SYNOPSIS
The future of head and neck lymphatic malformation (HNLM) evaluation and treatment is changing due to two decades of clinical research and recent basic science investigation Basic science investigation using cellular biology and molecular genetics has revealed the genetic etiology of some HNLM, which has created the possibility of medical treatment specific to HNLM. This article summarizes clinical and basic science research that will likely influence the future of HNLM assessment and treatment.
The future of head and neck lymphatic malformation (HNLM) evaluation and treatment is changing due to two decades of clinical research and recent basic science investigation. HNLM are not a result of disrupted vasculogenesis, but arise from sporadic genetic abnormalities in specific cells.1,2 Clinical research into HNLM has focused on nomenclature, diagnosis, assessment of natural history, and evaluation of invasive treatment efficacy.3–8 Due to the rarity and clinical variability of HNLM, the evidence created by this research is low quality (levels 2–4), but the gap between experience based decision making and evidence based practice is closing.8 Basic science investigation using cellular biology and molecular genetics has revealed the genetic etiology of some HNLM, which has created the possibility of medical treatment specific to HNLM.1,9 This article summarizes clinical and basic science research that will likely influence the future of HNLM assessment and treatment.
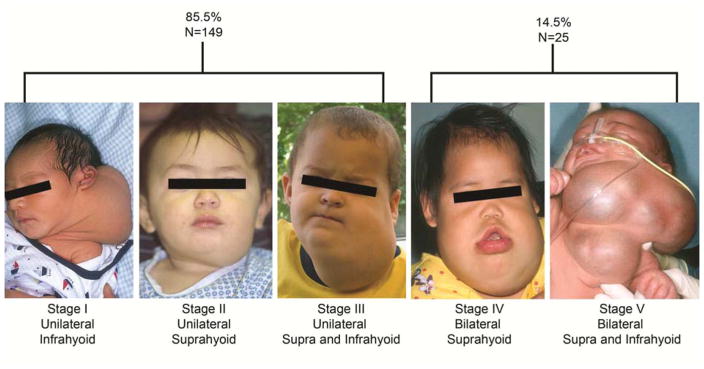
Nomenclature for all vascular anomalies (VA) has slowly evolved based on clinical phenotypic observation and availability of improved high resolution imaging. The descriptive terms of “cystic hygroma”, for large fluid filled neck masses, and “lymphangioma”, for infiltrative lymphatic channels seen in oral and oropharyngeal LM, have been changed to the more inclusive “common lymphatic malformations”, by the International Society for the Study of Vascular Anomalies (ISSVA) (Table 1).10,11 The ISSVA nomenclature for all VA enabled providers to better distinguish different congenital vascular lesions, particularly congenital and acquired lymphatic disease. Careful categorization of VA and lymphatic diseases allowed for improvements in clinical research and provided a framework to direct basic science investigation. Categorization of HNLM based on anatomical location and laterality led to a staging/grading system for intraoral and head and neck lesions (Figure 1).12,13 Treatment outcomes have been measured comparing differences between HNLM stages.5,7,14 Further refinement of our understanding of HNLM has been accomplished with radiographic imaging that categorizes these lesions as “macrocystic” and “microcystic” This information often directs the type of invasive treatment.15,16,17
Table 1.
ISSVA Vascular Anomalies classification scheme from 1996.
Data from: International Society for the Study of Vascular Anomalies
| Vascular Malformation | Vascular Tumor |
|---|---|
| Single Vessel Type | Hemangioma |
|
| |
| Capillary | Hemangioma of Infancy |
| Venous | |
| Lymphatic | Congenital Hemangioma |
| Common Malformation | Rapidly Involuting Congenital Hemangioma (RICH) |
| Generalized LA | |
| Gorham-Stout Disease | Non-Involuting Congenital Hemangioma (NICH) |
| Conducting Anomalies | |
| Lymphedema | Lobular Hemangioma (pyogenic granuloma) |
| Arteriovenous | |
|
| |
| Combined/Complex Malformations | Vascular Neoplasm |
|
| |
| Lymphaticovenous | Kaposiform Hemangioendothelioma |
| Capillary-venous | Angiosarcoma |
| Capillary-lymphaticovenous | Hemangiopericytoma |
| Capillary-arteriovenous | Tufted Angioma |
| Miscellaneous | |
Figure 1.
The deSerres head and neck lymphatic malformation staging system used to improve treatment outcome measurement and allow for quantitative data analysis. In a series of 174 head and neck lymphatic malformations 85.5% were stages 1–3, 14.5% were stage 4 or 5 and that in lower stage lesions surgery and sclerotherapy had the same efficacy.
From: Balakrishnan K, Menezes MD, Chen BS, Magit AE, Perkins JA, et al. Primary surgery vs primary sclerotherapy for head and neck lymphatic malformations. JAMA Otolaryngol Head Neck Surg. 2014;140(1):41–45; with permission, and de Serres LM, Sie KC, Richardson MA, et al. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck. 1995;121(5):577–582; with permission.
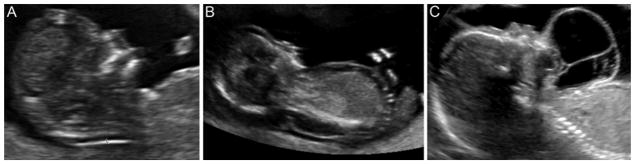
Prenatal diagnosis of HNLM is frequently made with in-utero ultrasound imaging. Lucency in the soft tissue of the posterior/dorsal neck with nuchal thickening is still called “cystic hygroma” in obstetrics literature. Radiolucent lesions in the nuchal region indicate increased risk for abnormal fetal karyotype, while radiolucent anterior or ventral neck lymphatic lesions do not confer this same risk(Figure 2).18 There are now highly sensitive and specific screening tests (non-invasive prenatal testing or NIPT) that allow direct sampling of fetal DNA from maternal blood, without the need for invasive amniocentesis or chorionic villus sampling.19 In-utero characterization of HNLM can be further functionally assessed with in-utero magnetic resonance (MRI) and three dimensional duplex imaging of the upper aerodigestive tract in mothers with polyhydramnios to guide high risk delivery planning and airway management.4
Figure 2.
In-utero ultrasound images demonstrating (A) nuchal thickening, (B) dorsal lymphatic malformation, and (C) ventral lymphatic malformation.
Postnatal HNLM diagnosis is either anticipated through prenatal diagnosis or clinical examination and radiographic characterization when presented after infancy. If the diagnosis is in question, histologic assessment of the lymphatic endothelium by identification of podoplanin (D2-40), can be used to clarify the diagnosis.20 Of note, the radiographic distinction of macrocystic and microcystic is not apparent histologically.21
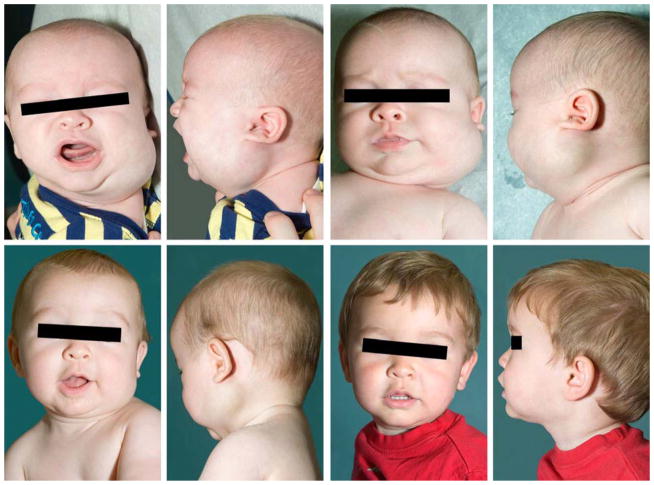
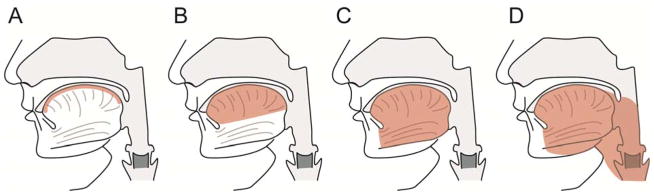
Evaluation of natural history and treatment efficacy in specific HNLM has been aided by staging or grading.12,17 Determining HNLM stage/grade has shown the normal distribution of these lesions. In a large two institution prospectively collected series where HNLM were treated primarily, lower stage 1–3 HNLM, lesions that are all unilateral, represented over eighty percent of all HNLM and had similar response to surgery and sclerotherapy (Figure 1).7 Interestingly stage 1 and 2 lesions, normally do not cause functional compromise (i.e. airway obstruction, dysphagia) have been reported to shrink without invasive therapy in up to 30% of cases (Figure 3).5 In contrast, higher stage 4 and 5 lesions, cause functional compromise, are bilateral and are usually predominately microcystic, possibly associated with lymphocytopenia and tertiary lymphoid organ formation, and are prone to persist and be recalcitrant to standard therapies.7,9,22 The same use of lesion staging has been applied to analysis of tongue LM natural history and treatment.13,23 Smaller, lower stage tongue lesions have a different history and treatment response compared to transmural malformations involving mucosa, muscle, and multiple anatomic spaces in and adjacent to the tongue (Figure 4).23 Assessment of differences in invasive HNLM therapy (i.e. surgery, sclerotherapy) efficacy is impossible based on extensive systematic review of existing medical literature.8 This is due to the lack of any comparative treatment trials, existing publications reporting treatment outcomes and/or safety from varied treatment philosophies, and lack of consistent reporting of pretreatment LM findings, and undefined treatment endpoints. In response to this systematic review, a multidisciplinary group representing differing treatment philosophies has published reporting guidelines for future reports of HNLM treatment, which will help create higher levels of evidence for treatment decisions.14
Figure 3.
Stage 1 HNLM demonstrating regression without therapy. From left to right top row, age 2.5 months and 3 months. From left to right bottom row, age 6 months and 17 months.
Figure 4.
Tongue lymphatic malformation staging used to describe treatment outcomes and strategies in malformations involving the tongue (shaded area is involved with lymphatic malformation). Malformations ranged from superficial to transmural. The more extensive the malformation the poorer the treatment outcome and malformation persistence.
From: Wiegand S, Eivazi B, Zimmermann AP, et al. Microcystic lymphatic malformations of the tongue: Diagnosis, classification, and treatment. Arch Otolaryngol Head Neck Surg. 2009;135(10):976–983; with permission.
Following completion of the Human Genome Project, the development of massively parallel DNA sequencing technology has enabled detailed exploration of molecular genetics causing rare conditions and contributing to neoplasms, including HNLM and other VA.24–26 This new molecular genetic knowledge has resulted in the ISSVA classification of VA to include known molecular genotypes (Table 2).27,28 In 2015, researchers discovered that the majority of “anterior or ventral” HNLM are caused by a gain-of-function postzygotic somatic gene mutation (phosphatyidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA)).1 Mutations in this gene have been detected in other types of tissue overgrowth.24–26,29 Somatic mutations differ from germline mutations in that the affected area is isolated to a specific cell type or anatomical region, which gives rise to cellular and phenotypic mosaicism (Figure 5). An important implication here is that the pathogenic mutations are not necessarily present in blood, the most frequently sampled tissue for genetic testing. In three different LM, one being HNLM, the PIK3CA somatic mutation was detected in the lymphatic endothelium.2 Evidence is emerging that cells containing somatic mutations influence phenotypic changes in adjacent cells with normal genomes, this results in the abnormal histologic appearance of malformation tissue (Figure 6). At this time it is unclear how genetic mosaicism in a single cell induces phenotypic mosaicism in complex tissues, or if it has not been discovered that multiple cell types are actually producing the histologic phenotype. PIK3CA gene mutations are associated with larger cell size, tissue overgrowth, and some malignant tumors.30,31 This explains the tissue overgrowth present in most HNLM (Figure 7). Exactly how these mutations induce tissue overgrowth and lymphatic malformation is unknown, but primary or adjunctive medical suppression of this gene and its pathway could be therapeutic for some recalcitrant HNLM. Rapamycin (Sirolimus), which suppresses the mTOR enzyme a component of the PIK3CA cellular signaling pathway, has been used with varied success for severe lymphatic conditions, including some HNLM.32,33 In presentations reporting the effect of Sirolimus on unselected HNLM, malformation induced mucosal and skin changes have improved, but reductions in HNLM size, is inconsistent.34 There has been no effect on tissue hypertrophy.
Table 2.
ISSVA Vascular Anomalies classification scheme from 2015.
Data from: International Society for the Study of Vascular Anomalies
| Capillary Malformations (CM) | |
|
| |
| Cutaneous and/or mucosal CM (port wine stain) | GNAQ |
| CM with bone and/or soft tissue hyperplasia | |
| CM with CNS and/or eye anomalies (Sturge-Weber) | GNAQ |
| CM of CM-AVM | RASA1 |
| Telangiectasia | |
| Hereditary hemorrhagic telangiectasia (HHT) | |
| HHT1 | ENG |
| HHT2 | ACVRL1 |
| HHT3 | |
| Others | |
| Cutis marmorata telangiectatica congentia (CMTC) | |
| Nevus simplex/Salmon patch | |
|
| |
| Lymphatic Malformations (LM) | |
|
| |
| Primary lymphedema | |
| Nonne-Milroy syndrome | FLT4/VEGRFR3 |
| Primary hereditary lymphedema | VEGFC |
| Primary hereditary lymphedema | GJC2/Connexin 47 |
| Lymphedema-distichiasis | FOXC2 |
| Hypotrichosis-lymphedema-telangiectasia | SOX18 |
| Primary lymphedema with mylodysplasia | GATA2 |
| Primary generalized lymphatic anomaly | CCBE1 |
| Microcephaly with/without chorioretinopathy | KIF11 |
| Lymphedema or mental retardation syndrome | |
| Lymphedema-choanal atresia | PTEN14 |
|
| |
| Venous Malformations (VM) | |
|
| |
| Common VM | TIE2 somatic |
| Familial VM cutaneo-mucosal (VMCM) | TIE2 |
| Blue rubber bleb nevus (Bean) syndrome VM | |
| Glomuvenous malformation (VM with glomus cells) | Glomulin |
| Cerebral cavernous malformation (CCM) | |
| CCM1 | KRIT1 |
| CCM2 | Malcavernin |
| CCM3 | PDCD10 |
|
| |
| Arteriovenous Malformations (AVM) | |
|
| |
| Sporadic in HHT | |
| HHT1 | ENG |
| HHT2 | ACVRL1 |
| JPHT (juvenile polyposis hem. telangiect.) | SMADA4 |
| CM-AVM | RASA1 |
|
| |
| Arteriovenous Fistulas (AVF) | |
|
| |
| Vascular Malformations Associated with other Anomalies | |
|
| |
| Klippel- Trenaunay syndrome | |
| Parkes Weber syndrome | RASA1 |
| Servelle-Martorell syndrome | |
| Sturge-Weber syndrome | GNAQ |
| Limb CM + congenital non-progressive limb overgrowth | |
| Maffucci syndrome | |
| Macrocephaly – CM (M-CM or MCAP) | PIK3CA |
| Microcephaly – CM (MICCAP) | STAMBP |
| CLOVES syndrome | PIK3CA |
| Proteus syndrome | AKT1 |
| Bannayan-Riley-Ruvalcaba syndrome | PTEN |
|
| |
| Provisionally Unclassified Vascular Anomalies | |
|
| |
| Verrucous hemangioma | |
| Multifocal lymphangioendotheliomatosis with thrombocytopenia/cutaneovisceral angiomatosis with thrombocytopenia (MLT/CAT) | |
| Kaposiform lymphangiomatosis (KLA) | |
| PTEN (type) hamartoma of soft tissue/“angiomatosis” of soft tissue | PTEN |
Figure 5.
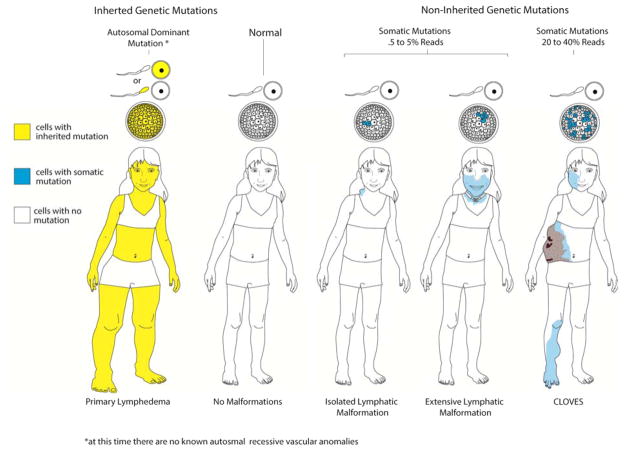
Schematic diagram of current theory of molecular genetics applied to germline and postzygotic somatic gene mutations creation of phenotype. On the left a person without malformations has a normal genome in all cells. In autosomal dominant germline inheritance all cells in the body have a mutation, shown as yellow. Somatic mutations occur after conception (i.e. zygote formation) and affect a variable number of cells in the blastomere, shown as blue. Cells with somatic mutations, by unknown mechanisms, affect one portion of the body as seen in blue. When 5–10% of cells, assuming “reads” are a surrogate measure for affected cells, are affected the involved area is small. The involved area becomes larger and more dysfunctional when more cells have that mutation.
Figure 6.
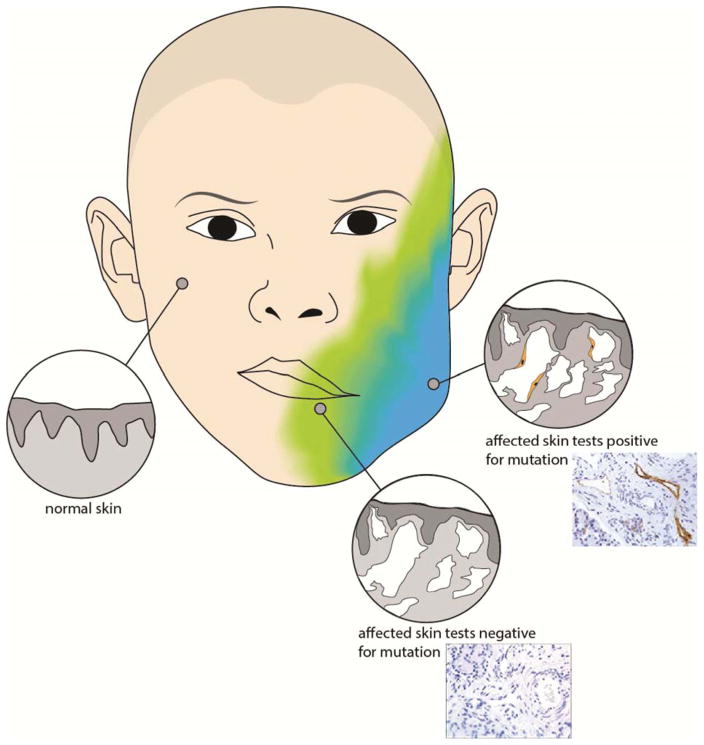
Adjacent cells interact, through mobile genomic sequences (i.e. transposable elements) and programmed cell death (i.e. apoptosis), creating an environment in which cells with somatic mutations cause neighboring genetically normal cells to exhibit mutant histologic phenotype. This may explain the occurrence and persistence of large areas of histologically abnormal lymphatic malformation tissue, schematically depicted and mirrored with HNLM tissue sections (top image with D2-40 immunostained lymphatic endothelium (brown)), while not all cells in the region have detectable mutations.
Figure 7.
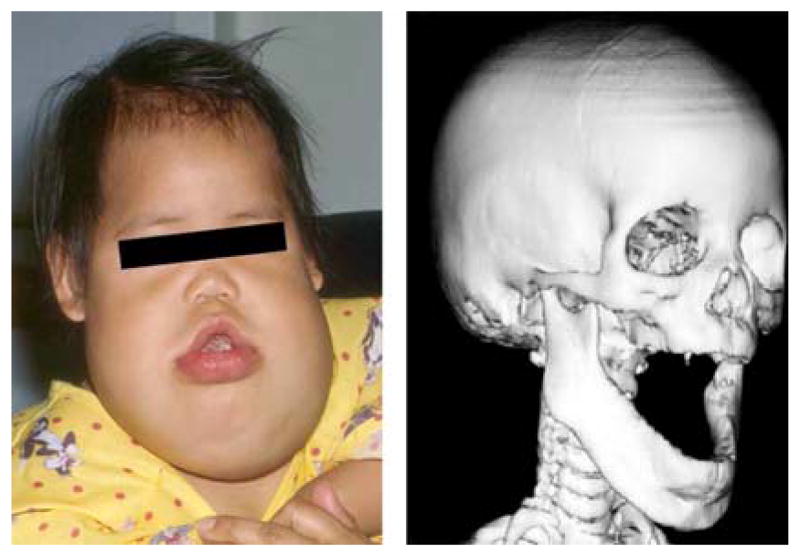
The gain-of-function post-zygotic somatic mutation in PIK3CA causes the persistent soft tissue and boney tissue overgrowth in this LM patient. Interestingly the one of the other known functions of the PIK3CA gene pathway is t-cell or lymphocyte differentiation by the mtor enzyme. This patient also has persistent lymphocytopenia which is probably related to disordered PIK3CA function.
Future innovations in HNLM assessment hinge on much of the work summarized in preceding paragraphs. As prenatal imaging capabilities improve to accurately assess the fetal airway and swallowing function, planning of HNLM patient delivery will be perfected to reduce any need for extensive delivery interventions (i.e. EXIT procedure). Additionally, prenatal molecular genetic diagnosis will refine the characterization and predictions regarding HNLM clinical presentation and behavior. Genetic characterization of HNLM is in its infancy, and as our understanding of and ability to detect somatic mutations improves, biologic reasons for varied HNLM clinical behavior (i.e. regression vs. persistence), distribution (i.e. stage or grade) and radiographic characteristics will be explained. Further study of the PIK3CA gene locus and other gene loci associated with lymphatic disease is warranted. Associating specific base pair rearrangements in this gene with HNLM clinical phenotype, natural history and treatment outcomes will probably change HNLM and other lymphatic disease nomenclature as it is doing in other conditions of tissue overgrowth. 25,29,35 The cost of sophisticated and sensitive means of automated genetic testing is decreasing and moving into the clinical arena. New information derived from widely available testing will enable development of treatment plans specific to an individual patient’s HNLM. Detection of malformation-causing somatic mutations in specific cells raises the possibility of targeting treatment to the destruction of these affected cells to predictably improve therapeutic outcomes. For example a localized HNLM may be best treated by complete removal followed by biologically driven therapy to eradicate remaining cells, whereas extensive infiltrative lesions may be best treated with medical therapy that suppresses PIK3CA activity. This will be a complete shift in treatment philosophy and strategy. It is anticipated that medications that completely suppress the whole PIK3CA pathway, rather than a downstream target (i.e. mTOR), will eliminate the possibility of treatment induced feedback mechanisms, so that medical therapy will be more consistently effective (Figure 8).24,26 The possibility of HNLM chronic medical therapy, either primary or adjunctive, opens the possibility of more comparative treatment trials, which in turn necessitates careful assessment of treatment efficacy and value. Parent and self report questionnaires will be essential in measuring treatment outcomes.36 Additionally, consideration of HNLM natural history based on differing lesion stages/grades needs to be included in treatment trials to reduce treatment of non-function threatening HNLM, invasive therapy and treatment costs. These trials would also provide a basis for evidence driven treatment approaches. Investigation into other methods of cell specific gene regulation (i.e. epigenetics) has been reported in other VA.37 These biologic mechanisms may reveal new relevant biomarkers for HNLM and reveal cell-specific targets for biologic therapy.26 In the next several decades, with further collaborative work, the treatment of HNLM will change significantly through application of new clinical investigation and cellular biologic discovery translated to the clinic.
Figure 8.
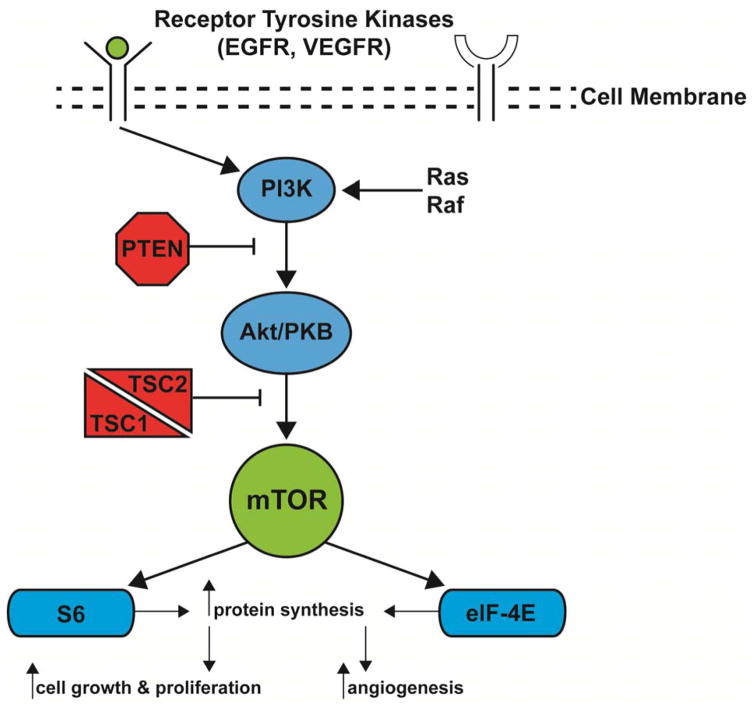
Schematic representation of PIK3CA cellular signaling pathway. Note, one of the principle functions of the mTOR enzyme is T-cell differentiation or programming.
KEY POINTS.
Head and neck lymphatic malformation (HNLM) are not a result of disrupted vasculogenesis, but arise from sporadic genetic abnormalities in specific cells.
Clinical research into HNLM has focused on nomenclature, diagnosis, assessment of natural history, and evaluation of invasive treatment efficacy.
Due to the rarity and clinical variability of HNLM, the evidence created by this research is low quality (levels 2–4), but the gap between experience based decision making and evidence based practice is closing.
Acknowledgments
We thank Carrie Capri for manuscript preparation and Eden Palmer for figure creation and assistance with figure preparation for publication.
Footnotes
DISCLOSURE STATEMENT
Johnathan A. Perkins has nothing he wishes to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. [Accessed 20150330];J Pediatr. 2015 166(4):1048–54. e1–5. doi: 10.1016/j.jpeds.2014.12.069. http://dx.doi.org/10.1016/j.jpeds.2014.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborn AJ, Dickie P, Neilson DE, et al. Activating PIK3CA alleles and lymphangiogenic phenotype of lymphatic endothelial cells isolated from lymphatic malformations. Hum Mol Genet. 2015;24(4):926–938. doi: 10.1093/hmg/ddu505. [DOI] [PubMed] [Google Scholar]
- 3.Longstreet B, Bhama PK, Inglis AF, Jr, Saltzman B, Perkins JA. Improved airway visualization during direct laryngoscopy using self-retaining laryngeal retractors: A quantitative study. Otolaryngol Head Neck Surg. 2011;145(2):270–275. doi: 10.1177/0194599811405429. [DOI] [PubMed] [Google Scholar]
- 4.Dighe MK, Peterson SE, Dubinsky TJ, Perkins J, Cheng E. EXIT procedure: Technique and indications with prenatal imaging parameters for assessment of airway patency. Radiographics. 2011;31(2):511–526. doi: 10.1148/rg.312105108. [DOI] [PubMed] [Google Scholar]
- 5.Perkins JA, Maniglia C, Magit A, Sidhu M, Manning SC, Chen EY. Clinical and radiographic findings in children with spontaneous lymphatic malformation regression. Otolaryngol Head Neck Surg. 2008;138(6):772–777. doi: 10.1016/j.otohns.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham stout syndrome (disappearing bone disease): Two additional case reports and a review of the literature. Arch Otolaryngol Head Neck. 2003;129(12):1340–1343. doi: 10.1001/archotol.129.12.1340. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan K, Menezes MD, Chen BS, Magit AE, Perkins JA. Primary surgery vs primary sclerotherapy for head and neck lymphatic malformations. JAMA Otolaryngol Head Neck Surg. 2014;140(1):41–45. doi: 10.1001/jamaoto.2013.5849. [DOI] [PubMed] [Google Scholar]
- 8.Adams MT, Saltzman B, Perkins JA. Head and neck lymphatic malformation treatment: A systematic review. [Accessed 20120927];Otolaryngol Head Neck Surg. 2012 147(4):627–639. doi: 10.1177/0194599812453552. http://KT8EW8CQ5H.search.serialssolutions.com/?sid=OVID:Ovid+MEDLINE%28R%29+%3C2011+to+June+Week+1+2015%3E&genre=article&id=pmid:22785242&id=doi:&issn=0194-5998&volume=147amp;issue=4amp;spage=627amp;pages=627-39amp;date=2012amp;title=Otolaryngology+-+Head+%26+Neck+Surgeryamp;atitle=Head+and+neck+lymphatic+malformation+treatment%3A+a+systematic+review.amp;aulast=Adamsamp;pid=%3Cauthor%3EAdams+MT%3C%2Fauthor%3E&%3CAN%3E22785242%3C%2FAN%3E. [DOI] [PubMed] [Google Scholar]
- 9.Kirsh AL, Cushing SL, Chen EY, Schwartz SM, Perkins JA. Tertiary lymphoid organs in lymphatic malformations. Lymphat Res Biol. 2011;9(2):85–92. doi: 10.1089/lrb.2010.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams D, Mulliken J, Azizkhan R, et al. Panel discussion on a consensus for lymphatic anomalies. International society for the study of vascular anomalies; Poster presentation at International Society for Study of Vascular Anomalies; 2012. (Poster abstracts) [Google Scholar]
- 11.Garzon MC, Enjolras O, Frieden IJ. Vascular tumors and vascular malformations: Evidence for an association. J Am Acad Dermatol. 2000;42(2 Pt 1):275–279. doi: 10.1016/S0190-9622(00)90138-5. [DOI] [PubMed] [Google Scholar]
- 12.de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck. 1995;121(5):577–582. doi: 10.1001/archotol.1995.01890050065012. [DOI] [PubMed] [Google Scholar]
- 13.Wiegand S, Eivazi B, Zimmermann AP, et al. Microcystic lymphatic malformations of the tongue: Diagnosis, classification, and treatment. Arch Otolaryngol Head Neck Surg. 2009;135(10):976–983. doi: 10.1001/archoto.2009.131. [DOI] [PubMed] [Google Scholar]
- 14.Balakrishnan K, Bauman N, Chun RH, et al. Standardized outcome and reporting measures in pediatric head and neck lymphatic malformations. Otolaryngol Head Neck Surg. 2015;152(5):948–953. doi: 10.1177/0194599815577602. [DOI] [PubMed] [Google Scholar]
- 15.Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004;15(5):431–445. doi: 10.1097/01.rvi.0000124949.24134.cf. [DOI] [PubMed] [Google Scholar]
- 16.Alomari AI, Karian VE, Lord DJ, Padua HM, Burrows PE. Percutaneous sclerotherapy for lymphatic malformations: A retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol. 2006;17(10):1639–1648. doi: 10.1097/01.RVI.0000239104.78390.E5. [DOI] [PubMed] [Google Scholar]
- 17.Smith MC, Zimmerman MB, Burke DK, Bauman NM, Sato Y, Smith RJ. Efficacy and safety of OK-432 immunotherapy of lymphatic malformations. Laryngoscope. 2009;119(1):107–115. doi: 10.1002/lary.20041. [DOI] [PubMed] [Google Scholar]
- 18.Bingham MM, Saltzman B, Vo NJ, Perkins JA. Propranolol reduces infantile hemangioma volume and vessel density. Otolaryngol Head Neck Surg. 2012;147(2):338–344. doi: 10.1177/0194599812451570. [DOI] [PubMed] [Google Scholar]
- 19.Norton ME, Rink BD. Changing indications for invasive testing in an era of improved screening. Semin Perinatol. 2016;40(1):56–66. doi: 10.1053/j.semperi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33(7):492–497. doi: 10.1111/j.1600-0560.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen EY, Hostikka SL, Oliaei S, Duke W, Schwartz SM, Perkins JA. Similar histologic features and immunohistochemical staining in microcystic and macrocystic lymphatic malformations. Lymphat Res Biol. 2009;7(2):75–80. doi: 10.1089/lrb.2009.0003. [DOI] [PubMed] [Google Scholar]
- 22.Tempero RM, Hannibal M, Finn LS, Manning SC, Cunningham ML, Perkins JA. Lymphocytopenia in children with lymphatic malformation. Arch Otolaryngol Head Neck. 2006;132(1):93–97. doi: 10.1001/archotol.132.1.93. [DOI] [PubMed] [Google Scholar]
- 23.Kim SW, Kavanagh K, Orbach DB, Alomari AI, Mulliken JB, Rahbar R. Long-term outcome of radiofrequency ablation for intraoral microcystic lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2011;137(12):1247–1250. doi: 10.1001/archoto.2011.199. [DOI] [PubMed] [Google Scholar]
- 24.Mei ZB, Duan CY, Li CB, Cui L, Ogino S. Prognostic role of tumor PIK3CA mutation in colorectal cancer: A systematic review and meta-analysis. Ann Oncol. 2016;27(10):1836–1848. doi: 10.1093/annonc/mdw264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469(2):125–134. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai Y, Dodhia S, Su GH. Dysregulations in the PI3K pathway and targeted therapies for head and neck squamous cell carcinoma. Oncotarget. 2017 doi: 10.18632/oncotarget.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkorian AY, Grossberg AL, Puttgen KB. Genetic basis for vascular anomalies. Semin Cutan Med Surg. 2016;35(3):128–136. doi: 10.12788/j.sder.2016.051. [DOI] [PubMed] [Google Scholar]
- 28.ISSVA classification for vascular anomalies-2014.
- 29.Keppler-Noreuil KM, Rios JJ, Parker VE, et al. PIK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A. 2015;167A(2):287–295. doi: 10.1002/ajmg.a.36836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchins AP, Pei D. Transposable elements at the center of the crossroads between embryogenesis, embryonic stem cells, reprogramming, and long non-coding RNAs. Sci Bull (Beijing) 2015;60(20):1722–1733. doi: 10.1007/s11434-015-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Garijo A, Steller H. Spreading the word: Non-autonomous effects of apoptosis during development, regeneration and disease. Development. 2015;142(19):3253–3262. doi: 10.1242/dev.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams DM, Trenor CC, 3rd, Hammill AM, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2):e20153257–3257. doi: 10.1542/peds.2015-3257. Epub 2016 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 34.Strychowsky J, Rahbar R, Padua H, Trenor C., III Sirolimus for the treatment of cervicofacial lymphatic malformations. Poster at Annual Meeting of the American Society of Pediatric Otolaryngology; 2015. [Google Scholar]
- 35.Loconte DC, Grossi V, Bozzao C, et al. Molecular and functional characterization of three different postzygotic mutations in PIK3CA-related overgrowth spectrum (PROS) patients: Effects on PI3K/AKT/mTOR signaling and sensitivity to PIK3 inhibitors. PLoS One. 2015;10(4):e0123092. doi: 10.1371/journal.pone.0123092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkham EM, Edwards TC, Weaver EM, Balakrishnan K, Perkins JA. The lymphatic malformation function (LMF) instrument. Otolaryngol Head Neck Surg. 2015;153(4):656–662. doi: 10.1177/0194599815594776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strub GM, Kirsh AL, Whipple ME, et al. Endothelial and circulating C19MC microRNAs are biomarkers of infantile hemangioma. JCI Insight. 2016;1(14):e88856. doi: 10.1172/jci.insight.88856. [DOI] [PMC free article] [PubMed] [Google Scholar]