Abstract
Background
The aldosterone inhibitor eplerenone (EPL) has been shown to reduce the incidence of AF in patients with systolic heart failure, but the mechanism is unknown.
Objectives
We hypothesized that by reducing atrial dilatation and fibrosis in the absence of heart failure EPL also reduces AF burden and prevents AF perpetuation.
Methods
We conducted a randomized-controlled study in 34 sheep that were atrially tachypaced (13±1 weeks). We compared daily oral EPL (N=19) versus sugar-pill (SP) treatment (N=15) from the start of tachypacing. The endpoint was a continuous 7-day stretch of persistent AF (N=29) or completion of 23-weeks tachypacing (N=5).
Results
EPL significantly reduced the rate of left atrial dilatation increase during AF progression. Atria from EPL-treated sheep had less smooth muscle actin protein, collagen-III expression, interstitial atrial fibrosis and cell hypertrophy than SP-treated sheep atria. However, EPL did not modify the AF-induced increase in the rate of dominant frequency and ion channel densities seen under SP treatment, but prolonged the time to persistent AF in 26% of animals. It also reduced the degree of fibrillatory conduction, AF inducibility and AF burden.
Conclusions
In the sheep model, EPL mitigates fibrosis and atrial dilatation, modifies AF inducibility and AF complexity, and prolongs the transition to persistent AF in 26% of animals, but it does not prevent AF-induced electrical remodeling or AF persistence. The results highlight structural remodeling as a central upstream target to reduce AF burden, and the need to prevent electrical remodeling to avert AF perpetuation.
Keywords: Atrial fibrillation progression, Aldosterone, Fibrosis, Upstream therapy, Atrial dilatation
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with an increased risk of stroke, heart failure and dementia (1–4). Non-paroxysmal AF (including persistent and permanent AF) increases the risk of thromboembolism and death, which calls for development of new upstream therapies to prevent AF progression (5). Atrial dilatation, fibrosis and electrical remodeling underlie the transition from paroxysmal to persistent AF (6) and contribute to AF perpetuation. Recently we demonstrated that targeting the pro-fibrotic protein galectin-3 (Gal-3) using a relatively low intravenous dose of the galactomannan GM-CT-01 (GMCT)(7) reduces both structural and electrical remodeling, as well as AF burden in a sheep model of persistent AF in the absence of co-morbidities (8). However, Gal-3 inhibition did not restore sinus rhythm in the long term (8). Nevertheless, our study provided a solid proof of concept in support of upstream AF prevention therapy.
Mineralocorticoid receptor blockers (MRBs) are beneficial in systolic heart failure (9–11). Specifically, the MRB eplerenone (EPL) has been shown to reduce new-onset AF and recurrent AF in heart failure patients (12). Both angiotensin II and aldosterone elevations may lead to atrial fibrosis and contribute to human AF (13). Experimental results suggest that aldosterone may cause a substrate for atrial fibrosis and AF (14). Aldosterone increases the expression of 11-β-hydroxysteroid dehydrogenase type-2 (11b-HSD2) leading to up-regulation of pro-fibrotic mediators and collagen synthesis, which is prevented by MRBs (15).
Here we have investigated whether EPL prevents structural and electrophysiological remodeling, and reduces AF burden in our sheep model of persistent AF (16). In addition, we have determined whether EPL is a potentially effective upstream therapy to prevent persistent AF.
Methods
An expanded methods section is available in the Online Supplement.
Protocol for the Blinded Randomized-Controlled Study in the Sheep
All procedures complied with National Institutes of Health guidelines. Thirty-four male sheep (35 – 40 kg) underwent subcutaneous pacemaker implantation with a lead attached to the right atrial (RA) appendage (8,16). A loop recorder (ILR) was inserted parasternally in close apposition to the left atrium (LA). After one month recovery, sheep were randomized to (i) sugar-pill (SP)-treated AF control (N=15), or (ii) EPL treated AF groups (100 mg/day orally, N=19). We used also 6 sham-operated animals from previous studies as a reference control.(8,16) The time from pacemaker implantation to terminal assessment was 108.4±11.4 days in SP-treated animals, 125.3±10.9 days in EPL-treated animals and 193±45.5 days in sham-operated animals. Investigators were blinded to the randomization. Animals were euthanized after 7 days of self-sustained AF without pacing (7d-PeAF) followed by 1-week observation and terminal ex-vivo and in-vitro experiments. Sheep that did not reach 7d-PeAF during 23 weeks of tachypacing were euthanized at week 24.
AF Progression and Follow-up
Data were collected weekly from pacemakers, ILRs and body surface ECG standard Lead II. Power spectral analysis was used to measure dominant frequency (DF) of atrial activation from the intracardiac RA lead and far-field LA signals during AF episodes. Atrial dimensions, mitral and tricuspid regurgitation, ejection fraction, and ventricular and septal dimensions were evaluated echocardiographically (echo) every two weeks.
Statistical Analyses
Normally distributed data are expressed as mean±standard error of the mean, where “N” represents the number of animals and “n” represents the number of cells. See online supplement for further details.
Results
AF progression in the sheep model is characterized by continuously increasing markers of electrophysiological and structural remodeling, as well as AF burden, until AF becomes persistent (8,16). To determine whether eplerenone interferes with such changes, we randomized 34 sheep into daily oral EPL (100 mg; N=19) or SP (N=15) (Online Figure 1). We defined five time points from the start of tachypacing: (a) first AF episode (1st-AF), (b) first 12-hour-AF episode (12h-AF), (c) first 1-day-AF episode (1d-AF), (d) first continuous 7-day stretch of persistent AF (7d-PeAF), and (e) terminal.
Body weight (BW) increased similarly with time in EPL-treated and SP-treated animals (Online Figure 2). No animals developed heart failure or stroke. On lead-II ECG, neither EPL nor SP-treatment altered the QT or QTc interval measured at baseline and at final evaluation (Online Figure 3).
EPL mitigates atrial dilatation and cell elongation during AF progression
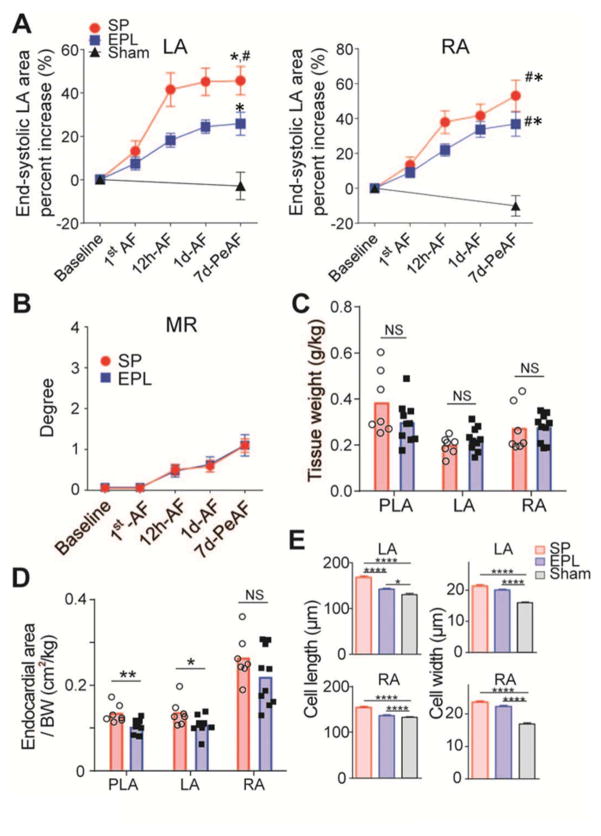
Echo measured LVEF and LV end-diastolic volume were unchanged with respect to baseline in both SP-treated and EPL-treated groups (Online Figures 4A–C). In contrast, the AF-associated increase in end-systolic LA area was significantly attenuated in EPL-treated compared to SP-treated animals at 12h-AF, 1d-AF, and 7d-PeAF (Figure 1A). At 7dPeAF end-systolic RA and LA areas were visibly smaller in the sham group than the other two groups. Throughout AF progression, mitral and tricuspid regurgitation in EPL- and SP-treated hearts were similar (Figure 1B, Online Figure 4D). At final evaluation, there was no significant difference in adjusted atrial tissue weight (Figure 1C), but adjusted endocardial LA and PLA areas measured directly in explanted hearts were reduced in EPL-treated compared to SP-treated group (Figure 1D). Myocyte length was significantly less in the EPL-treated group than the SP-treated group, but myocyte widths were similar (Figure 1E). Lengths of RA and LA myocytes from the sham group were consistently smaller than the other two groups.
Figure 1. EPL mitigates structural remodeling.
(A) Echocardiographically measured atrial areas adjusted by body weight at 5 different time points from SP-treated (N=15), EPL-treated (N=19) and sham-operated (N=6) groups. *p < 0.05 versus sham; #p < 0.05 vs EPL-treated group. LA=left atrium; RA=right atrium. (B) Mitral regurgitation (MR) at 5 different time points. (C) Atrial tissue weight adjusted by body weight at the end of the experiment. (D) Atrial areas obtained by direct measurement of the tissue at the end of experiment. (E) Single isolated cell length and width in Sham (N/n=4/192), SP-treated (N/n=4/144) and EPL-treated groups (N/n=5/244). *P<0.05, **P<0.01, ****P<0.0001. N = number of animals, n = number of cells.
EPL reduces atrial fibrosis
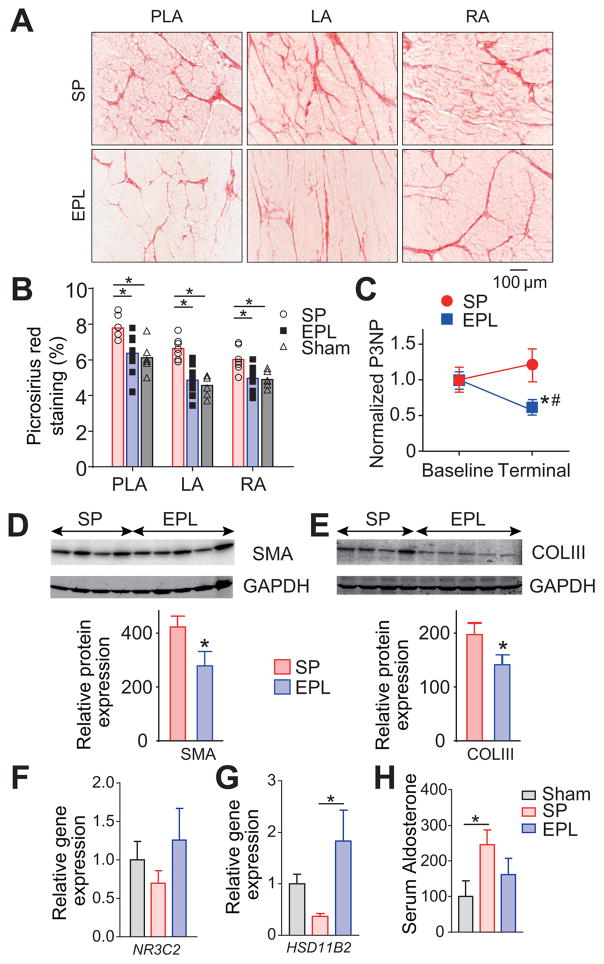
Fibrosis in LA, PLA and RA was significantly less in EPL-treated than SP-treated tissues as determined by picrosirius staining (Figures 2A–B). The results correlated well with decreased terminal serum levels of Procollagen-III N-Terminal Propeptide (P3NP) in EPL-treated compared to SP-treated animals (Figure 2C) (17). Further, on western blotting of atrial tissue lysates, smooth muscle actin (SMA) protein and COLIII expression were also 32% and 35% lower, respectively, in the EPL-treated than SP-treated sheep (Figures 2D–E). Finally, in cultured sheep atrial myofibroblasts, proliferation assay data show that aldosterone increased the number of atrial myofibroblasts at two different concentrations (Online Figure 5).
Figure 2. EPL reduces fibrosis development during AF progression.
(A) Representative picrosirius red staining of PLA, LA and RA. (B) Interstitial fibrosis for sham operated (N=6), SP-treated (N=7) and EPL-treated groups (N=10). Ten pictures per slide were randomly selected and analyzed. *P<0.05, **P<0.01. (C) EPL mitigates the increase of serum Procollagen III N-Terminal Propeptide (P3NP) during AF progression. *P<0.05 vs. Baseline, #P<0.05 vs. EPL. (D, E) Western blots for smooth muscle actin (SMA) and collagen III (COLIII) in atrial homogenates relative to GAPDH from SP-treated (N=4) and EPL-treated groups (N=5). (F,G) Mineralocorticoid receptor (NR3C2) and 11b-HSD2 (HSD11B2) gene expression in the LA. (H) serum aldosterone in sham operated, AF sheep treated with SP and AF sheep treated with EPL. For F–H, Sham (N=7), SP-treated (N=8) and EPL-treated groups (N=5). N=number of animal. *P<0.05. (B, F–H) One-way ANOVA followed by post hoc Bonferroni’s test. (C) Two-way ANOVA followed by post hoc Bonferroni’s test.
EPL-induced mitigation of atrial dilatation and fibrosis is not mediated through altered MR expression
Previous studies have shown upregulation of MR in AF (13). The MR is the nuclear aldosterone receptor responsible for the genomic effects of aldosterone. 11b-HSD2 inactivates cortisol and corticosterone, allowing aldosterone to activate the MR (18). We investigated whether AF or MR blockade by EPL altered MR (NR3C2) or 11b-HSD2 (HSD11B2) gene expression in sheep left atrial tissue, or aldosterone levels in serum of sham operated, SP-treated and EPL-treated sheep. In Figure 2F, NR3C2 expression was slightly increased in EPL-treated compared to SP-treated animals, but the difference was not significant. HSD11B2 was significantly increased in EPL-treated compared to SP-treated sheep (Figure 2G). 11b-HSD2 converts active cortisol to inactive cortisone, which is thought to reduce MR activation like EPL. Thus, in EPL-treated animals, EPL likely inhibited MR activation via two different ways: 1) by directly binding competitively to MR; 2) by increasing HSD11B2 expression thus increasing 11b-HSD2 protein, which increased cortisone binding to and inhibiting the activity of MR. Finally, as expected (19), serum aldosterone levels were increased in SP-treated AF sheep compared to sham-operated sheep (Figure 2G). EPL treatment tended to decrease the serum aldosterone level, yet there was no significant difference between the SP-treated and EPL-treated animals.
EPL fails to prevent electrical remodeling during AF progression
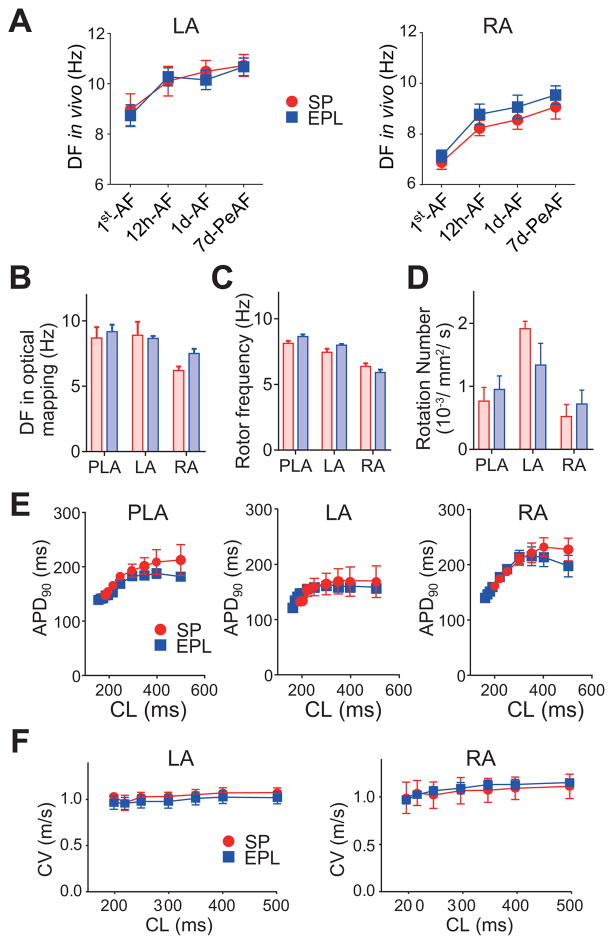
The rate of increase in DF in the EPL-treated group was similar to the SP-treated group both in RA and LA (Figure 3A). At final ex-vivo evaluation by optical mapping, AF was successfully re-induced by electrical pacing in all Langendorff-perfused hearts and did not spontaneously cardiovert to SR before the end of experiment. Regional maximum DF (DFmax) was recorded optically every 2 minutes from the PLA, LA and RA. As expected, DFmax was always larger in the PLA and the LA than the RA, but was similar for EPL-treated and SP-treated groups in all atrial regions (Figure 3B). In phase movies we counted the number of phase singularity points (SPs), the number of visible rotors per second and the frequency of rotations. Rotor frequency and number of rotors were similar in EPL-treated and SP-treated hearts (Figures 3C–D).
Figure 3. EPL does not change electrical remodeling.
(A) Dominant frequency (DF) measured in-vivo in LA and RA at 4 different time points during AF progression in SP-treated (N=15) and EPL-treated groups (N=19). (B–E) Data from optical mapping experiments: DF (B), rotor frequency (C), number of rotors (D), action potential duration (APD90) (E) and conduction velocity (CV) (F) recorded on the LA and RA from SP-treated (N=3) and EPL-treated sheep hearts (N=5). N=number of animals.
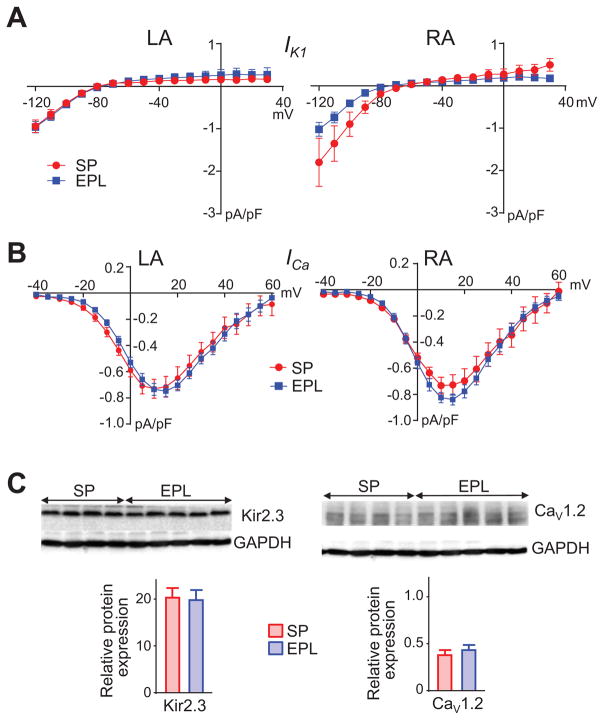
Upon restoration of stable SR by electrical shock in the Langendorff-perfused hearts we conducted action potential duration (APD) restitution experiments to determine whether EPL had prevented the previously demonstrated persistent AF-induced shortening of the optically mapped action potential (8). Mean APD at 90% repolarization (APD90) was similar in EPL-treated and SP-treated hearts at all cycle lengths tested (Figure 3E). In addition, conduction velocity (CV) and wavelength (APD90 x CV) during pacing were indistinguishable between EPL-treated and SP-treated groups in both LA and RA (Figure 3F, Online Figures 6 and 7). Moreover, in isolated LA and RA myocytes from persistent AF animals, inward rectifier potassium current (IK1) and L-type calcium current (ICaL) were not significantly different between EPL-treated and SP-treated animals (Figures 4A–B). IK1 and ICaL data were consistent with similar Kir2.3 and CaV1.2 protein expression levels in both groups. Thus, EPL-treatment had no effect on the previously demonstrated persistent AF-induced changes in Kir2.3 and CaV1.2 protein expression (8) when compared with changes seen in SP-treated animals (Figure 4C). Altogether, these results demonstrate that EPL-treatment does not modify persistent AF-related electrical remodeling at the whole animal, organ, cellular or molecular levels.
Figure 4. EPL does not reduce ion channel remodeling during AF progression.
(A) Left, Inward rectifier potassium (IK1) current-voltage relationships for LA cells from SP treated (N/n=3/7) and EPL-treated (N/n=5/14) animals. Right, IK1 current-voltage relationships for RA cells from for SP-treated (N/n=2/7) and EPL-treated (N/n=5/13) animals. (B) Left, current-ICaL voltage relationships for LA from SP treated (N/n=4/11) and EPL-treated (N/n=5/24) animals. Right, current-voltage relationships for RA from SP treated (N/n=4/17) and EPL-treated ICaL (N/n=5/26) animals. Two-way ANOVA with post hoc Bonferroni’s test. (C) Western blots for Kir2.3 (left) and Cav1.2 (right) in LA tissue lysate. Protein expression of both ion channel types was similar for SP-treated (N=4) and EPL-treated groups (N=5).
EPL-treatment reduces AF wave complexity
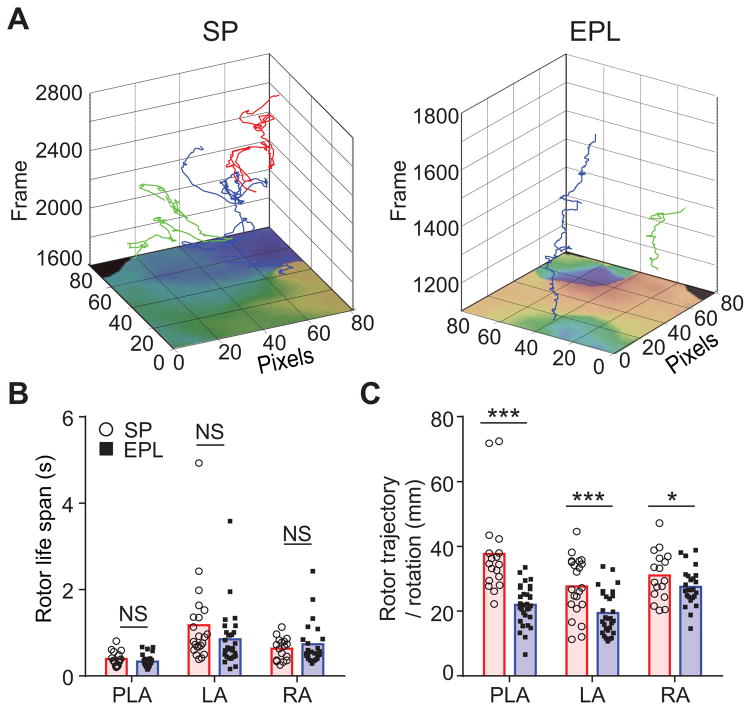
Both atrial stretch and fibrosis are known to be associated with persistent AF (20,21). In addition, the patterns of wave propagation in the fibrotic atria with persistent AF are more complex than in atria undergoing paroxysmal AF (8). Therefore, we hypothesized that by effectively reducing both atrial dilatation and interstitial fibrosis EPL modifies AF source dynamics in such a way as to reduce the complexity of wave propagation (fibrillatory conduction). We therefore used phase movies (22) to analyze AF dynamics in the PLA, LA and RA by counting the number of singularity points, the number of their rotations, their life spans and the length of their trajectories (Figure 5) (23). As shown above, the rotor frequency was similar in SP-treated and EPL-treated hearts (Figure 3C). In addition, the number of SPs (Online Figure 8) and their life spans (Figure 5B) were also similar between treatment groups. However, the average lengths of the individual rotor trajectories adjusted to the number of rotations were significantly smaller in EPL-treated than SP-treated hearts (Figure 5C). Altogether, the foregoing results suggest strongly that by preventing interstitial fibrosis throughout the atria, EPL-treatment contributed to a substrate where rotors could anchor to inscribe simpler trajectories. However, by failing to prevent electrical remodeling, the drug failed to alter the number, rotation frequency, and life span of AF sources.
Figure 5. EPL reduces AF wave propagation complexity.
(A) Representative 3-dimensional plots of spatial trajectories of rotors at consecutive movie frame numbers (500 μm2 pixels, 600 frames/s) for SP-treated (left) and EPL-treated (right) hearts. Note the reduced complexity in the trajectories of two rotors recorded from persistent AF hearts in the EPL treated compared with the SP treated hearts. (B and C) Quantification of lifespan (B) and trajectory length per rotation (C) of rotors recorded in optical mapping experiments from the PLA, LA and RA of SP-treated and EPL-treated hearts. Rotor lifespans were similar, but rotor trajectories were significantly shorter and less complex for EPL-treated than SP-treated hearts.
EPL prolongs the progression of AF, and reduces AF burden and inducibility
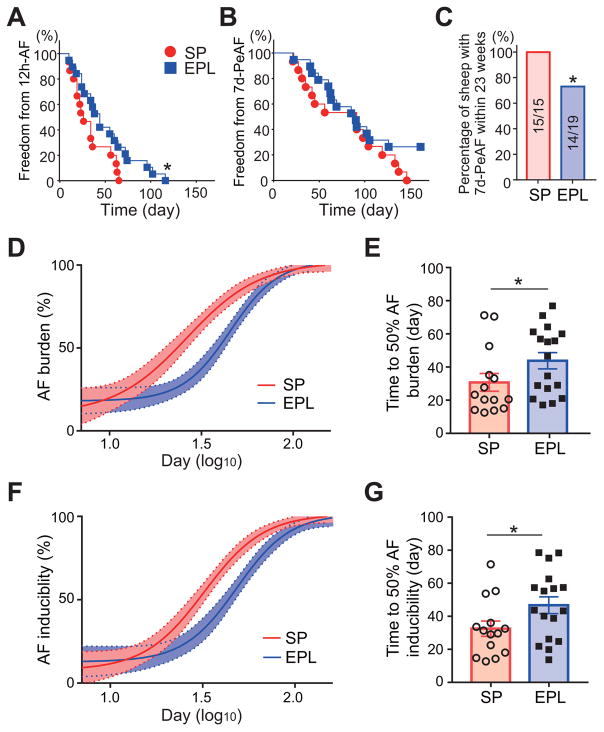
EPL-treatment significantly prolonged the time from start of tachypacing to 12h-AF in EPL-treated compared to SP-treated animals (Figure 6A, P=0.041). EPL-treatment also reduced the percentage of animals with sustained AF for more than 7 days within 23 weeks from the onset of tachypacing by 26% (Figure 6C, P=0.04, Fisher’s exact test).
Figure 6. EPL prolonged the time to 12h-AF, and reduced AF inducibility and AF burden.
Kaplan–Meier plot for freedom from 12h-AF (A) and 7d-PeAF (B) in SP-treated (N=15) and EPL-treated groups (N=19). Log-rank test (P=0.04 in 12hr-AF and P=0.15 in 7d-PeAF). (C) Percentage of sheep with 7d-PeAF within 23 weeks in SP-treated (N=15/15) and EPL-treated groups (N=14/19). Fisher’s exact test (P=0.04). Time course of AF burden (D) and AF inducibility index (F) in-vivo fitted with Hill equation for SP-treated (N=15) and EPL-treated (N=19 groups throughout AF progression. Time to 50% AF burden (E) and AF inducibility index (G), calculated individually fitted with Hill equation, for SP-treated (N=15) and EPL-treated (N=19) groups. Double sided t-test. *P<0.05. N=number of animals.
We define AF burden as the percentage of self-sustained AF over a given time. AF burden during AF progression follows a Hill regression in both treatment groups (R2=0.52 and 0.59, respectively, Figure 6D). EPL-treatment substantially shifted the curve to the right, indicating relief of AF burden. Time to 50% AF burden, calculated individually by sigmoidal fitting, was 42.5% greater in EPL-treated than SP-treated sheep (P=0.027, Figure 6E). AF inducibility index, which was calculated as the inverse of the number of pacing attempts to reinduce AF, also followed a Hill regression (R2=0.62 and 0.55, respectively). EPL-treatment also shifted the curve of AF inducibility index to right (Figure 6F), and increased the time to 50% AF inducibility index by 43.7% (P=0.021, Figure 6G), indicating a reduction in inducibility. These results indicate that EPL-treatment significantly changed vulnerability to AF during AF progression. EPL-treatment tended to prolong the total pacing time throughout the study compared to SP-treated animals, but did not change the total sustained AF time without tachypacing (Online Figure 9).
Discussion
The most important results of this study are (Central illustration): 1) EPL-treatment mitigated structural remodeling including atrial dilatation, myocyte hypertrophy, serum P3NP, tissue levels of SMA and COLIII and fibrosis during the transition to tachypacing-induced persistent AF; 2) EPL-treatment failed to prevent or even reduce the increase in DF, the decrease in APD and Cav1.2 (ICaL) and the increase in Kir2.3 (IK1) that reflect electrical remodeling during AF progression (8); 3) EPL-treatment decreased AF wave complexity, AF inducibility and AF burden, but delayed the onset of persistent AF only in 26% of animals.
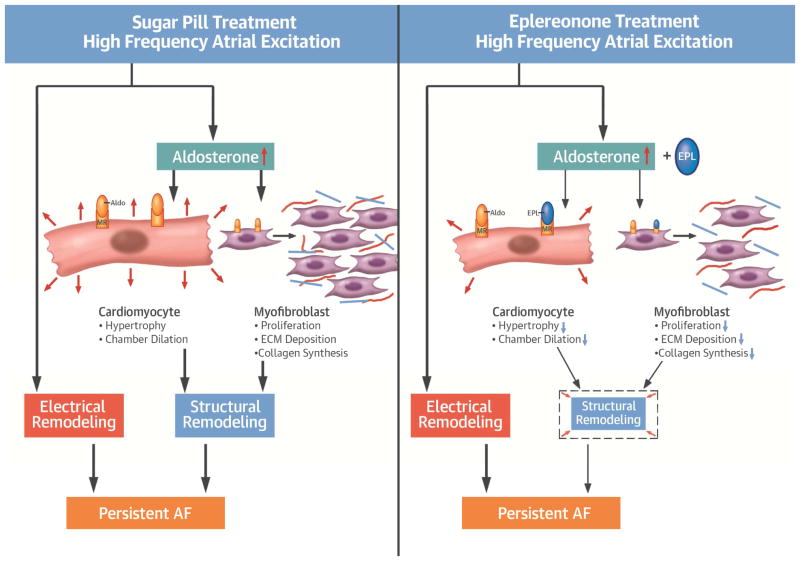
Central Illustration. High frequency atrial excitation leads to persistent AF by inducing electrical and structural remodeling.
Left, increased aldosterone during high frequency stimulation contributes to structural remodeling via atrial cardiomyocyte hypertrophy, atrial dilatation, myofibroblast proliferation, increased collagen synthesis and extracellular matrix (ECM) remodeling. Right, by blocking aldosterone, eplerenone (EPL), minimizes structural but not electrical remodeling.
The renin-angiotensin-aldosterone system (RAAS) is involved in atrial electroanatomical remodeling and is likely to mediate the development of atrial fibrosis and the progression of AF to more permanent forms (6) For example, angiotensin converting enzyme inhibition (ACEI) was shown to suppress atrial fibrosis and the development of persistent AF in an animal model of atrial tachypacing and left ventricular dysfunction (24). Also clinical studies have reported a lower incidence of AF in selected patient populations treated with RAAS inhibitors compared to controls (25). AF is also less likely to recur after cardioversion in patients treated with RAAS inhibitors than controls. New-onset AF in patients with significant underlying heart disease was reduced with ACEI or angiotensin II receptor blockers (ARBs) treatment, but there is a lack of robust evidence in patients with mild to moderate structural heart disease (26).
In addition to the traditional ACEIs and ARBs, MRBs also have been shown to effectively reduce the incidence of new-onset AF in systolic heart failure and to have beneficial effects on mortality in patients with cardiac disease (12). In the ventricular pacing-induced CHF dog model, MRB suppresses inducibility of sustained atrial tachyarrhythmias and attenuates LV diastolic remodeling (27). Our experimental results show clearly that EPL significantly mitigates structural remodeling during the transition to persistent AF. Animals treated with EPL had significantly smaller atrial size, and less cellular hypertrophy and fibrosis than SP-treated animals. Atrial size is an important determinant of clinical AF. Myocyte hypertrophy was also diminished by EPL. Our data are consistent with studies in mouse models in which deletion or inactivation of the MR gene attenuated left ventricular dilatation, hypertrophy, and development of heart failure (HF), whereas MR overexpression in cardiomyocytes induced ventricular remodeling, HF and arrhythmias (28,29).
EPL treatment did not have any substantial effect on electrical remodeling reflected by DF increase and APD shortening during AF progression. The results are different from those previously obtained by the use of the Gal-3 inhibitor GMCT (8), which unlike EPL, prevented the sustained AF-induced APD shortening in both RA and LA, prevented the sustained AF-induced increase in Kir2.3 expression and IK1 density in the same model of tachypacing induced persistent AF. On the other hand, similar to EPL, Gal-3 inhibition mitigated structural remodeling, and both drugs significantly reduced AF complexity, AF inducibility and AF burden. (see (8) and Figure 6).
The question of which is the most important factor underlying the pathophysiology of AF progression and perpetuation is still being debated. In this study, EPL treatment significantly attenuated structural remodeling of the LA (Figure 4), whereas electrical remodeling progressed equivalently at corresponding time points in both treatment groups during AF progression (Figure 3). Since 7d-PeAF was delayed only in 26% of animals (Figure 6B), the results presented here, together with the slowing of ion channel remodeling demonstrated previously in GMCT treated animals, (8) highlight the significance of electrical remodeling in AF perpetuation. However, it is important to note that while structural remodeling was attenuated by EPL treatment, it was not completely prevented, indicating that there might still exist a minimum requirement of structural remodeling, which together with advanced electrical remodeling contributes to AF perpetuation. In addition, the reduced AF burden and AF inducibility promoted by EPL (Figures 6D–G) clearly support the contention that structural remodeling is important. Therefore, while our results do not fully resolve the issue of which of these two factors is the most important, the data strongly suggest that both contribute and act in synergy to promote AF perpetuation. The target for the antifibrotic action of EPL is different from GMCT. In the case of EPL, the downstream pathways that translate MR activation into tissue inflammation, fibrosis and dysfunction are still being elucidated (30). Recent studies using cell-selective MR-null or MR-overexpressing mice indicate that selective regulation by the MR of matrix metallopeptidase 2/matrix metallopeptidase 9 activity and the transforming growth factor beta (TGF-β)–connective tissue growth factor (CTGF) profibrotic pathway in cardiomyocytes may be potential mechanisms for the cardioprotective effects of the genetic or pharmacologic loss of MR signaling (30). Significant upregulation of the TGFβ-CTGF inhibitor decorin in ventricular cardiomyocytes from MR-null mice suggests that lower collagen levels may reflect an increase in inhibitors of the fibrotic process (30). Such effects have yet not been demonstrated in atrial myocytes.
On the other hand, we have shown previously that GMCT reduces both Gal-3 and TGF-β1-induced sheep atrial fibroblast migration and proliferation in vitro (8). In addition, a low dose of GMCT applied intravenously to sheep twice per week prevented the increase in serum P3NP seen during progression to persistent AF, and also mitigated atrial dilatation, myocyte hypertrophy, fibrosis, and reduced the rate of DF increase. Moreover, TGF-β1 upregulates in the atria of persistent AF sheep, but GMCT-treated animals had significantly less TGF-β1-Smad2/3 signaling pathway activation and expression of SMA and COLIII than saline-treated animals. Similar to TGF-β1, platelet derived growth factor (PDGF) also mediates cardiac fibrosis (31). Recent data showed that myofibroblast release of the PDGF AB isoform reduced atrial ICa,L, shortened APD and produced calcium handling dysfunction in cultured atrial cardiac myocytes (32). These alterations are consistent with the electrical remodeling that has been shown to occur in myocytes during AF (16), and that was mitigated by GMCT but not EPL.
Nevertheless, EPL treatment did reduce the AF burden, prolonged the time to 12h-AF and increased the number of sheep resistant to persistent AF (Figure 6). Such results suggest that, even without modifying electrical remodeling during AF progression, reducing structural remodeling is beneficial. Moreover, by preventing dilatation and interstitial fibrosis throughout the atria, EPL treatment reduced the heterogeneity of the anatomical substrate and allowed the AF sources to evolve over simpler trajectories (Figure 5A). This, together with the previously demonstrated mitigation of electrical remodeling by Gal-3 inhibition, suggests the possibility that adding adjuvant upstream EPL therapy to concomitant traditional antiarrhythmic therapy might increase the possibility of substantially reducing AF burden in AF patients.
Study limitations
It is unknown whether EPL affected AF initiation by modulating focal atrial ectopic discharges or PV arrhythmogenicity, which is critical in the genesis and maintenance of human AF (33). The mechanisms of AF in the sheep model and human patients with AF may have nuanced differences. In the sheep, intense burst pacing is likely to be more aggressive compared to intrinsic AF-initiating events during the natural progression of paroxysmal to persistent AF in humans, where AF re-initiation may be much more infrequent. However, the fibrosis level achieved in the sheep model at 7 days persistent AF is substantially lower compared to human persistent AF (8,34). Another limitation is that while EPL can benefit patients with mild to moderate arterial hypertension (35), we have not evaluated the effects on arterial blood pressure in our model. Importantly, except for comparing the effects of eplerenone versus sugar pill, the experimental conditions were practically the same as in the previous two papers (8,16). Thus we have used data from six sham-operated sheep from those studies to provide a control reference. As discussed for Figure 1, end-systolic RA and LA areas in the sham group are visibly smaller than persistent AF sheep groups with or without EPL treatment. Similarly, the lengths of RA and LA myocytes from the sham group were consistently smaller than the EPL- and SP-treated persistent AF groups. Nevertheless, we acknowledge this use of historical sham-operated controls as a potential limitation of our study. Finally, the mechanisms underlying AF may include not only electrical and structural factors but also inflammation and oxidative stress. EPL may mitigate inflammation and ROS, but we have not investigated them in our model.
Conclusion
The results highlight structural remodeling as a central upstream target to reduce AF burden, and the need to prevent electrical remodeling to avert AF perpetuation.
Supplementary Material
Clinical Perspectives.
Clinical Competencies
Aldosterone elevation contributes to atrial dilatation and fibrosis in AF. Eplerenone mitigates fibrosis and atrial dilatation, and decreases AF inducibility and burden, delaying somewhat the progression to persistent AF, but it does not affect electrical remodeling.
Translational Outlook
The results raise the importance of structural remodeling as an upstream target to reduce AF burden. In addition to being helpful hemodynamically, using MRB therapy concomitantly with antiarrhythmic therapy might be beneficial in preventing persistent AF patients.
Acknowledgments
SOURCES OF FUNDING
Supported in part by National Heart, Lung and Blood Institute R01 grant HL122352 NIH/NHLBI (JJ); the Leducq Foundation: Transatlantic Network of Excellence Program on “Structural Alterations in the Myocardium and the Substrate for Cardiac Fibrillation” (JJ, and OB); University of Michigan Health System – Peking University Health Science Center Joint Institute for Translational and Clinical Research (UMHS-PUHSC) project: Molecular Mechanisms of Fibrosis and the Progression from Paroxysmal to Persistent Atrial Fibrillation (JJ). YT was supported by Uehara Memorial Foundation and postdoctoral fellowship from the American Heart Association (14POST18220000). OS was supported by Martin Escudero Foundation.
We thank Cicero Willis, MD, Christopher Zerr, Patrick Wolfer, Rahul Mehta, Bharath Jakka, Danillo Z. de O. Souza, Sicong Wang, and Daniel Garcia Iglesias for their assistance in the experiments. We also thank St. Jude Medical and Medtronic for assistance with implantable devices.
Abbreviation list
- AF
Atrial fibrillation
- SR
Sinus rhythm
- GMCT
GM-CT-01
- MRB
Mineralocorticoid receptor blocker
- EPL
Eplerenone
- SP
Sugar pill
- APD
Action potential duration
- CV
Conduction velocity
- IK1
Inward rectifier potassium current
- ICaL
L-type calcium current
Footnotes
DISCLOSURES
JJ is on the Scientific Advisory Board of Abbott EP. OB is the Scientific Officer for Rhythm Solutions Inc. Both JJ and OB receive research grant support from Medtronic, Inc. All other authors have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 2.Bunch TJ, Weiss JP, Crandall BG, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7:433–7. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Magnani JW, Rienstra M, Lin H, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–93. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 5.Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. European heart journal. 2016;37:1591–602. doi: 10.1093/eurheartj/ehw007. [DOI] [PubMed] [Google Scholar]
- 6.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 7.Traber PG, Chou H, Zomer E, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takemoto Y, Ramirez RJ, Yokokawa M, et al. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC Basic Transl Sci. 2016;1:143–154. doi: 10.1016/j.jacbts.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure.Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 12.Swedberg K, Zannad F, McMurray JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CT, Chiang FT, Tseng CD, et al. Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J Am Coll Cardiol. 2010;55:758–70. doi: 10.1016/j.jacc.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Reil JC, Hohl M, Selejan S, et al. Aldosterone promotes atrial fibrillation. European heart journal. 2012;33:2098–108. doi: 10.1093/eurheartj/ehr266. [DOI] [PubMed] [Google Scholar]
- 15.Lavall D, Selzer C, Schuster P, et al. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014;289:6656–68. doi: 10.1074/jbc.M113.519256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins RP, Kaur K, Hwang E, et al. Dominant Frequency Increase Rate Predicts Transition from Paroxysmal to Long-Term Persistent Atrial Fibrillation. Circulation. 2014;129:1472–1482. doi: 10.1161/CIRCULATIONAHA.113.004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uusimaa P, Risteli J, Niemela M, et al. Collagen scar formation after acute myocardial infarction: relationships to infarct size, left ventricular function, and coronary artery patency. Circulation. 1997;96:2565–72. doi: 10.1161/01.cir.96.8.2565. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4:965–94. doi: 10.1002/cphy.c130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–92. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 20.Corradi D, Callegari S, Manotti L, et al. Persistent lone atrial fibrillation: Clinicopathologic study of 19 cases. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–46. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 22.Jalife J, Pandit SV. Ionic mechanisms of wavebreak in fibrillation. Heart Rhythm. 2005;2:660–3. doi: 10.1016/j.hrthm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 24.Anne W, Willems R, Holemans P, et al. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J Mol Cell Cardiol. 2007;43:148–58. doi: 10.1016/j.yjmcc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur heart J. 2006;27:512–8. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 26.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace. 2011;13:610–25. doi: 10.1093/europace/eur023. [DOI] [PubMed] [Google Scholar]
- 27.Shroff SC, Ryu K, Martovitz NL, Hoit BD, Stambler BS. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006;17:534–41. doi: 10.1111/j.1540-8167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 28.Fraccarollo D, Berger S, Galuppo P, et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 2011;123:400–8. doi: 10.1161/CIRCULATIONAHA.110.983023. [DOI] [PubMed] [Google Scholar]
- 29.Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111:3025–33. doi: 10.1161/CIRCULATIONAHA.104.503706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young MJ, Rickard AJ. Mineralocorticoid receptors in the heart: lessons from cell-selective transgenic animals. J Endocrinol. 2015;224:R1–13. doi: 10.1530/JOE-14-0471. [DOI] [PubMed] [Google Scholar]
- 31.Fan Z, Guan J. Antifibrotic therapies to control cardiac fibrosis. Biomater Res. 2016;20:13. doi: 10.1186/s40824-016-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musa H, Kaur K, O’Connell R, et al. Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts. Heart rhythm. 2013;10:1044–51. doi: 10.1016/j.hrthm.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. NEngl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 34.Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–32. doi: 10.1016/j.jacc.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 35.Pelliccia F, Rosano G, Patti G, Volterrani M, Greco C, Gaudio C. Efficacy and safety of mineralocorticoid receptors in mild to moderate arterial hypertension. Int J Cardiol. 2014 doi: 10.1016/j.ijcard.2014.10.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.