Abstract
Background
Technological advances can improve care and outcomes but are a primary driver of health care spending growth. Understanding diffusion and use of new oncology therapies is important, given substantial increases in prices and spending on such treatments.
Objectives
Examine diffusion of bevacizumab, a novel (in 2004) and high-priced biologic cancer therapy, among U.S. oncology practices during 2005–2012 and assess variation in use across practices.
Research Design
Population-based observational study.
Setting
2329 U.S. practices providing cancer chemotherapy.
Participants
Random 20% sample of 236,304 Medicare fee-for-service beneficiaries aged >65 in 2004–2012 undergoing infused chemotherapy for cancer.
Measures
Diffusion of bevacizumab (cumulative time to first use and 10% use) in practices, variation in use across practices overall and by higher vs. lower-value use. We used hierarchical models with practice random effects to estimate the between-practice variation in the probability of receiving bevacizumab and to identify factors associated with use.
Results
We observed relatively rapid diffusion of bevacizumab, particularly in independent practices and larger versus smaller practices. We observed substantial variation in use; the adjusted odds ratio (95% confidence interval) of bevacizumab use was 2.90 higher (2.73–3.08) for practices one standard deviation above vs. one standard deviation below the mean. Variation was less for higher-value (odds ratio=2.72 [2.56–2.89]) than lower-value uses (odds ratio=3.61 [3.21–4.06]).
Conclusions
Use of bevacizumab varied widely across oncology practices, particularly for lower-value indications. These findings suggest that interventions targeted to practices have potential for decreasing low-value use of high-cost cancer therapies.
Keywords: variation, diffusion, cancer treatment
Introduction
Technological advances have provided substantial improvements in health that are recognized to be of reasonable value,1 and evidence suggests that rapid adoption of cost-effective innovations is associated with better outcomes.2 Nevertheless, technological advances are generally accepted as the primary driver of health care spending growth,3–5 and thus, understanding variations in diffusion and use of new technologies are instructive in light of efforts to improve the value of health care.
Understanding diffusion of new cancer therapies is of particular interest, given substantial increases in the prices of cancer drugs newly approved by the Food and Drug Administration (FDA)6 and rising spending on cancer drugs by insurers and patients.7,8 Moreover, new infused cancer drugs typically are covered by insurers, and reimbursement is often greater for higher-priced drugs; for example, oncology practices are currently reimbursed 104.3% of the average sales price for infused drugs for Medicare beneficiaries.9
Prior research has documented wide variation across oncology practices in Medicare expenditures for cancer care, including chemotherapy,10 suggesting broad opportunities for providers to improve the value of cancer care delivered, defined as health outcomes achieved per dollar spent.11 The American Society of Clinical Oncology12,13 and others14 now encourage providers to consider value in cancer treatment decisions by evaluating the clinical benefit, toxicity, and cost of treatments.12 Moreover, recent efforts seeking to improve the delivery of high-value cancer care, including the CMS Oncology Care Model, have been targeted at practices.15–17 Nevertheless, few studies have explored practice-level variation in use and diffusion of new cancer treatments; such information could inform the likelihood of success of policies and interventions targeting providers.
We studied diffusion of one of the first widely-used biologic cancer therapies—bevacizumab—across oncology practices. Bevacizumab was a novel treatment when initially approved by the FDA for advanced-stage colorectal cancer in February 2004 and since has been approved for treating advanced cancers of the lung, breast, brain, kidney, cervix, and ovary, allowing assessment of diffusion by indication (see Supplemental Materials Table 1 for exact indications). Moreover, the benefits of bevacizumab vary by cancer type; it has been shown to improve median overall survival for patients with metastatic colorectal cancer (20.3 months vs. 15.6 months for regimens with vs. without bevacizumab)18 and lung cancer (12.3 months vs. 10.3 months),19 but improves only progression-free survival, not overall survival, for patients with breast,20–23 kidney,24 glioblastoma of the brain,25,26 and ovarian cancers.27,28
Using Medicare data from 2004–2012, we assessed differences in diffusion of bevacizumab in oncology practices treating Medicare patients across the U.S. overall and by cancer type. In addition, we sought to understand variation in bevacizumab use across oncology practices overall and for higher vs. lower-value indications. Finally, we identified patient and practice characteristics associated with use of bevacizumab.
Methods
Data and patients
We used Medicare data for a random 20% sample of fee-for-service Medicare beneficiaries aged ≥65 years in 2004 through 2012 (the most recent data available at the time of the study). We excluded Medicare beneficiaries aged <65 because they are eligible based on disability and their patterns of care may differ from other patients of the same age and older Medicare beneficiaries. We identified individuals with at least 2 outpatient office visits (30 days apart) with a cancer diagnosis in a calendar year during 2005–2012 who received outpatient infused chemotherapy (Supplemental Digital Content Table 2). Using International Classification of Disease, 9th Edition (ICD9) diagnosis codes, we characterized cancer type as colorectal, lung, breast, brain, kidney, ovary, and other (all other cancer types) based on visits in the 6 months before and after the first chemotherapy claim in a calendar year (if more than one cancer type in that period, they were assigned to the diagnosis coded most frequently, or most recently if a tie). Cervical cancer, for which bevacizumab is also approved, is infrequent among Medicare beneficiaries and was categorized with other cancers. The study was approved by the Harvard Medical School Human Subjects Committee.
Bevacizumab and other intravenous chemotherapy
For each patient in each month, we documented receipt of infused chemotherapy (Supplemental Digital Content Table 2). We identified an “episode” of chemotherapy as long as there was no break of >3 months; patients who had >3 months without chemotherapy and started again were considered to have a second episode. Bevacizumab use was defined by Healthcare Common Procedure Coding System code J9035 at any time during an episode. Although bevacizumab was approved in 2004, its use could not be observed until January 2005 when the billing code was available. To avoid identifying bevacizumab for ophthalmic use, we required a cancer diagnosis code on the bevacizumab claim.
We characterized bevacizumab use as “higher value” when used for cancers for which an overall survival benefit had been demonstrated. Higher-value indications included colorectal cancer throughout the study period and lung cancer after presentation of trial findings demonstrating an overall survival benefit with bevacizumab in May 200529 (results were similar when using the publication date of the trial findings (December 2006)19 and are not presented). Although the overall survival benefits are modest (i.e., 2 months improvement in median survival for patients with non-small cell lung cancer), they are consistent with other widely adopted-therapeutic improvements in oncology. Use for other cancer types or for lung cancer before May 2005 was considered lower-value. We also characterized bevacizumab use as approved vs. not approved based on FDA approval for that cancer type; this is distinct from higher-value use because in some cases FDA approval was based on progression-free survival benefit without overall survival benefit. FDA-approved indications included colorectal cancer at all times, lung cancer after October 2006, breast cancer from February 2008 through November 2011, glioblastoma after May 2009, and kidney cancer after July 2009. All other indications were non-approved, including ovarian cancer (FDA-approved in 2014). We approximated “higher-value” and FDA-approved use based on cancer type, recognizing that we lacked information on stage, line of therapy, and histology to assess use exactly according to the FDA-labeled indication.
Identifying practices
Each patient was assigned for each year to an oncology practice (identified using tax identification numbers [TINs]) based on the plurality of office visits (evaluation and management codes 99201-99215, 99241-99245, G0402, G0438, G0438) with physicians with specialty codes for medical oncology, hematology/oncology, or gynecologic oncology. Patients with equal numbers of visits to more than one practice were assigned to the practice with the greater sum of allowed charges. We excluded very-low-volume practices, restricting our sample to practices providing chemotherapy to ≥8 patients in our 20% Medicare data per year, representing an average of 40 Medicare patients per practice. These practices included 55% of physicians and 95% of patients (Supplemental Digital Content Table 3).
Practice and patient characteristics
We identified practices affiliated with an academic medical center based on work by Welch and Bindman;30 other practices were classified as independent or hospital-owned (a time-varying variable where practices were considered hospital-owned in a year if >75% of the practices’ claims in that year had a place of service code for the outpatient hospital; practices can bill in this manner whether on- or off-campus).31 We also characterized the proportion of a practice’s patients residing in high-poverty zip codes using 2010 Census data. Finally, for each practice we determined the number of physicians, the number of oncologists, and the ratio of oncologists to all physicians billing in the practice over all patients in the 20% Medicare fee-for-service sample. We hypothesized that diffusion would be faster in academic practices because of their engagement in testing novel therapies in clinical trials. We hypothesized that use of bevacizumab would be greater in independent versus hospital-based practices because of the greater financial incentives for use.
For each patient in each episode, we characterized age, race/ethnicity, sex, cancer type, diagnosis codes suggesting metastatic cancer (ICD9 codes 196-199.0), year, Charlson comorbidity score,32,33 Census division, and zip-code level measures of median household income, proportion of residents living in poverty, and proportion of residents who graduated from high school. We also characterized whether the episode was the first versus 2nd or later episode. Patient and practice-level variables were categorized as in Table 1. Patients with missing race/ethnicity data were grouped with the “other” category. 242 patients (0.1%) with missing zip codes were excluded from analyses.
Table 1.
Characteristics of patients undergoing chemotherapy, N=236,304 who have 307,744 episodes; cared for by physicians in 2329 oncology practices
| Characteristic | N (%) patient episodes* | Proportion or mean (SD) Among patients not using bevacizumab | Proportion or mean (SD) among patients using bevacizumab | Adjusted Odds Ratio (95% CI) of Bevacizumab Use from Hierarchical Model | P value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
|
| |||||
| Age in years, mean (SD) | 74.1 (6.1) | 74.2 (6.1) | 72.9 (5.7) | 0.98 (0.97–0.98) | <.001 |
| Sex, N (%) | |||||
| Male | 146,092 (47) | 133,601 (47) | 12,491 (48) | – | – |
| Female | 161,652 (53) | 147,990 (53) | 13,662 (52) | 0.98 (0.95–1.01) | 0.27 |
| Race/ethnicity, N (%) | |||||
| Non-Hispanic white | 277,427 (90) | 254,229 (90) | 23,198 (89) | – | – |
| Non-Hispanic black | 20,236 (7) | 18,250 (6) | 1,986 (8) | 1.10 (1.03–1.16) | 0.003 |
| Hispanic | 2,796 (1) | 2,550 (1) | 246 (1) | 1.03 (0.87–1.22) | 0.77 |
| Other/Unknown | 7,285 (2) | 6,562 (2) | 723 (3) | 1.02 (0.91–1.13) | 0.77 |
| Cancer type, N (%) | |||||
| Colorectal | 37,429 (12) | 24,032 (9) | 13,397 (51) | Reference | – |
| Lung | 69,318 (23) | 62,295 (22) | 7,023 (27) | 0.19 (0.18–0.20) | <.001 |
| Breast | 34,716 (11) | 32,466 (12) | 2,250 (9) | 0.11 (0.10–0.12) | <.001 |
| Kidney | 4,207 (1) | 3,754 (1) | 453 (2) | 0.19 (0.17–0.21) | <.001 |
| Brain | 910 (<1) | 347 (<1) | 563 (2) | 3.32 (2.78–3.98) | <.001 |
| Ovary | 14,522 (5) | 13,245 (5) | 1,277 (5) | 0.15 (0.14–0.17) | <.001 |
| Other | 146,642 (48) | 145,452 (52) | 1,190 (5) | 0.01 (0.01–0.02) | <.001 |
| Charlson comorbidity score, N (%) | |||||
| 0 | 142,558 (46) | 129,316 (46) | 13,242 (51) | Reference | – |
| 1 | 87,584 (28) | 80,122 (28) | 7,462 (29) | 0.83 (0.80–0.86) | <.001 |
| 2 | 41,574 (14) | 38,391(14) | 3,183 (12) | 0.77 (0.73–0.81) | <.001 |
| 3 | 19,154 (6) | 17,831(6) | 1,323 (5) | 0.69 (0.65–0.75) | <.001 |
| 4+ | 16,874 (5) | 15,931 (6) | 943 (4) | 0.56 (0.52–0.60) | <.001 |
| Census division, N (%) | |||||
| New England | 16,575 (5) | 15,378 (5) | 1,197 (5) | 0.74 (0.65–0.85) | <.001 |
| Mid Atlantic | 40,921 (13) | 37,645 (13) | 3,276 (13) | 0.77 (0.70–0.86) | <.001 |
| East North Central | 54,865 (18) | 50,200 (18) | 4,665 (18) | 0.84 (0.76–0.93) | <.001 |
| West North Central | 23,747 (8) | 21,817 (8) | 1,930 (7) | 0.85 (0.75–0.96) | 0.009 |
| South Atlantic | 66,650 (22) | 60,918 (22) | 5,732 (22) | 0.86 (0.78–0.95) | 0.003 |
| East South Central | 21,509 (7) | 19,627 (7) | 1,882 (7) | 0.80 (0.70–0.91) | <.001 |
| West South Central | 34,568 (11) | 31,484 (11) | 3,084 (12) | 0.91 (0.81–1.03) | 0.13 |
| Mountain | 16,485 (5) | 15,013 (5) | 1,472 (6) | 0.87 (0.75–0.99) | 0.041 |
| Pacific | 31,867 (10) | 29,010 (10) | 2,857 (11) | Reference | – |
| Median household income of zip code of residence, mean (SD) | $56,943 ($22,898) | $56,969 ($22,918) | $56,673 ($22,681) | 1.002 (1.0004–1.003)‡ | 0.007 |
| Proportion of residents in zip code living in poverty, mean (SD) | 10.1 (7.3) | 10.1 (7.3) | 10.3 (7.4) | 1.000 (0.997–1.004)¶ | 0.89 |
| Proportion of residents in zip code who graduated from high school, mean (SD) | 87.0 (8.3) | 87.1 (8.3) | 86.9 (8.5) | 1.002 (0.999–1.005)¶ | 0.28 |
| “Episodes” of chemotherapy during study period, N (%)* | |||||
| 1 | 186,245 (61) | 170,106 (60) | 16,139 (62) | – | – |
| >1 | 121,499 (39) | 111,485 (40) | 10,014 (38) | 1.07 (1.04–1.11) | <.001 |
| Metastatic cancer, N (%) | |||||
| No | 234,484 (76) | 218,492 (78) | 15,992 (61) | – | – |
| Yes | 73,260 (24) | 63,099 (22) | 10,161 (39) | 2.05 (1.98–2.12) | <.001 |
| Year of first chemotherapy episode, median (25th, 75th) | 2008 (2006, 2011) | 2009 (2006, 2011) | 2008 (2006, 2010) | 1.003 (0.995–1.012) | 0.45 |
|
| |||||
| Practice characteristics | |||||
|
| |||||
| Practice type, N (%) | |||||
| Independent | 239,001 (78) | 217,911 (77) | 21,090 (81) | Reference | – |
| Hospital-owned (non-academic) | 31,114 (10) | 28,686 (10) | 2,428 (9) | 0.82 (0.75–0.90) | <.001 |
| Academic | 37,629 (12) | 34,994 (12) | 2,635(10) | 0.84 (0.75–0.95) | 0.006 |
| Number of physicians in practice, median (25th/75th) | 17 (5,89) | 17 (5, 92) | 15 (5, 67) | Not included† | – |
| Number of oncologists in practice, median (25th/75th) | 7 (3,17) | 7 (3, 17) | 6 (3, 15) | 1.001 (0.999–1.003) | 0.54 |
| Ratio of oncologists to all physicians, median (25th/75th) | 0.77 (0.10,1.00) | 0.77 (0.10,1.00) | 0.80 (0.16, 1.00) | 1.009 (1.001–1.017) | 0.027 |
| Proportion of individuals treated in practice living in area with proportion living in poverty above the median, mean (SD) | 9.5 (12.4) | 9.5 (12.4) | 9.6 (12.4) | 0.999 (0.997–1.001)¶ | 0.30 |
An episode is defined as a new initiation of chemotherapy after 3 months without any chemotherapy.
Omitted from model because collinear with number of oncologists and ratio of oncologists to all physicians, which were both included.
Reflects change per each $1000 increase above the mean.
Reflects change per 10% increase above the mean.
All continuous variables are centered at the mean, so differences reflect increases per unit above the mean.
Diffusion of bevacizumab
We described diffusion, defined as the cumulative percentage of bevacizumab adopters over time, following Rogers’ diffusion of innovation theory 34 and focusing on practices as the unit of interest. Similar to others,35–38 we examined the pace of bevacizumab adoption in practices at a given time. For each practice, we assessed time to first use of bevacizumab and time to 10% use. The latter was defined as the time from January 2005 to the month in which bevacizumab was used in 10% of cumulative patients receiving chemotherapy in the practice.36 Time to 10% use may represent more routine use and avoids characterizing diffusion rates based on a single infusion in a practice; we would not expect substantially greater use in most practices because only a subset of cancer patients receiving chemotherapy are eligible for bevacizumab. We also assessed time to 50% use; very few practices treated ≥50% of potentially-eligible patients based on cancer type with bevacizumab, and thus we focused on time to first use and time to 10% use.
Analyses
Diffusion
We plotted cumulative time to first use and 10% use in practices by month for patients with each cancer type.31 We also plotted diffusion stratified by academic vs. hospital-owned vs. independent practices and large practices (≥20 Medicare patients treated per year in our 20% Medicare sample) vs. small practices (8–20 Medicare patients treated in a year in our 20% sample).
Modeling speed of diffusion (time to use) across practices
In a subset of practices with at least one patient with colorectal, lung, and breast cancer in the 20% Medicare sample in 2005, we estimated frailty models with multivariate event times to determine the extent to which a practice’s time to first use for one type of cancer is correlated with its time to first use for other cancer types.39 These models included a random effect for each practice and permit modeling of multivariate, dependent, “failure” times. We used Kendall’s Tau to estimate the correlation between bevacizumab use for different cancer types within the same practice. We also assessed the association of practice characteristics described above with time to bevacizumab adoption via hazard ratios.
Bevacizumab use and between-practice variation
We used hierarchical models with practice random effects to estimate the between-practice variation in the probability of a patient receiving bevacizumab in a given episode, net of sampling error. Models adjusted for all patient and practice characteristics described above. We characterized the variation by exponentiating differences in the random intercepts (log odds of bevacizumab use) for a practice one standard deviation above the mean (higher-use practice) versus a practice one standard deviation below the mean (lower-use practice).40 We also assessed whether the between-practice variation in bevacizumab use differed for patients receiving bevacizumab for higher-value versus lower-value indications, for FDA-approved versus non-FDA-approved indications, and for larger vs. smaller practices.
Because practice variation in use may differ once practices reach a “steady state” of use, we assessed if models examining bevacizumab use differed between the early study period (2005–2008) and late study period (2009–2012). Results were similar to our primary analyses and are not presented.
Sensitivity analyses
Bevacizumab was first approved in 2004, but there was no procedure code for bevacizumab until 2005; rather before 2005 practices used the nonspecific J9999 code (antineoplastic drugs not otherwise classified) for bevacizumab. To assess if diffusion curves would have been similar if we had reliable data on its use in 2004, we recalculated the diffusion curves beginning in 2004 using code J9999 among patients with colorectal cancer, who comprised the vast majority of patients for whom this non-specific code was used in 2004.
Two-sided P values <0.05 were considered statistically significant. Analyses were performed using SAS Statistical Software, Version 9.4 (Cary, N.C.), R version 3.3.2, and RStudio version 1.0.136.
Results
The study population included 236,304 patients who received infused chemotherapy during 2005–2012 and were treated in 2329 practices. These patients had 307,746 chemotherapy episodes. The mean patient age was 74.1 years (standard deviation=6.1), 53% were female, and 90% were white (Table 1). The practices included 156,839 physicians of whom 10,196 were oncologists. The median (interquartile [IQ] range) number of physicians in the practices was 6 (2–48), the median (IQ range) number of oncologists was 3 (1–5), the mean proportion of oncologists was 82% (range=7%–100%). Of the 2329 practices, 6% were academic, 14% were non-academic hospital-owned, and 80% were independent. Approximately three-quarters (78%) of patients were in non-academic, independent practices.
Overall, patients received bevacizumab in 8.5% of chemotherapy episodes. Use was greatest for episodes with cancer types with evidence for overall survival benefit (20.0% of episodes) and for cancer types that were FDA-approved without overall survival benefit (10.8% of episodes) or for cancer types that would be or previously were FDA-approved (6.3% of episodes) than for other cancer types (0.8% of episodes).
Diffusion of bevacizumab
Figure 1 displays time to first use of bevacizumab in oncology practices by cancer type beginning in January 2005 through 2012. Practices were fastest to use bevacizumab for patients with colorectal cancer (green triangles), the first approved indication; nearly half of practices used bevacizumab for at least one patient by January 2006. Use by practices for at least one patient with lung, breast, ovarian, kidney, and brain cancers all preceded FDA approvals by many months. More than 30% of practices had used bevacizumab for at least 10% of colorectal cancer patients by July 2005 (Figure 2). The proportion of practices using bevacizumab for 10% of patients was lower for other cancers, particularly breast, kidney, and ovarian cancers, all lower-value indications. An exception was glioblastoma of the brain (a cancer with few approved treatments), where more than 50% of practices had used bevacizumab for 10% of patients receiving chemotherapy within 30 months of FDA-approval.
Figure 1. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2005.
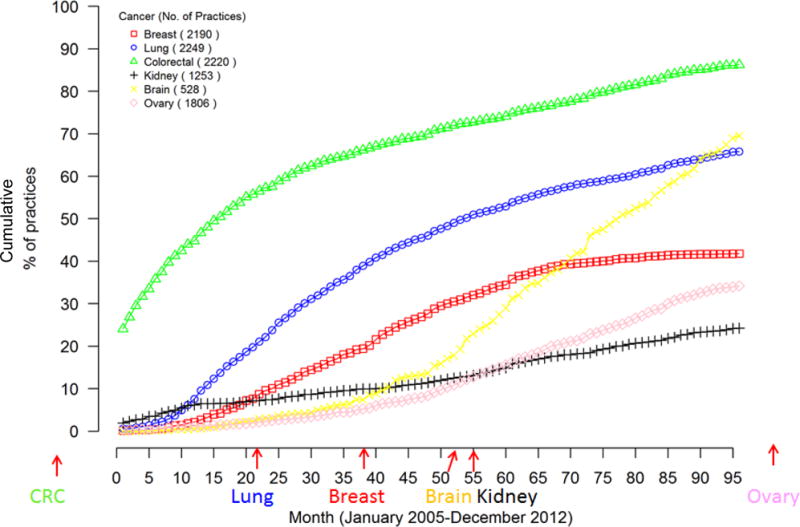
The lines reflect the cumulative proportion of practices that have used bevacizumab at least once for a patient with colorectal (green), lung (blue), breast (red), ovarian (pink), kidney (black) or glioblastoma/brain (yellow) cancers. The x axis reflects month of first use, starting in January 2005 (month 0) through December 2012 (month 95). The date of approval for each indication by the Food and Drug Administration is depicted with arrows along the x axis. The number in parentheses in the key reflects the number of practices treating at least one patient with this cancer type.
Figure 2. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2005.
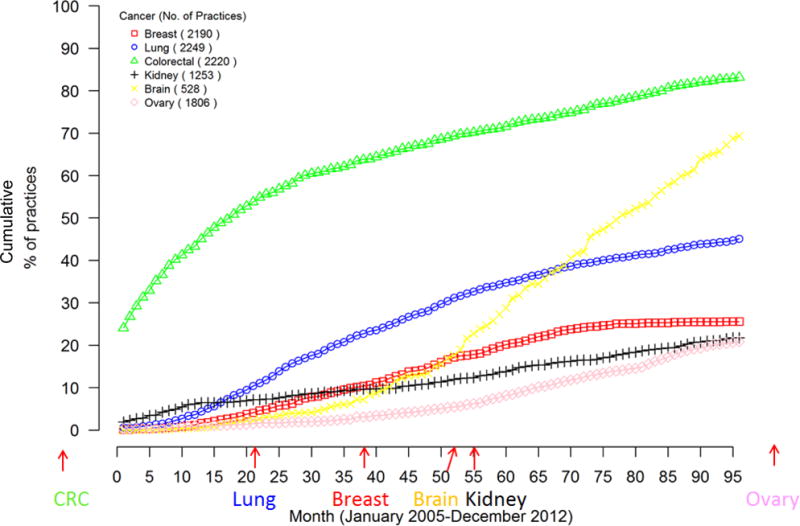
The lines reflect the cumulative proportion of practices that have used bevacizumab for at least 10% of patients for whom they have treated with chemotherapy for colorectal (green), lung (blue), breast (red), ovarian (pink), kidney (black) or glioblastoma/brain (yellow) cancers. The x axis reflects month of 10% use, starting in January 2005 (month 0) through December 2012 (month 95). The date of approval for each indication by the Food and Drug Administration is depicted with arrows along the x axis. The number in parentheses in the key reflects the number of practices treating at least one patient with this cancer type.
Time to first use of bevacizumab was more rapid among academic and independent than hospital-owned practices (Supplemental Digital Content Figure 1); time to 10% use was most rapid among independent practices (Supplemental Digital Content Figure 2). Time to first use and time to 10% use was faster in larger practices, which have more patients and thus more opportunities to use the drug (Supplemental Digital Content Figures 3 and 4).
Modeling speed of diffusion across practices
The frailty models confirmed that time to first bevacizumab use was faster for colorectal cancer than breast or lung cancer (Table 2). The Kendall’s tau was 0.29, suggesting moderate correlation between bevacizumab use for different cancer types within the same practice. Speed of diffusion was faster among practices with more oncologists, a higher ratio of oncologists to other physicians, and independent versus academic practices.
Table 2.
Factors associated with faster speed of adoption across practices
| Cancer type and practice characteristics | Hazard ratio (95% CI) | P value |
|---|---|---|
|
| ||
| Cancer type | ||
| Colorectal cancer | Reference | |
| Lung cancer | .076 (.065 to .088) | <.001 |
| Breast cancer | .032 (.026 to .038) | <.001 |
| Practice type, N (%) | ||
| Independent | Reference | |
| Hospital-owned (non-academic) | .89 (.67 to 1.17) | .39 |
| Academic | .48 (.33 to .68) | <.001 |
| Number of oncologists in practice (for each additional doctor above mean) | 1.05 (1.04 to 1.06) | <.001 |
| Ratio of oncologists to all physicians (for each 10 percentage point increase above mean) | 1.03 (1.01 to 1.05) | 0.004 |
| Proportion of individuals treated in practice living in high-poverty areas (for each 10 percentage point increase above the mean) | .98 (.93 to 1.03) | .48 |
Using Frailty models to estimate time to adoption in the 1128 practices with at least one patient with colorectal, lung, and breast cancer in 2005. Models adjusted for all practice characteristics in the table. Kendall’s Tau—which estimates the correlation between use of bevacizumab for different cancer types within the same practice—was .29, suggesting moderate correlation.
Use of bevacizumab and between-practice variation
Our hierarchical models identified substantial variation in the probability of bevacizumab use across practices. The odds ratio (95% confidence interval) of bevacizumab use was 2.90 (2.73–3.08) for a practice one standard deviation above the mean vs. a practice one standard deviation below the mean (after adjusting for patient and practice characteristics) (Table 3). Variation was less for higher-value uses (odds ratio=2.72 [2.56–2.89]) than lower-value uses (odds ratio=3.61 [3.21–4.06]). Similarly, variation was less for approved (odds ratio=2.68 [2.52–2.85]) versus non-approved (odds ratio=4.13 [3.60–4.74]) cancer types.
Table 3.
Variation in use of Bevacizumab across practices: odds of use for a patient seen in a moderately-high-use practice (+1 SD) versus a moderately-low-use practice (−1 SD)
| Cohort | Odds Ratio (95% CI) of Bevacizumab Use in Moderately-High-Use Practice vs. a Moderately-Low-Use Practice |
|---|---|
|
| |
| All cancers, all practices | 2.90 (2.73–3.08) |
| All cancers, higher-value use* | 2.72 (2.56–2.89) |
| All cancers, lower-value use* | 3.61 (3.21–4.06) |
| All cancers, approved cancer type† | 2.68 (2.52–2.85) |
| All cancers, non-approved cancer type† | 4.13 (3.60–4.74) |
| All cancers, large practices | 2.47 (2.35–2.61) |
| All cancers, small practices | 4.04 (3.47–4.70) |
| Colorectal cancers | 2.39 (2.23–2.55) |
| Breast cancers | 3.35 (2.83–3.97) |
| Lung cancers | 4.01 (3.54–4.54) |
| Brain cancers (glioblastoma) | 3.52 (1.45–8.59) |
| Kidney cancers | 3.33 (2.11–5.25) |
| Ovarian cancers | 3.74 (2.92–4.78) |
Higher-value use defined as use for cancers after evidence of an overall survival benefit has been documented. This was all times during the study period for colorectal cancer patients and after May 2005, when evidence of benefit was presented at the American Society of Clinical Oncology Annual Meeting. Lower-value use is all other use.
Approved cancer type reflects use during a time period when the drug was approved by the FDA for that cancer type. Non-approved use is all other cancer types.
Compared with independent practices, patients treated at academic and non-academic hospital-owned practices had lower odds of receiving bevacizumab (Table 1, right columns). Patients at practices with a higher ratio of oncologists to all physicians had higher odds of receiving bevacizumab. Older patients, patients with cancers other than colorectal and brain cancers, and those with greater comorbidity had lower odds of use. Bevacizumab use was more likely for non-Hispanic black vs. non-Hispanic white patients as well as patients living in areas with higher median incomes, patients in a second or later episode, patients with metastatic codes, patients living in the Pacific region, and patients treated more recently.
In sensitivity analyses using the nonspecific J9999 code to identify bevacizumab use for patients with colorectal cancer in 2004, diffusion curves were similar (Supplemental Digital Content Figures 5 and 6).
Discussion
We observed relatively rapid diffusion of bevacizumab across 2329 practices providing infused chemotherapy to Medicare beneficiaries with cancer, particularly for patients with colorectal cancer, the first cancer for which it was FDA-approved. Diffusion was also somewhat rapid for treatment of other cancer types, even before FDA approval for those cancers.
The relatively high off-label diffusion of bevacizumab is consistent with other evidence of frequent off-label drug use in oncology.41 Diffusion was more rapid for lung cancer (a higher-value use) than for breast or kidney cancers, consistent with the documented overall survival benefit for lung cancer. Other evidence of declining use of bevacizumab following the FDA’s withdrawal of its breast cancer indication42 also supports physicians’ responsiveness to emerging evidence. Nevertheless, bevacizumab continues to be included in breast cancer guidelines,43 and off-label use is typically covered by insurers, including Medicare. Policies requiring evidence development as a condition for insurance coverage of off-label chemotherapy use might inform our understanding of use and outcomes of these drugs in non-trial patient populations.
The substantial variation in bevacizumab use across oncology practices suggests that the likelihood of a patient receiving bevacizumab depends on the practice where they obtain care. This variation was particularly evident for lower-value versus higher-value indications and for non-approved versus approved cancer types. This substantial between-practice variation suggests that practices influence treatment decisions, practice patterns diffuse within practices, or oncologists sort to practices exhibiting similar practice patterns. Thus, interventions aimed at practices have potential for increasing high-value use and decreasing low-value use of high-cost therapies by prompting changes in practice management or focusing efforts to improve care delivery on inefficient practices. Initiatives such as Accountable Care Organizations and the CMS Oncology Care Model,17 which provides episode payments for chemotherapy combined with performance-based payments for meeting spending and quality targets, may be successful if they encourage practices to focus on appropriate use of high-cost therapies. Expanded use of clinical pathways that prioritize use of chemotherapy regimens that maximize clinical benefit and value is one promising strategy.44 Nevertheless, some45 have argued against the inclusion of drug costs in alternate payment models such as the Oncology Care Model due to concern about inadequate clinical data to assure patients with targetable mutations are treated appropriately with targeted therapies and because spending on chemotherapy explains substantially less of the variation in cancer care expenditures than acute hospital care and post-acute care.46
Finally, we documented differences in bevacizumab use by practice and patient characteristics. We observed greater use of bevacizumab in independent versus hospital-owned and academic practices. Hospital-based physicians are less likely than other physicians to report financial incentives for prescribing chemotherapy,47 likely because any profits from drug purchase and resale are realized by hospitals rather than physicians directly. Approximately one-third of oncology practice revenue reflects net drug revenue (total revenue from drugs less the cost of purchasing drugs)48 and evidence suggests that oncologists responded to the Medicare Modernization Act’s 2003 reduced payment rates for oncology drugs by decreasing use of less-profitable drugs and increasing use of more profitable (often higher-priced) drugs,49 although these differences varied across geographic regions.50 Another study of patients with colorectal cancer found increased adoption of newly approved agents (including bevacizumab) for individuals with metastatic colorectal cancer following the Medicare Modernization Act, which was accelerated in physician offices relative to hospital outpatient department settings.51 An alternate explanation is that independent practices may be able to respond more rapidly to evolving evidence.
We found greater use of bevacizumab among younger patients, those with less comorbidity, and those likely to have metastatic cancers, reflecting use among patients for whom the benefit to harm ratio is greatest. We observed greater use among black versus white patients, possibly reflecting more advanced stage of disease,52 for which we were unable to adjust because we lacked reliable information on cancer stage. The greater use among individuals in high-income areas suggests that non-clinical factors are also influencing use of new therapies.
Our study has several limitations. We lacked information about patients’ cancer stage, histology, and performance status, limiting our ability to precisely understand which patients received bevacizumab according to FDA indications, and we were unable to determine the extent to which diffusion was prompted by FDA approvals, release of promising clinical data, or physicians’ experimentation with off-label drugs in hopes of benefit. Nevertheless, the greater use for indications with stronger evidence of benefit suggests that physicians were responding to evidence. The lack of rich clinical data also limited our ability to adjust for differences in case mix across practices (although adjustment for patient-level covariates did not decrease the between-practice variation). We used diagnosis codes to infer metastatic stage, which may be inaccurate.53 We identified practices based on TINs, which may be imperfect markers of physicians who practice together, and we had limited information about practice characteristics. For example, we lacked information about whether any of the academic or hospital-owned practices were eligible for discounts under the 340B program or whether independent practices were affiliated with eligible hospitals, which could encourage their affiliated practices to refer patients to hospital-based infusion suites for chemotherapy.54 Finally, we studied only older patients enrolled in fee-for-service Medicare; patterns of care may differ for younger or privately-insured patients. However, because nearly all oncology practices accept Medicare and because the solid tumors for which bevacizumab is indicated are common in older individuals, our analysis represents a generalizable approach to characterizing bevacizumab diffusion.
In conclusion, we observed relatively rapid diffusion of bevacizumab and substantial variation of bevacizumab use across oncology practices, particularly for lower-value uses and non-approved indications of the drug. These findings suggest that interventions targeted at the practice level have potential for decreasing overuse of high-cost, and potentially lower-value, therapies.
Supplementary Material
Supplemental Digital Content Table 1. Food and Drug Administration Actions for Bevacizumab.
Supplemental Digital Content Table 2. Use of intravenous chemotherapy—agents included and not included.
Supplemental Digital Content Table 3. Number of practices and patients in those practices receiving chemotherapy, 2005–2012.
Supplemental Digital Content Figure 1. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2005, by academic vs. hospital-owned vs. independent practice.
Supplemental Digital Content Figure 2. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2005, by academic vs. hospital-owned vs. independent practice.
Supplemental Digital Content Figure 3. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2005, by large versus small practice.
Supplemental Digital Content Figure 4. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2005, by large versus small practice.
Supplemental Digital Content Figure 5. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2004.
Supplemental Digital Content Figure 6. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2004.
Acknowledgments
We are grateful to Katya Zelevinsky and Hocine Azeni for expert programming assistance and to Lauren Riedel and Ayan Elmi, all from the Department of Health Care Policy, Harvard Medical School, for administrative assistance. We are also grateful for W. Pete Welch, PhD, in the Office of the Assistant Secretary for Planning and Evaluation in the Department of Health and Human Services, for sharing information about academic practices.
This work was funded by U01MH103018 from the National Institute of Mental Health. Dr. Keating is also supported by K24CA181510 from the National Cancer Institute.
Footnotes
The authors have no potential conflicts of interest to report.
Supplemental Digital Content
File type: docx
File name: Supplemental Digital Content_Diffusion of Bevacizumab Across Oncology Practices_Medical Care
Contents:
References
- 1.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960–2000. N Eng J Med. 2006 Aug 31;355(9):920–927. doi: 10.1056/NEJMsa054744. [DOI] [PubMed] [Google Scholar]
- 2.Skinner J, Staiger D. Technology diffusion and productivity growth in health care. Rev Econ Stat. 2015;97(5):951–964. doi: 10.1162/REST_a_00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newhouse J. Medical care costs: how much welfare loss? J Econ Perspect. 1992;6(3):3–21. doi: 10.1257/jep.6.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs V. Economics, values, and healthcare reform. Am Econ Rev. 1996;86(1):1–24. [PubMed] [Google Scholar]
- 5.Ginsburg P, editor. Foundation RWJ. High and rising health care costs: demystifying U.S. health care spending. Princeton, NJ: 2008. (Research Synthesis Report no. 18). [PubMed] [Google Scholar]
- 6.Price & Value of Cancer Drug. 2016 https://www.mskcc.org/research-areas/programs-centers/health-policy-outcomes/cost-drugs. Accessed November 29, 2016.
- 7.Shih YC, Smieliauskas F, Geynisman DM, Kelly RJ, Smith TJ. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J Clin Oncol. 2015 Jul 1;33(19):2190–2196. doi: 10.1200/JCO.2014.58.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000–2014. JAMA Oncol. 2016 Jul 1;2(7):960–961. doi: 10.1001/jamaoncol.2016.0648. [DOI] [PubMed] [Google Scholar]
- 9.Polite BN, Ward JC, Cox JV, et al. Payment for oncolytics in the United States: a history of buy and bill and proposals for reform. J Oncol Pract. 2014 Nov;10(6):357–362. doi: 10.1200/JOP.2014.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clough JD, Patel K, Riley GF, Rajkumar R, Conway PH, Bach PB. Wide variation in payments for Medicare beneficiary oncology services suggests room for practice-level improvement. Health Aff (Millwood) 2015 Apr;34(4):601–608. doi: 10.1377/hlthaff.2014.0964. [DOI] [PubMed] [Google Scholar]
- 11.Porter ME. What is value in health care? N Engl J Med. 2010 Dec 23;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 12.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015 Aug 10;33(23):2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016 May 31;34(24):2925–2934. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 14.Memorial Sloan Kettering Cancer Center. Evidence driven drug pricing project. 2017 www.drugabacus.org. Accessed March 17, 2017.
- 15.Newcomber LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322–326. doi: 10.1200/JOP.2014.001488. [DOI] [PubMed] [Google Scholar]
- 16.Malin J, Nguyen A, Ban S, et al. Impact of enhanced reimbursement on provider participation a cancer care quality program and adherence to cancer treatment pathways in a commercial health plan. J Clin Oncol. 2015;33 (suppl; abstr 6571) [Google Scholar]
- 17.Centers for Medicare & Medicaid Services. Oncology Care Model. 2017 https://innovation.cms.gov/initiatives/oncology-care/. Accessed August 2, 2017.
- 18.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 03;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 21.Pivot X, Schneeweiss A, Verma S, et al. Efficacy and safety of bevacizumab in combination with docetaxel for the first-line treatment of elderly patients with locally recurrentl or metastatic breast cancer: results from AVADO. Eur J Cancer. 2011;47(16):2387–2395. doi: 10.1016/j.ejca.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011 Apr 1;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 23.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011 Nov 10;29(32):4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010 May 1;28(13):2144–2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 25.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009 Oct 1;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 26.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine inpatients with recurrent glioblastoma (BELOB trial): a randomized controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 27.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014 May 1;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 28.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015 Aug;16(8):928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandler AB, Gray R, Brahmer J, et al. Randomized phase II/III trial of paclitaxel (P) plus carboplatin (C) with or without bevacizumab (NSC #704865) in patients with advanced non-squamous non-small cell lung cancer (NSCLC): An Eastern Cooperative Oncology Group (ECOG) Trial –E4599. J Clin Oncol. 2005;23(16 Suppl) [Google Scholar]
- 30.Welch WP, Bindman AB. Town and gown differences among the 100 largest medical groups in the United States. Acad Med. 2016 Jul;91(7):1007–1014. doi: 10.1097/ACM.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 31.Neprash HT, Chernew ME, Hicks AL, Gibson T, McWilliams JM. Association of financial integration between physicians and hospitals with commercial health care prices. JAMA Intern Med. 2015 Dec;175(12):1932–1939. doi: 10.1001/jamainternmed.2015.4610. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 34.Rogers EM. Diffusion of Innovations. 5th. New York: Free Press; 2003. [Google Scholar]
- 35.Ryan B, Gross N. The diffusion of hybrid seed corn in two Iowa communities. Rural Sociology. 1943;8:15–24. [Google Scholar]
- 36.Griliches Z. Hybrid corn: An exploration in the economics of technological change. Econometrica. 1957;27:501–522. [Google Scholar]
- 37.Mansfield E. Technical change and the rate of imitation. Econometrica. 1961;29(4):741–766. [Google Scholar]
- 38.Bain A. The growth of demand for new commodities. J Royal Stat Soc, Series A. 1963;126(2):258–299. [Google Scholar]
- 39.Kelly PJ, Lim LL. Survival analysis for recurrent event data: an application to childhood infectious diseases. Stat Med. 2000 Jan 15;19(1):13–33. doi: 10.1002/(sici)1097-0258(20000115)19:1<13::aid-sim279>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. John Wiley & Sons, Ltd; 2004. pp. 168–169. [Google Scholar]
- 41.Conti RM, Bernstein AC, Villaflor VM, Schilsky RL, Rosenthal MB, Bach PB. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol. 2013 Mar 20;31(9):1134–1139. doi: 10.1200/JCO.2012.42.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conti RM, Dusetzina SB, Herbert AC, Berndt ER, Huskamp HA, Keating NL. The impact of emerging safety and effectiveness evidence on the use of physician-administered drugs: the case of bevacizumab for breast cancer. Med Care. 2013 Jul;51(7):622–627. doi: 10.1097/MLR.0b013e318290216f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NCCN. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 2.2016. 2016 https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 30, 2017.
- 44.Newcomer LN, Malin JL. Payer View of High-Quality Clinical Pathways for Cancer. J Oncol Pract. 2017 Mar;13(3):148–150. doi: 10.1200/JOP.2016.020503. [DOI] [PubMed] [Google Scholar]
- 45.Polite B, Ward JC, Cox JV, et al. A Pathway Through the Bundle Jungle. J Oncol Pract. 2016 Jun;12(6):504–509. doi: 10.1200/JOP.2015.008789. [DOI] [PubMed] [Google Scholar]
- 46.Brooks GA, Li L, Uno H, Hassett MJ, Landon BE, Schrag D. Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff (Millwood) 2014 Oct;33(10):1793–1800. doi: 10.1377/hlthaff.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malin JL, Weeks JC, Potosky AL, Hornbrook MC, Keating NL. Medical oncologists’ perceptions of financial incentives in cancer care. J Clin Oncol. 2013;31(5):530–535. doi: 10.1200/JCO.2012.43.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towle EL, Barr TR, Senese JL. The National Practice Benchmark for oncology, 2014 report on 2013 data. J Oncol Pract. 2014 Nov;10(6):385–406. doi: 10.1200/JOP.2014.001826. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson M, Earle CC, Price M, Newhouse JP. How Medicare’s payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010 Jul;29(7):1391–1399. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians’ responses to a reimbursement change. N Engl J Med. 2011 Dec 01;365(22):2049–2052. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsons HM, Schmidt S, Tenner LL, Davidoff AJ. Trends in antineoplastic receipt after Medicare payment reform: Implications for future oncology payment design. J Cancer Policy. 2017 doi: 10.1016/j.jcpo.2016.09.008. In press (epub ahead of print accessed at http://www.sciencedirect.com/science/article/pii/S221353831630025X on August 7, 2017) [DOI] [PMC free article] [PubMed]
- 52.Cancer facts and figures for African Americans, 2016–2018. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 53.Chawla N, Yabroff KR, Mariotto A, McNeel TS, Schrag D, Warren JL. Limited validity of diagnosis codes in Medicare claims for identifying cancer metastases and inferring stage. Ann Epidemiol. 2014 Sep;24(9):666–672. 672 e661–662. doi: 10.1016/j.annepidem.2014.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conti RM, Bach PB. Cost consequences of the 340B drug discount program. JAMA. 2013 May 15;309(19):1995–1996. doi: 10.1001/jama.2013.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content Table 1. Food and Drug Administration Actions for Bevacizumab.
Supplemental Digital Content Table 2. Use of intravenous chemotherapy—agents included and not included.
Supplemental Digital Content Table 3. Number of practices and patients in those practices receiving chemotherapy, 2005–2012.
Supplemental Digital Content Figure 1. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2005, by academic vs. hospital-owned vs. independent practice.
Supplemental Digital Content Figure 2. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2005, by academic vs. hospital-owned vs. independent practice.
Supplemental Digital Content Figure 3. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2005, by large versus small practice.
Supplemental Digital Content Figure 4. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2005, by large versus small practice.
Supplemental Digital Content Figure 5. Cumulative percentage of oncology practices using bevacizumab in at least one patient by month from January 2004.
Supplemental Digital Content Figure 6. Cumulative percentage of oncology practices using bevacizumab in at least 10% of patient receiving chemotherapy by month from January 2004.


