Abstract
BACKGROUND
Opioid analgesics are commonly prescribed on an as needed (PRN) basis for acute painful conditions. Uncertainty of how patients actually take PRN opioids, coupled with a desire to completely cover pain, leads to variable and overly generous opioid prescribing practices, resulting in a surplus of opioids. This opioid surplus becomes a source for diversion and nonmedical opioid use. Understanding patterns of actual opioid ingestion after acute painful conditions can help clinicians counsel patients on safe opioid use, and allow timely recognition and intervention when escalating opioid self-dosing occurs, to prevent tolerance and addiction.
METHODS
We used a novel oxycodone digital pill system (ingestible biosensor within a standard gelatin capsule combined with 5mg oxycodone) that when ingested, is activated by the chloride ion gradient in the stomach thereby emitting a radiofrequency signal captured by a wearable Reader. The Reader relays ingestion data to a cloud-based server that displays ingestion events to the study team. We deployed the oxycodone digital pill among opioid naïve individuals discharged from the emergency department (ED) with acute fracture pain. Participants were trained on digital pill operation and discharged with 21 5mg oxycodone digital pills. They were instructed to take digital pills PRN for pain upon discharge. We conducted a brief interview 7 days after study enrollment, at which point participants returned the digital pill system. We identified oxycodone ingestion events in real time by data from the digital pill system, and performed pill counts at return visit to validate digital pill reporting of medication ingestion.
RESULTS
In this study, 26 individuals were approached; 16 enrolled with 15 completing the study. Participants ingested a median of 6 (3–9.5) oxycodone digital pills over the course of 7 days, with 82% of the oxycodone dose ingested in the first 3 days. In individuals who required operative repair, 86% (N=6) continued to ingest opioids at 1 week. There was substantial variability in ingestion patterns between individuals.
CONCLUSIONS
The utilization patterns of individuals with acute fracture pain could be captured using a digital pill system and revealed a median opioid ingestion of 45mg morphine equivalents for acute pain over 7 days. Seven participants ceased using opioids within 4 days following discharge from the emergency department, although operative repair was associated with longer use. This digital pill system was able to measure changes in and patterns of opioid self-dosing, which varied between patients.
1. Introduction
Opioids are commonly prescribed on an as needed (or “PRN”) basis, meaning that in the outpatient setting, patients make all decisions regarding opioid dose and frequency relatively independently, and not according to any predefined schedule. Data on how patients actually take PRN opioids (eg, the timing, number and frequency of dosage units ingested) is unknown. Wide variation exists in how clinicians prescribe opioids in response to acute pain, and providers often err on the side of too many in order to avoid the inconvenience of refilling prescriptions, a practice that contributes to excess opioid dosage units in the community.1 Prior investigations suggest that 42–72% of prescription opioids are left unused after outpatient surgical procedures. 1–6 Although several investigations have demonstrated a large portion of prescribed opioids are unused, previously employed methods consist of pill counts (often at a single timepoint), prohibiting any description of the patterns by which individuals ingest PRN opioids.
Current methods to measure medication ingestion are indirect; they rely on pill counts, pharmacy refills, Micro-Electro-Mechanical Systems (MEMS) caps that detect opening of pill bottles, patient diaries and urine drug screens.7–9 Long term opioid adherence monitoring uses urine or hair drug screening, methods that suffer from difficulty in interpretation, varying analytic techniques, and inaccurate results depending on the relationship between opioid ingestion and sample acquisition.10,11 These monitoring methods describe opioid use in aggregate, are easily manipulated, and fraught with user bias. Aggregate measures of opioid ingestion miss important dynamic information such as the dose per ingestion as well as changes in dosing interval. Real-time changes in dosing interval, or increased dose per ingestion, may indicate problems with pain control or important changes in the nature of opioid use, and thus represent an opportunity for assessment and early intervention—a phenomenon that is difficult to detect through current measures.
Digital pills—where a medication is incorporated into a gelatin capsule containing a biosensor that activates in the stomach—can provide direct and definitive evidence of medication ingestion.12–17 Iterative improvements in data transmission and signal strength have made digital pills feasible and acceptable to pateints.13 Formative data on the use of digital pills have demonstrated the ability to detect ingestion of antidiabetic agents, antipsychotics, and antituberculosis medications.14,18,19 Although used to measure medication adherence, direct ingestion monitoring through digital pills creates a new opportunity to measure more than just adherence. In the setting of opioid use, knowledge of real-time ingestion can produce relevant information regarding the opioid dose (number of pills ingested) as well as the timing interval between opioid ingestions. Patterns of escalating dosage or increased interval dosing or conversely, patterns of decreased dosing and tapering can help clinicians managing acute exacerbations of pain. In the present study, we report data on opioid ingestion patterns detected by a digital pill in emergency department (ED) patients discharged after treatment for acute fractures. We additionally report qualitative user acceptance of digital pills to monitor opioid ingestion patterns after acute fracture pain.
2. Methods
Institutional Review Board and Patient Consent
This study was approved by our institutional review board (IRB); written informed consent was obtained from all study participants.
Digital Pill technology
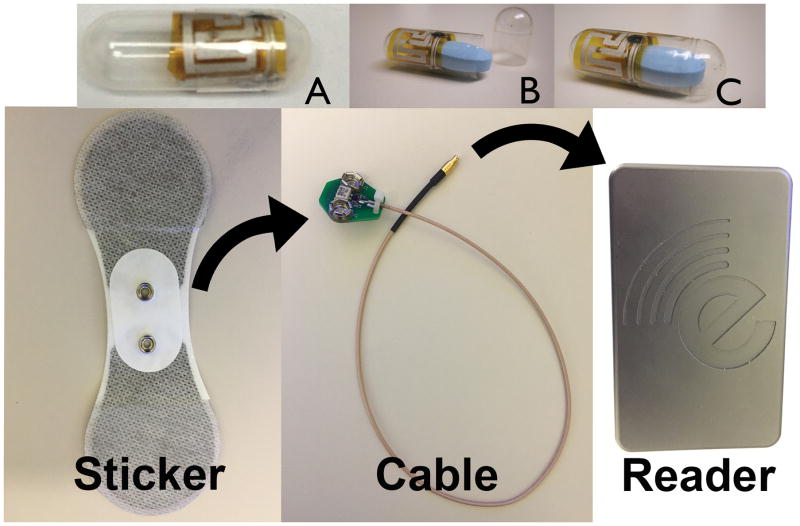
We utilized a digital pill system (eTectRx, Newbury, FL) (Figure 1) to measure opioid ingestion patterns. The digital pill system is an investigational device, classified by the Food and Drug Administration as a nonsignificant risk device. The digital pill comprises an ingestible radiofrequency emitter and a standard gelatin capsule sized to accept a single 5mg oxycodone tablet. Each oxycodone digital pill contains its own unique radiofrequency emitter. When the digital pill is ingested, the gelatin capsule is dissolved, liberating the oxycodone tablet. Chloride ions energize the ingestible radiofrequency emitter which transmits a unique signal containing ingestion time and pre-programmed pill identification number. This signal is acquired by a cutaneous patch adhered to the abdominal wall. The patch is attached by a cable to a Reader (approximately the size of an iPod) which stores and relays ingestion data through cellular signaling to a cloud-based server that collates and displays ingestion events. Upon capturing the radiofrequency signal, the reader also sends a text message to the investigational staff and study participants confirming the ingestion event. The Reader only needs 2–3 seconds to capture the transmitted signal from the digital pill. The digital pill has been utilized without evidence of retention of the radiofrequency transmitter in the gut, and no adverse events have been reported in previous safety trials.16 Digital pills were packaged in a standard blister pack; there was no particular order in which participants were required to take digital pills. A total of 21 dosage units (eg. 21 5-mg oxycodone digital pills) were dispensed to each study participant.
Figure 1.
The digital pill comprised of an ingestible radiofrequency emitter combined with a gelatin capsule (A) sized to accept an oxycodone tablet (B) creating an oxycodone digital pill (C). The reader is composed of an adherent sticker attached by a cable to the Reader that records ingestion events from the digital pill.
Design
This manuscript adheres to the applicable TREND guidelines.20 We performed a descriptive study of opioid-naïve adults discharged from a tertiary care emergency department (ED) between February 2016 to November 2016 with a diagnosis of acute fracture. We excluded individuals who had previous psychiatric and drug abuse histories, were admitted for fracture care, lived outside our catchment area and therefore would be unable to follow up with us, or had multiple sites of injury. Trained research assistants (RA) used the electronic medical record to screen ED patients for inclusion/exclusion criteria before approaching them for participation in the study. Eligible participants were approached in the ED and enrolled on a convenience basis pending the availability of the RA, and an investigator. Participants were only able to be recruited during the times the investigational pharmacy was open (8AM–5PM, weekdays) as digital pill oxycodone was dispensed by our investigational pharmacy for this study. After providing written informed consent, participants prior to discharge received a 15-minute training session from the RA on the operation of the oxycodone digital pill prior to discharge. A study investigator (PRC, EWB or SC) filled out a prescription for oxycodone digital pills in order for the hospital investigational pharmacy to dispense oxycodone digital pills to participants prior to their discharge from the ED. Participants then demonstrated use of each component; we verified successful use of the technology once participants had ingested the digital pill and received a text message confirming ingestion.
We instructed participants to use oxycodone (1–2 five milligram oxycodone digital pills every 6–8 hours) as needed for pain, and ensured that study participants did not receive additional prescriptions for opioid analgesics at discharge from the emergency department, orthopedic services, or their primary care physician. Participants returned equipment and unused digital pills to our clinical research center at the end of seven days or at the time of their follow up orthopedic clinic visit. During this visit, we reviewed ingestion data with participants and conducted a pill count to assess the validity of the digital pill measurement. We also asked participants about their subjective quality of pain control and their decision-making process regarding whether and when to taper oxycodone use. Participants were remunerated for their participation in the study.
Statistical Analysis
Oxycodone ingestions were measured by the digital pill system and collated on a Research Electronic Data Capture (REDCap) database. Results were downloaded and basic descriptive statistics were calculated on Microsoft Excel (Redmont, WA). Ingestion of the oxycodone digital pill associated with system training in the ED was marked time as 0; the study period ran for the next 7 days from this timepoint. If a patient reported adequate pain management at time of system training, time 0 for the study period was time of patient discharge. Ingestions were defined as taking place on Day 1 if they were recorded between time 0 and 24 hours, Day 2 from 25–48 hours, Day 3 from 49–72 hours, etc. Ingestion of oxycodone digital pills were recorded for 7 days; we calculated dose amount and dosing interval using ingestion data downloaded from our study server.
Patients were prescribed one to two 5 mg oxycodone digital pills every 6–8 hours as needed for pain. We calculated dosing interval by dividing 24 hours by the average number of doses taken in a day across all participants. A dose was defined as taking one or two 5 mg oxycodone digital pills at the same time. If a participant took two 5 mg oxycodone digital pills at one time, we considered that one dose of 10 mg. Because of the sample size, we calculated the median dose ingestion over the study period, and median dose ingestions per day for the entire set of participants. We separated participants into those who required operative management of their fracture and those who were managed nonoperatively. We then calculated median ingestion amount and dosing interval for each group.
3. Results
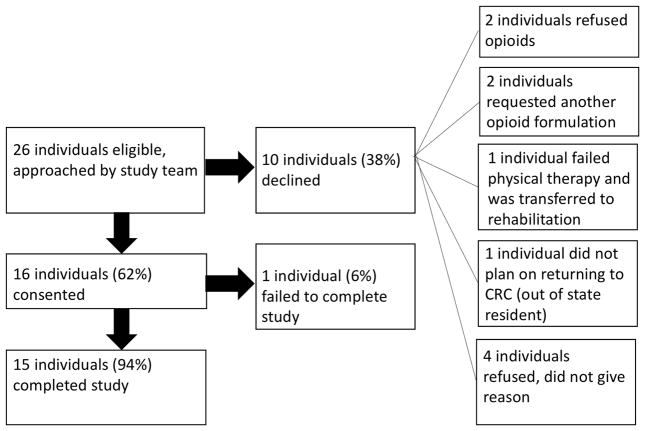
During the study period, 26 individuals were eligible and approached for the study (Figure 2). Ten individuals declined to participate; the majority of non-consenting individuals reported they preferred a different opioid formulation that we did not compound with the digital pill (N=2), that they did not want opioids on discharge (N=2), that they did not intend to participate in the follow up interview (N=1), or that they did not reside in our region (N=1). None refused participation based upon concerns related to ingesting a biosensor. There were no deviations from the study protocol. Of the sixteen individuals consented, 15 completed the study. The one individual who did not complete the study reported that excessive pain prevented him from recharging the battery of the Reader. The mean age of study participants was 45; 60% (N=9) identified as male while 40% (N=7) identified as female (Table 1). Seven participants ultimately required operative repair of their fracture while eight participants were treated nonoperatively.
Figure 2.
Study schematic and enrollment pattern.
Table 1.
Demographics, fracture pattern, and final treatment of study participants (N=15)
| Participant | Age (years) | Sex | Injury | Definitive Therapy |
|---|---|---|---|---|
| 2002 | 32 | M | Lisfranc fracture | Surgical fixation |
| 2003 | 48 | F | Distal radius ulna fracture | Surgical fixation |
| 2004 | 55 | M | Trimalleolar fracture | Surgical fixation |
| 2005 | 55 | M | Bimalleolar fracture | Surgical fixation |
| 2006 | 63 | F | Tibial plateau, proximal fibula fracture | Knee immobilizer |
| 2007 | 21 | M | C6 right pedicle fracture, L2+L3 transverse process fracture | Cervical collar |
| 2008 | 44 | F | Distal fibula fracture | Controlled ankle motion boot |
| 2009 | 66 | F | Distal fibula fracture | Short leg cast |
| 2010 | 27 | F | Distal fibula fracture | Short leg cast |
| 2011 | 55 | M | Distal radius fracture | Wrist splint |
| 2012 | 21 | F | Distal radius fracture, ulnar-styloid fracture | Surgical fixation |
| 2013 | 50 | M | L3 superior endplate fracture | Thoracolumbar orthosis |
| 2014 | 54 | M | Calcaneal fracture | Surgical fixation |
| 2015 | 55 | M | Bimalleolar fracture | Surgical fixation |
| 2016 | 55 | F | Distal radius fracture | Wrist splint |
| 2017 | 32 | M | Lisfranc fracture | Surgical fixation |
The digital pill recorded a total of 112 ingestion events (Table 2); pill counts demonstrated a total of 134 ingestions (84% accuracy). All missed ingestion events were isolated to two study participants who ingested digital pills without wearing the Reader, or did not recharge the Reader due to severe pain. The digital pill system detected 34 simultaneous ingestion events where participants ingested two pills at one time. Eighteen of these episodes (52%) occurred in the first 48 hours after discharge.
Table 2.
Accuracy of the digital pill recording ingestion events.
| Subject | Ingestion events recorded | Pills taken (based on pill count) | System accuracy (%) |
|---|---|---|---|
| 2002 | 16 | 16 | 100.00% |
| 2003 | 14 | 14 | 100.00% |
| 2004 | 14 | 20 | 70.00% |
| 2005 | 9 | 9 | 100.00% |
| 2007 | 16 | 16 | 100.00% |
| 2008 | 12 | 20 | 60.00% |
| 2009 | 6 | 6 | 100.00% |
| 2010 | 0 | 0 | 100.00% |
| 2011 | 2 | 2 | 100.00% |
| 2012 | 0 | 0 | 100.00% |
| 2013 | 8 | 8 | 100.00% |
| 2014 | 4 | 4 | 100.00% |
| 2015 | 5 | 13 | 38.46% |
| 2016 | 0 | 0 | 100.00% |
| 2017 | 6 | 6 | 100.00% |
| Total | 112 | 134 | 84% |
Participants ingested a median of 6 (3, 9) oxycodone digital pills (45mg morphine equivalents) during the study period (Figure 3). The majority—82%—of cumulative oxycodone dose per participant was ingested in the first 72 hours. After 3 days, 46% (N=7) participants had stopped taking oxycodone. Most participants self-tapered their oxycodone dosing intervals over the first 72 hours after discharge (Figure 4). In the first 24 hours after discharge, participants ingested oxycodone digital pills on average every 12 hours, then typically lengthened their dosing interval to one ingestion daily at 48 hours after discharge. Participants returned a mean of 12 oxycodone digital pills at the end of the study period.
Figure 3.
Median oxycodone ingestion events in all study participants (A), in participants who ultimately underwent operative repair (B) and in participants who ultimately underwent nonoperative repair (C).
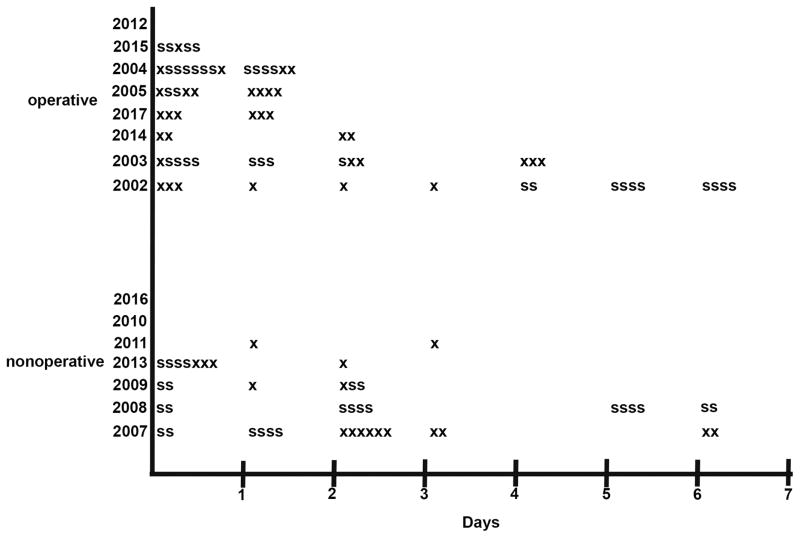
Figure 4.
Ingestion events recorded by the oxycodone digital pill. “X” denotes recorded ingestion event; “S” denotes recorded ingestion event that is part of a simultaneous (ingested 2 pills at one time) ingestion event recorded by the digital pill.
Participants who required operative repair (N=7) ingested a median of 8 (range:6, 11) oxycodone digital pills (60mg morphine equivalents); 84% of the cumulative dose was ingested in the first 72 hours. Six of the 7 participants (86%) with operative fractures remained on oxycodone at one week, while one participant did not ingest opioids during the study period. Most participants with operative fractures ingested oxycodone digital pills every 8 hours for the first 24 hours before tapering down to every 24 hours at 48 hours after discharge.
Participants with nonoperative fractures (N=8) ingested a median of 5 (range:1.5–7) oxycodone digital pills (37.5mg morphine equivalents); 80% of cumulative dose was ingested in the first 72 hours. On average, participants ingested oxycodone digital pills once daily for the first 24 hours, and had stopped taking oxycodone digital pills 96 hours after their injury. No participants who were managed nonoperatively continued to ingest opioids at the end of the study period.
Most participants (N=12) self-reported that their pain was well controlled during the study. Ten participants (66%) reported pain that was severe (eg, in the range of 7–10/10), prompting them to use the oxycodone digital pill for the first 72 hours. Although most participants reported they continued to feel pain after 3 days, 10 reported that the intensity of pain had decreased until it was adequately managed with NSAIDs and acetaminophen. Of note, 6 participants reported even after their pain had subsided, they continued to ingest oxycodone digital pills in the evening prior to bedtime as “prophylaxis” against potential episodes of pain in the morning.
4. Discussion
The findings of this pilot study indicate that most opioid naïve patients self-administer opioids to manage the pain from acute fracture for only a brief period, even among patients with fractures requiring surgical management. Furthermore, most patients stopped self-administering opioids in advance of exhausting their opioid supply. The point at which most of our patients completely stopped taking opioid analgesics following fracture—96 hours—is far shorter than the week-long duration of opioid analgesia for the management of acute pain often prescribed by physicians.5,6 In addition, we observed that the majority of participants, whether they had operative and non-operative fractures, tapered (increasing the length of intervals in their dosing schedule) the dose of opioid analgesic. These findings therefore may be used to improve the precision with which physicians prescribe opioids. Rather than prescribing medications on a PRN basis, we propose that clinicians consider instructing patients to begin tapering the dose of opioid analgesics beginning at 24 hours following injury, which would also reduce the number of pills dispensed, and ultimately wasted or made available for diversion.
Interestingly, patients only ingested a median of 6 pills, despite being given 21 pills to each participant, suggesting that even a week-long duration of therapy following fracture may represent overprescribing opioids. Overprescribing may lead to several important problems. First, it may encourage increased opioid use by that patient. A recent study of postoperative patients showed an association between a larger number of tablets dispensed and the number consumed, independent of patient characteristics or pain, suggesting that patients may take more pills just because they are given more, and that this does not lead to improved pain control or satisfaction.21,22 Our results showing that most patients stopped use within 3 days are especially significant in light of data from the Centers for Disease Control that correlate opioid therapy of greater than three days’ duration with long-term nonmedical opioid use. 23,24. Limiting the availability of unused opioids through evidence-based prescribing is an effective strategy in preventing downstream opioid abuse 25.
Investigators have proposed limiting the number of opioid dosage units to the amount truly needed for a specific clinical indication.26,27 Unfortunately, previous methods of assessing medication-taking behavior (which only infer but do not measure ingestion) are easily manipulated, suffer from user bias, provide aggregated information regarding opioid ingestion, and thus cannot provide information on patterns of opioid ingestion. Consequently, the precise number of dosage units and duration of therapy has remained undefined. Our data suggest that approximately six 5mg oxycodone tablets, when properly tapered, may be sufficient to control pain from acute fractures in the majority of patients, and may serve to support governmental policies directed toward limiting the number of prescribed dosage units of opioid analgesics. Additionally, the mismatch between what was prescribed and what was self-administered underscores the importance of directed teaching to promote non-opioid analgesia (NSAIDs and acetaminophen) amongst providers, despite it being potentially time consuming in a busy ED setting. Physicians should consider providing patients with anticipatory guidance regarding the expected duration of opioid use and the amount of opioids required. Understanding that patients likely need considerably fewer opioid dosage units that are commonly dispensed is important in preventing both downstream long-term opioid use and opioid diversion.23 Our data regarding actual opioid utilization is consistent with other studies demonstrating that physicians significantly overprescribe opioids in response to acute pain.1,5,28
Participants’ practice of ingesting opioid analgesics prior to sleep is concerning. Patients reported the concern of waking up at night with pain as the rationale for ingesting opioids prior to sleep, and indeed, this is a common informal instruction that patients receive from providers in recovery. This approach carries considerable risk, however, because of the respiratory depressant effect of opioids, which may exacerbate preexisting obstructive sleep apnea. Discharge instructions given to patients receiving opioid prescriptions should therefore include explicit warnings regarding the risk of new or worsening sleep apnea that may culminate in death.
Our ultimate goal is not to prevent physicians from prescribing opioids. Instead, our intent is to provide guidelines, based upon direct observation, on how to best manage acute pain while preventing overdose and long-term misuse. Pain is not merely distressing; it is also therapeutic. Acute pain promotes healing by limiting an individual’s activity following injury. A careful balance must be struck between functional analgesia and opioid prescribing to avoid long term nonmedical opioid use. Our data from digital pills that can detect patterns of opioid use is a novel and important step towards responsible and evidence-based opioid prescribing.
Limitations
This study has several limitations. First, it was conducted at a single academic tertiary emergency department. The demographics of individuals presenting to our emergency department, and their preferences for opioid analgesics may not be generalizable to other emergency departments in different settings. Second, the small number of participants enrolled in the study limit the ability to draw conclusions regarding the median number of opioid analgesics required to control acute pain. Despite this, our data suggests that individuals ingesting opioids for acute pain may use less opioids than previously thought. Future investigations will investigate opioid ingestion patterns following more homogenous painful conditions (eg. acute pain after a specific surgical procedure). Third, this study focused on opioid -naïve individuals with acute fractures. Individuals with a history of opioid use may respond differently to acute exacerbations of pain producing different ingestion patterns. Fourth, we selected a single painful condition—acute fractures. Our results, while important may be different in individuals with other painful conditions.
Implications for Further Research
With increasing restrictions on opioid prescribing, detecting opioid ingestion patterns in individuals provide greater precision in determining the numbers of opioid dosage units needed to treat pain while minimizing the threat of misuse. As an investigational tool, the digital pill provides not only a direct measure of opioid ingestion, but also evidence of more dynamic changes in medication-taking behavior. Additionally, use of a digital pill technology, when linked to ecologic momentary assessments, can define affective and environmental contexts that surround opioid-taking behavior, such as whether opioid ingestion is linked to subjective notions of increasing pain, or periods of stress.
We recognize the technology associated with the digital pill described here may appear cumbersome and difficult to use. Despite this, we demonstrated that individuals, with short instruction in the ED, can operate digital pills in the real-world. Iterative improvements in the technology will make digital pills more seamless; we project that there will be two major advances. First, we have witnessed, even during our study period, iterative improvement in energy harvesting in the digital pill. This is significant because improved energy harvesting allows the digital pill to transmit ingestion data across a larger distance. Second, because of improved detection capabilities of the digital pill, the most cumbersome components of the digital pill (eg. the Reader and associated patch) will miniaturize and integrate into other on-body technology like a smartwatch or the patient’s smartphone. We believe that these advances will improve the overall acceptability of digital pills, and allow expansion of this technology to observe opioid ingestion in disparate populations. The improved technology can also be employed in future trials to observe differences in opioid use between different age groups, individuals with disparate risk factors and among various types of surgical procedures.
Clinically, the wireless delivery of opioid ingestion information allows interventions to be delivered to patients at the precise moment they are needed. The discovery of behaviors consistent with the development of tolerance—either an escalation of opioid dose or an increase in dosing frequency—can trigger interventions by clinicians in real time. The development of real-time interventions that are linked to specific patterns of opioid ingestion is an area of urgent need.
Key Points.
Question: How do discharged emergency department opioid naïve patients take opioids prescribed on an as-needed basis after acute fracture pain?
Findings: Using a digital pill medication ingestion monitor, emergency department patients ingested a median of 6 oxycodone digital pills in the 7 days after sustaining an acute fracture.
Meaning: Opioid naïve emergency department patients with acute fracture pain ingest less opioids over a shorter period of time than previously thought.
Footnotes
Disclosures: This work was supported by the National Institutes of Health [5K24DA037109 (PI: EWB), 1KL2TR001455 (SC), P30AI060354 (PRC)]
Contributions:
PRC: This author conceived the study, recruited participants, and completed data analysis in this study. This author was principally responsible for the study as a whole and drafted the manuscript.
SC: This author conceived and completed data analysis in this study. This author was also instrumental in providing key edits to the manuscript.
BJI: This author recruited participants, was primarily responsible for data analysis in the study, and provided key edits in the manuscript.
BC: This author was responsible for recruiting participants, completed data analysis and provided key edits in the manuscript.
KLS: This author helped draft the manuscript and provided key edits in revisions of the manuscript. This author also provided technical assistance in preparing figures and visualizing data for the manuscript.
RRE: This author helped draft the manuscript and provided key edits in revisions of the manuscript.
AJC: This author helped draft key portions of the manuscript and provided key edits in the revisions of the manuscript.
EWB: This author conceived the study, recruited participants and mentored PRC and SC in data analysis and drafting of the manuscript. This author also provided the principle funding through 5K24DA037109 to acquire materials to perform the study.
References
- 1.Kumar K, Gulotta LV, Dines JS, Allen AA, Cheng J, Fields KG, YaDeau JT, Wu CL. Unused Opioid Pills After Outpatient Shoulder Surgeries Given Current Perioperative Prescribing Habits. Am J Sports Med. 2017;61:363546517693665. doi: 10.1177/0363546517693665. [DOI] [PubMed] [Google Scholar]
- 2.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185:551–5. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 3.Lewis ET, Cucciare MA, Trafton JA. What do patients do with unused opioid medications? Clin J Pain. 2014;30:654–62. doi: 10.1097/01.ajp.0000435447.96642.f4. [DOI] [PubMed] [Google Scholar]
- 4.Kim N, Matzon JL, Abboudi J, Jones C, Kirkpatrick W, Leinberry CF, Liss FE, Lutsky KF, Wang ML, Maltenfort M, Ilyas AM. A Prospective Evaluation of Opioid Utilization After Upper-Extremity Surgical Procedures: Identifying Consumption Patterns and Determining Prescribing Guidelines. J Bone Joint Surg Am. 2016;98:e89. doi: 10.2106/JBJS.15.00614. [DOI] [PubMed] [Google Scholar]
- 5.Maughan BC, Hersh EV, Shofer FS, Wanner KJ, Archer E, Carrasco LR, Rhodes KV. Unused opioid analgesics and drug disposal following outpatient dental surgery: A randomized controlled trial. Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Hill MV, McMahon ML, Stucke RS, Barth RJ. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg. 2017;265:709–14. doi: 10.1097/SLA.0000000000001993. [DOI] [PubMed] [Google Scholar]
- 7.Woster P, Ko M, Smith T. (207) Predictors of medication adherence assessed by urine drug monitoring in patients prescribed opioid medications: relationship with opioid dose. J Pain. 2016;17:S27. [Google Scholar]
- 8.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 9.Tacke U, Uosukainen H, Kananen M, Kontra K, Pentikanen H. A pilot study about the feasibility and cost-effectiveness of electronic compliance monitoring in substitution treatment with buprenorphine-naloxone combination. J Opioid Manag. 2009;5:321–9. doi: 10.5055/jom.2009.0032. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz DA, George MP, Bluth MH. Toxicology in Pain Management. Clin Lab Med. 2016;36:673–84. doi: 10.1016/j.cll.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Compton P. The Role of Urine Toxicology in Chronic Opioid Analgesic Therapy. Pain Manag Nurs. 2007;8:166–72. doi: 10.1016/j.pmn.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Chai PR, Castillo-Mancilla J, Buffkin E, Darling C, Rosen RK, Horvath KJ, Boudreaux ED, Robbins GK, Hibberd PL, Boyer EW. Utilizing an Ingestible Biosensor to Assess Real-Time Medication Adherence. J Med Toxicol. 2015:1–6. doi: 10.1007/s13181-015-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai PR, Carreiro S, Innes BJ, Rosen RK, O'Cleirigh C, Mayer KH, Boyer EW. Digital Pills to Measure Opioid Ingestion Patterns in Emergency Department Patients With Acute Fracture Pain: A Pilot Study. J Med Internet Res. 2017;19(1):e19. doi: 10.2196/jmir.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browne SH, Behzadi Y, Littlewort G. Let Visuals Tell the Story: Medication Adherence in Patients with Type II Diabetes Captured by a Novel Ingestion Sensor Platform. J mHealth uHealth. 2015;3:e108–19. doi: 10.2196/mhealth.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J Med Internet Res. 2017;19:e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores GP, Peace B, Carnes TC, Baumgartner SL, Buffkin E, Euliano N, Smith LN. Performance, Reliability, Usability, and Safety of the ID-Cap System for Ingestion Event Monitoring in Healthy Volunteers: A Pilot Study. Innov Clin Neurosci. 2016;13:1–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Chai P, Rosen RK, Boyer EW. Ingestible Biosensors for Real-Time Medication Adherence Monitoring: MyTMed. Proc Annu Hawaii Int Conf Syst Sci. 2016 Jan;2016:3416–3423. doi: 10.1109/HICSS.2016.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, DiCarlo L, Reves R. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PloS one. 2013;8:e53373. doi: 10.1371/journal.pone.0053373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane JM, Perlis RH, DiCarlo LA, Au-Yeung K, Duong J, Petrides G. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. The J Clin Psych. 2013;74:e533–40. doi: 10.4088/JCP.12m08222. [DOI] [PubMed] [Google Scholar]
- 20.Jarlais Des DC, Lyles C, Crepaz N TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361–6. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateman BT, Franklin JM, Bykov K, Avorn J, Shrank WH, Brennan TA, Landon JE, Rathmell JP, Huybrechts KF, Fischer MA, Choudhry NK. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1–353.e18. doi: 10.1016/j.ajog.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu M, McQuaid-Hanson E, Hopp S, Kaimal A, Leffert L, Bateman BT. 817: Shared decision-making for opioid prescribing after cesarean delivery. Am J Obstet Gynecol. 2017;216:S469. doi: 10.1097/AOG.0000000000002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006–2015. MMWR Morbidity and mortality weekly report. 2017;66:265–9. doi: 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health 2015. 2016. [PubMed] [Google Scholar]
- 25.Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305:1346–7. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 26.Cicero TJ, Ellis MS, Harney J. Shifting Patterns of Prescription Opioid and Heroin Abuse in the United States. N Engl J Med. 2015;373:1789–90. doi: 10.1056/NEJMc1505541. [DOI] [PubMed] [Google Scholar]
- 27.Dart RC, Bronstein AC, Spyker DA, Cantilena LR, Seifert SA, Heard SE, Krenzelok EP. Poisoning in the United States: 2012 emergency medicine report of the National Poison Data System. Ann Emerg Med. 2015;65:416–22. doi: 10.1016/j.annemergmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Harris K, Curtis J, Larsen B, Calder S, Duffy K, Bowen G, Hadley M, Tristani-Firouzi P. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149:317–21. doi: 10.1001/jamadermatol.2013.1871. [DOI] [PubMed] [Google Scholar]