≥50% as compared with the preoperative force of stream. A PVR measurement was then assessed on all of the patients. No patients in the study had an unexpected emergency department or clinic visit for urinary retention.23 It is important to note that their criteria for passing the void trial was a PVR <500 mL, which is far more lenient than the void trial cutoffs used in our study. Given that none of the patients in study by Ingber and colleagues returned with urinary retention when using a much more lenient PVR volume, we are further reassured that our algorithm is conservative and safe. Tunitsky-Bitton et al performed a prospective study of 108 women undergoing midurethral sling surgery randomly selected for a standard backfill-assisted void trial compared with a subjective force-of-stream void trial.24 To pass the standard void trial, participants had to void two-thirds of the instilled volume. To pass the force-of-stream void trial, participants had to report a force of stream of ≥50% of baseline. The two groups were found to have a similar rate of void trial failure, approximately 25%, and subjective force of stream was moderately correlated (Spearman ρ 0.5, P < 0.001) with voided volume.24 Although this method of assessing subjective force of stream could avoid a PVR measurement, there are several limitations in both of these studies. The force-of-stream approach requires additional patient and nurse training and allows for potential patient bias (ie, patients may be motivated to report an increased force of stream knowing that their assessment will influence the decision to be discharged with or without a catheter). Furthermore, in patients with an altered force of stream before surgery (ie force of stream altered secondary to prolapse), the ability of the patient to judge normal force of stream after prolapse repair and in the acute postoperative setting may be limited. The effect of prolapse repair on force-of-stream perception has not been studied given that the studies by Ingber et al and Tunitsky-Bitton and colleagues only included patients undergoing midurethral sling surgery. Ingber et al still assessed PVR in all patients, and Tunitsky-Bitton et al did not report the percentage of patients who required a PVR assessment; as such, we are not able to determine whether a force-of-stream void trial approach does indeed reduce how often PVR assessment is performed.
We found a higher void trial pass rate among participants who underwent midurethral sling procedures as compared with those who did not. This differs from other studies reporting that patients who undergo a midurethral sling are at a higher risk of postoperative voiding dysfunction.23,26,27 Book et al did find that patients undergoing midurethral sling had significantly higher void trial pass rates than did patients following posterior colporrhaphy and conjectured that this difference may have been related to less postoperative pain after a midurethral sling alone.18 The discrepancy seen in our data could be related to differences in postoperative pain or in criteria set for passing a void trial, or it could be simply a statistical anomaly. Regardless, the voided volume cutoffs remained unchanged when analyzing patients who underwent a sling procedure as well as those who did not, thereby demonstrating that the algorithm is applicable to patients undergoing reconstructive pelvic surgery, regardless of concomitant midurethral sling.
The strengths of our study include the use of prospectively gathered rigorous data in a relatively large patient population. It is important to note that the patients included in this study underwent a variety of urogynecologic procedures, making this algorithm generalizable to patients undergoing a variety of urogynecologic surgeries. Because this was an ancillary study using data collected as part of a larger RCT, it is important to consider that the data were not collected with the intent of studying void trial outcomes; however, the void trial and resultant data were collected per research study standards in a prospective fashion. One limitation is that the algorithm is based on results from a backfill void trial method and may not be applicable to other void trial methods. Furthermore, the calculated PPVs and NPVs depend on the prevalence of voiding dysfunction in the population being studied, and therefore may be unique to our institution. In addition, patients with low creatinine clearance were excluded from the parent RCT, making our data potentially less generalizable to older women with a lower body mass index.
As with any algorithm that is based on PPVs and NPVs, there will be outliers. For example, if a patient voids ≥200 mL, then she is discharged without catheterization. There is a chance ultimately that she could have postoperative urinary retention requiring further intervention. This is the most feared outcome in this scenario and therefore our study used a more conservative upper limit of voided volume in the algorithm to minimize this risk (ie, 200 mL instead of 180 mL, as suggested by ROC analysis). The results of Ingber and colleagues further support this conservative threshold because they had no patients with urinary retention, despite tolerating PVR volumes up to 500 mL.23 Urinary retention is still possible in patients who are discharged after undergoing a backfill-assisted void trial with routine PVR assessment; therefore, all patients should be counseled regarding the signs and symptoms of urinary retention and instructed to call for or seek evaluation if they are not emptying sufficiently following surgery. More important, although urinary retention is a significant concern, it also must be considered that being too conservative on void trial cutoffs increases the risk of UTI because of increasing exposure to and duration of catheterization. As such, finding a way to maximize efficiency while minimizing unnecessary catheterization is of high clinical importance, especially as catheter-associated UTI becomes a more prominent issue. In addition, short-term bladder catheterization has an impact on quality of life, and a questionnaire has been developed to evaluate short-term catheter burden.28 Future studies, including a prospective analysis of the proposed algorithm, are needed to validate the results, confirm that this is a safe alternative, and evaluate patient satisfaction.
The proposed algorithm for a PVR-minimizing postoperative void trial provides a more streamlined approach to the traditional backfill postoperative void trial for patients undergoing urogynecologic surgery. By avoiding PVR measurement in the majority of patients, this algorithm has the potential to reduce nursing workload and make a common postoperative process more efficient and more satisfying for the patient.
Key Points.
Postoperative voiding dysfunction is still a significant concern, with a reported incidence after pelvic reconstructive surgery ranging from 11% to 84%.
We propose an algorithm for void trials after urogynecologic surgery. After backfilling the bladder: if voided volume is ≥200 mL, the void trial is successful and no postvoid residual volume (PVR) is needed; if voided volume is between 100 and 199 mL, the void trial is indeterminate and PVR is recommended; and if voided volume is <100 mL, the void trial is unsuccessful and catheterization is needed.
By avoiding PVR measurement in the majority of patients, this algorithm has the potential to reduce nursing workload and make a common postoperative process more efficient and more satisfying for the patient.
Footnotes
To purchase a single copy of this article, visit sma.org/smj-home. To purchase larger reprint quantities, please contact Reprintsolutions@wolterskluwer.com.
N.Y.S. has received compensation from the National Institute of Diabetes and Digestive and Kidney Diseases, Medtronic, the Institute for Surgical Excellence, and Intuitive Surgical. C.L.A. has received compensation from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors did not report any financial relationships or conflicts of interest.
References
- 1.Oliphant SS, Wang L, Bunker CH, et al. Trends in stress urinary incontinence inpatient procedures in the United States, 1979–2004. Am J Obstet Gynecol. 2009;200:521e1–e6. doi: 10.1016/j.ajog.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieter AA, Wilkins MF, Wu JM. Epidemiological trends and future care needs for pelvic floor disorders. Curr Opin Obstet Gynecol. 2015;27:380–384. doi: 10.1097/GCO.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobak WH, Walters MD, Piedmonte MR. Determinants of voiding after three types of incontinence surgery: a multivariable analysis. Obstet Gynecol. 2001;97:86–91. doi: 10.1016/s0029-7844(00)01103-0. [DOI] [PubMed] [Google Scholar]
- 4.Sze EH, Miklos JR, Karram MM. Voiding after Burch colposuspension and effects of concomitant pelvic surgery: correlation with preoperative voiding mechanism. Obstet Gynecol. 1996;88(4 Pt 1):564–567. doi: 10.1016/0029-7844(96)00238-4. [DOI] [PubMed] [Google Scholar]
- 5.Brady CM, Ahmed I, Drumm J, et al. A prospective evaluation of the efficiency of early postoperative bladder emptying after the Stamey procedure or pubovaginal sling for stress urinary incontinence. J Urol. 2001;165:1601–1604. [PubMed] [Google Scholar]
- 6.Bidmead J, Cardozo L. Retropubic urethropexy (Burch colposuspension) Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:262–265. doi: 10.1007/s001920170050. [DOI] [PubMed] [Google Scholar]
- 7.Smith RN, Cardozo L. Early voiding difficulty after colposuspension. Br J Urol. 1997;80:911–914. doi: 10.1046/j.1464-410x.1997.00480.x. [DOI] [PubMed] [Google Scholar]
- 8.Norton PA, Nager CW, Chai TC, et al. Risk factors for incomplete bladder emptying after midurethral sling. Urology. 2013;82:1038–1041. doi: 10.1016/j.urology.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375. doi: 10.1002/14651858.CD006375.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Natale F, La Penna C, Saltari M, et al. Voiding dysfunction after anti-incontinence surgery. Minerva Ginecol. 2009;61:167–172. [PubMed] [Google Scholar]
- 11.Vierhout ME. Prolonged catheterization after vaginal prolapse surgery. Acta Obstet Gynecol Scand. 1998;77:997–999. [PubMed] [Google Scholar]
- 12.Hakvoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG. 2011;118:1055–1060. doi: 10.1111/j.1471-0528.2011.02935.x. [DOI] [PubMed] [Google Scholar]
- 13.Foster RT, Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627e1–e4. doi: 10.1016/j.ajog.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Wohlrab KJ, Erekson EA, Korbly NB, et al. The association between regional anesthesia and acute postoperative urinary retention in women undergoing outpatient midurethral sling procedures. Am J Obstet Gynecol. 2009;200:571e1–571.e5. doi: 10.1016/j.ajog.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partoll LM. Efficacy of tension-free vaginal tape with other pelvic reconstructive surgery. Am J Obstet Gynecol. 2002;186:1292–1298. doi: 10.1067/mob.2002.123736. [DOI] [PubMed] [Google Scholar]
- 16.Geller EJ. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Womens Health. 2014;6:829–838. doi: 10.2147/IJWH.S55383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter PJ, Dieter AA, Siddiqui NY, et al. Perioperative anticholinergic medications and risk of catheterization after urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2014;20:163–167. doi: 10.1097/SPV.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 18.Book NM, Novi B, Novi JM, et al. Postoperative voiding dysfunction following posterior colporrhaphy. Female Pelvic Med Reconstr Surg. 2012;18:32–34. doi: 10.1097/SPV.0b013e31824041a4. [DOI] [PubMed] [Google Scholar]
- 19.Hakvoort RA, Burger MP, Emanuel MH, et al. A nationwide survey to measure practice variation of catheterisation management in patients undergoing vaginal prolapse surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:813–818. doi: 10.1007/s00192-009-0847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulvino JQ, Duecy EE, Buchsbaum GM, et al. Comparison of 2 techniques to predict voiding efficiency after inpatient urogynecologic surgery. J Urol. 2010;184:1408–1412. doi: 10.1016/j.juro.2010.05.096. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney DD, Leng WW. Treatment of postoperative voiding dysfunction following incontinence surgery. Curr Urol Rep. 2005;6:365–370. doi: 10.1007/s11934-005-0055-9. [DOI] [PubMed] [Google Scholar]
- 22.Delorme E, Droupy S, de Tayrac R, et al. Transobturator tape (Uratape): a new minimally-invasive procedure to treat female urinary incontinence. Eur Urol. 2004;45:203–207. doi: 10.1016/j.eururo.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Ingber MS, Vasavada SP, Moore CK, et al. Force of stream after sling therapy: safety and efficacy of rapid discharge care pathway based on subjective patient report. J Urol. 2011;185:993–997. doi: 10.1016/j.juro.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 24.Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597e1–597.e9. doi: 10.1016/j.ajog.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96–103. doi: 10.1097/AOG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 26.Al-Badr A, Ross S, Soroka D, et al. Voiding patterns and urodynamics after a tension-free vaginal tape procedure. J Obstet Gynaecol Can. 2003;25:725–730. doi: 10.1016/s1701-2163(16)31001-5. [DOI] [PubMed] [Google Scholar]
- 27.Mutone N, Brizendine E, Hale D. Factors that influence voiding function after the tension-free vaginal tape procedure for stress urinary incontinence. Am J Obstet Gynecol. 2003;188:1477–1483. doi: 10.1067/mob.2003.453. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter JS, Heit M, Rand KL. Development and psychometric properties of a measure of catheter burden with bladder drainage after pelvic reconstructive surgery. Neurourol Urodyn. 2017;36:1140–1146. doi: 10.1002/nau.23077. [DOI] [PubMed] [Google Scholar]


