Abstract
Objective
To evaluate endometrial BCL6 expression as a prognostic biomarker for In Vitro Fertilization (IVF) outcome in women with unexplained infertility (UI) prior to embryo transfer.
Design
Prospective cohort study.
Setting
University associated infertility clinic.
Patients
Women with UI for greater than 1 year.
Interventions
We studied women with UI who underwent testing for endometrial BCL6, in an LH-timed mid-luteal phase biopsy and completed an IVF cycle and embryo transfer.
Main Outcome Measure(s)
Clinical pregnancy rate (CPR) and live birth rate (LBR) per transfer was compared for women positive or negative for BCL6 expression. An abnormal BCL6 result was defined by an HSCORE (> 1.4).
Results
Women with normal and abnormal BCL6 and those who conceived or not had similar characteristics. Women with low levels of BCL6 expression had a significantly higher CPR (11/17; 64.7%; 95%CI: 41.3 to 82.6), compared to women with abnormal (high) BCL6 expression (9/52; 17.3%; 95%CI: 9.3 to 30.8). These results yield a relative risk (RR) of 0.267 (95%CI: 0.13 to 0.53; p = 0.0004) for those with normal BCL6 expression, an absolute benefit (AB) of 47.4% (95%CI: 22.5 to 72). LBR was also significantly higher in women with low BCL6 expression(10/17; 58.8; 95%CI: 36 to 78.4), compared to women with abnormal BCL6 expression (6/52; 11.5%; 95%CI: 5.4 to 23). The RR was 0.19 (95%CI: 0.08 to 0.45; p = 0.0002), yielding an AB of 47.3% (95%CI: 21.8 to 67.8).
Conclusions
Aberrant BCL6 expression (> 1.4 HSCORE) was strongly associated with poor reproductive outcomes in IVF cycles in women with UI.
Keywords: Endometrium, BCL6, prognosis, IVF, pregnancy
BACKGROUND
The first In Vitro Fertilization (IVF) pregnancy resulted in the birth of Louise Joy Brown. Today, nearly 40 years later, there are nearly 200,000 cycles performed annually in the US alone (1), but success rates for live birth remains below 50%. Even when Preimplantation Genetic Screening (PGS)-defined euploid embryos are transferred, nearly half fail to implant (2). This observation strongly suggests the presence of an undiagnosed endometrial deficit that contributes to IVF success or failure. While studies have suggested that endometriosis does not alter IVF outcomes (3), the diagnosis of endometriosis is listed in only 3–4% of cases in the most recent SART database (4), well below the expected prevalence in an infertile population. Indeed, endometriosis is likely present in up to 50% of infertile women (5) and up to 70% of patients with endometriosis will not have a live birth (6). Older studies have pointed to oocyte quality as a cause of IVF failure associated with endometriosis (7). A more recent, larger study, using fertilized sibling oocytes transferred into women with and without endometriosis, demonstrated reduced implantation, clinical pregnancy rate (CPR), ongoing pregnancy rate and live birth rate in women with endometriosis, in support of defects in endometrial receptivity as a cause of IVF failure (8). Given how common this disease is in the infertile population, the question remains whether endometriosis is a hidden cause of implantation failure in IVF (9).
Undiagnosed endometriosis was the focus of a study by Littman and Guidice who studied women with unexplained IVF failure (10). At laparoscopy, most were found to have endometriosis, and many conceived naturally without IVF, once the disease was identified and treated. Such studies recognize a need to identify less invasive, non-surgical methods to predict endometriosis before starting IVF, especially in women with unexplained infertility (UI).
We recently reported that a majority of women with UI over-express the protein BCL6, a new biomarker for the presence of endometriosis (11). BCL-6 is a proto-oncogene and transcriptional repressor that contributes to cell cycle control and differentiation, as well as apoptosis inhibition (12,13). The over-expression has been associated with increased cellular proliferation (14) and BCL6 is stabilized by STAT3 activation and stimulates cytokine expression, including IL-1, IL-6 and IL-18 (15–17). We recently showed that endometrial BCL6 pairs with a histone deacetylase sirtuin-1 (SIRT1), which is also aberrantly expressed in response to activated KRAS in endometriosis (18). Together, SIRT1/BCL6 complex binds to and inactivates key regulators of progesterone action in the endometrium such as Gli in women with endometriosis. Progesterone is essential for establishment of pregnancy and BCL6 appears to be a central cause of progesterone resistance.
Aberrant BCL6 expression above a 1.4 HSCORE cut-off has a high sensitivity and specificity for the diagnosis of all stages of endometriosis (11). The objective of this study was to use BCL6 as a surrogate biomarker for endometriosis and to determine if aberrant BCL6 predicts IVF outcome in a population of women with otherwise unexplained difficulty conceiving.
MATERIALS AND METHODS
Study design/Setting
This cohort study was conducted (recruitment, exposure, follow-up, and data collection) between January 1, 2008 and December 31, 2016, at the Fertility Center of the Carolinas in Greenville Health System, South Carolina, USA. Institutional Review Board was approved by the Committee for the Protection of Human Subjects (GHS #00013885).
Participants
Ovulatory women with normal male partners with at least 1 year of infertility were invited for this cohort study. To be included, each woman was required to have regular cyclic menses (25 to 32 days apart), partners with normal sperm parameters according to the World Health Organization (19), and at least one patent fallopian tube. All patients underwent an LH-timed endometrial biopsy 7 to 10 days after ovulation, performed within 6 months prior to IVF. Only fresh IVF cycles with embryo transfer were included, and none received surgical or medical suppression of endometriosis prior to their IVF cycle. Exclusion included the discovery of significant fibroids (> 4 cm), male factor infertility, endometritis on endometrial biopsy or lack of adequate tissue for analysis on the biopsy result.
Endometrial biopsies
Endometrial biopsy was performed in all participants using a pipelle device (Cooper Surgical, Trumbull, CT), 7 to 10 days after a urinary LH surge. Endometrial biopsies were placed in 10% buffered formalin and transported to the Pathology Laboratory for paraffin embedding and sectioning and immunostaining. The menstrual cycle stage was determined according to Noyes et al. (20).
Immunohistochemistry
Immunohistochemistry was performed on an automated system by a certified Pathologist (Pathology Associates, GHS, Greenville, South Carolina) using the Bond immunostainer platform (Leica Biosystems, Buffalo Grove, IL). Sections of endometrium from archived blocks were stained for BCL6, using an automated system, with clone LN22 as primary antibody (Leica Biosystems), as previously described (11). Lymph nodes served as a positive external controls. Adequacy of the endometrium sample was a requirement for inclusion in this study.
BCL6 expression was assigned a histological score (HSCORE), which ranged from 0 to 4. HSCORE was calculated using the following equation: HSCORE =Σ Pi (i+1)/100, where i= intensity of staining with a value of 1, 2, or 3, (weak, moderate or strong, respectively) and Pi is the percentage of stained epithelial cells for each intensity, varying from 0–100% as previously described (21). All HSCOREs were assigned in a blinded fashion without knowledge of the clinical history or outcome.
Variables
The following variables were analyzed: age, body mass index (BMI), peak estradiol, days of stimulation, number of oocytes retrieved, fertilization rate, number of embryos transferred, clinical pregnancy rate (CPR), live birth rates (LBR) and median values of BCL6 expression as a continuous variable. Positive and negative BCL6 expression were based on HSCORE results (normal ≤1.4; overexpressed > 1.4). CPR was defined as a pregnancy documented by ultrasound that shows a gestational sac in the uterus with a cardiac activity. A positive and rising hCG with an early loss without evidence an intrauterine sac on ultrasound (biochemical pregnancy) was not counted as a pregnancy.
Data sources/measurement
Data from the variables were obtained from SART database and medical records. They were analyzed as mean (± standard deviation) or as median (range), depending if they had passed the normality test for normal distribution. BCL6 positivity was judged as abnormal if the HSCORE was > 1.4, as defined by Receiving Operating Characteristic (ROC) curve analysis for the diagnosis of endometriosis (11).
Bias
Two researchers (BAL, KRB) verified the electronic records (SART database) independently to reduce bias. The biopsies were read by a single blinded pathologist without knowledge of IVF outcome. The pregnancy tests and ultrasound results were performed without knowledge of the BCL6 results.
Study size
Calculation of sample size as a prognostic factor for pregnancy was performed according to the literature (22). All calculations considered an alpha error of 5% and a power of 80%. We expected that 80% of patients will have an abnormal over-expression of BCL6. We considered a clinically significant relative risk (i.e., RR≤0.2), as suggested in the literature (23), for those that have normal expression of BCL6; with these values, it would expect at least 19 cases of pregnancy in the cohort; with a mean follow-up of 6 months, a cumulative pregnancy rate of 60% and 20% in those with normal and abnormal BCL6, respectively, it would be necessary to have at least 65 subjects in the cohort, 52 in the group with abnormal BCL6 expression and 13 in the group with normal BCL6.
In comparing the proportion of BCL6 between two groups, we expected that aberrant BCL6 expression would be present in 80% of the population, and 20% would have a normal (low) expression of BCL6 (11). With this in mind, sample size was calculated according to the literature (24) and it was verified that at least 14 patients (7 normal and 7 over-expressed BCL6) were required to have a 80% chance of detecting, as significant at the 5% level, an over-expression of BCL6 in 80% of the non-pregnant group, while BCL6 would be over-expressed in 20% in the normal (pregnant) group.
Quantitative variables
Age (years-old and months), BMI and peak estradiol were analyzed as continuous variables. Days of stimulation, number of oocytes retrieved, fertilized oocytes, number of embryos transferred were analyzed as nominal variables. CPR and LBR were analyzed as percentages, while positive or negative BCL6 were analyzed as categorical variable. BCL6 expression (normal/abnormal) was compared to the outcome of CPR (pregnant, non-pregnant) or LBR. Groups were divided into normal and aberrant BCL6 because they were considered as prognostic factors for CPR and LBR.
Statistical methods
Fisher’s exact test, relative risk and 95% confidence intervals were used for comparisons of categorical data. Parametric data were compared between groups using Student t-test if data had a Gaussian distribution. Gaussian distribution was verified by D’Agostino & Pearson omnibus normality test. Mann-Whitney U test was used if Gaussian distribution was not present. Statistical analysis was performed with GraphPad Prism version 6.00 for Mac, (GraphPad Software, La Jolla California, USA).
RESULTS
Participants and descriptive data
A total of 70 patients met the inclusion criteria and 69 completed fresh IVF cycle with follow-up and were analyzed. Age, BMI, days of stimulation, peak estradiol, median number of oocyte retrieved, fertilized and transferred were similar between pregnant (n=20) and not pregnant (n=49) groups (Table 1). Results were also examined based on BCL6 expression (Table 2 and Figure 1).
Table 1.
Characteristics of the sample population based on outcome (clinical pregnancy rate)
| Characteristics | Pregnant n=20 |
Not Pregnant n=49 |
p |
|---|---|---|---|
| Age - years (mean ± SD) | 36.3 ± 3.2 | 34.5 ± 3.9 | 0.05a |
| BMI - median (range) | 24.3 (18.6 – 44.6) | 23.9 (17.9 – 36) | 0.8b |
| BCL6 expression median (range) | 0.9 (0 – 4) | 2.1 (0.5 – 4) | 0.01b |
| Cycle characteristics | |||
| Days of stimulation - mean ± SD | 10.4 ± 1.9 | 10.4 ± 1.8 | 0.9a |
| Peak estradiol pg/mL - median (range) | 1387(341 – 5000) | 1945 (477 – 5000) | 0.7b |
| Oocyte retrieved - median (range) | 14 (2 – 35) | 11 (3 –56) | 0.3 |
| Oocytes fertilized - median (range) | 7 (1 – 24) | 6 (1 – 21) | 0.4 |
| Embryos transferred - median (range) | 2 (1 – 4) | 2 (1 – 3) | 0.2b |
Student t-test
Mann-Whitney test
Table 2.
Characteristics of the sample population based on BCL6 expression
| Characteristics | Normal n=17 |
Abnormal n=52 |
p |
|---|---|---|---|
| Age - years (mean ± SD) | 35.6 ± 3.1 | 34.8 ± 3.9 | 0.05a |
| BMI - median (range) | 24.8 (18.6 – 36.4) | 23.6 (17.9 – 44.6) | 0.9b |
| Clinical pregnancy rate (CPR) | 11 (64.7%) | 9 (17.3%) | 0.0004c |
| Relative risk (95% CI) for normal BCL6 | 0.26 (0.13 to 0.53) | ||
| Live birth rate (LBR) | 10 (58.8%) | 6 (11.5%) | 0.0002c |
| Relative risk (95% CI) for normal BCL6 | 0.19 (0.08 to 0.45) | ||
| Cycle characteristics | |||
| Days of stimulation - mean ± SD | 10.4 ± 2.1 | 10.4 ± 1.7 | 0.9a |
| Peak estradiol pg/mL - median (range) | 1331(341 – 3096) | 1650 (477 – 5000) | 0.3b |
| Oocyte retrieved - median (range) | 11 (2 – 35) | 12.5 (2 – 56) | 0.8b |
| Oocytes fertilized - median (range) | 6 (1 – 24) | 7 (1 – 21) | 0.6b |
| Embryos transferred - median (range) | 2 (1 – 3) | 2 (1–4) | 0.9b |
Student t-test
Mann-Whitney
Fisher’s exact test
Figure 1.
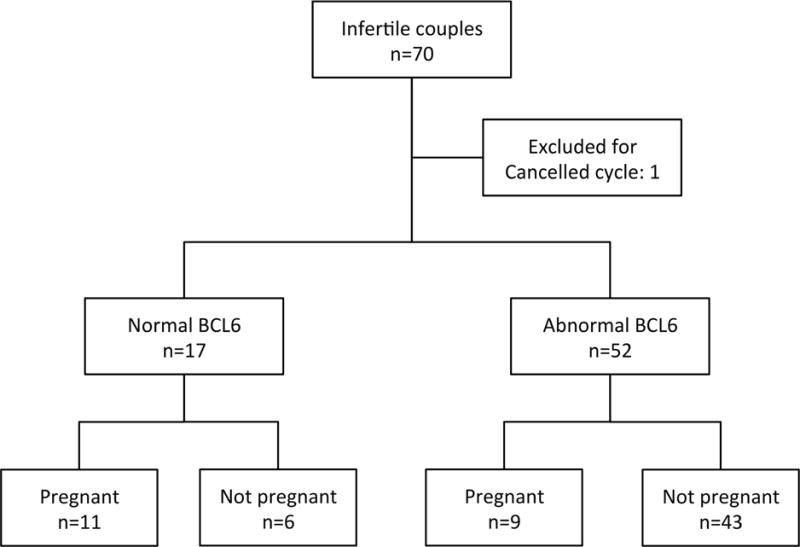
Flow diagram of the study
Outcome data and main results
Subjects that were pregnant or not pregnant were similar in terms of age, BMI, peak estradiol, days of stimulation and embryo transferred (Table 1). The overall CPR in 69 cycles of IVF was 29% (95%CI 19.6 to 40.5%). BCL6 HSCORE was greater than the 1.4 cut-off value in 52/69 (75.3%; 95%CI: 64 to 84) of cycles. Staining for BCL6 was predominantly localized in the nucleus (Figure 2A and B). Normal BCL6 expression had low-level staining (≤ 1.4) (Figure 2A). Abnormal samples exhibited aberrant expression of BCL6 (> 1.4) (Figure 2B). Lymph node was used as a positive control and exhibited high BCL6 expression (inset; Figure 2A). Based on BCL6 results alone, women with normal BCL6 expression had a significantly higher CPR (11/17; 64.7%; 95%CI: 41.3 to 82.6), compared to women with elevated BCL6 (9/52; 17.3%; 95%CI: 9.3 to 30.8), as shown in Figure 2C. These results yield a relative risk of 0.267 (95%CI: 0.13 to 0.53; p = 0.0004) for those with normal BCL6 expression; an absolute benefit of 47.4% (95%CI:22.5 to 72.2) was found. LBR was also significantly higher in women with low BCL6 expression (10/17; 58.8; 95%CI: 36 to 78.4), compared to women with abnormal BCL6 expression (6/52; 11.5%; 95%CI: 5.4 to 23). The RR was 0.19 (95%CI: 0.08 to 0.45; p = 0.0002), yielding an absolute benefit of 47.3% (95%CI: 21.8 to 67.8).
Figure 2.
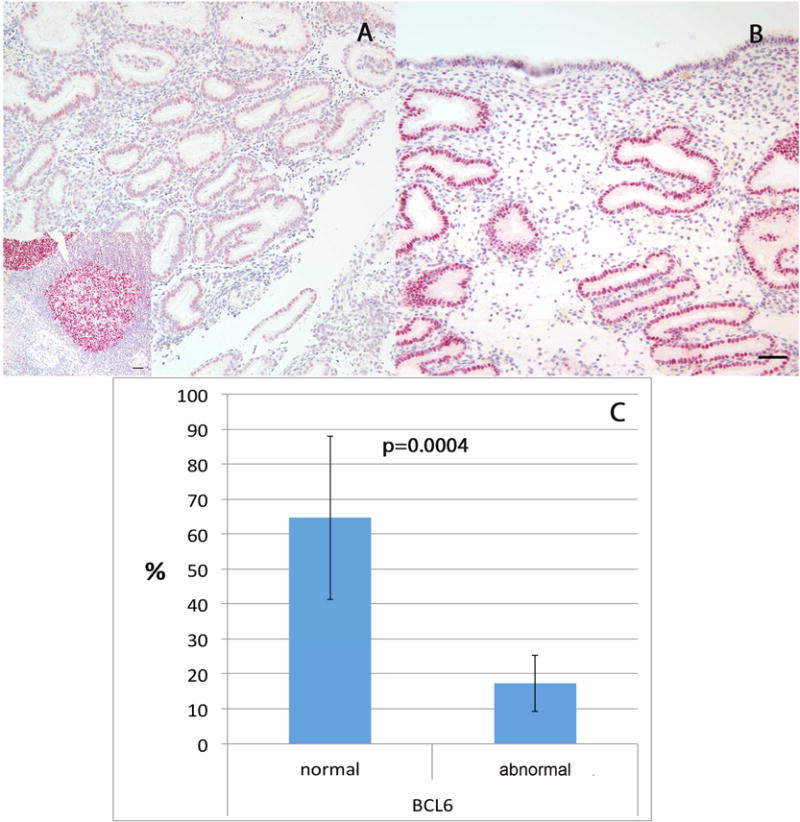
Expression patterns of BCL6 in A: Normal, in phase endometrium; inset: Lymph node (positive control). B: Endometrium from IVF failure patient with endometriosis. Bars represent 50μm. C: Percentage of pregnant women (bars represent 95% confidence interval) according to BCL6 expression: normal (≤1.4 HSCORE) or abnormal (overexpressed) of BCL6 (> 1.4 HSCORE).
CONCLUSIONS
Abnormal expression of BCL6 (> 1.4 HSCORE) was strongly associated with poor reproductive outcomes in IVF cycles. Our previous study reported that elevated expression of BCL6 is a validated biomarker for detection of endometrial inflammation and it is most commonly associated with endometriosis in women with UI (11). In the current study, we find that 75.3% of UI patients tested positive for BCL6, similar to the previous report (11). This is the first paper to examine BCL6 as a prognostic marker for IVF outcome and suggests that endometriosis may be commonly associated with IVF failure in UI. Aberrantly elevated BCL6 expression is analogous to what others have reported for abnormal aromatase expression in women with IVF failure (25). Interestingly, BCL6 is associated with the similar inflammatory pathways involved in aromatase overexpression (26). Other alterations in endometrial receptivity have been described in endometriosis and UI, including reduced leukemia inhibitor factor (LIF) expression in UI (27–31) and IVF failure (32). LIF is essential for establishment of pregnancy, and defects in LIF expression have been associated with endometriosis and adenomyosis (33–36) and tubal disease with hydrosalpinx (37), similar to reports on aberrant BCL6 overexpression (11).
Abnormal BCL6 expression in the endometrium of women with UI is associated with an endometrial progesterone (P) resistance (38), which can help explain the association with poor IVF outcome. Progesterone is essential for the establishment of pregnancy, so reduction in progesterone action would logically be associated with multiple down-stream changes in gene expression in the endometrium. In addition, inflammation is associated with P-resistance and an immune-regulated impact on the endometrium (39,40). BCL6 pairs with the histone deacetylase SIRT1, is centrally associated with epigenetic alterations in endometrial gene expression associated with endometriosis and likely has multiple effects on other key down-stream progesterone-regulated genes (18).
The mean CPR published by SART for unknown factor for all ages, i.e., 27.1% (41), is similar to the overall CPR of our study (20/69, 28.5%;95%CI: 19 to 41.3%) found in our study; thus, a majority of subjects were not successful at achieving pregnancy in women with UI. While overall IVF success rates have improved over the past 10 years, they remain below 50% per cycle for most centers. There has been a reduction in the use of laparoscopy in infertile women (42) and the proportion of women in the SART database with endometriosis as their diagnosis has steadily decreased, while the proportion of women with unspecified diagnoses has increased over time (3). Further, those women previously diagnosed have all had surgery for endometriosis and may no longer be representative of a larger proportion of women with undiagnosed (active) endometriosis still present within the population.
This study has a few weaknesses. The prevalence of aberrant endometrial BCL6 was high in our study population (75.3%), raising concerns about the external validity. We previously reported, however, that BCL6 was elevated in 80% of women with UI (11), and our population reflects this diagnosis. The effect of endometrial scratching may have had benefit on implantation rates before IVF, although data on this topic are controversial (43,44), but since scratching was performed in all subjects this should not be a confounder.
There are clear strengths in this study. This is a prospective cohort and subjects were recruited at a common point in their evaluation. Secondly, we used two clinically relevant outcomes (CPR and LBR). The follow-up for all patients was uniform and complete, using non-biased assessment for pregnancy outcome. The pregnancy tests and ultrasound results were performed without knowledge of the biopsy results. The biopsies were read by a blinded observer (DPS) without knowledge of IVF outcome. We reported a 0.26 relative beneficial risk (Table 1) and this should call attention for other centers to try to reproduce these data (23), since an abnormal BCL6 expression in UI population prior IVF reduces the chance of being unsuccessful IVF treatment in 74% of the population. The number of subjects with UI in our study was large enough and allowed us to perform a post-hoc power analysis for the association of BCL6 expression and pregnancy outcome. The significant difference found in those who did and did not get pregnant based on BCL6 immunohistochemical positivity had a power of 97.4%. We expect our data to have external validity, since our results are similar to those published at the SART database. Finally, the BCL6 test has been validated in women with UI and shown previously to be associated with both endometriosis and progesterone resistance (11,18), lending credence to the results obtained.
In conclusion, the aberrant expression of endometrial BCL6 is associated with poor reproductive outcomes in subsequent IVF cycles. As a biomarker for endometriosis, high levels of BCL6 expression in this cohort suggests that undiagnosed endometriosis may be a common factor that needs to be considered in women prior to undergoing IVF. More research is required to identify the factor(s) involved in implantation defects and to determine the best treatments prior to IVF treatment for women with abnormal BCL6 expression.
Capsule.
Aberrant epithelial BCL6 expression in endometrium of women with unexplained infertility is a useful prognostic factor for IVF outcome.
Acknowledgments
Funding: This study was supported by NICHD/NIH R01 HD067721and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior: Grant 99999.003035/2015-08 (BAL) and by CAPES/PROAP (RFS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Summary Report [Internet] [cited 2017 Jul 21]; Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2014.
- 2.Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 3.Senapati S, Sammel MD, Boudhar S, Morse CB, Barnhart KT. The impact of endometriosis on IVF: an evaluation using the society of assisted reproductive technologies (SART) database. Fertil Steril. 2014;102(3):e48–9. doi: 10.1016/j.fertnstert.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Summary Report [Internet] [cited 2017 Jul 21];Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2014.
- 5.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92(1):68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 6.Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–7. doi: 10.1007/s10815-010-9436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz I, Navarro J, Blasco L, Simón C, Pellicer A, Remohí J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril. 2000;74(1):31–4. doi: 10.1016/s0015-0282(00)00570-7. [DOI] [PubMed] [Google Scholar]
- 8.Prapas Y, Goudakou M, Matalliotakis I, Kalogeraki A, Matalliotaki C, Panagiotidis Y, et al. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod Biomed Online. 2012;25(5):543–8. doi: 10.1016/j.rbmo.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Navarro PA de A de S, de Sall Navarro PA de A, de Melo AS, Ferriani RA. Subtle Endometriosis and Unexplained Infertility. Unexplained Infertility. 2015:203–9. [Google Scholar]
- 10.Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–8. doi: 10.1016/j.fertnstert.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 11.Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, et al. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women With Endometriosis. Reprod Sci. 2016;23(9):1234–41. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai T, Miki T, Kikuchi M, Fukuda T, Miyasaka N, Kamiyama R, et al. The proto-oncogene Bc16 inhibits apoptotic cell death in differentiation-induced mouse myogenic cells. Oncogene. 1999;18(2):467–75. doi: 10.1038/sj.onc.1202306. [DOI] [PubMed] [Google Scholar]
- 13.Kojima S, Hatano M, Okada S, Fukuda T, Toyama Y, Yuasa S, et al. Testicular germ cell apoptosis in Bcl6-deficient mice. Development. 2001;128(1):57–65. doi: 10.1242/dev.128.1.57. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13(2):199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 15.Yu RY-L, Wang X, Pixley FJ, Yu JJ, Dent AL, Broxmeyer HE, et al. BCL-6 negatively regulates macrophage proliferation by suppressing autocrine IL-6 production. Blood. 2005;105(4):1777–84. doi: 10.1182/blood-2004-08-3171. [DOI] [PubMed] [Google Scholar]
- 16.Takeda N, Arima M, Tsuruoka N, Okada S, Hatano M, Sakamoto A, et al. Bcl6 Is a Transcriptional Repressor for the IL-18 Gene. The Journal of Immunology. 2003;171(1):426–31. doi: 10.4049/jimmunol.171.1.426. [DOI] [PubMed] [Google Scholar]
- 17.Chaouat G, Dubanchet S, Ledée N. Cytokines: Important for implantation? J Assist Reprod Genet. 2007;24(11):491–505. doi: 10.1007/s10815-007-9142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo J-Y, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C, et al. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci Rep. 2017;7(1):6765. doi: 10.1038/s41598-017-04577-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 2010 [Google Scholar]
- 20.Noyes RW, Hertig AT, Rock J. Dating the Endometrial Biopsy. Fertil Steril. 1950;1(1):3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 21.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–25. [PubMed] [Google Scholar]
- 22.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. [PubMed] [Google Scholar]
- 23.Grimes DA. Epidemiologic research with administrative databases: red herrings, false alarms and pseudo-epidemics. Hum Reprod. 2015;30(8):1749–52. doi: 10.1093/humrep/dev151. [DOI] [PubMed] [Google Scholar]
- 24.Seldrup J, Pocock SJ. Clinical Trials. A Practical Approach. Statistician. 1985;34(3):337. [Google Scholar]
- 25.Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19(2):352–6. doi: 10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- 26.Fox C, Morin S, Jeong J-W, Scott RT, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105(4):873–84. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39(2):137–43. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 28.Tawfeek MA, Eid MA, Hasan AM, Mostafa M, El-Serogy HA. Assessment of leukemia inhibitory factor and glycoprotein 130 expression in endometrium and uterine flushing: a possible diagnostic tool for impaired fertility. BMC Womens Health. 2012;12:10. doi: 10.1186/1472-6874-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai HD, Chang CC, Hsieh YY, Lo HY. Leukemia inhibitory factor expression in different endometrial locations between fertile and infertile women throughout different menstrual phases. J Assist Reprod Genet. 2000;17(8):415–8. doi: 10.1023/A:1009457016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aghajanova L, Altmäe S, Bjuresten K, Hovatta O, Landgren B-M, Stavreus-Evers A. Disturbances in the LIF pathway in the endometrium among women with unexplained infertility. Fertil Steril. 2009;91(6):2602–10. doi: 10.1016/j.fertnstert.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod. 1997;12(3):569–74. doi: 10.1093/humrep/12.3.569. [DOI] [PubMed] [Google Scholar]
- 32.Mariee N, Li TC, Laird SM. Expression of leukaemia inhibitory factor and interleukin 15 in endometrium of women with recurrent implantation failure after IVF; correlation with the number of endometrial natural killer cells. Hum Reprod. 2012;27(7):1946–54. doi: 10.1093/humrep/des134. [DOI] [PubMed] [Google Scholar]
- 33.Xiao Y, Sun X, Yang X, Zhang J, Xue Q, Cai B, et al. Leukemia inhibitory factor is dysregulated in the endometrium and uterine flushing fluid of patients with adenomyosis during implantation window. Fertil Steril. 2010;94(1):85–9. doi: 10.1016/j.fertnstert.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Mikolajczyk M, Wirstlein P, Skrzypczak J. Leukaemia inhibitory factor and interleukin 11 levels in uterine flushings of infertile patients with endometriosis. Hum Reprod. 2006;21(12):3054–8. doi: 10.1093/humrep/del225. [DOI] [PubMed] [Google Scholar]
- 35.Mikołajczyk M, Skrzypczak J, Szymanowski K, Wirstlein P. The assessment of LIF in uterine flushing–a possible new diagnostic tool in states of impaired fertility. Reprod Biol. 2003;3(3):259–70. [PubMed] [Google Scholar]
- 36.Dimitriadis E, Stoikos C, Stafford-Bell M, Clark I, Paiva P, Kovacs G, et al. Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006;69(1):53–64. doi: 10.1016/j.jri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Xu B-F, Chen Q-J, Sun X-X. Effects of hydrosalpinx on pinopodes, leukaemia inhibitory factor, integrin beta3 and MUC1 expression in the peri-implantation endometrium. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):171–5. doi: 10.1016/j.ejogrb.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Fox CW, Young SL, Jeong J, Palomino WA, Lessey BA. BCL6 AND SIRT1 expression in unexplained infertility versus unexplained recurrent pregnancy loss. Fertil Steril. 2016;106(3):e219. [Google Scholar]
- 39.Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril. 2016;106(6):1420–31.e7. doi: 10.1016/j.fertnstert.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lessey BA, Julie Kim J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 2017;108(1):19–27. doi: 10.1016/j.fertnstert.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Summary Report [Internet] [cited 2017 Jul 21];Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2014.
- 42.Feinberg EC, Levens ED, DeCherney AH. Infertility surgery is dead: only the obituary remains? Fertil Steril. 2008;89(1):232–6. doi: 10.1016/j.fertnstert.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Gibreel A, Badawy A, El-Refai W, El-Adawi N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res. 2013;39(3):680–4. doi: 10.1111/j.1447-0756.2012.02016.x. [DOI] [PubMed] [Google Scholar]
- 44.Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum Reprod. 2014;29(11):2474–81. doi: 10.1093/humrep/deu213. [DOI] [PubMed] [Google Scholar]


