Abstract
Purpose of Review
Herpesvirus latency has been viewed as a binary state where replication is either on or off. During latency, gene expression is thought to be restricted to non-coding RNAs or very few proteins so that the virus avoids detection by the immune system. However, a number of recent studies across herpesvirus families call into question the existence of a binary switch for latency, and suggest that latency is far more dynamic than originally presumed. These studies are the focus of this review.
Recent Findings
Highly sensitive and global approaches to investigate viral gene expression in the context of latency have revealed low level viral transcripts, and in some cases protein, from each of the three kinetic gene classes during the latent alpha and beta herpesvirus infection either in vitro or in vivo. Further, low level, asymptomatic virus shedding persists following acute infection. Together, these findings have raised questions about how silent the latent infection truly is.
Summary
Emerging evidence suggests that viral gene expression associated with latent states may be broader and more dynamic than originally presumed during herpesvirus latency. This is an important possibility to consider in understanding the molecular programs associated with the establishment, maintenance and reactivation of herpesvirus latency. Here, we review these findings and detail how they contribute to the emergence of a biphasic model of reactivation.
Keywords: herpesvirus, latency, reactivation, cytomegalovirus, herpes simplex virus
INTRODUCTION
Long-term persistence in the host often arises from a truce whereby the virus limits its replication and pathogenesis and avoids immune clearance. Herpesviruses have evolved an exquisite ability to persist in the host through latency, where viral genomes are maintained in the absence of virus production. Essential to herpesvirus persistence is an ability to reactivate in response to changes in the host state (e.g., stress, disease). Intense focus on latency in the herpesvirus field has yielded important insights into viral and cellular determinants of latency. However, much remains to be understood about the programs of gene expression and the virus-host interactions underlying entry into, maintenance of, and exit from latency.
All herpesviruses target long-lived cellular reservoirs for the latent infection; however, each herpesvirus family establishes latency in a unique reservoir. Members of the alpha herpesvirus family, including HSV-1 and VZV, persist latently in neurons [1, 2]. Beta herpesviruses, such as CMV, establish latency in hematopoietic progenitor cells and cells committed to the myeloid lineage [3]. The gamma herpesviruses, such as EBV and KSHV, reside in lymphoid cells, and in the long-lived resting B cells in the case of EBV [4, 5]. Studies in the gamma herpesvirus field have painted a complex picture of latency, with these viruses having evolved several distinct transcriptional programs for establishing and maintaining latency in lymphoid cell subpopulations. With the development of more sensitive techniques and more advanced animal and in vitro models for studying alpha and beta herpesvirus latency [6–17], a greater understanding of the molecular details regulating the switch between lytic and latent infection are emerging, and are, in turn, challenging the classical definition of herpesvirus latency.
A CLASSICAL VIEW OF HERPESVIRUS LATENCY
Early work in the herpesvirus field described latency as a quiescent infection in which the viral genome is maintained in the absence of viral lytic gene expression and detectable infectious virus (reviewed in [18]). This classical definition of latency effectively divided viral genes into two categories (lytic vs. latent) to differentiate patterns of viral gene expression associated with replicative and latent states of HSV, VZV, and EBV infection. An inability to detect broad viral gene expression led to a model whereby latency is maintained by a binary system of gene expression, where the lytic gene expression program is either “on” (and a full replicative cycle leads to the production of progeny virions) or “off” (and an overwhelmingly silent latency is established) [19]. Indeed, it has been presumed that viral gene expression during latency is restricted to a subset of RNAs or proteins that promote the latent infection or restrict replication. Examples of these in HSV-1 and HCMV latency include the latency-associated transcripts (LATs) that have long been thought of as the molecular hallmark of HSV latency [20, 21], viral gene products encoded in the ULb’ region of the HCMV genome (e.g., the UL133–UL138 locus) [22–24], and HCMV LUNA (Latency Unique Natural Antigen) [25, 26]. The specific role(s) of these genes in the establishment of longer term maintenance of latency is not yet fully understood. Whereas some genes may function directly in the establishment and maintenance of latency, others may contribute indirectly by promoting cell survival, mediating cellular stress pathways, or otherwise tweaking the host cell to maintain an optimal environment for persistence of the virus or to modulate the progression to full reactivation. In addition, it is clear that host cell or viral miRNAs will contribute to latency by dampening viral gene expression, but also likely the host response to infection (reviewed in [27]).
This binary paradigm of herpesviral latency emerged from studies using predominantly in situ hybridization and immunohistochemistry and either viruses with defects in replication or treatment with inhibitors of replication in the case of alpha herpesviruses [28]. Further, there was no approach to globally analyze viral gene expression and, as such, primary focus was placed around a small number of “lytic genes” such as the immediate early and the emerging “latency” genes.
A MORE DYNAMIC VIEW OF HERPESVIRUS LATENCY
Expression of productive cycle genes during latency
Latent infection has long been perceived as being marked by the absence of expression of productive cycle or “lytic” genes [19], such as immediate early genes, involved in transactivating the viral program of replication (e.g., ICP0, ICP4, and ICP27 in HSV-1 and IE1 and IE2 in HCMV). As such, immediate early gene expression is often used as a proxy for the reactivation, while its absence is evidence for a latent state. However, the increase in sensitivity and breadth of gene expression analysis made possible over the last decade or so has begun to define broader patterns of gene expression associated with the latent infection [11, 29–35].
Detection of viral gene expression once designated as “lytic” in models of HSV-1 latency have blurred the distinction between latent and lytic states of infection [34, 35]. Recent studies have expanded on these observations to begin to understand the mechanisms by which viral gene expression is controlled during latency. During latent HSV-1 infection in rabbits, expression of the LATs is associated with increased expression of lytic genes, suggesting that the LAT may function to poise cells for reactivation [36]. Further, Proenca et al. found that a large subpopulation of neurons in HSV-1-infected mice had encountered IE promoter activation at some point between the initial infection and the establishment of latency [37], arguing against the idea that herpesviruses “select” a lytic or latent gene expression program based on cell type immediately following infection. Consistent with the possibility that broad gene expression persists during the maintenance of latency, studies by the Tscharke group found that approximately 66% of the neurons containing HSV-1 DNA from latently infected mice expressed one or more lytic transcripts [38] and, more recently, proteins [39]. Further, the classically defined HSV-1 immediate early lytic gene, ICP0, is not only expressed during latency [37, 40–42], but also influences the latent environment, promoting the establishment of and reactivation from latency [43, 44] by stimulating the expression of LAT and altering the chromatin state of the latent genome [45]. Therefore, we must reconsider the roles of viral genes previously classified as lytic- or latent-acting, and by extension, the paradigm that latency is a static state devoid of broader viral gene expression.
Similar to HSV-1 latency, immediate early and some classically defined early and late genes are detected in the early stages following HCMV infection of latency-associated cells, although productive viral replication does not ensue [11, 29, 33, 46]. These findings are mirrored in clinical studies where latently infected cells from healthy carriers sporadically express IE genes, but fail to produce detectable progeny virus ex vivo [47]. Together, these findings suggest that gene expression associated with CMV latency also may be substantially more dynamic than originally presumed. One caveat to this interpretation is borne from the models available to study HCMV latency. Due to the strict species tropism for beta herpesviruses, in vivo models are restricted to the murine strain of CMV [16, 48, 49] or HCMV infection in humanized mice [15]. Further, in vitro models include hematopoietic cell lines or cultured CD14+ or CD34+ primary hematopoietic cells. Cell lines have the problem of being a limited reflection of latency, while primary cell populations are heterogeneous, consisting of a number of different subpopulations at various stages of differentiation and lineage-commitment and, as such, likely do not support a uniform latent program. The heterogeneity of primary hematopoietic cell populations leaves open the possibility that genes detected represent an average of different gene expression programs present in distinct cell subpopulations. Therefore, while some cells may maintain a true latency, other cells may support distinct gene expression profiles. These possibilities challenge a binary system for cytomegalovirus latency that warrants further investigation to understand the true nature of cytomegalovirus persistence.
Sporadic Reactivation and Shedding
Latent alpha herpesvirus infection was conventionally described as long periods of quiescence marked by occasional periods of reactivation that were clinically linked to the appearance of oral or genital lesions. However, a number of studies over the last two decades have provided a more complete clinical picture, demonstrating asymptomatic shedding of both HSV-1 and HSV-2 in the absence of clinical lesions. The earliest studies of HSV-2 shedding found that infectious virus could be detected following genital swabbing at a frequency of 20 to 25% of all days tested, including days during which no clinical lesions were detected [50, 51]. Additional studies performed following the development of more sensitive PCR techniques have demonstrated that the frequency of asymptomatic shedding is even higher, with HSV-2 DNA detected during 20 of the 30 days out of each month [52]. Similar frequencies of asymptomatic shedding of HSV-1 from the oral cavity have been reported [53]. These findings are consistent with the high frequency of shedding observed in the animal models [54] and with a report by Margolis et al., in which infectious virus was detected in latently infected mouse neurons, and appeared to be indicative of spontaneous reactivation events that occur frequently [55]. In addition to fueling almost continuous viral shedding, HSV-1 spread through neurons continues beyond the peak of acute infection [39], although it has yet to be determined if continued shedding is due to a protracted period of chronic replication in neurons or sporadic reactivation.
A similar clinical situation may exist during HCMV infection. Following a typical primary infection, children can shed CMV in saliva and urine for months to years, most often in the context of an asymptomatic infection [56, 57]. During clinical latency, low subclinical levels of virus may be chronically shed from productively infected epithelial cells in the salivary glands or kidneys, for example, or produced following sporadic reactivation of latently infected cells [57]. The extent to which the HCMV genome is quiescent or active during latency has not, as of yet, been clearly demonstrated, in part because global gene expression studies have only been performed in heterogeneous populations of cells infected in vitro. Certainly, the high number of circulating T cells in CMV-seropositive, latently infected individuals [58, 59] suggests that HCMV antigens may be expressed more sporadically and frequently than in other herpesvirus infections [60]. Taken together, these studies support the notion that alpha and beta herpesvirus latency is a dynamic state, poised for frequent spontaneous reactivation leading to asymptomatic shedding.
Epigenetic regulation of latent herpesvirus gene expression
Epigenetic switches are largely thought to control the initial transition of the viral genome into and out of latency. During the establishment of latency, herpesvirus gene expression is restricted by chromatinization of the genome and modification of histones with repressive marks [reviewed in [61–63]]. While epigenetic silencing is clearly central to the establishment or maintenance of viral latency, it is now becoming clear that the chromatin state is dynamic to allow silencing to be readily reversed [64–66]. This dynamic state of chromatin silencing likely contributes to the possibility of “leaky” gene expression during latency.
In the case of HSV-1 latency, early studies revealed that epigenetic markers associated with active transcription are enriched at the LAT locus compared to the loci of several lytic genes [67–69], consistent with higher expression levels of the LATs during latency. Subsequent studies further suggested that the LAT plays a role in suppressing lytic gene expression, as infection of mice with a LAT-negative mutant resulted in heightened expression of lytic genes compared to wildtype infection [70–74]. The repression of lytic genes by the LAT was later attributed, at least in part, to the LAT’s ability to alter the chromatin state of the viral genome for latency, as LAT expression was found to be positively correlated with an increase in both constitutive (non-reversible) and facultative (readily reversible) repressive heterochromatin marks on an number of lytic genes [64, 75]. Interestingly, infection with the LAT-mutant virus also resulted in decreased explant reactivation in the mouse model [70, 74], indicating that the LAT may also play a role in modulating viral reactivation following latency. These findings are consistent with a report that LAT expression is associated with an increase rather than a decrease in lytic gene expression in the rabbit model [36]. Although there is some conflict as to whether LAT expression decreases or increases lytic gene expression, a recent study by Kwiatkowsi et al. found that the genome of LAT-mutant virus contains a significant enrichment in facultative heterochromatin compared to the wildtype virus, while constitutive heterochromatin does not vary. These findings suggest a role for the LATs in maintaining a “softer” or less restricted heterochromatic state of the latent genome [65]. In this way, the LAT may both contribute to viral genome silencing, while ensuring that the epigenetic restriction is readily reversible for reactivation.
Studies of CMV latency indicate that beta herpesviruses share many features of latency-associated chromatin regulation with HSV. As is the case with HSV, the DNA is not methylated during latent infection, rather gene expression appears to be controlled by repressive histone modifications [76, 77]. In studies of both HCMV [78] and MCMV [79], the viral genome becomes associated with repressive histone modifications at early times post infection. Early findings indicated that the HCMV IE promoter is transcriptionally silent and is heterochromatinized, whereas the active LUNA promoter is euchromatic [80, 81]. The CMV genome has also shown to be silenced, in part, by the polycomb repressive complex 2 (PRC2) that catalyzes the formation of facultative heterochromatin with the trimethylation of histone H3 on lysine 27 [82]. Further, as is the case with latent HSV infection, a number of studies in MCMV latently infected mice indicate that IE transcripts can be detected during latency, although full viral replication does not ensue [16, 48, 83–85]. Therefore, it follows that the CMV genome is also maintained in a dynamic state capable of low level gene expression and poised for reactivation.
A NEW MODEL FOR REACTIVATION OF HSV-1 FROM LATENCY
A large body of evidence in the HSV-1 field now exists demonstrating readily reversible or leaky epigenetic silencing of latent viral genomes, detection of “lytic” gene expression during latency, and frequent or chronic viral shedding in the absence of clinical symptoms. These findings create a puzzling scenario when considered in the context of a canonically silent latency program where viral gene expression is restricted to a small subset of latency-associated gene products. A recently proposed model describing a biphasic reactivation of HSV-1 from latency sheds some light on the matter while potentially redefining the way in which we think about herpesvirus latency. During the first phase, characterized as the “animation” phase, broad and non-sequential expression of viral transcripts from all three kinetic classes occurs [86, 87]. Early and late gene expression is observed in the absence of viral protein synthesis [86, 88, 89], and late gene expression is not dependent on replication of the viral DNA [86]. In addition, the first phase of lytic gene expression appears to be independent of the viral tegument protein VP16, which serves as a transcriptional activator during de novo gene expression following primary infection. The second phase, or “synthesis” phase, more closely resembles the pattern of gene expression seen in de novo infection, as it is characterized by a coordinated cascade of IE, E, and L gene expression and results in the production of infectious virus [86]. It is proposed that the initial burst of lytic gene expression during Phase I serves to produce quantities of key viral proteins (such as VP16) that are sufficient to cross a threshold that triggers the coordinated gene expression and virus production associated with Phase II. It is conceivable that Phase I is often abortive, and that while it contributes to viral gene expression during latency, it may not always reach the threshold required to initiate Phase II and full reactivation. This would result in a more dynamic state of gene expression during latency and would account for the expression of replicative cycle genes discussed above. This model also fits with the dynamic nature of chromatinization of the latent genome. Recent studies have shown that the first phase of reactivation occurs when the viral genome is still in its repressive chromatinized state [90] and, presumably, also occurs in the absence of viral proteins such as VP16 [42, 86]. Together, these findings indicate that Phase I of reactivation is likely initiated in response to changes in the cellular environment. Indeed, a recent study by Cliffe et al. shows that activation of the c-Jun N-terminal kinase (JNK) cellular stress response pathway triggers Phase I of HSV-1 reactivation by activating cellular transcription factors and altering the chromatin state of the latent genome so that it is permissive for transcription [90]. Interestingly, the epigenetic changes described during Phase I do not include removal of the repressive histone lysine modifications, but involve a histone methyl/phospho switch whereby a neighboring serine residue is phosphorylated to provide, in essence, a shortcut that allows simultaneous Phase I viral gene expression of immediate early, early, and late genes. Viral regulatory factors produced during Phase I, such as VP16, which has a known role in chromatin remodeling [66], can then remove repressive heterochromatin marks to allow for ordered IE, E, and L gene expression and the production of progeny virions during Phase II of reactivation. Given the aforementioned potential for frequent, but abortive Phase I reactivation, it is not yet clear if the viral gene expression detected during latency is the result of leaky gene expression during latency or if it is the result of sporadic and perhaps abortive Phase I reactivation.
CONCLUSIONS AND FUTURE DIRECTIONS
Clinical latency in a host organism is characterized by long-term maintenance of viral genomes and sporadic and frequent virus shedding. While a stable and static latent state may exist in some cell types, it is becoming clear that latency may have a more dynamic nature than previously appreciated. Three observations in HSV-1 underlie this conclusion: 1) the detection of “lytic” transcripts during the establishment and maintenance phases of latency, 2) the readily reversible nature of the repressed chromatin state of the latent viral genome, and 3) the frequency of spontaneous viral reactivation and chronic shedding in the host. These findings indicate that the viral chromosome is restricted, but not entirely silent during latency. Further, rather than a distinct binary switch, reactivation may be phased where distinct mechanisms control (i) the expression of viral genes initially triggered by changes in host signaling (Phase I) and (ii) the initiation of full reactivation and the canonical cascade of gene expression (Phase II). Phase I reactivation may occur frequently, but abortively in response to minor insults (e.g., stress, changes in host cell signaling), and contribute to the non-productive expression of lytic genes in latent infection.
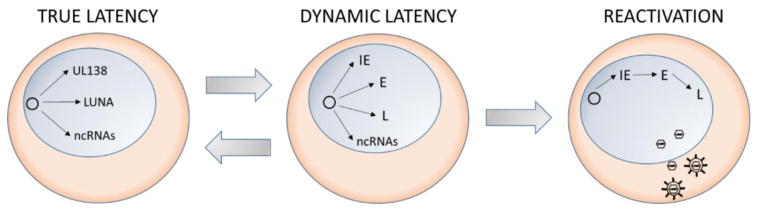
HCMV latency is similarly also associated with low-level, but broad viral gene expression [11, 29, 33, 46], suggesting that it may non-productively express viral genes during latency and also undergo phased reactivation (Figure 1). Genes expressed during a period of latency may contribute to the establishment or maintenance of latency, or may be the products of abortive reactivation events. As HCMV reactivation has been linked with differentiation of an infected cell along the myeloid lineage [47, 80, 91–93], HCMV reactivation is undoubtedly triggered in response to changes in host signaling accompanying differentiation. Similarly to HSV-1, inhibition of growth factor signaling and inhibition of PI3K results in reactivation of HCMV [94, 95]. It is then critical to understand how the virus “senses” and responds to changes in the host cell to impact the epigenetic state of the virus and the balance between latent and replicative states of HCMV infection.
Figure 1. A dynamic view of HCMV latency-a hypothetical model.
Latency has been classically defined as a predominantly silent program where viral gene expression is restricted to very few proteins (e.g., UL138 and LUNA) or non-coding RNAs (both long non-coding and micro RNAs) (left). However, studies over the last decade have detected the expression of additional replicative cycle genes in the context of latency. Based on the studies in HSV-1, where the biphasic viral reactivation program is thought to result in frequent, but abortive viral gene expression, we propose a similar multiphasic reactivation where IE, E, and L genes would be expressed sporadically during the latent infection (center). While a static latent state may exist in some contexts, emerging evidence supports a dynamic latent state characterized by low level viral gene expression that occurs in the absence of virus progeny production. Commitment to full reactivation and a switch to the canonical cascade of IE, E, then L gene expression, resulting in the production of viral progeny (right) would require viral gene expression crossing a threshold and possibly other stimuli to induce additional changes in host signaling and differentiation.
Our work has identified the UL138 viral gene as important for the latent infection. UL138 is encoded within a genetic locus containing 3 other genes, UL133, UL135, and UL136 [24]. Disruption of UL138 results in viral replication in latency-associated CD34+ hematopoietic progenitor cells (HPCs), which is due at least in part to replication-promoting activities of UL135 and UL136 [22–24, 30, 96]. We have shown that UL138 actively promotes the maintenance of surface levels of epidermal growth factor receptor (EGFR) to maintain its activity [94]. Reduction of EGFR or downstream PI3K activity by chemical inhibition results in increased frequencies of reactivation when coupled to a stimulus for myeloid differentiation. Intriguingly, UL135, which is required for reactivation, targets EGFR for turnover. This regulation may profoundly affect cellular stress, differentiation, and survival pathways controlled by EGFR. The opposing regulation of EGFR by UL135 and UL138 may alter the cellular state, and, in turn, impact programs of viral gene expression contributing to virus-mediated control of latency and reactivation. Consistent with this notion, UL138 has also been shown to impede demethylation of histones associated with the viral genome required for expression of IE genes [97], suggesting that UL138 regulates signaling cascades which will ultimately impact the chromatin state of the viral genome. Therefore, control over a homeostatic regulator like EGFR may effectively allow the virus to hardwire itself into the host cell to sense and respond to changes in the state of the cell.
While significant progress in understanding the mechanisms controlling herpesvirus latency and reactivation has been made over the past two decades, contemporary studies aimed at defining the complex mechanisms underlying herpesvirus latency and reactivation have produced as many new questions as answers, and have challenged existing paradigms. The possibility of broader gene expression during the latent program increases the complexity of the biology of latency and opens up a number of new opportunities and challenges. Defining the nature of the dynamic latent state and the roles of genes expressed will reveal important and novel virus-host interactions important to latency and will advance our molecular understanding of latency in the host. One challenge will be to differentiate meaningful gene expression important for establishing and maintaining or exiting latency from sporadic leaky gene expression that neither aids latency nor results in full reactivation. It will also be important to differentiate the distinct programs of gene expression important to latency, a challenge that requires in depth single cell analysis.
Footnotes
COMPLIANCE WITH ETHICS GUIDELINES
Donna Collins-McMillen and Felicia D. Goodrum declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Roizman B, Knipe DM, Whitely RJ. Herpes Simplex Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 1823–97. [Google Scholar]
- 2.Arvin AM, Gilden D. Varicella-Zoster Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 2015–57. [Google Scholar]
- 3.Mocarski Jr E, Shenk T, Griffiths PD, et al. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 1960–2014. [Google Scholar]
- 4.Longnecker RM, Kieff E, Cohen JI. Epstein-Barr Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 1898–959. [Google Scholar]
- 5.Damania BA, Cesarman E. Kaposi’s Sarcoma-Associated Herpesvirus. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 2080–128. [Google Scholar]
- 6.Wilcox CL, Johnson EM., Jr Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. Journal of virology. 1987;61(7):2311–5. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox CL, Johnson EM., Jr Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. Journal of virology. 1988;62(2):393–9. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi M, Kim JY, Camarena V, et al. A primary neuron culture system for the study of herpes simplex virus latency and reactivation. Journal of visualized experiments : JoVE. 2012;(62) doi: 10.3791/3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson AC, Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends in microbiology. 2012;20(12):604–11. doi: 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertke AS, Swanson SM, Chen J, et al. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. Journal of virology. 2011;85(13):6669–77. doi: 10.1128/jvi.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodrum FD, Jordan CT, High K, et al. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc Natl Acad Sci USA. 2002;99(25):16255–60. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albright ER, Kalejta RF. Myeloblastic cell lines mimic some but not all aspects of human cytomegalovirus experimental latency defined in primary CD34+ cell populations. Journal of virology. 2013;87(17):9802–12. doi: 10.1128/jvi.01436-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcangeletti MC, Vasile Simone R, Rodighiero I, et al. Human cytomegalovirus reactivation from latency: validation of a “switch” model in vitro. Virology journal. 2016;13(1):179. doi: 10.1186/s12985-016-0634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor CM, Murphy EA. A myeloid progenitor cell line capable of supporting human cytomegalovirus latency and reactivation, resulting in infectious progeny. Journal of virology. 2012;86(18):9854–65. doi: 10.1128/jvi.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MS, Goldman DC, Bailey AS, et al. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell host & microbe. 2010;8(3):284–91. doi: 10.1016/j.chom.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurz SK, Rapp M, Steffens HP, et al. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. Journal of virology. 1999;73(1):482–94. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddehase MJ, Simon CO, Seckert CK, et al. Murine model of cytomegalovirus latency and reactivation. Current topics in microbiology and immunology. 2008;325:315–31. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- 18.Stevens JG. Human herpesviruses: a consideration of the latent state. Microbiological reviews. 1989;53(3):318–32. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellett PE, Roizman B. Herpesviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 1802–22. [Google Scholar]
- 20.Spivack JG, Fraser NW. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. Journal of virology. 1987;61(12):3841–7. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens JG, Wagner EK, Devi-Rao GB, et al. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science (New York, NY) 1987;235(4792):1056–9. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 22.Caviness K, Bughio F, Crawford LB, et al. Complex Interplay of the UL136 Isoforms Balances Cytomegalovirus Replication and Latency. mBio. 2016;7(2):e01986. doi: 10.1128/mBio.01986-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umashankar M, Rak M, Bughio F, et al. Antagonistic determinants controlling replicative and latent states of human cytomegalovirus infection. J Virol. 2014;88(11):5987–6002. doi: 10.1128/JVI.03506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umashankar M, Petrucelli A, Cicchini L, et al. A novel human cytomegalovirus locus modulates cell type-specific outcomes of infection. PLoS Pathog. 2011;7(12):e1002444. doi: 10.1371/journal.ppat.1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bego M, Maciejewski J, Khaiboullina S, et al. Characterization of an antisense transcript spanning the UL81–82 locus of human cytomegalovirus. Journal of virology. 2005;79(17):11022–34. doi: 10.1128/jvi.79.17.11022-11034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bego MG, Keyes LR, Maciejewski J, et al. Human cytomegalovirus latency-associated protein LUNA is expressed during HCMV infections in vivo. Archives of virology. 2011;156(10):1847–51. doi: 10.1007/s00705-011-1027-7. [DOI] [PubMed] [Google Scholar]
- 27.Piedade D, Azevedo-Pereira JM. The Role of microRNAs in the Pathogenesis of Herpesvirus Infection. Viruses. 2016;8(6) doi: 10.3390/v8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom DC. Alphaherpesvirus Latency: A Dynamic State of Transcription and Reactivation. Advances in virus research. 2016;94:53–80. doi: 10.1016/bs.aivir.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Cheung AK, Abendroth A, Cunningham AL, et al. Viral gene expression during the establishment of human cytomegalovirus latent infection in myeloid progenitor cells. Blood. 2006;108(12):3691–9. doi: 10.1182/blood-2005-12-026682. [DOI] [PubMed] [Google Scholar]
- 30.Goodrum F, Reeves M, Sinclair J, et al. Human cytomegalovirus sequences expressed in latently infected individuals promote a latent infection in vitro. Blood. 2007;110(3):937–45. doi: 10.1182/blood-2007-01-070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohrs RJ, Gilden DH. Varicella zoster virus transcription in latently-infected human ganglia. Anticancer research. 2003;23(3a):2063–9. [PubMed] [Google Scholar]
- 32.Kosz-Vnenchak M, Coen DM, Knipe DM. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. Journal of virology. 1990;64(11):5396–402. doi: 10.1128/jvi.64.11.5396-5402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetto CC, Tarrant-Elorza M, Pari GS. Cis and trans acting factors involved in human cytomegalovirus experimental and natural latent infection of CD14 (+) monocytes and CD34 (+) cells. PLoS Pathog. 2013;9(5):e1003366. doi: 10.1371/journal.ppat. Epub 2013 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer MF, Chen SH, Knipe DM, et al. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. Journal of virology. 1998;72(2):1177–85. doi: 10.1128/jvi.72.2.1177-1185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer MF, Coen DM. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. Journal of virology. 1995;69(3):1389–99. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giordani NV, Neumann DM, Kwiatkowski DL, et al. During herpes simplex virus type 1 infection of rabbits, the ability to express the latency-associated transcript increases latent-phase transcription of lytic genes. Journal of virology. 2008;82(12):6056–60. doi: 10.1128/jvi.02661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proenca JT, Coleman HM, Connor V, et al. A historical analysis of herpes simplex virus promoter activation in vivo reveals distinct populations of latently infected neurones. The Journal of general virology. 2008;89(Pt 12):2965–74. doi: 10.1099/vir.0.2008/005066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •38.Ma JZ, Russell TA, Spelman T, et al. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS pathogens. 2014;10(7):e1004237. doi: 10.1371/journal.ppat.1004237. This study uses single cell transcription analysis to reveal the highly dynamic nature of HSV latency. It is the first to show that lytic HSV-1 gene expression occurs frequently in the majority of latently infected neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Russell TA, Tscharke DC. Lytic Promoters Express Protein during Herpes Simplex Virus Latency. PLoS pathogens. 2016;12(6):e1005729. doi: 10.1371/journal.ppat.1005729. A follow up study to Ma et al (2014), this study shows that lytic protein is expressed during latent HSV-1 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SH, Lee LY, Garber DA, et al. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. Journal of virology. 2002;76(10):4764–72. doi: 10.1128/JVI.76.10.4764-4772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maillet S, Naas T, Crepin S, et al. Herpes simplex virus type 1 latently infected neurons differentially express latency-associated and ICP0 transcripts. Journal of virology. 2006;80(18):9310–21. doi: 10.1128/jvi.02615-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proenca JT, Coleman HM, Nicoll MP, et al. An investigation of herpes simplex virus promoter activity compatible with latency establishment reveals VP16-independent activation of immediate-early promoters in sensory neurones. The Journal of general virology. 2011;92(Pt 11):2575–85. doi: 10.1099/vir.0.034728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leib DA, Coen DM, Bogard CL, et al. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. Journal of virology. 1989;63(2):759–68. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halford WP, Schaffer PA. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. Journal of virology. 2001;75(7):3240–9. doi: 10.1128/jvi.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •45.Raja P, Lee JS, Pan D, et al. A Herpesviral Lytic Protein Regulates the Structure of Latent Viral Chromatin. mBio. 2016;7(3) doi: 10.1128/mBio.00633-16. This study shows that the HSV lytic protein ICP0 is functional during latent infection and influences the latent environment by driving LAT expression and regulating viral chromatin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodrum F, Jordan CT, Terhune SS, et al. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood. 2004;104(3):687–95. doi: 10.1182/blood-2003-12-4344. [DOI] [PubMed] [Google Scholar]
- 47.Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68:1597–604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grzimek NK, Dreis D, Schmalz S, et al. Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. Journal of virology. 2001;75(6):2692–705. doi: 10.1128/jvi.75.6.2692-2705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurz SK, Reddehase MJ. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. Journal of virology. 1999;73(10):8612–22. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corey L, Wald A, Davis LG. Subclinical shedding of HSV: its potential for reduction by antiviral therapy. Advances in experimental medicine and biology. 1996;394:11–6. doi: 10.1007/978-1-4757-9209-6_2. [DOI] [PubMed] [Google Scholar]
- 51.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. The New England journal of medicine. 2000;342(12):844–50. doi: 10.1056/nejm200003233421203. [DOI] [PubMed] [Google Scholar]
- 52.Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. Jama. 2011;305(14):1441–9. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller CS, Danaher RJ. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2008;105(1):43–50. doi: 10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Berman EJ, Hill JM. Spontaneous ocular shedding of HSV-1 in latently infected rabbits. Investigative ophthalmology & visual science. 1985;26(4):587–90. [PubMed] [Google Scholar]
- 55.Margolis TP, Elfman FL, Leib D, et al. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. Journal of virology. 2007;81(20):11069–74. doi: 10.1128/jvi.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pass RF, Hutto SC, Reynolds DW, et al. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics. 1984;74(1):121–6. [PubMed] [Google Scholar]
- 57.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Current topics in microbiology and immunology. 2008;325:417–70. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 58.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of experimental medicine. 2005;202(5):673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chidrawar S, Khan N, Wei W, et al. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clinical and experimental immunology. 2009;155(3):423–32. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krishna BA, Lau B, Jackson SE, et al. Transient activation of human cytomegalovirus lytic gene expression during latency allows cytotoxic T cell killing of latently infected cells. Scientific reports. 2016;6:24674. doi: 10.1038/srep24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen HS, Lu F, Lieberman PM. Epigenetic regulation of EBV and KSHV latency. Current opinion in virology. 2013;3(3):251–9. doi: 10.1016/j.coviro.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lieberman PM. Keeping it quiet: chromatin control of gammaherpesvirus latency. Nature reviews Microbiology. 2013;11(12):863–75. doi: 10.1038/nrmicro3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinclair J. Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection. Biochimica et biophysica acta. 2010;1799(3–4):286–95. doi: 10.1016/j.bbagrm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Cliffe AR, Garber DA, Knipe DM. Transcription of the herpes simplex virus latency-associated transcript promotes the formation of facultative heterochromatin on lytic promoters. Journal of virology. 2009;83(16):8182–90. doi: 10.1128/jvi.00712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwiatkowski DL, Thompson HW, Bloom DC. The polycomb group protein Bmi1 binds to the herpes simplex virus 1 latent genome and maintains repressive histone marks during latency. Journal of virology. 2009;83(16):8173–81. doi: 10.1128/jvi.00686-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill JM, Quenelle DC, Cardin RD, et al. Inhibition of LSD1 reduces herpesvirus infection, shedding, and recurrence by promoting epigenetic suppression of viral genomes. Science translational medicine. 2014;6(265):265ra169. doi: 10.1126/scitranslmed.3010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubat NJ, Amelio AL, Giordani NV, et al. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. Journal of virology. 2004;78(22):12508–18. doi: 10.1128/jvi.78.22.12508-12518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kubat NJ, Tran RK, McAnany P, et al. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. Journal of virology. 2004;78(3):1139–49. doi: 10.1128/JVI.78.3.1139-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumann DM, Bhattacharjee PS, Giordani NV, et al. In vivo changes in the patterns of chromatin structure associated with the latent herpes simplex virus type 1 genome in mouse trigeminal ganglia can be detected at early times after butyrate treatment. Journal of virology. 2007;81(23):13248–53. doi: 10.1128/jvi.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leib DA, Bogard CL, Kosz-Vnenchak M, et al. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. Journal of virology. 1989;63(7):2893–900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garber DA, Schaffer PA, Knipe DM. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. Journal of virology. 1997;71(8):5885–93. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devi-Rao GB, Bloom DC, Stevens JG, et al. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. Journal of virology. 1994;68(3):1271–82. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen SH, Kramer MF, Schaffer PA, et al. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. Journal of virology. 1997;71(8):5878–84. doi: 10.1128/jvi.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicoll MP, Hann W, Shivkumar M, et al. The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons. PLoS pathogens. 2016;12(4):e1005539. doi: 10.1371/journal.ppat.1005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang QY, Zhou C, Johnson KE, et al. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16055–9. doi: 10.1073/pnas.0505850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hummel M, Yan S, Li Z, et al. Transcriptional reactivation of murine cytomegalovirus ie gene expression by 5-aza-2′-deoxycytidine and trichostatin A in latently infected cells despite lack of methylation of the major immediate-early promoter. The Journal of general virology. 2007;88(Pt 4):1097–102. doi: 10.1099/vir.0.82696-0. [DOI] [PubMed] [Google Scholar]
- 77.Murphy JC, Fischle W, Verdin E, et al. Control of cytomegalovirus lytic gene expression by histone acetylation. The EMBO journal. 2002;21(5):1112–20. doi: 10.1093/emboj/21.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nitzsche A, Paulus C, Nevels M. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. Journal of virology. 2008;82(22):11167–80. doi: 10.1128/jvi.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu XF, Yan S, Abecassis M, et al. Biphasic recruitment of transcriptional repressors to the murine cytomegalovirus major immediate-early promoter during the course of infection in vivo. Journal of virology. 2010;84(7):3631–43. doi: 10.1128/jvi.02380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reeves MB, MacAry PA, Lehner PJ, et al. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(11):4140–5. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reeves MB, Sinclair JH. Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. The Journal of general virology. 2010;91(Pt 3):599–604. doi: 10.1099/vir.0.015602-0. [DOI] [PubMed] [Google Scholar]
- 82.Abraham CG, Kulesza CA. Polycomb repressive complex 2 silences human cytomegalovirus transcription in quiescent infection models. Journal of virology. 2013;87(24):13193–205. doi: 10.1128/jvi.02420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henry SC, Hamilton JD. Detection of murine cytomegalovirus immediate early 1 transcripts in the spleens of latently infected mice. The Journal of infectious diseases. 1993;167(4):950–4. doi: 10.1093/infdis/167.4.950. [DOI] [PubMed] [Google Scholar]
- 84.Yu Y, Henry SC, Xu F, et al. Expression of a murine cytomegalovirus early-late protein in “latently” infected mice. The Journal of infectious diseases. 1995;172(2):371–9. doi: 10.1093/infdis/172.2.371. [DOI] [PubMed] [Google Scholar]
- 85.Yuhasz SA, Dissette VB, Cook ML, et al. Murine cytomegalovirus is present in both chronic active and latent states in persistently infected mice. Virology. 1994;202(1):272–80. doi: 10.1006/viro.1994.1343. [DOI] [PubMed] [Google Scholar]
- 86.Kim JY, Mandarino A, Chao MV, et al. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS pathogens. 2012;8(2):e1002540. doi: 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Penkert RR, Kalejta RF. Tegument protein control of latent herpesvirus establishment and animation. Herpesviridae. 2011;2(1):3. doi: 10.1186/2042-4280-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS pathogens. 2009;5(3):e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du T, Zhou G, Roizman B. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(46):18820–4. doi: 10.1073/pnas.1117203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •90.Cliffe AR, Arbuckle JH, Vogel JL, et al. Neuronal Stress Pathway Mediating a Histone Methyl/Phospho Switch Is Required for Herpes Simplex Virus Reactivation. Cell host & microbe. 2015;18(6):649–58. doi: 10.1016/j.chom.2015.11.007. This study is the first report of a cellular reactivation stimulus activating silenced promoters to induce HSV lytic gene expression. It describes a mechanism by which JNK signaling activates a histone methyl/phospho switch to bypass repressive lysine methylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soderberg-Naucler C, Streblow DN, Fish KN, et al. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol. 2001;75:7543–54. doi: 10.1128/JVI.75.16.7543-7554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91(1):119–26. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 93.Reeves MB, Sinclair JH. Circulating dendritic cells isolated from healthy seropositive donors are sites of human cytomegalovirus reactivation in vivo. Journal of virology. 2013;87(19):10660–7. doi: 10.1128/jvi.01539-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buehler J, Zeltzer S, Reitsma J, et al. Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication. PLoS pathogens. 2016;12(5):e1005655. doi: 10.1371/journal.ppat.1005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Camarena V, Kobayashi M, Kim JY, et al. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell host & microbe. 2010;8(4):320–30. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petrucelli A, Rak M, Grainger L, et al. Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus. Journal of virology. 2009;83(11):5615–29. doi: 10.1128/jvi.01989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SH, Caviness K, Albright ER, et al. Long and Short Isoforms of the Human Cytomegalovirus UL138 Protein Silence IE Transcription and Promote Latency. Journal of virology. 2016;90(20):9483–94. doi: 10.1128/jvi.01547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]