Abstract
Objective
To evaluate the impact of the Neonatal Resuscitation Program (NRP) recommended low oxygen strategy on neonatal morbidities, mortality and neurodevelopmental outcomes in preterm neonates.
Study design
In March 2011, Parkland Hospital changed from a high oxygen strategy (HOX) of resuscitation with initial 100% O2 and targeting 85–94% SpO2 for delivery room (DR) resuscitation to a low oxygen strategy (LOX) with initial 21% O2 and titrating O2 to meet NRP recommended transitional target saturations. Neonates ≤28 weeks gestational age (GA) born between August 2009 and April 2012 were identified. In this retrospective, observational study, neonates exposed to LOX versus HOX were compared for short-term morbidity, mortality and long-term neurodevelopmental outcomes. Regression analysis was performed to control for confounding variables.
Results
Of 199 neonates, 110 were resuscitated with HOX and 89 with LOX. Compared with HOX, LOX neonates had lower O2 exposure in the DR (5.2 ± 1.5 vs 7.8 ± 2.8 (ΣFiO2 × Time min), P < .01), spent fewer days on O2 (30 (5, 54) vs 46 (11, 82), p=0.01) and had lower odds of developing bronchopulmonary dysplasia (BPD) (adjusted odds ratio 0.4(0.2, 0.9)). There was no difference in mortality (17(20%) vs 20(18%)), but LOX neonates had higher motor composite scores on Bayley III assessment (91 (85, 97) vs 88 (76, 94), p<0.01).
Conclusion
The NRP recommended LOX strategy was associated with improved respiratory morbidities and neurodevelopmental outcomes with no increase in mortality. Prospective trials to confirm the optimal oxygen strategy for the resuscitation of preterm neonates are needed.
Keywords: Bronchopulmonary Dysplasia, Delivery room, Neonate, Neurodevelopmental Outcomes, Oxygen, Preterm, Resuscitation
Oxygen is an essential fuel source and plays a major role in numerous oxidative metabolic reactions and physiologic processes.[1] However, excess oxygen exposure can result in the production of oxygen free radicals. Unchecked, such reactive oxygen species can damage lipids, proteins and DNA resulting in tissue injury and cell death.[2–4] Birth is an oxidative challenge to the newborn as it adapts from a low oxygen intrauterine environment to the higher oxygen extra-uterine environment.[1] Preterm neonates are especially vulnerable to oxidative stress due to decreased enzymatic and non-enzymatic oxygen defenses and the frequent need for resuscitation with oxygen exposure at birth.[3, 5, 6] Although multiple small randomized controlled trials have examined various initial oxygen concentrations for preterm resuscitation at birth, the optimal oxygen strategy for preterm neonatal resuscitation remains unknown.[7–13] An optimal oxygen strategy would avoid both hypoxia and hyperoxia. Hyperoxemia during resuscitation results in oxidative stress and is associated with various neonatal morbidities such as bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP).[4, 8, 12–19] Although newborns with high fetal hemoglobin and high cardiac output should physiologically be able to tolerate lower oxygen saturations during transition[1], exposure to prolonged hypoxia also results in increased neonatal morbidities such as intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL) and increased mortality.[13, 20, 21]
Prior to 2011, the American Heart Association/American Academy of Pediatrics Neonatal Resuscitation Program (NRP) recommended that preterm neonates receive 100% oxygen as the preferred gas during delivery room resuscitation/stabilization.[22] Based on the 2010 International Liaison Committee on Resuscitation (ILCOR) Consensus on Science and Treatment recommendations[23], the 2011 NRP recommended starting with lower oxygen concentrations (21% to 30%) for preterm delivery room resuscitation. The oxygen concentration was titrated with an oxygen blender to achieve goal oxygen saturations based on the approximated median transitional saturations observed in healthy term neonates. The most recent ILCOR[24] scientific review and NRP[25] guidelines continue these recommendations that emphasize the need to provide sufficient oxygen to correct any hypoxic state while trying to avoid excess oxygen exposure.
There is ample evidence that resuscitation of preterm neonates with a low oxygen strategy starting with 21% oxygen is feasible. [8, 9] However, several studies, systematic reviews and meta-analyses have given conflicting results about the impact of a low versus high initial oxygen strategy on short-term clinical outcomes and mortality in preterm neonates.[7–13, 19, 21, 26–28] Little evidence is available regarding the effect of delivery room oxygen strategies on long-term neurodevelopmental outcomes.[28] The primary objective of this study was to evaluate the impact of the change from the long-standing initial 100% oxygen strategy to the initial 21% oxygen strategy on morbidity, mortality and long-term neurodevelopmental outcomes in preterm neonates.
Methods
A retrospective cohort study was conducted examining preterm neonates ≤28 weeks’ gestational age (GA) born between August 2009 to April 2012 at Parkland Hospital, Dallas, Texas. The study was approved by University of Texas Southwestern Medical Center Institutional Review Board.
At Parkland Hospital prior to March 2011, in compliance with the 2006 NRP guidelines[22], preterm neonates were resuscitated in the delivery room with a high oxygen strategy (HOX) where stabilization was initiated with 100% oxygen and the oxygen concentration was adjusted to achieve pre-ductal goal saturations of 85–94%. In March 2011, the new 2011 NRP recommendations of using an initial low oxygen strategy (LOX) for resuscitation of preterm neonates was adopted.[25] With LOX, resuscitation was initiated with 21% oxygen and oxygen was titrated to achieve the transitional pre-ductal NRP recommended goal saturations. These goal saturations are approximated median pre-ductal saturations observed in healthy term neonates.[29, 30]
This primary objective of the study was to compare short- and long-term outcomes of neonates resuscitated with the HOX versus LOX strategy. All preterm neonates 23–28 weeks GA born during the study period were identified from the Parkland Neonatal Resuscitation Registry. Neonates enrolled in a competing randomized control trial [8], prenatally diagnosed cyanotic congenital heart disease and those with planned comfort care only were excluded. The cohort was divided into those resuscitated with the HOX versus LOX strategy based on their date of birth and verified by chart review.
Baseline maternal and infant characteristics, resuscitation details, morbidities and mortality were collected for comparison. Data were obtained from the electronic medical record, the Parkland Neonatal Resuscitation Registry and the Parkland Neonatal Intensive Care Unit (NICU) Database, which prospectively collects data on all neonates admitted to the Parkland NICU. Prolonged rupture of membrane was defined as rupture of membranes ≥ 18 hours before birth. The National Institute of Child Health and Human Development expert panel definition for chorioamnionitis was used.[31] In April 2010, Parkland Hospital adopted a policy of giving antenatal magnesium for neuroprotection to all preterm neonates < 28 weeks GA. For the current study, antenatal magnesium given either for neuroprotection or for maternal preeclampsia was recorded. Intrauterine growth restriction was defined by Ponderal Index < 10th percentile for GA. All deliveries of infants ≤ 28 weeks GA at Parkland hospital are attended by the neonatal resuscitation team. An obstetric circulating nurse joins the team to record details of the resuscitation, including the infant’s vital signs, oxygen saturations and interventions such as changes in FiO2 on a resuscitation record every 30 seconds during the stabilization. This delivery room resuscitation record becomes part of the patient’s medical record. Resuscitation data such as heart rate, FiO2, SpO2 during first 10 minutes after birth, prolonged positive pressure ventilation > 1 minute, need for Continuous Positive Airway Pressure, intubation, chest compressions and epinephrine administration in the delivery room, were abstracted from these records. Oxygen load (Inspired oxygen) for first 10 minutes after birth was calculated using the equation: ΣFiO2 × Timemin as previously described.[8]
Morbidities such as respiratory distress syndrome (RDS), pneumothorax, pulmonary arterial hypertension (PAH), BPD, sepsis, severe IVH, necrotizing enterocolitis (NEC), clinically significant patent ductus arteriosus (PDA), severe ROP, length of hospitalization and death during NICU stay were recorded from the NICU database. RDS, PAH and clinically significant PDA were recorded if the clinician documented these morbidities as present in the patient’s electronic chart. BPD was defined as the need for supplemental oxygen at 36 weeks postmenstrual age. Bacteremia/sepsis were recorded only if the blood culture was positive for a pathogenic organism. Severe IVH was defined as grade III or higher on any head ultrasounds unilaterally or bilaterally as per Papile criteria.[32] Necrotizing enterocolitis was defined ≥ Stage 2 based on the modified Bell criteria.[33] Severe ROP was defined as Stage 3 or higher based on the international classification of retinopathy of prematurity.[34]
Neonates ≤ 28 weeks GA who are born at Parkland Hospital undergo systematic standardized neurologic assessment including the Bayley Scales of Infant and Toddler Development – Third edition (Bayley III) [35, 36] at the Children’s Medical Center Dallas THRIVE clinic at 22–26 months corrected age. The providers at the follow-up clinic were not aware of the initial oxygen concentration used during resuscitation at birth. The gross motor functional classification system (GMFCS) score was used to classify functional impairment in children with cerebral palsy. Hearing and visual impairment were also assessed. Moderate to severe neurodevelopmental impairment (NDI) was defined as the presence of any one of the following: cerebral palsy with a GMFCS score ≥ 2, Bayley III cognitive or motor score < 85, visual impairment or permanent hearing loss that does not permit the child to understand directions from the examiner and communicate with or without amplification. Because a Bayley III motor composite score of <85 may overestimate neurodevelopmental impairment, a modified moderate to severe NDI was also calculated where the only difference was a Bayley III motor composite score of < 73 instead of <85.[37] Severe NDI was defined as any of the following. Severe cerebral palsy with GMFCS score ≥ 4, Bayley III cognitive and motor score < 70, bilateral blindness or no functional hearing with amplification.[36]
The composite of death and/or moderate to severe NDI was the primary outcome of the study. Pre-specified secondary outcomes included death, severe IVH, PVL, BPD, severe ROP, NEC, moderate to severe NDI, severe NDI, and individual components of the neurodevelopmental assessment. These outcomes were chosen because oxidative stress has been implicated in the pathogenesis of these clinical morbidities in preterm neonates.[3]
Statistical Analyses
SAS version 9.2 (SAS Institute Inc, Cary, North Carolina.) was used to perform statistical analyses. Descriptive statistics were calculated to compare LOX and HOX neonates. Categorical variables were analyzed by the Pearson Chi square or Fisher exact test as applicable. Continuous variables were analyzed by the Student t-test or Wilcoxon rank sum test. A 2-sided 0.05 level of significance was used for all analyses. For the outcomes of BPD and NDI, stepwise forward multiple logistic regression was performed to account for confounders. In addition to those pre-specified variables known to be associated with the outcomes of interest, all variables with P values < 0.1 were included in the logistic regression. For neurodevelopmental outcomes, GA, antenatal corticosteroids and antenatal magnesium administration were included in the logistic regression. Confounding variables included for BPD as an outcome were GA, chorioamnionitis, preeclampsia, antenatal corticosteroids, sex, intubation in the delivery room, surfactant use, symptomatic PDA and intrauterine growth restriction.
Results
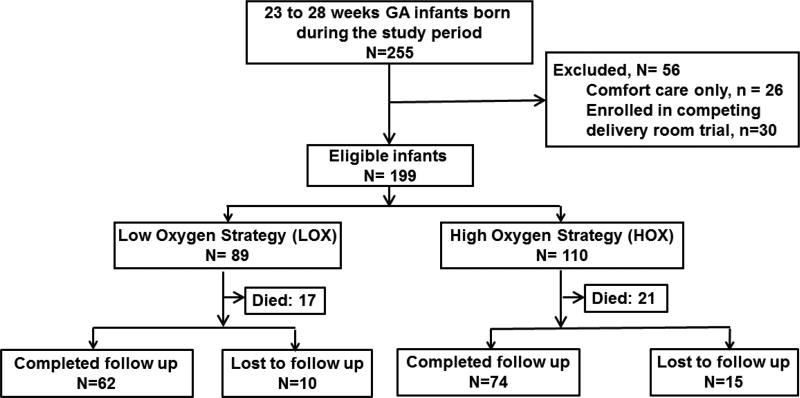
During the study period, 255 neonates were born at 23–28 weeks GA (Figure). After excluding neonates who received only comfort care at the request of their family and those who were enrolled in a delivery room randomized control trial of different oxygen strategies[8], 199 neonates were included in the study. Of these, 89 were resuscitated with LOX and 110 were resuscitated with HOX. After accounting for mortality before neurodevelopmental assessment and loss to follow up, 87% of the study cohort completed the primary outcome assessment.
Figure.
Flow diagram of study population
There were no differences in baseline maternal characteristics between the two groups including maternal age, receipt of antenatal corticosteroids, maternal diabetes, preeclampsia, prolonged rupture of membranes, chorioamnionitis, abruption, placenta previa and cesarean delivery. However, antenatal magnesium was given more often in the LOX group (Table I).
Table 1.
Maternal/infant characteristics and delivery room interventions
| HOX N=110 |
LOX N= 89 |
P Value |
|
|---|---|---|---|
| Maternal | |||
| Age, y, mean ± SD | 27 ± 6 | 26 ± 5 | NS |
| Antenatal steroids, n (%) | 60 (54%) | 45 (51%) | NS |
| Maternal diabetes, n (%) | 15 (14%) | 14 (16%) | NS |
| Preeclampsia, n (%) | 28 (25%) | 26 (29%) | NS |
| Antenatal magnesium, n (%) | 39 (35%) | 50 (56%) | 0.04 |
| Prolonged rupture of membranes, n (%) | 22 (20%) | 18 (20%) | NS |
| Chorioamnionitis, n (%) | 9 (8%) | 7 (8%) | NS |
| Abruption, n (%) | 5 (4%) | 4 (4%) | NS |
| Placenta Previa, n (%) | 4 (3%) | 3(3%) | NS |
| Cesarean section, n (%) | 74 (67%) | 59 (66%) | NS |
| Infant | |||
| Male sex, n (%) | 58 (53%) | 43 (48%) | NS |
| Obstetric gestation age, wk, mean ± SD | 26 ± 1 | 26 ± 1 | NS |
| Birth weight, g, mean ± SD | 939 ± 255 | 983 ± 224 | NS |
| Intrauterine growth restriction, n (%) | 4 (4%) | 4 (4%) | NS |
| Multiple gestations, n (%) | 23 (21%) | 22 (25%) | NS |
| Arterial cord pH, mean ± SD | 7.23 ± 0.1 | 7.25 ± 0.1 | NS |
| Arterial cord base deficit, mEq/L, mean ± SD | 7 ± 5 | 6 ± 3 | NS |
| Delivery Room Interventions and Apgar scores | |||
| Initial FiO2 | 0.21 | 1.0 | |
| Final FiO2, median (Interquartile range) | 0.40 (0.30, 0.50) | 0.40 (0.30, 0.70) | NS |
| Oxygen Load (ΣFiO2 × Timemin), mean ± SD | 7.8 ± 2.8 | 5.2 ± 1.5 | < 0.01 |
| Positive pressure ventilation > 1 minute, n (%) | 85 (77%) | 79 (89%) | NS |
| Intubation in the delivery room, n (%) | 64 (58%) | 62 (70%) | NS |
| Chest compressions, n (%) | 1 (1%) | 2 (2%) | NS |
| Epinephrine, n (%) | 1 (1%) | 2 (2%) | NS |
| Apgar scores | |||
| 1 minute, median (Interquartile range) | 5 (3,7) | 5 (2,6) | NS |
| 5 minute, median (Interquartile range) | 7 (6,8) | 7 (6,8) | NS |
There were no differences in sex distribution, obstetric GA, birth weight, intrauterine growth restriction, multiple gestations, arterial cord pH or base deficit. By definition LOX and HOX infants had different initial concentrations of FiO2 during resuscitation (Table 1). Although HOX and LOX neonates had a similar FiO2 on admission to NICU, HOX neonates were exposed to higher oxygen load in the delivery room (Table 1). The number of neonates requiring positive pressure ventilation for > 1 minute, intubation, chest compressions and epinephrine were similar between groups. There were no differences in Apgar scores.
LOX and HOX neonates had similar rates of RDS, surfactant use, pneumothorax, PAH, days on the ventilator, and days on continuous positive airway pressure (Table 2). However, LOX neonates had lower rates of BPD and fewer days spent on oxygen during their NICU stay. There was no difference in sepsis, severe IVH, NEC, symptomatic PDA, severe ROP, length of hospitalization or death before discharge. Respiratory failure followed by late onset sepsis and that followed by NEC and IVH were the major causes of mortality in both groups. There was no statistical difference in cause of death between both groups. Using multiple logistic regression, compared with HOX neonates, LOX neonates had a lower incidence of BPD even after controlling for GA, chorioamnionitis, preeclampsia, antenatal corticosteroids, sex, intubation in delivery room, surfactant administration, rates of symptomatic PDA and rates of intrauterine growth retardation (Odds Ratio 0.4, 95% CI 0.2–0.9, P=0.03).
Table 2.
Neonatal short- and long-term clinical outcomes (Unadjusted)
| HOX N=110 |
LOX N= 89 |
P Value |
|
|---|---|---|---|
| Respiratory Distress Syndrome, n (%) | 98 (89%) | 79 (89%) | NS |
| Surfactant use, n (%) | 80 (73%) | 64 (72%) | NS |
| Pneumothorax, n (%) | 13 (12%) | 8 (9%) | NS |
| Pulmonary arterial hypertension, n (%) | 9 (8%) | 5 (6%) | NS |
| Bronchopulmonary dysplasia (BPD), n (%) | 36 (33%) | 14 (16%) | 0.01 |
| Days on ventilator, median (interquartile range) | 6 (1,26) | 4 (1,14) | NS |
| Days on continuous positive airway pressure, mean ± SD | 22 (6,36) | 24 (12,33) | NS |
| Days on oxygen, mean ± SD | 46 (11,82) | 30 (5,54) | 0.01 |
| Sepsis, n (%) | 35 (32%) | 27 (30%) | NS |
| Severe intraventricular hemorrhage (IVH), n (%) | 21 (19%) | 10 (11%) | NS |
| Necrotizing enterocolitis, n (%) | 7 (6%) | 7 (8%) | NS |
| Symptomatic patent ductus arteriosus, n (%) | 46 (42%) | 27 (30%) | NS |
| Severe retinopathy of prematurity, n (%) | 14 (13%) | 4 (4%) | NS |
| Length of hospitalization, median (interquartile range) | 94 (65,120) | 87 (71,107) | NS |
| Death before discharge, n (%) | 20 (18%) | 17 (20%) | NS |
| Death or BPD, n (%) | 50 (45%) | 29 (33%) | 0.02 |
Neurodevelopmental assessment was available in 85% of neonates alive at 22–26 months corrected age (Table 3). Neonates who were lost to follow up (n=25) were more mature than those who had their follow-up assessment done (GA 27 ± 0.5 weeks vs 26 ± 1.5 weeks, p <0.01). In the neonates, who had their follow-up assessment done, there were no differences in rates of cerebral palsy, GMFCS scores ≥ 2, vision impairment, permanent deafness, or need for hearing aids between HOX and LOX neonates. In addition, there were no differences in moderate to severe NDI or death. Although the Bayley III composite cognitive and language scores were similar between the LOX and HOX groups, motor composite scores were higher in LOX neonates (Table 3). This difference persisted even after controlling for GA, antenatal corticosteroids and antenatal magnesium (P<0.01). There was no difference in modified moderate to severe NDI where the Bayley III motor composite score cutoff was changed from <85 to <73.[37] Although there was a lower rate of severe NDI in LOX neonates with unadjusted analysis, this difference was no longer statistically significant when adjusted for GA.
Table 3.
Neurodevelopmental outcomes at 22–26 month corrected age follow up
| HOX N= 74 |
LOX N= 62 |
P Value |
|
|---|---|---|---|
| Age at follow up assessment, months, median (interquartile range) | 23 (22,24) | 24 (22,25) | NS |
| Cerebral Palsy, n (%) | 8 (11%) | 5(8%) | NS |
| GMFCS score ≥2, n (%) | 6 (8%) | 1 (2%) | NS |
| Vision impairment, n (%) | 4 (5%) | 0 | NS |
| Permanent Deafness, n (%) | 2 (3%) | 2 (3%) | NS |
| Need hearing aid, n (%) | 4 (5%) | 0 | NS |
| Bayley III cognitive composite score, median (interquartile range) | 85 (75,90) | 85 (80,95) | NS |
| Bayley III motor composite score, median (interquartile range) | 88 (76,94) | 91 (85,97) | 0.01 |
| Bayley III language composite score, median (interquartile range) | 79 (71,86) | 83 (74,90) | NS |
| Neurodevelopmental impairment, n (%) | 41 (55%) | 25 (40%) | NS |
| Modified neurodevelopmental impairment† n (%) | 32 (43%) | 21 (34%) | NS |
| Severe neurodevelopmental impairment, n (%) | 13 (18%) | 3 (5%) | 0.04 |
| HOX N=110* | LOX N=89* | ||
| Death before follow up assessment‡, n (%) | 21 (19%) | 17 (19%) | NS |
| NDI or Death*, n (%) | 62 (54%) | 42 (47%) | NS |
| Severe NDI or Death*, n (%) | 34 (31%) | 20 (22%) | NS |
Modified NDI was calculated with Bayley III motor composite score cut off <73 instead of <85 as used in NDI definition
One infant died in HOX group after discharge from NICU. No death in LOX group after discharge from NICU
For primary outcome, denominator changed to total study population
Discussion
This study demonstrates that preterm neonates resuscitated with the NRP recommended LOX strategy were exposed to lower oxygen load in the delivery room, had fewer days on oxygen in the NICU and a lower incidence of BPD even after adjusting for confounding variables. Infants resuscitated with LOX also had no increase in mortality, and had higher Bayley III motor composite scores at 2-year follow-up.
It is possible that the reduced oxygen load in the delivery room resulted in a lower content of oxygen free radicals as a potential mechanism for the improved neonatal outcomes observed with the LOX strategy.[38, 39] In animal models, even a brief exposure to 100% O2 results in very high PaO2 levels in the brain and other tissues.[40, 41] Further, multiple animal and human studies have found adverse effects of hyperoxemia during resuscitation resulting in tissue injury and worse outcomes.[2, 3, 9, 12, 16, 17, 19, 40–47] On the other hand, several studies comparing initial O2 concentrations and outcomes in preterm neonates have reported conflicting findings.[7–10, 12, 13, 21, 25, 26, 28, 48]
Our current finding of reduced respiratory morbidities in the LOX group is similar to a small randomized controlled trial conducted at this institution [8], as well as a trial of low versus high initial O2 strategy conducted by Vento et al.[12] A recent meta-analysis of the available trials showed no differences in rates of BPD between low and high initial O2 strategies.[9] However, the meta-analysis compared multiple randomized control trials with varying oxygen titration strategies, and thus with varying exposure to oxygen loads in the DR. Given that our study’s HOX group started with 100% O2 and targeted 85–94% saturation from the first minute of life, it is possible that this group of infants had higher O2 exposure compared with some of the randomized control trials included in the meta-analysis resulting in bigger differences between groups. It is also possible that the observed reduction in the incidence of BPD might be due to type I error or a result of unrecognized change in NICU practice during the course of the study. To the best of our knowledge, no major practice change or quality improvement initiative occurred at Parkland NICU during the study period to account for the difference in respiratory morbidity seen between LOX and HOX neonates.
The current study found no change in mortality with adoption of the LOX strategy, similar to the previous trial from this institution.[8] In contrast, the recent To2rpido trial, in which preterm neonates < 29 weeks that were resuscitated with an initial O2 concentration of 21% had a higher mortality compared with those initially resuscitated with 100% O2.[13] The differences in outcomes between the current study and To2rpido may also be due to differences in the oxygen titration strategies. In the To2rpido trial, O2 was adjusted by increasing FiO2 by ≤ 10% every minute for SpO2 < 65% for the first 5 minutes of life and SpO2 < 80% after 5 minutes, as well as decreasing FiO2 by 10% for SpO2 ≥ 95% at any time. In the current study, O2 for the LOX group was titrated by 10–20% every 30 seconds in order to reach the NRP recommended goal saturations which were higher than the targets used in the To2rpido trial. In the To2rpido trial, the observation of increased mortality in preterm < 29 weeks infants was a post-hoc analysis on significantly underpowered subgroups (21% O2 group n= 46, 100% O2 group n = 54). Further, when including all enrolled infants in the analyses, the 21% O2 group needed fewer days of respiratory support and did not have a higher mortality than the 100% O2 group. Although To2rpido is the largest randomized controlled trial comparing 21% versus 100% O2 as the initial O2 concentration for resuscitation, the study was limited by early termination (stopped after reaching only 15% of target enrollment) because of inadequate enrollment. This was due to a prevailing bias in medical providers’ preference for the low O2 strategy resulting in a large number of eligible infants not being enrolled.
A retrospective cohort study from 2004–2009 from the Canadian Neonatal Network by Rabi et al. demonstrated an increased incidence of death and/or severe IVH/PVL following a change in Canadian policy to titrate oxygen from an initial 21% O2 compared with the previous initial 100% O2 used for preterm infants < 28 weeks GA.[21] In contrast, our study found no increases in IVH/PVL or mortality following the change to LOX. The difference between these studies could be due to differences in the O2 titration strategies and their implementation. In addition, a recent meta-analysis of all randomized controlled trials showed no difference in mortality between an initial low O2 concentration versus an initial high O2 concentration during resuscitation.[9] It also demonstrated that studies conducted with low initial O2 concentrations (21–30%) after publication of NRP guidelines had lower risk of mortality compared with earlier studies.
This observation may reflect a learning curve for titration of oxygen. There was also higher mortality in masked studies compared with unmasked studies. There are limited data on the impact of the delivery room O2 strategies on neurodevelopmental outcomes. A meta-analysis of 3 available studies found no differences in neurodevelopmental outcomes in late preterm and term infants resuscitated with an initial low O2 strategy.[49] A randomized trial by Boronat et al, compared 30% to 60% initial O2 and found no difference in neurodevelopmental outcomes in preterm neonates.[28] This study used target O2 saturations that were different from those currently recommended by NRP.[25] Our findings of improved neurodevelopmental outcomes could be due to differences in oxygen load between HOX and LOX groups compared with those achieved by Boronat et al. It is also possible that our findings may reflect a type 1 error.
The strengths of the current study are that we studied a large cohort of <29 weeks GA preterm neonates, used pre-specified and uniform definitions of outcomes, and long-term neurodevelopmental outcomes were available with standardized assessments and sufficient rates of follow-up. To our knowledge, this is the first study to report neurodevelopmental outcomes for infants resuscitated with the current NRP-recommended low O2 strategy. Although this is a single center, retrospective cohort study, the resuscitation details were collected prospectively for the Parkland Neonatal Resuscitation Registry. Importantly, the study included all < 29 week GA preterm neonates who are vulnerable to the effects of early hypoxia and hyperoxia, and the sample size of 199 infants allowed examination of the impact of the initial O2 strategy on neonatal morbidity and mortality.
This study has several limitations. OB circulating nurses manually recorded changes in oxygen saturation and oxygen concentration during delivery room resuscitation in LOX and HOX neonates. As direct download of pulse oximetry data was not available, total time spent outside goal saturations in the delivery room, compliance with the LOX strategy and its effect on neonatal outcomes were not evaluated. Although regression analysis was conducted to account for all known confounding variables, oxygen strategy-associated improvements in BPD and neurodevelopmental outcomes could have been affected by unknown confounders. Due to the retrospective nature of the study, changes in NICU practice during the course of the study could have influenced neonatal outcomes. However, to the best of our knowledge, no major changes in practice occurred in the Parkland NICU during the study period. Further, as retrospective studies cannot establish causality, caution must be taken when interpreting the results of this study.
In conclusion, the currently recommended NRP LOX strategy was feasible and was associated with improvement in BPD and neurodevelopmental outcomes without increased mortality in preterm neonates < 29 weeks GA. Larger, adequately powered randomized control trials focused not only on different initial O2 concentrations but also on different target saturations and titration strategies, are needed to definitively establish the impact of the initial delivery room O2 strategies on neonatal outcomes.
Acknowledgments
Supported by NICHD/NIH 1K23HD083511-01A1 (to V.K.).
We would like to acknowledge Gaston Ofman, MD, Maricel Maxey, NNP, and Vaishali Mashruwala, MD for assistance in collecting data for the study.
Abbreviations
- Bayley III
Bayley Scales of Infant and Toddler Development – Third edition
- BPD
Bronchopulmonary Dysplasia
- DR
Delivery room
- GA
gestational age
- GMFCS
Gross motor Functional Classification System
- HOX
High oxygen strategy
- ILCOR
International Liaison Committee on Resuscitation
- IVH
Intraventricular hemorrhage
- LOX
low oxygen strategy
- NDI
neurodevelopmental impairment
- NEC
Necrotizing enterocolitis
- NICU
Neonatal intensive care unit
- NRP
Neonatal Resuscitation Program
- O2
Oxygen
- PAH
Pulmonary arterial hypertension
- PDA
Patent ductus arteriosus
- PVL
Periventricular leukomalacia
- RDS
Respiratory distress syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.McNamara PJ, El-Khuffash A. 71 - Oxygen Transport and Delivery A2 - Polin, Richard A. In: Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and Neonatal Physiology. Fifth. Elsevier; 2017. pp. 724–37.e2. [Google Scholar]
- 2.Munkeby BH, Borke WB, Bjornland K, Sikkeland LI, Borge GI, Halvorsen B, et al. Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatr Res. 2004;56:783–90. doi: 10.1203/01.PDR.0000141988.89820.E3. [DOI] [PubMed] [Google Scholar]
- 3.O'Donovan DJ, Fernandes CJ. Free radicals and diseases in premature infants. Antioxidants & redox signaling. 2004;6:169–76. doi: 10.1089/152308604771978471. [DOI] [PubMed] [Google Scholar]
- 4.Trindade CEP, Rugolo LMSS. Free Radicals and Neonatal Diseases. NeoReviews. 2007;8:e522–e32. [Google Scholar]
- 5.Chen Y, Whitney PL, Frank L. Comparative responses of premature versus full-term newborn rats to prolonged hyperoxia. Pediatr. Res. 1994;35:233–7. doi: 10.1203/00006450-199402000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15:191–5. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Escrig R, Arruza L, Izquierdo I, Villar G, Saenz P, Gimeno A, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics. 2008;121:875–81. doi: 10.1542/peds.2007-1984. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics. 2013;132:e1488–96. doi: 10.1542/peds.2013-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oei JL, Vento M, Rabi Y, Wright I, Finer N, Rich W, et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2016 doi: 10.1136/archdischild-2016-310435. [DOI] [PubMed] [Google Scholar]
- 10.Rabi Y, Singhal N, Nettel-Aguirre A. Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR study. Pediatrics. 2011;128:e374–81. doi: 10.1542/peds.2010-3130. [DOI] [PubMed] [Google Scholar]
- 11.Rook D, Schierbeek H, Vento M, Vlaardingerbroek H, van der Eijk AC, Longini M, et al. Resuscitation of Preterm Infants with Different Inspired Oxygen Fractions. J Pediatr. 2014 doi: 10.1016/j.jpeds.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–49. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 13.Oei JL, Saugstad OD, Lui K, Wright IM, Smyth JP, Craven P, et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics. 2017;139 doi: 10.1542/peds.2016-1452. [DOI] [PubMed] [Google Scholar]
- 14.Buczynski BW, Maduekwe ET, O'Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Semin Perinatol. 2013;37:69–78. doi: 10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 16.Naumburg E, Bellocco R, Cnattingius S, Jonzon A, Ekbom A. Supplementary oxygen and risk of childhood lymphatic leukaemia. Acta Paediatr. 2002;91:1328–33. doi: 10.1080/08035250216105. [DOI] [PubMed] [Google Scholar]
- 17.Spector LG, Klebanoff MA, Feusner JH, Georgieff MK, Ross JA. Childhood cancer following neonatal oxygen supplementation. J Pediatr. 2005;147:27–31. doi: 10.1016/j.jpeds.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Vento M, Sastre J, Asensi MA, Vina J. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med. 2005;172:1393–8. doi: 10.1164/rccm.200412-1740OC. [DOI] [PubMed] [Google Scholar]
- 19.Peters TRT, Delmore P, Ahlers-Schmidt C, Bloom B. Oxygen Therapy during Resuscitation of Preterm Infants: A Retrospective Analysis. The e-journal of neonatal research. 2012;2:118–25. [Google Scholar]
- 20.Di Fiore JM, Martin RJ, Li H, Morris N, Carlo WA, Finer N, et al. Patterns of Oxygenation, Mortality, and Growth Status in the Surfactant Positive Pressure and Oxygen Trial Cohort. The Journal of Pediatrics. doi: 10.1016/j.jpeds.2017.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabi Y, Lodha A, Soraisham A, Singhal N, Barrington K, Shah PS. Outcomes of preterm infants following the introduction of room air resuscitation. Resuscitation. 2015;96:252–9. doi: 10.1016/j.resuscitation.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kattwinkel Je. Textbook of Neonatal Resuscitation. 5. American Academy of Pediatrics; 2006. [Google Scholar]
- 23.Wyllie J, Perlman JM, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2010;81:e260–e87. doi: 10.1016/j.resuscitation.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132:S204–S41. doi: 10.1161/CIR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 25.Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S543–S60. doi: 10.1161/CIR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 26.Brown JV, Moe-Byrne T, Harden M, McGuire W. Lower versus higher oxygen concentration for delivery room stabilisation of preterm neonates: systematic review. PLoS One. 2012;7:e52033. doi: 10.1371/journal.pone.0052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CL, Anderson C, Leone TA, Rich W, Govindaswami B, Finer NN. Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics. 2008;121:1083–9. doi: 10.1542/peds.2007-1460. [DOI] [PubMed] [Google Scholar]
- 28.Boronat N, Aguar M, Rook D, Iriondo M, Brugada M, Cernada M, et al. Survival and Neurodevelopmental Outcomes of Preterms Resuscitated With Different Oxygen Fractions. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1405. [DOI] [PubMed] [Google Scholar]
- 29.Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340–7. doi: 10.1542/peds.2009-1510. [DOI] [PubMed] [Google Scholar]
- 30.Mariani G, Dik PB, Ezquer A, Aguirre A, Esteban ML, Perez C, et al. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J Pediatr. 2007;150:418–21. doi: 10.1016/j.jpeds.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IA, Watterberg K, et al. Evaluation and Management of Women and Newborns With a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet Gynecol. 2016;127:426–36. doi: 10.1097/AOG.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papile L-A, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 33.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal Necrotizing Enterocolitis - Therapeutic Decisions Based Upon Clinical Staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 35.Albers CA, Grieve AJ. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2007;25:180–90. [Google Scholar]
- 36.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Epi MS, et al. Are Outcomes of Extremely Preterm Infants Improving? Impact of Bayley Assessment on Outcomes. The Journal of pediatrics. 2012;161:222–8.e3. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan AF, Bann C, Boatman C, Hintz SR, Vaucher YE, Vohr BR, et al. Do currently recommended Bayley-III cutoffs overestimate motor impairment in infants born <27 weeks gestation? J Perinatol. 2015;35:516–21. doi: 10.1038/jp.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres-Cuevas I, Parra-Llorca A, Sánchez-Illana A, Nuñez-Ramiro A, Lozano S, Kuligowski J, et al. Oxygen and oxidative stress in the perinatal period. Redox Biology. doi: 10.1016/j.redox.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Cuevas I, Cernada M, Nuñez A, Escobar J, Kuligowski J, Chafer-Pericas C, et al. Oxygen Supplementation to Stabilize Preterm Infants in the Fetal to Neonatal Transition: No Satisfactory Answer. Frontiers in Pediatrics. 2016;4:29. doi: 10.3389/fped.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-de-Sa V, Cunha-Goncalves D, Nordh A, Hansson S, Larsson A, Ley D, et al. High brain tissue oxygen tension during ventilation with 100% oxygen after fetal asphyxia in newborn sheep. Pediatr Res. 2009;65:57–61. doi: 10.1203/PDR.0b013e31818a01a4. [DOI] [PubMed] [Google Scholar]
- 41.Perrone S, Tataranno LM, Stazzoni G, Ramenghi L, Buonocore G. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.796170. [DOI] [PubMed] [Google Scholar]
- 42.Higgins RD, Bancalari E, Willinger M, Raju TN. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119:790–6. doi: 10.1542/peds.2006-2200. [DOI] [PubMed] [Google Scholar]
- 43.Kapadia VS, Chalak LF, DuPont TL, Rollins NK, Brion LP, Wyckoff MH. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J Pediatr. 2013;163:949–54. doi: 10.1016/j.jpeds.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 44.Presti AL, Kishkurno SV, Slinko SK, Randis TM, Ratner VI, Polin RA, et al. Reoxygenation with 100% oxygen versus room air: late neuroanatomical and neurofunctional outcome in neonatal mice with hypoxic-ischemic brain injury. Pediatr Res. 2006;60:55–9. doi: 10.1203/01.pdr.0000223766.98760.88. [DOI] [PubMed] [Google Scholar]
- 45.Saugstad OD, Rootwelt T, Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics. 1998;102:e1. doi: 10.1542/peds.102.1.e1. [DOI] [PubMed] [Google Scholar]
- 46.Solberg R, Andresen JH, Escrig R, Vento M, Saugstad OD. Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidation. Pediatr Res. 2007;62:559–63. doi: 10.1203/PDR.0b013e318156e8aa. [DOI] [PubMed] [Google Scholar]
- 47.Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr. 2003;142:240–6. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 48.Aydemir O, Akar M, Uras N, Eras Z, Erdeve O, Oguz SS, et al. Total antioxidant capacity and total oxidant status in perinatal asphyxia in relation to neurological outcome. Neuropediatrics. 2011;42:222–6. doi: 10.1055/s-0031-1295480. [DOI] [PubMed] [Google Scholar]
- 49.Saugstad OD, Vento M, Ramji S, Howard D, Soll RF. Neurodevelopmental outcome of infants resuscitated with air or 100% oxygen: a systematic review and meta-analysis. doi: 10.1159/000333346. [DOI] [PubMed] [Google Scholar]