Summary
The macromolecular kinetochore functions to generate interactions between chromosomal DNA and spindle microtubules [1]. To facilitate chromosome movement and segregation, kinetochores must maintain associations with both growing and shrinking microtubule ends. It is critical to define the proteins and their properties that allow kinetochores to associate with dynamic microtubules. The kinetochore-localized human Ska1 complex binds to microtubules and tracks with depolymerizing microtubule ends [2]. We now demonstrate that the Ska1 complex also autonomously tracks with growing microtubule ends in vitro, a key property that would allow this complex to act at kinetochores to mediate persistent associations with dynamic microtubules. To define the basis for Ska1 complex interactions with dynamic microtubules, we investigated the tubulin-binding properties of the Ska1 microtubule binding domain. In addition to binding to the microtubule lattice and dolastatin-induced protofilament-like structures, we demonstrate that the Ska1 microtubule binding domain can associate with soluble tubulin heterodimers and promote assembly of oligomeric ring-like tubulin structures. We generated mutations on distinct surfaces of the Ska1 microtubule binding domain that disrupt binding to soluble tubulin, but do not prevent microtubule binding. These mutants display compromised microtubule tracking activity in vitro and result in defective chromosome alignment and mitotic progression in cells using a CRISPR/Cas9-based replacement assay. Our work supports a model in which multiple surfaces of Ska1 interact with diverse tubulin substrates to associate with dynamic microtubule polymers and facilitate optimal chromosome segregation.
Keywords: Mitosis, Kinetochore, Microtubule, Tubulin, Chromosome Segregation
eTOC
Monda and Whitney et al. demonstrate that the kinetochore-localized Ska1 complex autonomously tracks with both growing and depolymerizing microtubule ends in vitro and that this activity requires multiple microtubule binding surfaces. Ska1 mutants that bind microtubules, but are unable to tip track, result in defective mitotic progression in cells.
Results and Discussion
The Ska1 microtubule binding domain oligomerizes tubulin around microtubules
We sought to define the properties of the Ska1 microtubule binding domain (MTBD; [2]) that are required for its activities in microtubule tracking and chromosome segregation. Our previous work using electron microscopy found that the human Ska1 complex binds to microtubules, forming an ill-structured decoration on the microtubule surface [2, 3] (Figure S1A). In contrast, when we incubated the monomeric human or C. elegans Ska1 microtubule binding domain with microtubule polymers, we found that it formed striking spiral structures that curled around the microtubule lattice (Figure 1A and B). To assess the nature of these oligomeric structures, we used cryo-EM and image analysis to generate a low-resolution reconstruction of the C. elegans SKA-1 microtubule binding domain bound to microtubules (Figure 1C; see Method Details). These reconstructions revealed prominent density for the microtubule lattice, an outer ring surrounding the microtubule, and additional density connecting the two rings. The outer ring has more density than could easily be accounted for by the Ska1 MTBD, which has a molecular weight of ~15 kDa. Instead this outer ring is consistent with a filament of tubulin based on its structure, conformation, and its diameter of ~5 nm (Figure 1C). The density connecting the microtubule and outer ring therefore likely corresponds to the SKA-1 microtubule binding domain.
Figure 1. The Ska1 microtubule binding domain associates with soluble tubulin dimer.
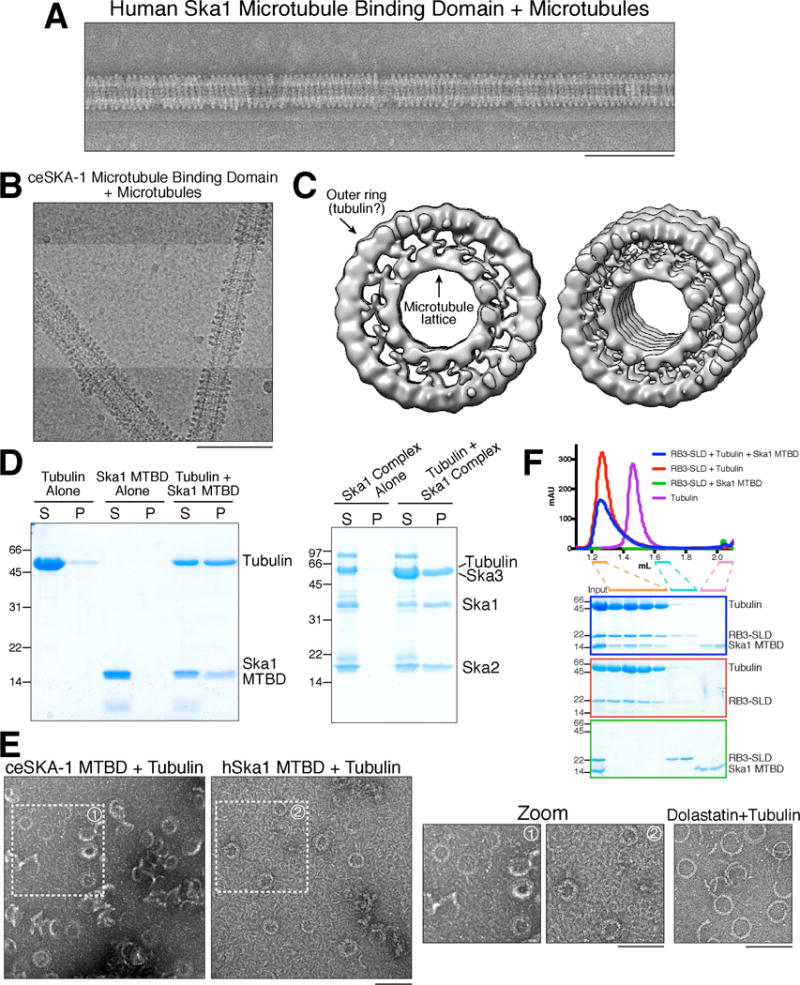
A. Negative stain transmission electron microscopy image of the human Ska1 microtubule binding domain forming oligomeric assemblies around a microtubule. B. Cryo-EM image of the C. elegans SKA-1 microtubule binding domain forming oligomeric assemblies around microtubules. C. 2 views of the cryo-EM reconstruction of the C. elegans SKA-1 microtubule binding domain bound to a microtubule showing the presence of the microtubule lattice, an outer oligomeric ring likely composed of tubulin, and density connecting these rings likely corresponding to the SKA-1 microtubule binding domain. D. Sedimentation assay demonstrating that incubation of soluble tubulin heterodimer with Ska1 MTBD or Ska1 complex results in the formation of large assemblies. Coomassie-stained gel showing supernatant (S) and pellet (P) fractions from the indicated conditions. E. Negative stain transmission electron microscopy images of the assemblies formed following incubation of the C. elegans SKA-1 MTBD or human Ska1 MTBD with soluble tubulin. Rings formed by the incubation of dolastatin-10 with tubulin are shown as a comparison. Scale bars, 100 nm. Also see Figure S1B. F. Size exclusion chromatography traces and Coomassie-stained gels of the peak fractions for the indicated samples demonstrating co-elution of the Ska1 MTBD with RB3-SLD-tubulin. Fractions run on the gel are indicated by the orange, blue and pink bars.
This behavior of the Ska1 MTBD is strikingly similar to that observed for the kinesin-13 MCAK, which also induces the formation of a protofilament-like structure encircling microtubules [4–6]. In the case of both Ska1 and MCAK, it is unlikely that such a spiral or ring-like assembly is formed in cells (as occurs for the fungal Dam1 complex, for example [7, 8]). Indeed, the full Ska1 complex did not generate similar spirals around microtubules (Figure S1A), suggesting that this ordered structure is constrained by the positioning of the MTBDs within the intact Ska1 complex. Intriguingly, we note that formation of a tubulin spiral around microtubules by the Ska1 MTBD suggests that the monomeric Ska1 MTBD is able to bind simultaneously to two tubulin subunits – in this case, tubulin subunits present in the microtubule lattice as well as soluble tubulin heterodimers.
Ska1 binds to tubulin dimer to form a higher order assembly
We next tested directly whether Ska1 is able to interact with unpolymerized, soluble tubulin heterodimers. We incubated the Ska1 MTBD with an equimolar concentration of GDP-tubulin heterodimers in the absence of added GTP to prevent microtubule polymerization. Surprisingly, this resulted in the formation of an opaque precipitate that pelleted after centrifugation (data not shown), suggesting that oligomeric assemblies were formed. We therefore assessed sedimentation through a glycerol cushion. In agreement with our prior results that the Ska1 microtubule binding domain behaves as a monomer in solution [2], no sedimentation was detected for the Ska1 microtubule binding domain alone (Figure 1D). In contrast, incubation of the Ska1 MTBD with tubulin heterodimer resulted in the formation of an assembly containing both tubulin and the Ska1 MTBD that sedimented by centrifugation (Figure 1D). Interestingly, similar assemblies were also observed following the addition of the full-length human Ska1 complex to soluble tubulin (Figure 1D), indicating that this property is preserved in the physiological complex.
To define the nature of the Ska1-tubulin assembly, we next used negative stain transmission electron microscopy to visualize the C. elegans and human Ska1 MTBD (Figure 1E; Figure S1B) or the human Ska1 complex (Figure S1B) following incubation with soluble tubulin heterodimer. We observed striking ring-like assemblies reminiscent of the ring-like protofilament structures induced by the incubation of tubulin with the compound dolastatin-10 (Figure 1E; Figure S1B; also see [2]). Although tubulin ring formation is not a common property of microtubule binding proteins, we note that similar assemblies have been observed following addition of the CAP-Gly domains of p150Glued or CLIP-170 to soluble tubulin [9], or following the addition of the motor domain of the kinesin-8 Kif18A to stabilized microtubules [10].
Because incubation of tubulin with the Ska1 MTBD results in large oligomeric assemblies, to directly test its binding to soluble tubulin we utilized the stathmin-like domain (SLD) of RB3, which associates with two tubulin heterodimers and maintains tubulin in an unpolymerized state [11]. We found that both the Ska1 MTBD (Figure 1F) and the Ska1 complex (Figure S1C) associated with the RB3-tubulin complex based on altered migration in size exclusion chromatography. Together, these results demonstrate that Ska1 associates with tubulin heterodimers in addition to its previously defined interaction with polymeric tubulin substrates.
Generation of separation of function mutations for microtubule/tubulin binding by targeting distinct surfaces on the Ska1 microtubule binding domain
To specifically probe the contributions of Ska1 microtubule tip tracking to chromosome segregation, it is critical to identify separation of function mutants that maintain the ability to bind microtubules. The Ska1 MTBD forms a winged-helix structure and has multiple distinct surfaces with positively-charged residues that contribute to microtubule binding (Figure 2A; [2, 12]). Prior work suggested that these distinct surfaces allow Ska1 to bind to microtubules in diverse orientations [12]. Although several models could explain Ska1-induced tubulin oligomerization, we hypothesized that this reflects Ska1 binding to multiple tubulin heterodimers in diverse orientations. Because it seems unlikely that microtubule binding would require multiple competent binding surfaces simultaneously, we sought to identify Ska1 mutants that bound to microtubules, but did not induce tubulin oligomerization. To do this, we generated a series of mutants targeting distinct surfaces of the Ska1 MTBD with single or double amino acid substitutions in positively-charged, surface-exposed residues known to contribute to microtubule interactions [2, 12]. As compared to a four-residue mutant (K183A K184A K223A K226A) previously shown to severely compromise microtubule binding (Figure 2B and S2A; [12]), these mutants largely retained the ability to associate with microtubule polymers (Figure 2B; Figure S2A). These Ska1 mutants also bound to dolastatin-induced protofilament-like tubulin rings, albeit with some reductions in apparent binding activity (Figure 2B; Figure S2A). Significantly, the majority of these mutations prevented formation of larger assemblies with tubulin heterodimer (Figure 2B and S2A) and also compromised binding to the RB3-tubulin complex (Figure 2C; Figure S2B). We additionally analyzed the K183A K184A mutant in the context of the full Ska1 complex. Similar to the K183A K184A mutant MTBD, binding to microtubules and dolastatin rings was maintained for the K183A K184A Ska1 complex, but tubulin assembly and binding to the RB3-tubulin complex was compromised (Figure S2C and D). Thus, multiple distinct surfaces on Ska1 are required for promoting assembly of soluble tubulin heterodimers, and this activity is separable from the binding of Ska1 to the cylindrical microtubule lattice (Figure 2D).
Figure 2. The Ska1 microtubule binding domain uses multiple distinct surfaces to interact with tubulin.
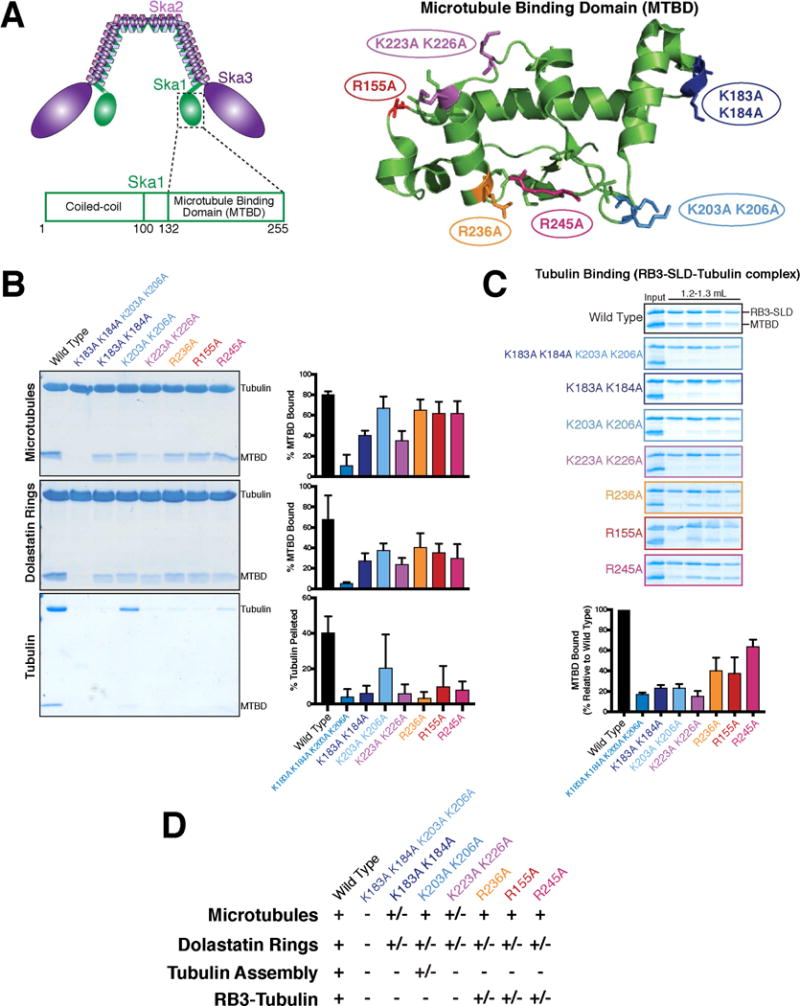
A. Left: Schematic of the human Ska1 complex highlighting the location of the Ska1 microtubule binding domain within the complex, and within the Ska1 protein. Right: Structure of the human Ska1 microtubule binding domain (4C9Y.pdb) [12] showing the locations of the indicated residues targeted for mutational analysis. B. Coomassie-stained gels showing the pellet fractions from representative co-sedimentation assays for the binding of the indicated Ska1 microtubule binding domain mutants to either microtubule polymers, dolastatin-induced rings, or soluble tubulin dimer. For microtubules and dolastatin-induced rings, quantifications indicate the percentage of MTBD bound to the substrate for 2 experiments, with the mean + SD shown. For tubulin, the quantification indicates the percentage of tubulin in the pellet for 7 independent experiments, with the mean + SD shown. See Figure S2A for the Coomassie-stained gels of the supernatant fractions. C. Coomassie-stained gels of the size exclusion chromatography fractions for binding of the Ska1 MTBD and mutants to an RB3-SLD-tubulin complex. The gel for wild type MTBD is duplicated from Figure 1F. RB3-SLD-tubulin peak fractions (see Figure 1F) are shown for each of the Ska1 MTBD mutants. Quantifications indicate the amount of Ska1 MTBD co-eluting with RB3-SLD relative to wild type for 2 experiments, with the mean + SD shown. All mutants show reduced or eliminated binding to RB3-SLD-tubulin. Also see Figure S2B. D. Table summarizing the binding data from this figure.
The Ska1 complex tracks with both depolymerizing and elongating microtubule plus ends
We next sought to test the behavior of the Ska1 complex in the presence of dynamic microtubules. We demonstrated previously that the Ska1 complex is able to track with the depolymerizing microtubule end in vitro following induced depolymerization by ablation of a stabilizing microtubule cap [2]. To test Ska1 interactions with dynamically growing and shrinking microtubules, here we utilized GMPCPP-stabilized microtubule seeds attached to a coverslip and altered the concentration of soluble tubulin to promote either microtubule depolymerization or elongation (Figure 3A; see Method Details). We observed binding and diffusion of GFP-labeled wild type Ska1 complex on the GDP-containing microtubule lattice (Figure 3B), as well as an enrichment of diffusing Ska1 complexes on the GMPCPP-containing microtubule seed (Figure S3A and B). Consistent with our prior work [2], when microtubule depolymerization was induced by the removal of soluble tubulin, the Ska1 complex showed an enrichment at the shortening microtubule ends. At low Ska1 complex concentration, we observed that individual molecules associated with and tracked the microtubule plus end only briefly, indicating that this tracking is not highly processive (Figure 3B). Additionally, although work from others has not detected plus end tracking of elongating microtubules by the Ska1 complex [13], we observed a notably increased brightness for GFP-Ska1 complex fluorescence at growing microtubule ends (Figure 3B; Figure S3C; Movie S1) indicative of plus end tracking with microtubule polymerization.
Figure 3. In vitro analysis of Ska1 interactions with dynamic microtubules.
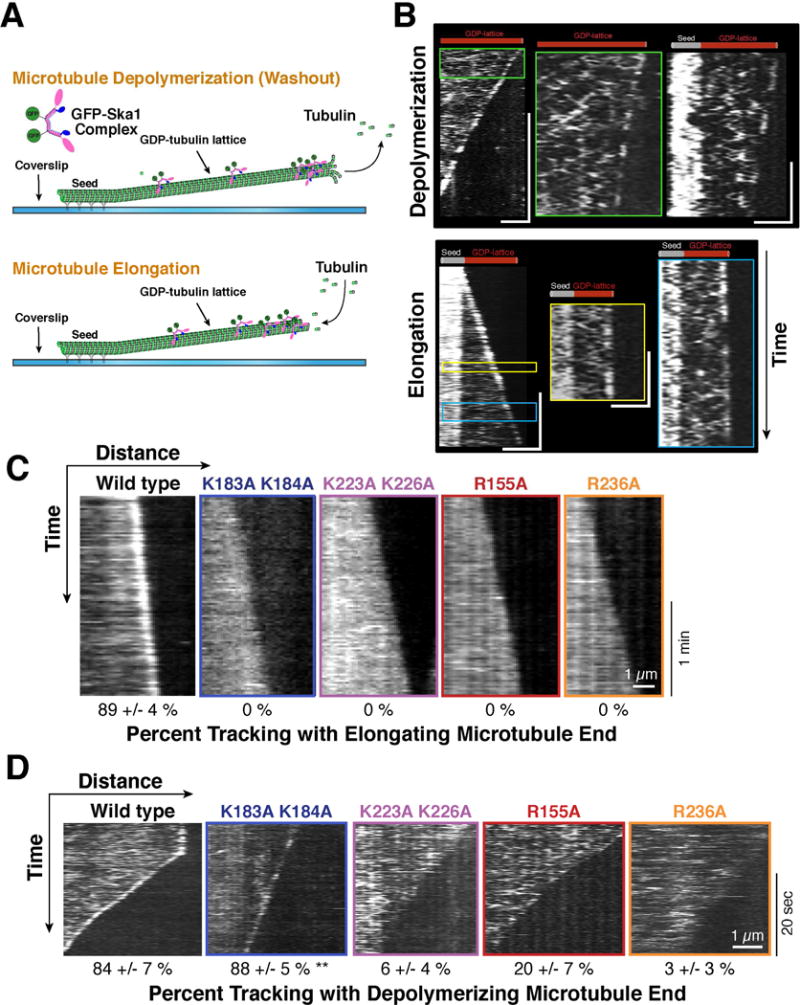
A. Schematic of the in vitro TIRF assay to visualize Ska1 complex associations with dynamic microtubules. B. Representative kymographs of GFP-Ska1 complexes on elongating and depolymerizing microtubules, as visualized with GFP fluorescence. Also see Movie S1. Vertical scale bar is 30 s, horizontal - 2 μm; microtubule plus ends point to the right. Enlarged images are shown for the green, yellow and blue boxed regions. The kymograph on the right of the depolymerization panel is an equivalently enlarged region from a distinct microtubule. All enlargements shown were chosen to highlight Ska1 complex diffusion near the elongating and depolymerizing ends, their encounter with ends, and brief tracking. For the enlargements, the vertical scale bar is 3 s, horizontal - 2 μm. C. Representative kymographs of the indicated GFP-Ska1 complexes on polymerizing microtubules. Tracking by wild type Ska1 appears as an almost continuous bright line because at this time scale, individual complexes cannot be discriminated. Microtubule plus ends point to the right. Percent of microtubules (mean ± SD) that display Ska1 complex tip-tracking is shown below each kymograph. Data are based on ≥ 38 microtubules for each protein. D. Representative kymographs of the indicated GFP-Ska1 complexes on depolymerizing microtubules. Also see Movie S2. Microtubule plus end points to the right. Percent of microtubules (mean ± SD) that display Ska1 complex tip-tracking is shown below each kymograph. Data are based on ≥ 29 microtubules for each protein. ** for the K183A K184A mutant indicates that this mutant shows a similar percentage of microtubules that display tracking, but the tracking signal is less robust relative to the wild type Ska1 complex. Vertical scale bar is 20 s, horizontal - 1 μm. Also see Figure S3.
Diverse plus tip tracking proteins associate with elongating microtubules in cells, but most of these represent “hitchhiker” molecules that associate with microtubule ends through the EB family of proteins, rather than acting as “autonomous tip trackers” [14]. Although the Ska1 complex has been proposed to interact with EB1 [15], our results indicate that the Ska1 complex is capable of EB1-independent tracking of polymerizing microtubules in vitro. The ability of the Ska1 complex to bind to soluble tubulin and track elongating microtubule plus ends is reminiscent of the autonomously tracking microtubule polymerase XMAP215 [16]. However, unlike XMAP215, the addition of Ska1 complex did not significantly change the microtubule polymerization rate (Figure S3D). Imaging at a high frame rate revealed that the enrichment of Ska1 complex fluorescence at the dynamic microtubule end persisted for an average of ~1 second on growing microtubules, similar to the tracking behavior detected on shrinking microtubules (Figure S3E). Thus, the Ska1 complex is an autonomous microtubule tip tracking protein with the ability to remain associated with both polymerizing and depolymerizing microtubule plus ends. This property has not been reported for other human kinetochore-localized microtubule-associated proteins [17], highlighting Ska1 as an important player for kinetochore tracking of microtubule ends.
Ska1 mutants defective in soluble tubulin binding show reduced microtubule end-tracking behavior in vitro
We next tested mutant Ska1 complexes containing a subset of the mutations characterized above that prevented MTBD-induced tubulin oligomerization and represent each of the distinct surfaces on the Ska1 MTBD (K183A K184A, K223A K226A, R155A, and R236A; Figure 2A). Based on fluorescent decoration along the entire microtubule polymer, each complex displayed similar binding to the microtubule lattice (Figure S3F). However, some mutants required a 2–3-fold increased concentration of GFP-Ska1 complex to achieve a similar level of microtubule decoration (see Method Details), indicating a slightly reduced affinity for microtubules, in agreement with the co-sedimentation assays described above.
Strikingly, we found that all four Ska1 mutant complexes failed to track with the plus end of elongating microtubules (Figure 3C), despite associating with the microtubule lattice. As we observed for the wild type Ska1 complex, the microtubule polymerization rate in the presence of the mutant complexes was unchanged (Figure S3D). These mutants additionally displayed defects in associating with depolymerizing microtubules. Three of the mutant Ska1 complexes tested (K223A K226A, R155A, and R236A) failed to exhibit tip tracking as evidenced by the lack of enhanced brightness at the shortening microtubule ends (Figure 3D; Movie S2). The Ska1 K183A K184A mutant complex did display increased GFP-intensity at the depolymerizing microtubule end relative to the adjacent microtubule lattice. However, the tracking for this mutant appeared less robust and persistent relative to the wild type Ska1 complex as judged by the brightness and continuity of the tracking signals (Figure 3D). These data indicate that multiple, distinct, positively-charged tubulin interaction surfaces on the Ska1 microtubule binding domain contribute to robust association with dynamic microtubule ends.
Multi-faceted Ska1-tubulin binding contributes to proper chromosome segregation
Prior work has examined the cellular consequences of Ska1 mutants with severely compromised microtubule binding activity [2, 12]. In contrast, the mutations characterized here disrupt soluble tubulin interactions and microtubule tracking without eliminating binding to the microtubule lattice. To test the effects of these precise mutations on kinetochore function and chromosome segregation, we next utilized an inducible CRISPR/Cas9-based replacement strategy in human HeLa cells [18]. Consistent with prior RNAi-based studies [2, 3, 12, 19–23], induction of the Cas9 nuclease in cells constitutively expressing a guide RNA (sgRNA) targeting Ska1 resulted in severe defects in chromosome alignment and mitotic progression that could be rescued by expression of a guide resistant version of GFP-Ska1 (Figure 4A–D; [18]). In agreement with our prior work [2], we found that the microtubule binding domain of Ska1 was required to fully rescue the loss of endogenous Ska1, as knockout of Ska1 in cells expressing GFP-Ska1 ΔMTBD (lacking residues 132–255) resulted in a mitotic delay and chromosome alignment defects (Figure 4A–D).
Figure 4. Ska1 microtubule binding domain mutants disrupt proper mitotic progression.
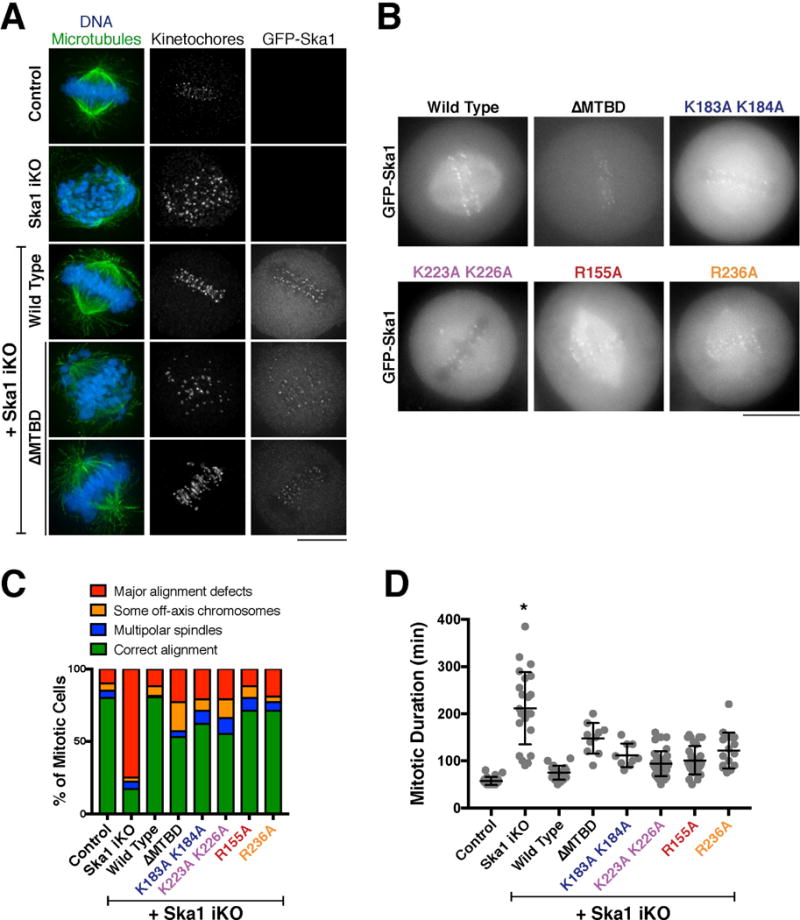
A. Immunofluorescence images of microtubules (DM1A), DNA (Hoechst), kinetochores (ACA), and GFP-Ska1 in the indicated conditions. The Ska1 inducible knockout (iKO) was induced with doxycycline for 4 days prior to imaging. The fluorescence is not scaled identically in each panel due to acquisition on different days. Scale bar, 10 μm. B. Fluorescent images of live cells stably expressing the indicated GFP-Ska1 constructs. Images are provided for a qualitative comparison of localization, but the GFP fluorescence is not scaled identically in each panel due to acquisition on different days. Scale bar, 10 μm. C. Quantification of mitotic phenotypes in control cells (uninduced Ska1 iKO), Ska1 iKO cells, and cells expressing the indicated GFP-tagged Ska1 constructs in the iKO background 4 days following induction of CRISPR-Cas9. n=100 cells/condition D. Quantification of mitotic duration (chromosome condensation to anaphase onset; mean +/− SD) in control cells and the indicated GFP-Ska1 cell lines 4 days following induction of CRISPR/Cas9. Control (n=22), Ska1 iKO (n=24), wild type (n=18), ΔMTBD (n=10), K183A K184A (n=9), K223A K226A (n=37), R155A (n=34), R236A (n=17). The mutants are statistically different from the wild type rescue condition and the Ska1 ΔMTBD mutant based on unpaired t tests (see Figure S4B). The asterisk for the Ska1 iKO indicates that most cells did not exit mitosis during the imaging such that this data under-represents the mitotic duration.
We next tested the consequences of expressing the four Ska1 mutants analyzed in the microtubule tip-tracking assay (K183A K184A, K223A K226A, R155A, and R236A; Figure S4A). In contrast to Ska1 ΔMTBD, which does not localize to spindle microtubules, all four mutants displayed detectable localization to microtubules, in addition to their kinetochore localization (Figure 4B). Interestingly, none of these mutants were able to fully compensate for the loss of endogenous Ska1, as evidenced by increased chromosome alignment defects (Figure 4C) and a mitotic delay (Figure 4D; Figure S4B). However, these defects were generally not as severe as those observed for Ska1 ΔMTBD (Figure 4C and D; Figure S4B). Based on the ability of these mutants to localize to kinetochores (Figure 4B) and associate with microtubules in vitro (Figure 2B; Figure S3F) and in cells (Figure 4D), it is tempting to speculate that the observed mitotic defects are the result of a reduced ability of these mutants to track dynamic microtubules.
Intriguingly, we note that we were able to isolate a viable stable cell line expressing Ska1 ΔMTBD, but apparently lacking endogenous Ska1 (Figure S4C; see Method Details). When compared to Ska1 ΔMTBD-expressing cells 4 days after induction of the Ska1 knockout, this stable cell line displayed similar defects in chromosome alignment and mitotic progression (Figure S4D and E), suggesting that the viability of the Ska1 ΔMTBD stable replacement cell line is not due to long term adaptation to the loss of endogenous Ska1. Therefore, although the Ska1 microtubule binding domain is not essential for viability in cell culture, it is required for efficient chromosome alignment and timely cell division, both of which critically depend on optimal kinetochore-microtubule interactions.
Together, our data indicate that multiple surfaces on the Ska1 microtubule binding domain are required for associations with soluble tubulin, bi-directional microtubule tip tracking activity in vitro, and optimal mitotic progression in cells.
Ska1 uses multiple distinct surfaces to interact with microtubules
Our work demonstrates that the Ska1 complex is able to associate with and autonomously track both depolymerizing ([2]; Figure 3B) and polymerizing (Figure 3B) microtubules. This contrasts with other key components of the kinetochore-microtubule interface, such as the Ndc80 complex, which at a single molecule level fails to associate with dynamic microtubule tips [2, 24]. Defining the mechanistic basis for the tip-tracking ability of the Ska1 complex and its contribution to the kinetochore-microtubule interface are important goals. Importantly, our work reveals several properties of the Ska1 microtubule binding domain that may contribute to its tip-tracking activity and interactions with microtubule ends at mitotic kinetochores. First, we find that Ska1 is able to interact with diverse tubulin substrates, including the cylindrical microtubule lattice, protofilament-like tubulin structures, soluble tubulin heterodimer, and an RB3-tubulin complex (Figure 2). Second, we identify an involvement of diverse surfaces of the Ska1 MTBD in tracking with dynamic microtubules in vitro (Figure 3), suggesting the potential for heterogeneity in the conformation with which Ska1 binds microtubules, consistent with prior crosslinking analyses [12]. The absence of a specific conformation by which the Ska1 MTBD interacts with the microtubule further contrasts it with the highly stereotypical binding displayed by the Ndc80 complex ([25, 26]; also see [12]).
The tubulin-binding properties that we have identified here likely facilitate the association of Ska1 with dynamic microtubule ends, a key requirement at kinetochores [17]. For example, we speculate that instead of “hopping” between specific binding sites on the tubulin subunits within the microtubule lattice in a manner that is susceptible to the loss of an interaction, the Ska1 microtubule binding domain could “roll” by simultaneously or sequentially engaging multiple surfaces of the Ska1 MTBD and tubulin to maintain their association. This mode of motility is consistent with the lack of a specific or regular microtubule-binding orientation for the Ska1 complex as observed by electron microscopy (Figure S1A; [2, 3, 26]). We note that a structurally unrelated microtubule-binding protein KLP10-A, a kinesin-13 family member, has also been suggested to diffuse along the microtubule lattice by engaging multiple, distinct microtubule-binding surfaces [27]. Finally, our cellular analysis suggests that Ska1-mediated tip tracking likely contributes to proper cell division. Thus, the features and properties of the Ska1 microtubule binding domain defined here contribute to its roles at the kinetochore-microtubule interface. In addition, although the Ska1 MTBD is necessary and sufficient for Ska1 complex microtubule binding and spindle localization [2], we note that recent work has also suggested a contribution to microtubule interactions from Ska3 [28]. Ultimately, these activities must be regulated [13, 29, 30] and integrated with other players at the kinetochore, including the Ndc80 complex [2, 31, 32], to ensure faithful chromosome segregation during every cell division.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Iain Cheeseman (icheese@wi.mit.edu). Plasmids are also available through Addgene (http://www.addgene.org/Iain_Cheeseman/).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human tissue culture cell lines used in this study are listed in the key resources table. All HeLa (female cervix adenocarcinoma cells, not authenticated) lines used in this study were cultured in Dulbecco’s modified Eagle medium supplemented with 10% tetracycline-free fetal bovine serum, 100 units/mL penicillin, 100 units/mL streptomycin, and 2 mM L-glutamine at 37°C with 5% CO2.
All recombinant protein expression was performed in BL21 (DE3) E. coli.
METHOD DETAILS
Molecular Biology
E. coli expression vectors for human Ska1 MTBD, C. elegans SKA-1 MTBD, and the polycistronic vector containing human GFP-Ska1 and human Ska2 were described in [2]. Human Ska3 was described in [3]. Human His-TEV-Ska1 and human Ska2 was cloned into pST39. The stathmin-like domain (SLD) of RB3 was generated by cloning into pBAT4 the cDNA encoding residues 49–189 with an additional alanine at its N-terminus and, at its C-terminus, a GGSG linker followed by a 6xHis tag. GFP-tagged Ska1 and Ska1 ΔMTBD for expression in human cells were described in [2] and here mutated by site-directed mutagenesis to harden against CRISPR-Cas9 by introducing the maximal number of basepair changes within the sequence recognized by the guide RNA while maintaining the amino acid sequence. Mutations in human Ska1 MTBD and human GFP-Ska1 complex were introduced by site-directed mutagenesis.
Protein Purification
All protein expression was done in BL21(DE3) E. coli. For purification of ceSKA-1 MTBD, human Ska1 MTBD and variants, expression was induced using 0.1 mM IPTG overnight at 18°C. Cells were lysed and sonicated i n 50 mM potassium phosphate (KPi), 300 mM NaCl, 10 mM imidazole, 0.1% TWEEN 20, pH 8.0 (His lysis buffer). Cleared supernatant was incubated with Ni-NTA agarose (Qiagen) for 1 hr at 4°C, washed three times with 50 mM KPi, 500 mM NaCl, 40 mM imidazole, 0.1% TWEEN 20, 5 mM beta-mercaptoethanol (βME), pH 8.0 (His wash buffer), followed by elution with 50 mM KPi, 500 mM NaCl, 250 mM imidazole, 5 mM βME, pH 7.0 (His elution buffer). The eluted protein was supplemented with PreScission protease at 4°C overnight to remove the 6x-His tag, and purified on a Superdex 200 16/600 column in 20 mM HEPES, 150 mM KCl, 1 mM DTT, pH 7.0 (Gel filtration buffer). Gel filtration fractions were concentrated in a Vivaspin 3 kDa MWCO spin concentrator (GE Healthcare).
For purification of untagged Ska1 complex (used in Figure 1D; S1C, S2C and D), all cultures were induced using 0.4 mM IPTG for 4 hours at 37°C. His-Ska1 complexed with Ska2 was purified by the same protocol used for Ska1 MTBD. The eluted protein was supplemented with TEV protease at 4°C overnight to remove the 6x-His tag, and purified on a Superdex 200 16/600 column in Gel filtration buffer. Gel filtration fractions were concentrated in a Vivaspin 10 kDa MWCO spin concentrator (GE Healthcare). Bacteria expressing GST-Ska3 were lysed and sonicated in PBS, 250 mM NaCl, 0.1% TWEEN 20 (GST lysis buffer). Cleared supernatant was incubated with glutathione agarose (Sigma Aldrich) for 1 hr at 4°C, washed three times with GST lysis buffer supplemented with 1 mM DTT (GST wash buffer), and eluted with 50 mM Tris, 75 mM KCl, 10 mM reduced glutathione, pH 8.0 (GST elution buffer). The eluted protein was supplemented with PreScission protease at 4°C overnight to remove the GST tag, and purified on a Superdex 200 16/600 column in Gel filtration buffer. Ska3 containing fractions were passed back over glutathione agarose, concentrated in a Vivaspin 10 kDa MWCO spin concentrator (GE Healthcare), and mixed equimolar with purified Ska1-Ska2, incubated on ice for 30 min and the resulting complex was purified on a Superdex 200 16/600 column in Gel filtration buffer. Gel filtration fractions were concentrated in a Vivaspin 50 kDa MWCO spin concentrator (GE Healthcare).
For purification of His-Ska1 complex (used in Figure S1A and B) and His-GFP-Ska1 complex and variants (used in Figure 3 and S3), all cultures were induced using 0.1 mM IPTG overnight at 18°C. His Ska1-Ska2 and Hi s-GFP-Ska1-Ska2 were purified with the same protocol used for Ska1 MTBD, without removal of the 6x-His tag. Bacteria expressing GST-Ska3 was lysed, sonicated, bound to beads and washed identically to the Ska3 purification above. Eluted His-Ska1-Ska2 or His-GFP-Ska1-Ska2 was then added to the glutathione bound GST-Ska3 and incubated at 4°C for 1 hour. The bound complex was washed with GST wash buffer and eluted by cleaving GST-Ska3 off of the beads with PreScission protease at 4°C overnight. The eluted complex was purified on a Superdex 200 16/600 column in gel filtration buffer and concentrated in a Vivaspin 50 kDa MWCO spin concentrator (GE Healthcare).
RB3-SLD was induced using 0.4 mM IPTG for 4 hours at 37°C and purified by the same His-tagged purification strategy used for Ska1 MTBD described above. The Ni-NTA eluate was desalted on a NAP-5 column (GE Healthcare) into BRB80 and concentrated in a Vivaspin 3 kDa MWCO spin concentrator (GE Healthcare).
Co-sedimentation Assays
GMPCPP-stabilized microtubules were generated by incubating 25 μM tubulin dimer (Cytoskeleton) with 500 μM GMPCPP (Jena Bioscience) at 37°C for 1 hr. Dolastatin-10-induced rings were generated by incubating 25 M tubulin dimer in BRB80 (80 mM PIPES, pH 6.8, 4 mM MgCl, and 1 mM EGTA) and 100 M dolastatin-10 (provided by the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute) for 1 hr at room temperature. Microtubules and rings were purified by pelleting through a cushion of BRB80 with 40% glycerol for 10 min at 80000 rpm at room temperature and resuspended in BRB80. The concentration of microtubules was determined by measuring the absorbance at 280 nm after depolymerizing an aliquot of the microtubules in BRB80 + 10 mM CaCl2 on ice for 15 minutes. The concentration of dolastatin rings was determined by Coomassie staining relative to a standard curve of tubulin on the same gel. For the tubulin assembly assays, soluble tubulin heterodimer was pre-cleared at 90000 rpm before mixing with the test protein. Co-sedimentation assays were conducted as described previously [2, 33]. Briefly, 10 M Ska1 MTBD was mixed with 10 M of the appropriate tubulin substrate at room temperature and sedimented through a 40% glycerol cushion in BRB80 for 10 min at 80000 rpm. Coomassie gels are stained with AcquaStain (Bulldog Bio). Scanned gels were converted to grayscale in Adobe Photoshop and band intensities were quantified in Image Studio Lite. The graphs, generated in GraphPad Prism, display the percentage of Ska1 MTBD or tubulin pelleted, as determined by dividing the intensity of the pellet sample by the total intensity of the supernatant and pellet for each MTBD construct.
Size Exclusion Chromatography Assays
RB3-SLD and tubulin heterodimer were mixed at a 1:2 molar ratio and incubated on ice for 1 hour. For the right panel of Figure S1C, ~30% less tubulin was incubated with RB3-SLD to limit the concentration of fully saturated tubulin-bound RB3-SLD. In all experiments, Ska1 MTBD or Ska1 complex was then added in an approximately equimolar ratio to RB3-SLD, with the final salt concentration of the reaction being 85 mM KCl. The samples were incubated on ice for an additional 30 minutes, spun at 13,000 rpm for 10 min, and loaded on a Superdex 200 3.2/300 column on an Akta Micro in 20 mM HEPES, 75 mM KCl, 1 mM DTT, pH 7.0. Peak elution fractions were analyzed on polyacrylamide gels stained with AcquaStain. Scanned gels were converted to grayscale in Adobe Photoshop and band intensities were quantified in Image Studio Lite. For each Ska1 MTBD construct, the intensity of the Ska1 MTBD was measured for each of the 4 peak RB3-SLD-tubulin fractions (the fractions displayed in Figure 2C). The sum of these intensities was divided by the sum of the RB3-SLD intensities in these lanes to correct for differences in RB3-SLD-tubulin concentrations in each experiment. The graphs display these RB3-SLD normalized intensities as percentages relative to wild type Ska1 MTBD.
Electron Microscopy
Bovine brain microtubules were prepared by polymerizing 5 mg/ml bovine brain tubulin (Cytoskeleton, Inc.) in polymerization buffer (80 mM PIPES, pH 6.8, 1 mM EGTA, 4 mM MgCl2, 2 mM GTP, 12% dimethyl sulfoxide) for 30 min at 36°C. Paclitaxel was added at 250 μM before further incubation of 30 min at 36°C. The polymerized microtubules were then incubated at room temperature for several hours before use. The C. elegans SKA-1 MTBD (13 μM) was mixed with 1 μM microtubules in BRB80 and after a 10 min incubation the sample were adsorbed to glow-discharged 400-mesh C-flat grids (Electron Microscopy Sciences, Hatfield, PA) containing 2.0- μm holes separated by 2.0- μm spacing. The cryosamples were prepared using a manual plunger. Images were acquired using a K2 direct detector (Gatan, Pleasanton, CA) and the Tecnai F-20 microscope (FEI, Hillsboro, OR). Selected segments from 45 images were processed using a single-particle approach and the iterative helical real-space reconstruction procedure [34] with multimodel projection matching of microtubules with various numbers of protofilaments [35]. The rings around the microtubules were not stacked in an ordered manner (Figure 1B), preventing us from constructing a high-resolution map. However, we were able to obtain a low-resolution reconstruction from selected segments of microtubules belonging to the 13 protofilament family. UCSF Chimera [36] was used to display the 3-D reconstruction.
To obtain ring-like structures with unpolymerized tubulin, bovine or porcine brain tubulin were mixed in equimolar ratios (1:1 with 1–20 μM of each component) with C. elegans Ska1 MTBD, human Ska1 MTBD or human Ska1 complex in BRB80 and incubated for 5–10 min. The sample was then adsorbed for 1 min onto carbon coated glow-discharged grids before blotting and negatively stained with 1% uranyl acetate. Images were acquired on a FEI Tecnai T12 Transmission Electron Microscope (TEM), using a 4K × 4K Teitz F416 camera.
Cell Culture and Cell Line Generation
Generation of the Ska1 CRISPR/Cas9-based inducible knockout cell line was described in [18]. Cells expressing guide resistant GFP-Ska1 constructs were generated by retroviral infection of the Ska1 inducible knockout cell line and sorted for clonal cell lines. Although the mutants are more highly expressed than endogenous Ska1, they display roughly similar expression levels to each other (see Figure S4A). To induce the knockout, cells were dosed with 1 μg/mL doxycycline at 0, 24 and 48 hrs and assayed at 96 hours (4 days). To generate the stable replacement Ska1 ΔMTBD cell line, single cell sorting was performed on the fourth day and clonal populations were screened by fluorescence microscopy to verify proper localization of the transgene, Western blot to verify loss of the endogenous protein, and genomic sequencing to confirm the presence of Cas9 induced frameshift mutations.
For Western blotting, cell pellets were incubated on ice for 25 min in urea lysis buffer (50 mM Tris, 150 mM NaCl, 0.5% NP-40, 0.1% SDS, 6.5 M urea, pH 7.5 supplemented with 1 mM PMSF, and Complete EDTA-free protease inhibitor cocktail (Roche). Following size separation on a 12% SDS-PAGE gel, samples were semi-dry transferred to nitrocellulose and blocked for 30 min in 5% skim milk power in TBS + 0.1% Tween-20 (Blocking buffer). Primary and secondary antibodies were diluted in blocking buffer. The Ska1 antibody was described in [3] and used at 1 μg/mL. DM1A (Sigma Aldrich) was used at 1:5,000. HRP conjugated secondary antibodies (GE Healthcare) were used at 1:10,000. All antibody incubations were performed at room temperature for 1 hour. Clarity (Bio-Rad) was used as the ECL substrate.
To assess the genomic locus in the stable Ska1 ΔMTBD replacement cell line, genomic DNA was extracted by lysing the cell pellet in 100 mM Tris, 5 mM EDTA, 200 mM NaCl, 0.2% SDS, pH 8.0, supplemented with Proteinase K (New England Biolabs) at a final concentration of 0.2 mg/mL at 55°C overn ight. DNA was precipitated with isopropanol, washed with 75% ethanol and resuspended with TE buffer. PCR primers were designed to bind within the introns surrounding the Ska1 sgRNA target site (5′-gtacggtaccgtggttagaaac-3′ and 5′-cgttatgcaaaaatctaaaattacctaag-3′ to amplify a 402 nucleotide region centered on the on the sgRNA target site or 5′-gattacctgggcccatttctt-3′ and 5′-cattaaggaacgcatgactgt-3′ to amplify an 806 nucleotide region centered on the sgRNA target site). The PCR products were run on an agarose gel for gel purification. For both sets of primers, the only detectable product was a single band of the expected size. Gel purified PCR products were cloned into pCR4-TOPO using the TOPO TA Cloning Kit For Sequencing (Invitrogen). Following transformation of the TOPO reaction product, plasmid DNA was isolated from single bacterial colonies by miniprep and the region of the plasmid containing the insert was sequenced by Sanger sequencing. A total of 23 independent plasmids were sequenced from 2 different preparations of the genomic DNA, each of which displayed the same deletion spanning nucleotides 9–16 of exon 1, resulting in a frameshift and a protein product predicted to terminate after the addition of 7 new amino acids. The absence of any wild type sequences or in-frame deletions/insertions supports the Western blot data indicating a lack of detectable endogenous Ska1 protein.
Fluorescence Microscopy in Cells
For immunofluorescence, cells were fixed with 3.7% formaldehyde in PHEM buffer. Microtubules were visualized using 1:3,000 DM1A (Sigma Aldrich) and centromeres were visualized using 1:300 anti-centromere antibody (ACA). DNA was visualized with 100 ng/ml Hoechst-33342 (Sigma Aldrich). Images were acquired on a DeltaVision Core deconvolution microscope (Applied Precision/GE Healthsciences) equipped with a CoolSnap HQ2 CCD camera with a 100× 1.40 NA Olympus U-PlanApo objective. Images were deconvolved and maximally projected. For time-lapse microscopy, the media was changed to CO2-independent media prior to imaging and imaging was performed at 37°C. 3 Z-sections were acquired with 5 μm spacing using fluorescent light at the lowest level usable for data collection. Images were collected at 5-min intervals for 12 hrs using a using a 40×, 1.35-NA U-PlanApo objective (Olympus).
In Vitro Microtubule Motility Assays
Tubulin was purified and labeled as in [37, 38]. Microtubule seeds were prepared from a mixture containing 7 parts of unlabeled tubulin and 3 parts of DIG-labeled tubulin supplemented with 1 mM GMPCPP (Jena Bioscience). Flow chambers were prepared as in [39] using silanized coverslips and double stick tape, then anti-DIG antibodies (Roche Applied Science) were adsorbed to the coverslip and the surface was blocked with 1% Pluronic F127. Dynamic microtubules were polymerized from these seeds by adding 5 μM tubulin mixture containing 10 parts of unlabeled tubulin and 1 part of Hilyte647-labeled tubulin supplemented with 1 mM Mg-GTP. To test tip tracking on elongating microtubules, wild type GFP-Ska1 was introduced at 10–25 nM and molecular motions were observed at 32°C in imaging buffer, which in addition to soluble tubulin and Mg-GTP contained 80 mM K-PIPES (pH 6.9), 1 mM EGTA, 4 mM MgCl2, 4 mg/ml BSA, 10 mM DTT, 6 mg/ml glucose, 80 μg/ml catalase, 0.1 mg/ml glucose oxidase. To examine tracking of shortening microtubule ends, disassembly was induced by removing soluble tubulin with imaging buffer containing GFP-Ska1 complex. Bidirectional tracking was also observed on microtubules undergoing spontaneous dynamic instability in the presence of soluble tubulin (Figure S3C). Some experiments were done in the presence of 0.5% of methyl cellulose to reduce microtubule thermal motions and improve resolution of single molecule motions. This modification did not affect the tip-tracking by Ska1, so results with and without methyl cellulose were combined. Experiments with wild type and mutant Ska1 complexes were done analogously by using 10–30 nM R155A, 10–30 nM R236A, 35–70 nM K223A K236A, and 35–85 nM K183A K184A. The number of independent experiments for each Ska1 complex mutant was ≥2.
Microscopy for the in vitro assays was conducted as described in [40]. We used a 488 nm diode laser (Coherent) as a light source to excite GFP fluorescence, and a CUBE 640 nm diode laser (Coherent) for Hilyte647. Imaging was carried out in TIRF mode with 30 ms exposure and rapid switching between 488-nm and 640-nm lasers via acousto-optical tunable filter (AA OPTO-ELECTRONIC). To analyze tracking, the microtubule visible via Hilyte647 fluorescence was fitted with a straight line using Metamorph software (Molecular Devices, LLC). Because the position of the dynamic microtubule can change due to thermal rotations, the width of the line was varied to include microtubule images from all frames in the time series. The microtubule-representing line was then transferred onto the GFP image, and the kymograph was plotted along this line. The kymographs with a visually detectable increase in GFP-intensity at the end of microtubule relative to the lattice were identified as tip-tracking events (Figure 3C, D, S3D). The mean and SD of the fraction of tip tracking events were calculated in assumption of binomial probability distribution with n equal to the total number of microtubules collected from n≥2 independent trials. The intensity of GFP signal associated with the microtubule end was determined using ImageJ by calculating mean pixel intensity along the straight line (2 px width) drawn along the microtubule end in a kymograph. The lattice and background intensities were calculated analogously by using the 2 px wide lines on the microtubule lattice and in the microtubule-free area of the same kymograph. GFP brightness of the microtubule (Figure S3E) was determined by calculating mean pixel intensity of the entire microtubule lattice area on the kymograph, and subtracting the mean intensity of the similar background area.
QUANTIFICATION AND STATISTICAL ANALYSIS
All quantification methods are described in the Method Details. Statistical parameters are described in the Figure legends.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-Tubulin (clone DM1A) | Sigma-Aldrich | Cat# T9026; RRID: AB_477593 |
| Human polyclonal anti-Centromere | Antibodies Incorporated | Cat# 15-234-0001; RRID:AB_2687472 |
| Rabbit polyclonal anti-Ska1 | Cheeseman lab [3] | N/A |
| Goat anti-rabbit IgG-HRP | GE Healthcare | Cat# RPN4301; lot: 9721201 |
| Sheep anti-mouse IgG-HRP | GE Healthcare | Cat# NA931V; lot: 9729340 |
| anti-Digoxigenin-AP (anti-DIG), Fab fragments from sheep | Roche Applied Science | Cat# 11093274910 |
| Bacterial and Virus Strains | ||
| E. coli: One Shot™ Mach1™ T1 Phage-Resistant Chemically Competent E. coli | Thermo Fisher Scientific | Cat# C862003 |
| E. coli: BL21(DE3) Competent E. Coli | Cheeseman lab | N/A |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Hoechst-33342 | Sigma Aldrich | Cat# B2261 |
| Urea | Millipore Sigma | Cat# 1084870500; CAS: 57-13-6 |
| Complete EDTA-free protease inhibitor cocktail | Sigma Aldrich | Cat# 4693159001 |
| Proteinase K | New England Biolabs | Cat# P8107 |
| PreScission protease (pGEX3C-PreScission) | Cheeseman lab | N/A |
| TEV protease (pRK793) | Cheeseman lab | N/A |
| GMPCPP | Jena Bioscience | Cat# NU-405 |
| Tubulin protein (>99% pure): porcine brain | Cytoskeleton | Cat# T240 |
| Dolastatin-10 | Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute | NSC# 376128 |
| Paclitaxel | Sigma Aldrich | Cat# T7402 |
| 400-mesh C-flat grids | Electron Microscopy Sciences | Cat# CF-2/2-4C-50 |
| Doxycycline | Sigma Aldrich | Cat# D9891 |
| Bovine tubulin | Purified as described in [37] | N/A |
| DIG-tubulin | Bovine tubulin labeled with Digoxigenin (Invitrogen) as described in [38] | Cat# A-2952 |
| Hilyte647-tubulin | Bovine tubulin labeled with Hilyte647 (Anaspec) as described in [38] | Cat# 81256 |
| Pluronic F127 | Sigma-Aldrich | Cat# P2443 |
| Mg-GTP | Sigma-Aldrich | Cat# A9187 |
| GMPCPP | Jena Bioscience | Cat# NU405-S |
| Catalase | Sigma-Aldrich | Cat# C-40 |
| Glucose oxidase | Sigma-Aldrich | Cat# G-2133 |
| Glucose | Sigma-Aldrich | Cat# G-8270 |
| BSA | Sigma-Aldrich | Cat# A7638 |
| DTT | Invitrogen | Cat# 15508-13 |
| Methyl cellulose | MP Biomedicals | Cat# 155492 |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Human: HeLa, female Cervix Adenocarcinoma Cells | Cheeseman lab | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA (cKM250.3) |
Cheeseman lab [18] | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA, GFP-Ska1 CRISPR/Cas9 hardened (cIW13.28) |
This paper | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA, GFP-Ska1 ΔMTBD CRISPR/Cas9 hardened (cIW14.23) |
This paper | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA, GFP-Ska1 K183A K184A CRISPR/Cas9 hardened (cIW19.1) |
This paper | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA, GFP-Ska1 K223A K226A CRISPR/Cas9 hardened (cIW20.F) |
This paper | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA, GFP-Ska1 R236A CRISPR/Cas9 hardened (cIW18.I) |
This paper | N/A |
| Human: HeLa, Inducible Cas9, Ska1 sgRNA, GFP-Ska1 R155A CRISPR/Cas9 hardened (cIW22.A) |
This paper | N/A |
| Human: HeLa, Induced Cas9, Ska1 sgRNA, GFP-Ska1 ΔMTBD CRISPR/Cas9 hardened (Stable endogenous knockout) (cIW14.23v2) |
This paper | N/A |
| Experimental Models: Organisms/Strains | ||
| Oligonucleotides | ||
| Forward primer for Ska1 genomic amplification: 5′-gtacggtaccgtggttagaaac-3′ | This paper | N/A |
| Forward primer for Ska1 genomic amplification: 5′-gattacctgggcccatttctt-3′ | This paper | N/A |
| Reverse primer for Ska1 genomic amplification: 5′-cgttatgcaaaaatctaaaattacctaag-3′ | This paper | N/A |
| Reverse primer for Ska1 genomic amplification: 5′-cattaaggaacgcatgactgt-3′ | This paper | N/A |
| Recombinant DNA | ||
| pET3aTr-His-Precission-human Ska1 MTBD (pJS249) |
Cheeseman lab [2] | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD K183A K184A K203A K206A (pIC400) |
This paper | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD K183A K184A (pIW28) |
This paper | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD K203A K206A (pIW39) |
This paper | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD K223A K226A (pDK616) |
This paper | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD R236A (pIW40) | This paper | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD R155A (pJS278) | This paper | N/A |
| pET3aTr-His-Precission-human Ska1 MTBD R245A (pJS281) | This paper | N/A |
| pET3aTr-C.elegans Ska1 MTBD-His (pJS263) |
Cheeseman lab [2] | N/A |
| pST39-His-GFP-human Ska1; human Ska2 (pJS183) |
Cheeseman lab [2] | N/A |
| pST39-His-GFP-human Ska1 K183A K184A; human Ska2 (pIW87) |
This paper | N/A |
| pST39-His-GFP-human Ska1 K223A K226A; human Ska2 (pIW90) | This paper | N/A |
| pST39-His-GFP-human Ska1 R236A; human Ska2 (pIW84) | This paper | N/A |
| pST39-His-GFP-human Ska1 R155A; human Ska2 (pIW95) | This paper | N/A |
| pGEX-6P-1-human Ska3 (pCB4) |
Cheeseman lab [3] | N/A |
| pST39-His-TEV-human Ska1; human Ska2 (pJS182) |
This paper | N/A |
| pST39-His-TEV-human Ska1 K183A K184A; human Ska2 (pIW86) |
This paper | N/A |
| pBAT4-RB3-SLD (pJM160) |
This paper | N/A |
| pBABEblast-GFP-human Ska1 CRISPR/Cas9 hardened (pIW69) |
This paper | N/A |
| pBABEblast-GFP-human Ska1 CRISPR/Cas9 hardened K183A K184A (pIW85) |
This paper | N/A |
| pBABEblast-GFP-human Ska1 CRISPR/Cas9 hardened K223A K226A (pIW88) |
This paper | N/A |
| pBABEblast-GFP-human Ska1 CRISPR/Cas9 hardened R236A (pIW82) |
This paper | N/A |
| pBABEblast-GFP-human Ska1 CRISPR/Cas9 hardened R155A (pIW93) |
This paper | N/A |
| pBABEblast-GFP-human Ska1 ΔMTBD CRISPR/Cas9 hardened (pIW70) |
This paper | N/A |
| Software and Algorithms | ||
| Photoshop CS5.1, version 12.1 | Adobe | http://www.adobe.com |
| Image Studio Lite Version 5.2.5 | LI-COR | http://www.licor.com |
| GraphPad Prism 7, Version 7.0c | GraphPad software | http://www.graphpad.com |
| UCSF Chimera | Molecular graphic images were produced using the Chimera package from Computer Graphics Laboratory, University of California, San Francisco. | https://www.cgl.ucsf.edu/chimera/ |
| Metamorph | Molecular Devices | http://www.moleculardevices.com |
| Other | ||
| Clarity Western ECL Substrate | Bio-Rad | Cat# 1705060 |
| TOPO TA Cloning Kit For Sequencing | Invitrogen | Cat# 450030 |
| Ni-NTA agarose | Qiagen | Cat# 30230 |
| Glutathione agarose | Sigma Aldrich | Cat# G4510 |
| HiLoad 16/600 Superdex 200 pg | GE Healthcare | Cat# 28989335 |
| NAP-5 column | GE Healthcare | Cat# 17-0853-01 |
| Vivaspin 3 kDa MWCO spin concentrator | GE Healthcare | Cat# 28-9323-58 |
| Vivaspin 10 kDa MWCO spin concentrator | GE Healthcare | Cat# 28-9323-60 |
| Vivaspin 50 kDa MWCO spin concentrator | GE Healthcare | Cat# 28-9323-62 |
| AcquaStain | Bulldog Bio | Cat# AS001000 |
| Superdex 200 Increase 3.2/300 | GE Healthcare | Cat# 28990946 |
| Nitrocellulose, Amersham Protran 0.2 NC | GE Healthcare | Cat# 10600001 |
Supplementary Material
Movie S1. Microtubule plus end tracking by the Ska1 complex on a dynamic microtubule. Related to Figure 3. Movie showing dynamic microtubule growing, then depolymerizing spontaneously in the presence of soluble tubulin (5 μM) and 15 nM wild type GFP-Ska1 complex. GFP-Ska1 decorates strongly the GMPCPP-containing seed and highlights the plus end of microtubule during both polymerization and depolymerization. This movie is played at 30 fps and events are shown 7.2 times faster than real. Scale bar is 2 μm.
Movie S2. Ska1 complex mutants show reduced or eliminated plus end tracking activity. Related to Figure 3. Movies showing depolymerizing microtubules (red) with GFP-Ska1 complexes (green). Four Ska1 constructs are shown: wild type GFP-Ska1 (25 nM), K183A K184A GFP-Ska1 (85 nM), R236A GFP-Ska1 (10 nM) and R155A GFP-Ska1 (20 nM). All GFP-Ska1 constructs strongly decorate the coverslip-attached GMPCPP seed, which marks the minus end. Individual GFP-Ska1 complexes are seen diffusing on the GDP-containing microtubule wall and dissociating from the wall frequently. In case of wild type and K183A K184A GFP-Ska1, the depolymerizing microtubule end is temporarily enriched in GFP-Ska1, these events are indicated by white arrowheads. The movie played at 30 fps and events are shown 1.8 times faster than real. Scale bars are 2 μm.
Highlights.
The Ska1 complex associates with and oligomerizes soluble tubulin heterodimers
Mutating specific Ska1 surfaces disrupts tubulin, but not microtubule binding
The Ska1 complex tracks the plus end of both growing and shrinking microtubules
Ska1 tubulin binding mutants prevent tip tracking and proper mitotic progression
Acknowledgments
We thank A. Zaytsev for technical assistance and members of the Cheeseman and Grishchuk labs for stimulating discussions and critical reading of the manuscript. This work was supported in part by grants from the NIH to ELG (GM098389), RAM & EMW-K (GM052468), and IMC (GM088313). ELG is supported in part by Research Scholar Grant RSG-14-018-01-CCG from the American Cancer Society, and EVT was supported in part by grant 16-14-00-224 from the Russian Science Foundation. This work was supported by a Scholar award to IMC from the Leukemia & Lymphoma Society and a National Science Foundation Graduate Research Fellowship under Grant No. (1122374) to JKM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, I.M.C., J.K.M., E.W-K., E.L.G.; Methodology, I.P.W., J.K.M., E.V.T., E.L.G., E.W-K., I.M.C.; Investigation, I.P.W., J.K.M, E.V.T, E.W-K., I.M.C.; Writing – Original Draft, I.M.C. and J.K.M.; Writing – Review & Editing, I.M.C, J.K.M., I.P.W., E.V.T., E.W-K., E.L.G.; Funding Acquisition, I.M.C. and E.L.G., E.W-K., R.M.; Supervision, I.M.C., E.L.G., and R.M.
References
- 1.Cheeseman IM. The kinetochore. Cold Spring Harb Perspect Biol. 2014;6:a015826. doi: 10.1101/cshperspect.a015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt JC, Arthanari H, Boeszoermenyi A, Dashkevich NM, Wilson-Kubalek EM, Monnier N, Markus M, Oberer M, Milligan RA, Bathe M, et al. The kinetochore-bound Ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev Cell. 2012;23:968–980. doi: 10.1016/j.devcel.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell. 2009;16:374–385. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moores CA, Cooper J, Wagenbach M, Ovechkina Y, Wordeman L, Milligan RA. The role of the kinesin-13 neck in microtubule depolymerization. Cell Cycle. 2006;5:1812–1815. doi: 10.4161/cc.5.16.3134. [DOI] [PubMed] [Google Scholar]
- 5.Tan D, Asenjo AB, Mennella V, Sharp DJ, Sosa H. Kinesin-13s form rings around microtubules. J Cell Biol. 2006;175:25–31. doi: 10.1083/jcb.200605194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Asenjo AB, Greenbaum M, Xie L, Sharp DJ, Sosa H. A second tubulin binding site on the kinesin-13 motor head domain is important during mitosis. PLoS One. 2013;8:e73075. doi: 10.1371/journal.pone.0073075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G. Formation of a dynamic kinetochore-microtubule interface through assembly of the Dam1 ring complex. Mol Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Miranda JJ, De Wulf P, Sorger PK, Harrison SC. The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol. 2005;12:138–143. doi: 10.1038/nsmb896. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Crevenna AH, Kunze I, Mizuno N. Structural basis for the extended CAP-Gly domains of p150(glued) binding to microtubules and the implication for tubulin dynamics. Proc Natl Acad Sci U S A. 2014;111:11347–11352. doi: 10.1073/pnas.1403135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters C, Brejc K, Belmont L, Bodey AJ, Lee Y, Yu M, Guo J, Sakowicz R, Hartman J, Moores CA. Insight into the molecular mechanism of the multitasking kinesin-8 motor. Embo J. 2010;29:3437–3447. doi: 10.1038/emboj.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 12.Abad MA, Medina B, Santamaria A, Zou J, Plasberg-Hill C, Madhumalar A, Jayachandran U, Redli PM, Rappsilber J, Nigg EA, et al. Structural basis for microtubule recognition by the human kinetochore Ska complex. Nat Commun. 2014;5:2964. doi: 10.1038/ncomms3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciejowski J, Drechsler H, Grundner-Culemann K, Ballister ER, Rodriguez-Rodriguez JA, Rodriguez-Bravo V, Jones MJK, Foley E, Lampson MA, Daub H, et al. Mps1 Regulates Kinetochore-Microtubule Attachment Stability via the Ska Complex to Ensure Error-Free Chromosome Segregation. Dev Cell. 2017;41:143–156 e146. doi: 10.1016/j.devcel.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16:711–726. doi: 10.1038/nrm4084. [DOI] [PubMed] [Google Scholar]
- 15.Thomas GE, Bandopadhyay K, Sutradhar S, Renjith MR, Singh P, Gireesh KK, Simon S, Badarudeen B, Gupta H, Banerjee M, et al. EB1 regulates attachment of Ska1 with microtubules by forming extended structures on the microtubule lattice. Nat Commun. 2016;7:11665. doi: 10.1038/ncomms11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grishchuk EL. Biophysics of Microtubule End Coupling at the Kinetochore. Prog Mol Subcell Biol. 2017;56:397–428. doi: 10.1007/978-3-319-58592-5_17. [DOI] [PubMed] [Google Scholar]
- 18.McKinley KL, Cheeseman IM. Large-Scale Analysis of CRISPR/Cas9 Cell-Cycle Knockouts Reveals the Diversity of p53-Dependent Responses to Cell-Cycle Defects. Dev Cell. 2017;40:405–420. doi: 10.1016/j.devcel.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaitanos TN, Santamaria A, Jeyaprakash AA, Wang B, Conti E, Nigg EA. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. Embo J. 2009;28:1442–1452. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auckland P, Clarke NI, Royle SJ, McAinsh AD. Congressing kinetochores progressively load Ska complexes to prevent force-dependent detachment. J Cell Biol. 2017;216:1623–1639. doi: 10.1083/jcb.201607096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daum JR, Wren JD, Daniel JJ, Sivakumar S, McAvoy JN, Potapova TA, Gorbsky GJ. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Current biology: CB. 2009;19:1467–1472. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanisch A, Sillje HH, Nigg EA. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. Embo J. 2006;25:5504–5515. doi: 10.1038/sj.emboj.7601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivakumar S, Janczyk PL, Qu Q, Brautigam CA, Stukenberg PT, Yu H, Gorbsky GJ. The human SKA complex drives the metaphase-anaphase cell cycle transition by recruiting protein phosphatase 1 to kinetochores. eLife. 2016:5. doi: 10.7554/eLife.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lampert F, Hornung P, Westermann S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J Cell Biol. 2010;189:641–649. doi: 10.1083/jcb.200912021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alushin GM, Ramey VH, Pasqualato S, Ball DA, Grigorieff N, Musacchio A, Nogales E. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;467:805–810. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson-Kubalek EM, Cheeseman IM, Milligan RA. Structural comparison of the Caenorhabditis elegans and human Ndc80 complexes bound to microtubules reveals distinct binding behavior. Mol Biol Cell. 2016;27:1197–1203. doi: 10.1091/mbc.E15-12-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee C, Benoit MP, DePaoli V, Diaz-Valencia JD, Asenjo AB, Gerfen GJ, Sharp DJ, Sosa H. Distinct Interaction Modes of the Kinesin-13 Motor Domain with the Microtubule. Biophys J. 2016;110:1593–1604. doi: 10.1016/j.bpj.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abad MA, Zou J, Medina-Pritchard B, Nigg EA, Rappsilber J, Santamaria A, Jeyaprakash AA. Ska3 Ensures Timely Mitotic Progression by Interacting Directly With Microtubules and Ska1 Microtubule Binding Domain. Sci Rep. 2016;6:34042. doi: 10.1038/srep34042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redli PM, Gasic I, Meraldi P, Nigg EA, Santamaria A. The Ska complex promotes Aurora B activity to ensure chromosome biorientation. J Cell Biol. 2016;215:77–93. doi: 10.1083/jcb.201603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Sivakumar S, Chen Y, Gao H, Yang L, Yuan Z, Yu H, Liu H. Ska3 Phosphorylated by Cdk1 Binds Ndc80 and Recruits Ska to Kinetochores to Promote Mitotic Progression. Curr Biol. 2017;27:1477–1484 e1474. doi: 10.1016/j.cub.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 31.Cheerambathur DK, Prevo B, Hattersley N, Lewellyn L, Corbett KD, Oegema K, Desai A. Dephosphorylation of the Ndc80 Tail Stabilizes Kinetochore-Microtubule Attachments via the Ska Complex. Dev Cell. 2017;41:424–437 e424. doi: 10.1016/j.devcel.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janczyk PL, Skorupka KA, Tooley JG, Matson DR, Kestner CA, West T, Pornillos O, Stukenberg PT. Mechanism of Ska Recruitment by Ndc80 Complexes to Kinetochores. Dev Cell. 2017;41:438–449 e434. doi: 10.1016/j.devcel.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The Conserved KMN Network Constitutes the Core Microtubule-Binding Site of the Kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 34.Egelman EH. Single-particle reconstruction from EM images of helical filaments. Curr Opin Struct Biol. 2007;17:556–561. doi: 10.1016/j.sbi.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D, Nogales E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell. 2014;157:1117–1129. doi: 10.1016/j.cell.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 37.Miller HP, Wilson L. Preparation of microtubule protein and purified tubulin from bovine brain by cycles of assembly and disassembly and phosphocellulose chromatography. Methods Cell Biol. 2010;95:3–15. doi: 10.1016/S0091-679X(10)95001-2. [DOI] [PubMed] [Google Scholar]
- 38.Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- 39.Volkov VA, Zaytsev AV, Grishchuk EL. Preparation of segmented microtubules to study motions driven by the disassembling microtubule ends. J Vis Exp. 2014 doi: 10.3791/51150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gudimchuk N, Vitre B, Kim Y, Kiyatkin A, Cleveland DW, Ataullakhanov FI, Grishchuk EL. Kinetochore kinesin CENP-E is a processive bidirectional tracker of dynamic microtubule tips. Nat Cell Biol. 2013;15:1079–1088. doi: 10.1038/ncb2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Microtubule plus end tracking by the Ska1 complex on a dynamic microtubule. Related to Figure 3. Movie showing dynamic microtubule growing, then depolymerizing spontaneously in the presence of soluble tubulin (5 μM) and 15 nM wild type GFP-Ska1 complex. GFP-Ska1 decorates strongly the GMPCPP-containing seed and highlights the plus end of microtubule during both polymerization and depolymerization. This movie is played at 30 fps and events are shown 7.2 times faster than real. Scale bar is 2 μm.
Movie S2. Ska1 complex mutants show reduced or eliminated plus end tracking activity. Related to Figure 3. Movies showing depolymerizing microtubules (red) with GFP-Ska1 complexes (green). Four Ska1 constructs are shown: wild type GFP-Ska1 (25 nM), K183A K184A GFP-Ska1 (85 nM), R236A GFP-Ska1 (10 nM) and R155A GFP-Ska1 (20 nM). All GFP-Ska1 constructs strongly decorate the coverslip-attached GMPCPP seed, which marks the minus end. Individual GFP-Ska1 complexes are seen diffusing on the GDP-containing microtubule wall and dissociating from the wall frequently. In case of wild type and K183A K184A GFP-Ska1, the depolymerizing microtubule end is temporarily enriched in GFP-Ska1, these events are indicated by white arrowheads. The movie played at 30 fps and events are shown 1.8 times faster than real. Scale bars are 2 μm.


