Abstract
Atrial fibrillation (AF) is a prominent risk factor for stroke and a leading cause of death and disability throughout Latin America. Contemporary evidence-based guidelines for the management of AF and stroke incorporate the use of practical and relatively simple scoring methods to estimate both stroke and bleeding risk, in order to assist in matching patients with appropriate interventions. This review examines consistencies and differences among guidelines for reducing stroke risk in patients with AF, assessing the role of user-friendly scoring methods to determine appropriate patients for anticoagulation and other treatment options. Current options include warfarin and direct oral anticoagulants such as dabigatran, rivaroxaban, apixaban, and edoxaban. These agents have been found to be superior or noninferior to standard vitamin K antagonist anticoagulation in large randomized trials. Potential benefits of these agents mainly include lower ischemic stroke rates, reduced intracranial bleeding, no need for regular monitoring, and fewer drug–drug and drug–food interactions. Expert opinions regarding clinical situations for which data are presently lacking, such as emergency bleeding and stroke in anticoagulated patients, are also provided. Enhanced attention and adherence to evidence-based guidelines are essential components for a strategy to reduce stroke morbidity and mortality across Latin America.
Keywords: evidence-based guidelines, Latin America, nonvalvular atrial fibrillation
Introduction
Stroke is a leading cause of mortality and disability in Latin America, although information regarding its epidemiology, subtypes, and risk factors in the region is limited.1–4 Economic status, health resources, and habits differ between and within countries in Latin America, so direct comparisons may be misleading. Stroke prevalence per 1000 people, based on door-to-door surveys, ranges from 1.7 among rural Bolivians to 7.7 among a predominantly urban Mexican population.5 In a series of older patients (aged ≥60 or ≥65 years), crude prevalence of stroke ranged from 18.2 per 1000 in Mexico to 46.7 per 1000 in Colombia,5 and in an Argentine survey, point prevalence of stroke was 8.7 cases per 1000 inhabitants (4.7 per 1000 age-adjusted to the worldwide population).6 Incidence rates of stroke reported in Latin American studies (all adjusted for Segi’s world population) have included 76.5 annual first-ever strokes per 100 000 in a 2013 to 2015 Argentine study,7 94 per 100 000 among a predominantly Hispano–Mestizo population in Chile,8 105 per 100 000 in Joinville, Brazil,9 and a hospitalization rate of 110 per 100 000 for first-ever stroke in Mexico.10 These incidence rates are in the low-to-average range of rates seen globally,11 while notably lower rates have been seen in registry data from locations such as Dijon, France (57.9 per 100 000)12 and Kurashiki, Japan (60.7 per 100 000).13
Positive trends have been observed in some areas of Latin America; the death rate associated with cerebrovascular disease in Brazil has decreased in recent decades, although stroke is still a leading cause of death.9,14,15 A clear association between stroke death and socioeconomic status has been shown, with mortality rates almost 3 times higher in the lowest versus highest human development index stratum.16
Atrial fibrillation (AF) is a significant risk factor for stroke, increasing the risk approximately 5-fold; however, as AF is often asymptomatic, this figure may be considerably underestimated.17 Data from national health-care systems for 7 Latin American countries showed a range of prevalence from 1.44% to 1.95% for AF in the general over-40 population, with nonvalvular AF (NVAF) accounting for over 85% of cases.18 Prevalence increases with age, ranging from 2.22% to 2.34% in people aged 60 to 69 years to 8.17% to 8.48% in those aged ≥80 years.18 In Brazil alone, an estimated 1.5 million people have AF, with associated elevated risks of stroke and heart failure and increased total mortality.19 In a series of patients with stroke in Brazil, the frequency of AF ranged from 9.5% to 17.5%.1,20,21
Strokes associated with AF are generally more severe and have worse outcomes than other strokes.22 The Mexican PREMIER registry reported a 30-day poststroke mortality of 22.0% in patients with AF versus 13.7% in those without; severe disability followed stroke in 69% of patients with AF versus 52% without.23–25 In a series of patients with stroke admitted to tertiary care in São Paulo, the rate of functional independence at discharge was 60.8% in patients with and 81% in patients without AF (P < .01).21
The risk of stroke in patients with AF increases with age and other risk factors, including hypertension, diabetes, heart failure, and previous stroke. It can be estimated using the scores from congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, and prior stroke or transient ischemic attack (CHADS2) or Congestive Heart failure, hypertension, Age ≥75, Diabetes, Stroke, Vascular disease, Age 65–74, and Sex-female (CHA2DS2-VASc) (Figure 1).26–28 The CHA2DS2-VASc scheme allows a more comprehensive stroke risk assessment and a greater ability to identify patients at very low risk who may not require anticoagulation.
Figure 1.
Stroke risk in patients with NVAF by 2 common scoring methods. A, Stroke risk by CHADS2 score in patients with NVAF. Based on data from Gage et al.26 B, Stroke risk by CHA2DS2-VASc score in patients with NVAF. Based on data from Lip et al.28 NVAF indicates nonvalvular atrial fibrillation; TIA, transient ischemic attack. aPrior myocardial infarction, peripheral artery disease, or aortic plaque.
Anticoagulation reduces stroke risk in patients with AF. Vitamin K antagonists (VKAs) reduce the risk of stroke by approximately 66% and the risk of death by approximately 28% versus no therapy; they are widely prescribed in Latin America, although perhaps still underused.29,30 Studies have consistently concluded that the benefit from anticoagulation significantly exceeds the risks for almost all patients with AF with a CHADS2 or CHA2DS2-VASc score ≥2.31,32
Despite the evidence showing its efficacy, anticoagulation is widely underused. One Mexican study reported that only 35.9% of patients with a history of AF and recurrent transient ischemic attack (TIA)/ischemic stroke and 24% of patients with a history of AF and first-ever TIA/ischemic stroke were receiving oral anticoagulation with a VKA; of these, only 13.1% and 4.0%, respectively, were maintained within an optimal therapeutic range (international normalized ratio [INR]: 2.0-3.0).24,25 In 1 Brazilian study, only 46.5% of eligible patients with AF were receiving warfarin, with just 15.6% maintained within the optimal INR range.33 In a survey of 7 countries (Argentina, Brazil, Chile, Colombia, Mexico, Peru, and Venezuela), more than half of patients with AF were receiving medical treatment but a significant proportion of patients were not receiving appropriate anticoagulation despite high stroke risk. Moreover, proportions of patients with AF receiving treatment within the national health-care system decreased with increasing age across all countries.18 Cost and lack of health infrastructure are major barriers to care.34 Additionally, even appropriate treatment has limits; an Argentine study found that only 35% of patients with AF who sustained ischemic strokes had received appropriate levels of anticoagulation (other stroke etiologies could partially explain this failure).35
Search Strategy and Selection Criteria
The PubMed database was searched for practice guidelines concerning stroke prevention in AF published within the last 5 years. In Latin America, physicians often follow European and/or US guidelines, as well as local guidelines, if available. The authors selected results based on applicability to Latin America and the practicing neurologist. Supporting evidence was retrieved based on reference lists for each guideline. Additional searches were performed to obtain Latin American epidemiologic and health-care quality data, as well as clinical trial data concerning therapies of ongoing research interest that were published after the most recent guideline updates. As not all local societies’ publications are indexed on PubMed, Google was used to identify additional Latin American guidelines.
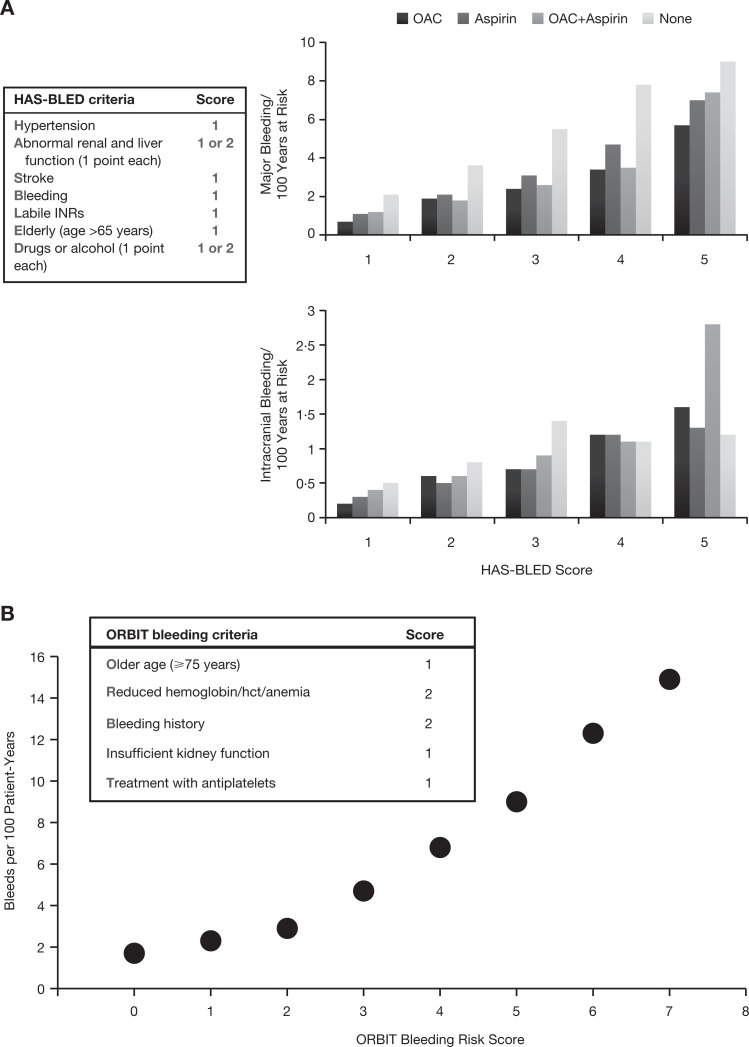
A key development that has been reflected in guidelines over the past 5 years is that additional agents—“novel” direct oral anticoagulants (DOACs)—have become available. Previously, oral anticoagulation options were limited to VKAs, which require frequent monitoring of anticoagulant effect, dose adjustments, and close attention to diet.36,37 Access barriers to monitoring, including distance and cost, may help explain why physicians hesitate to prescribe warfarin for patients with limited resources.36,37 Aspirin is a widely available alternative but has consistently and substantially been found less effective in reducing thromboembolic risk than warfarin in patients with AF with a CHADS2 score ≥1.38–40 Vitamin K antagonists are associated with an increased risk of major bleeding including intracranial hemorrhage (ICH); indeed, physician concerns about major bleeding represent a key barrier to optimal anticoagulation use in AF. Therefore, assessment of bleeding risk should be part of patient evaluation before starting anticoagulation. Available scores to assess bleeding risk include the hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly (HAS-BLED) score (Figure 2A).41 The (older age [75+ years], reduced haemoglobin/haematocrit/history of anaemia, bleeding history, insufficient kidney function, and treatment with antiplatelet (ORBIT) bleeding score (older age [75+ years], reduced haemoglobin/haematocrit/history of anaemia, bleeding history, insufficient kidney function, and treatment with antiplatelet) is a new, user-friendly score that may be more widely applicable than existing schemes (Figure 2B).42
Figure 2.
Bleeding risk in patients with NVAF estimated by 2 scoring methods. A, Bleeding risk according to HAS-BLED score. Based on data from Friberg et al.41 B, Bleeding risk according to ORBIT score. Based on data from O’Brien et al.42 Hct indicates hematocrit; INR, international normalized ratio; NVAF, nonvalvular atrial fibrillation; OAC, oral anticoagulant.
The DOACs dabigatran, rivaroxaban, apixaban, and edoxaban (approved in the United States, Japan, and Europe) have predictable pharmacokinetic and pharmacodynamic profiles, have fewer drug–drug interactions than warfarin, and do not require regular monitoring. (However, it bears mentioning that the DOACs are not without potential risk of interactions, including P-glycoprotein inducers or inhibitors with dabigatran, P-glycoprotein inducers with edoxaban, or dual P-glycoprotein and strong CYP3A4 inducers or inhibitors with either rivaroxaban or apixaban.)43–45 The DOAC therapy has been compared with VKA treatment for reducing the risk of stroke in patients with NVAF in 4 phase III trials45–50 (and compared with aspirin in 1 phase III trial51); results are summarized in Table 1. All trials included patients from Latin America as well as other regions; results from Latin American subgroups of Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Comparedwith Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF), Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF–TIMI 48), and Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) are shown in Table 2. Meta-analysis determined that DOACs reduced stroke or systemic embolic events versus warfarin (relative risk [RR] = 0.81; P < .0001) while also reducing ICH (RR = 0.48; P < .0001).52 Although not every guideline includes each one of the DOACs due to the time of update and the status of evidence for each DOAC at the time, the agents are included in recommendations from the European Society of Cardiology (ESC), the ESC branch European Heart Rhythm Association (EHRA), the American Heart Association/American Stroke Association (AHA/ASA), the AHA/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS), the American Academy of Neurology (AAN), the Brazilian Society of Cardiology (BSC), the Mexican Social Security Institute (MSSI), and the Argentine Society of Cardiology (ASC).43,44,53–59
Table 1.
Results of Trials of DOACs for Stroke Prevention in NVAF.a
| RE-LY Dabigatran 110 mg BID46,49,50 | RE-LY Dabigatran 150 mg BID46,49,50 | ROCKET AF Rivaroxaban 20 mg QD48 | ENGAGE AF–TIMI 48 Edoxaban 30 mg QD45 | ENGAGE AF–TIMI 48 Edoxaban 60 mg QD45 | ARISTOTLE Apixaban 5 mg BID47 | AVERROES Apixaban 5 mg BID51 | |
|---|---|---|---|---|---|---|---|
| Comparator | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Aspirin 80-324 mg |
| Total N | 18 113 | 14 264 | 21 105 | 18 201 | 5599 | ||
| Latin American patients (n) | 1134 (South America; ITT, both efficacy and safety) | 1878 (ITT); 1877 (SOT) | 2661 (ITT); 2651 (SOT) | 3468 (ITT); 3460 (SOT) | 1185 (ITT, efficacy and safety) | ||
| Efficacy | |||||||
| Stroke or systemic embolism (noninferiority) | 1.54 vs 1.71, RRR = 10%, P < .001 | 1.11 vs 1.71, RRR = 35%, P < .001 | PP: 1.7 vs 2.2, RRR = 21%, P < .001 | mITT: 1.61 vs 1.50, RRI = 7%, P = .005 | mITT:1.18 vs 1.50, RRR= 21%, P < .001 | 1.27 vs 1.60, RRR = 21%, P < .001 | |
| Stroke or systemic embolism (superiority) | 1.54 vs 1.72, RRR = 11%, P = .27 | 1.12 vs 1.72, RRR = 35%, P < .001 | 2.1 vs 2.4, RRR = 12%, P = .12, OT: 1.7 vs 2.2, RRR = 21%, P = .02 | 2.04 vs 1.80, RRI = 13%, P = .10 | 1.57 vs 1.80, RRR = 13%, P = .08 | 1.27 vs 1.60, RRR = 21%, P = .01 | 1.6 vs 3.7, RRR = 55%, P < .001 |
| Ischemic stroke | Ischemic or nonspecified 1.34 vs 1.22, RRI = 10%, P = .42 | Ischemic or nonspecified 0.93 vs 1.22, RRR = 24%, P = .03 | SOT: 1.34 vs 1.42, RRR = 6%, P = .581 | 1.77 vs 1.25, RRI = 41%, P < .001 | 1.25 vs 1.25, RRR = 0%, P = 0.97 | Ischemic or nonspecified, 0.97 vs 1.05, RRR = 8%, P = .42 | 1.1 vs 3.0, RRR = 63%, P < .001 |
| Hemorrhagic stroke | 0.12 vs 0.38, RRR = 69%, P < .001 | 0.10 vs 0.38, RRR = 74%, P < .001 | SOT: 0.26 vs 0.44, RRR: 41%, P =.024 | 0.16 vs 0.47, RRR = 67%, P < .001 | 0.26 vs 0.47, RRR = 46%, P < .001 | 0.24 vs 0.47, RRR = 49%, P < .001 | 0.2 vs 0.3, RRR = 33%, P = .45 |
| All-cause mortality | 3.75 vs 4.13, RRR = 9%, P = .13 | 3.64 vs 4.13, RRR = 12%, P = .051 | SOT: 1.87 vs 2.21, RRR = 15%, P = .073 | 3.80 vs 4.35, RRR = 13%, P =.006 | 3.99 vs 4.35, RRR = 8%, P = .08 | 3.52 vs 3.94, RRR = 11%, P = .047 | 3.5 vs 4.4, RRR = 21%, P = .07 |
| Safety | |||||||
| Major bleeding | 2.92 vs 3.61, RRR = 20%, P = .003 | 3.40 vs 3.61, RRR = 6%, P = .41 | SOT: 3.6 vs 3.4, RRI = 4%, P = .58 | SOT: 1.61 vs 3.43, RRR = 53%, P < .001 | SOT: 2.75 vs 3.43, RRR = 20%, P <.001 | SOT: 2.13 vs 3.09, RRR = 31%, P < .001 | 1.4 vs 1.2, RRI = 13%, P = .57, SOT: 1.4 vs 0.9, RRI = 54%, P = .07 |
| Intracranial hemorrhage | 0.23 vs 0.76, RRR = 70%, P < .001 | 0.32 vs 0.76, RRR = 59%, P < .001 | SOT: 0.5 vs 0.7, RRR = 33%, P = .02 | SOT: 0.26 vs 0.85, RRR = 70%, P < .001 | SOT: 0.39 vs 0.85, RRR = 53%, P < .001 | SOT: 0.33 vs 0.80, RRR = 58%, P < .001 | 0.4 vs 0.4, RRR = 15%, P = .69 |
Abbreviations: BID, twice daily; DOAC, direct oral anticoagulant; INR, international normalized ratio; ITT, intent to treat; mITT, modified intent to treat; NVAF, nonvalvular atrial fibrillation; OT, on treatment; PP, per protocol; QD, once daily; RRI, relative risk increase; RRR, relative risk reduction; SOT, safety on-treatment.
aBoth RRRs and RRIs are calculated from the published hazard ratios for ROCKET AF, ENGAGE AF–TIMI 48, ARISTOTLE, and AVERROES and from the published relative risks from RE-LY. All columns show DOAC versus warfarin, except AVERROES, which compared apixaban with aspirin. All data are presented as annual rates per 100 patients, except as noted. All analyses were performed on ITT populations unless otherwise specified. Adapted with permission of Dove Medical Press Ltd, from Foody JM. Clin Int Aging. 2017;12:175-187; permission conveyed through Copyright Clearance Center, Inc.
Table 2.
Results of Trials of DOACs for Stroke Prevention in NVAF (Latin American Subgroups).a
| RE-LY Dabigatran 110 mg BID46 | RE-LY Dabigatran 150 mg BID46 | ROCKET AF Rivaroxaban 20 mg QD48 | ENGAGE AF–TIMI 48 Edoxaban 30 mg QD45 | ENGAGE AF–TIMI 48 Edoxaban 60 mg QD45 | ARISTOTLE Apixaban 5 mg BID47 | |
|---|---|---|---|---|---|---|
| Comparator | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 | Warfarin target INR, 2.0-3.0 |
| Total N | 18 113 | 14 264 | 21 105 | 18 201 | ||
| Latin American patients (n) | 1134 (South America; ITT, both efficacy and safety) | 1878 (ITT); 1877 (SOT) | 2661 (ITT); 2651 (SOT) | 3468 (ITT); 3460 (SOT) | ||
| Efficacy | ||||||
| Stroke or systemic embolism | 1.82 vs 1.68 | 0.91 vs 1.68 | 3.9 vs 4.8 | 2.15 vs 2.50 | 1.61 vs 2.50 | 1.4 vs 1.8 |
| Safety | ||||||
| Major bleeding | 1.66 vs 3.74 | 2.65 vs 3.74 | 2.1 vs 3.5 | |||
| Major and CRNM bleeding | 17.78 vs 19.72 | |||||
Abbreviations: BID, twice daily; CRNM, clinically relevant nonmajor; DOAC, direct oral anticoagulant; INR, international normalized ratio; ITT, intent to treat; NVAF, nonvalvular atrial fibrillation; QD, once daily; SOT, safety on-treatment.
aAll columns show DOAC versus warfarin. All data are presented as annual rates per 100 patients.
Current Guidelines Available for the Management of Stroke in Patients With AF
Selection of Medical Therapy for Primary and Secondary Prevention
There is broad acceptance in guidelines of the role of oral anticoagulant therapy for patients with AF and a CHA2DS2-VASc score ≥2. The ESC recommends considering oral anticoagulation for women with a CHA2DS2-VASc score of 2 and men with a CHA2DS2-VASc score of 1, while noting the importance of balancing the expected stroke reduction with individual characteristics such as bleeding risk and patient preference; a strong class I recommendation (indicating evidence and/or general agreement that the treatment is beneficial, useful, and effective) is made for oral anticoagulation for patients at higher risk levels. The DOACs and VKAs are both effective treatment options, with DOACs recommended over VKAs or aspirin therapy in patients eligible to receive them (class I recommendation).58
While the ESC guidelines acknowledge the usefulness of bleeding risk scores such as HAS-BLED, ORBIT, and ABC (age, biomarkers, clinical history), they do not describe a high bleeding score as a contraindication for anticoagulation but rather as a prompt to treat those risk factors that can be corrected.41,42,58,60
The ESC notes that the evidence for stroke prevention with aspirin is very limited and that antiplatelet therapy cannot be recommended for stroke prevention in patients with AF.61 The AHA/ASA recommends oral anticoagulation for primary prevention of stroke in patients with NVAF, a CHA2DS2-VASc score ≥2, and an “acceptably low risk” of hemorrhagic complications (class I recommendation, indicating benefit clearly outweighs risk).55 Clinicians should select from options including warfarin (INR: 2.0-3.0), dabigatran, rivaroxaban, and apixaban on the basis of patient risk factors (particularly ICH risk), cost, tolerability, patient preference, potential for drug–drug interactions, and other clinical characteristics, including (for patients taking warfarin) whether a therapeutic INR is consistently maintained. These factors are also to be taken into account when determining anticoagulation for patients with NVAF, a CHA2DS2-VASc score of 1, and an acceptably low risk of hemorrhagic complications (although anticoagulation is a weaker class IIb recommendation in such patients).
The AHA/ACC/HRS guidelines similarly recommend warfarin, dabigatran, rivaroxaban, or apixaban in patients with NVAF and prior stroke or TIA, or a CHA2DS2-VASc score ≥2, but do not make recommendations for use of the HAS-BLED or other bleeding scores.44 In the AHA/ASA guidelines for secondary stroke prevention, VKA therapy (class I; level of evidence A), apixaban (class I; level of evidence A), and dabigatran (class I; level of evidence B) are indicated for the prevention of recurrent stroke in patients with paroxysmal or permanent NVAF. Rivaroxaban is a reasonable choice for such patients (class IIa; level of evidence B).54
The AAN recommends warfarin, dabigatran, rivaroxaban, and apixaban to reduce stroke risk in patients with NVAF judged to require oral anticoagulation; specific recommendations include warfarin in patients who are well controlled on warfarin already and dabigatran, rivaroxaban, and apixaban in those with high risk of ICH or who are unwilling or unable to submit to INR testing.53 Where oral anticoagulation is unavailable, the AAN suggests combined aspirin and clopidogrel.
As only dabigatran and rivaroxaban were approved in Brazil at the time of the 2013 BSC guidelines on antiplatelet and anticoagulant agents in cardiology, recommendations for the use of DOACs in patients with NVAF are limited to these agents. Anticoagulation is recommended in patients with a CHA2DS2-VASc score ≥2, and anticoagulation or aspirin is recommended in patients with a CHA2DS2-VASc score of 1; dabigatran and rivaroxaban are described as alternatives to warfarin in patients needing anticoagulation.56 Similarly, the ASC guidelines offer recommendations on the use of dabigatran and rivaroxaban, with the additional option of apixaban.59 Brazilian guidelines note that selection of antithrombotic therapy should be based on risk of embolic events as per CHA2DS2-VASc score and risk of bleeding as per HAS-BLED and based on RR benefit for each individual patient, particularly among individuals. The MSSI guidelines, published in 2012, mention only dabigatran and identify it as an alternative to VKA.57
Emergency Bleeding
Although the short half-life of DOACs may decrease the need for immediate reversal, in cases of urgent bleeding or overdose, warfarin may have a perceived advantage as its activity can be reversed by vitamin K. The dabigatran antidote idarucizumab has recently become available in the United States,62 and phase III trials of andexanet alfa for reversal of apixaban and rivaroxaban have been published63; aripazine, an agent for reversal of all DOACs, is also in development.64 It should be noted that onset of vitamin K reversal of warfarin’s anticoagulant effects may take hours after infusion; sometimes over a day is needed for an effective response.43,65 In cases of major bleeding related to VKA administration, prothrombin complex concentrate (PCC) may be required in addition to vitamin K (fresh frozen plasma is another strategy but is associated with potential allergic reaction or infection and with longer time to prepare; more evaluation is needed for the use of recombinant factor VIIa).43,66 The AHA/ASA note the limitations of warfarin’s purported advantages in reversal, citing the high mortality rates of warfarin-related ICH despite the availability of reversal agents.55 The 2015 EHRA practical guide43 aligns with the ESC recommendations for anticoagulation in patients with NVAF, with specific practical clinical scenarios such as the need for emergency reversal.43 The EHRA practical guide advises consideration of PCC or activated PCC (aPCC) for emergency reversal of bleeding in a patient who has taken a DOAC (or idarucizumab, if available, for a patient who has taken dabigatran).43
The BSC guidelines indicate that PCC can be used to reverse the activity of factor Xa inhibitors.56 Prothrombin complex concentrate and aPCC are available in Latin American countries for anticoagulation reversal; however, because of their higher cost versus fresh frozen plasma, they are not routinely used, especially in public hospitals.67 The ASC guidelines note that prothrombin factor complex has been found to restore coagulation in patients treated with rivaroxaban, but not those treated with dabigatran (while not making a formal recommendation for reversing DOAC-induced anticoagulation).59
Compared with VKAs, the risk of ICH with DOACs is reduced, but not eliminated, and ICH is still associated with high rates of death and disability.52,68,69 Because patients with AF who survive ICH continue to have increased risk of ischemic stroke,41 clinicians are tasked with weighing the risk–benefit of resuming or discontinuing oral anticoagulation therapy for anticoagulated patients presenting with ICH.
Nielsen et al identified patients with AF receiving warfarin or a DOAC with incident ICH.70 In 1752 patients, after 1 year since the ICH, the rate of ischemic stroke/systemic embolism (SE) and all-cause mortality (per 100 person-years) was 13.6 for oral anticoagulation-treated patients compared with 27.3 for untreated patients and 25.7 for patients receiving antiplatelets; the adjusted hazard ratio (HR) for the combined end point of ischemic stroke/SE and all-cause mortality was 0.55 (95% confidence interval: 0.39-0.78) for oral anticoagulation versus no treatment. The EHRA considers that DOACs may be restarted 4 to 8 weeks after an ICH, if the risk of another ICH is considered to be low and cardioembolic risk is high.43 The BSC guidelines advocate restarting anticoagulation 10 to 30 weeks after an event of acute cerebral hemorrhage.56
Analyses from RE-LY showed similar ICH distributions across anatomic sites between dabigatran and warfarin, while absolute rates at all sites and fatal and traumatic ICH rates were lower for dabigatran.71 However, direct comparisons of DOACs versus warfarin following ICH are lacking. A trial assessing apixaban versus no anticoagulation in patients with AF and recent ICH during anticoagulation treatment is ongoing.72
Treatment Interruption
Although the need for a temporary cessation of anticoagulation is quite common (in large clinical trials of DOACs versus warfarin, approximately one-quarter to one-third of patients required such cessation), guidelines offer inconsistent recommendations for its management.73,74 The EHRA practical guide recommends that apixaban, edoxaban, and rivaroxaban be stopped ≥48 hours before elective surgery in those undergoing procedures with high bleeding risk and ≥24 hours before in those undergoing procedures with low bleeding risk (≥36 hours before in case of creatinine clearance [CrCl]15-30 mL/min). In those undergoing procedures with low bleeding risk, dabigatran should be stopped ≥24, ≥36, and ≥48 hours beforehand in those with CrCl ≥80 mL/min, 50 to 80 mL/min, and 30 to 50 mL/min, respectively (dabigatran is not indicated in patients with CrCl 15-30 mL/min); these times are doubled for procedures with high bleeding risk. The EHRA does not recommend bridging therapy with another anticoagulant.43 This contrasts with the AHA/ASA, who note the possibility of increased risk of stroke after abrupt discontinuation of the DOACs and thus recommend consideration of bridging therapy for those taking a DOAC, as well as recommending bridging for those taking VKA at high risk of thromboembolism and considering bridging for those at moderate risk.54 The AHA/ACC/HRS guidelines acknowledge the lack of data but regard bridging therapy with heparin in those at high thromboembolic risk who are taking a VKA to be common practice, while DOACs can be simply withheld for 1 day before the procedure.44 Brazilian guidelines recommend bridging therapy with heparin in patients taking VKA for whom cardiac surgery is planned (with discontinuation of unfractionated heparin 4 hours before or low-molecular-weight heparin 24 hours before in those at high risk of thromboembolism). In those with normal renal function undergoing cardiac surgery, dabigatran should be discontinued 48 hours before surgery (24 hours before a procedure with low bleeding risk) and rivaroxaban 24 hours before surgery, with no bridging therapy recommended in either case.56 The ESC holds that most cardiovascular interventions can be safely performed without interrupting anticoagulation and that when interruption is necessary bridging therapy is not beneficial in patients without mechanical heart valves.58 The ASC guidelines identify bridging therapy with heparin as a reasonable strategy for those being treated with a VKA; administration of dabigatran should be suspended 24 hours before surgery in those with CrCl >50 mL/min and 2 to 5 days beforehand in those with CrCl <50 mL/min, while rivaroxaban and apixaban should be suspended 24 hours before surgery.59
Management of Acute Ischemic Stroke in Anticoagulated Patients
Despite the benefits of VKAs and DOACs to reduce stroke in patients with AF, approximately 1.0% to 2.0% of treated patients are still likely to experience an acute ischemic stroke each year.45–48,50,51
Thrombolytic therapy with recombinant tissue-type plasminogen activator is appropriate when given shortly after the onset of ischemic stroke (≤4.5 hours).43 However, current anticoagulation is a contraindication to thrombolysis. Clinicians must determine the patient’s current anticoagulation status and estimate any corresponding increase in the risk of hemorrhage with reperfusion. Clinically important anticoagulant effect can be ruled out by detecting normal values on the thrombin time or ecarin clotting time, the Hemoclot Thrombin Inhibitor assay, ≥4 hours after the last dose of dabigatran, or a normal antifactor Xa assay ≥5 hours after the last dose of rivaroxaban or apixaban.75 Absent reliable point-of-care tests, the EHRA recommends avoiding thrombolysis in patients who have received DOAC therapy within 24 to 48 hours or in whom there is uncertainty regarding anticoagulation status; however, this recommendation is arbitrary and untested.43
Recent clinical trial evidence has shown that arterial thrombectomy can be an effective treatment for patients with acute ischemic stroke with large artery occlusions.76–80 These trials, unlike previous evaluations of endovascular therapy, tested the addition of the endovascular approach to standard intravenous thrombolysis, required documentation of occlusion, had low median onset-to-groin times, and generally employed stent retrievers that were able to achieve faster and more complete recanalization. Recent investigation has found no increased risk of symptomatic ICH associated with the use of oral anticoagulants among patients undergoing arterial thrombectomy.81 Thus, this approach may be an option in patients with acute ischemic stroke who are receiving oral anticoagulants.
With no prospective data indicating the ideal time to resume anticoagulation after ischemic stroke, the decision is largely based on clinical judgment. The EHRA practical guide advises that DOAC continuation after ischemic stroke is linked to infarct volume.43 If the infarct size is unlikely to increase the risk of early secondary intracerebral bleeding, DOAC use is similar to usual practice with VKAs. While acknowledging the lack of data, the guidelines mention the 1-3-6-12-day principle, which advises resumption of anticoagulation after 1 day following a TIA; 3 days following a small, nondisabling infarct; 5 to 7 days following a moderate stroke; and 12 to 14 days after a large infarct.43 The AHA/ASA guidelines recommend initiating anticoagulation within 14 days after the onset of neurologic symptoms (class IIa; level of evidence B).54 The BSC guidelines recommend antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke.56
Left Atrial Appendage Closure
The ESC recommends left atrial appendage closure only for those patients in whom anticoagulation is contraindicated, citing limited evidence comparing this approach to anticoagulation.58 A meta-analysis cited in the guidelines including 2406 patients with NVAF found reduced rates of hemorrhagic strokes (HR = 0.22; P = .004), cardiovascular/unexplained death (HR = 0.48; P = .006), and nonprocedural bleeding (HR = 0.51; P = .006) with the WATCHMAN device versus warfarin, although the increased ischemic stroke rate with the device meant a similar event rate for all-cause stroke or SE (HR = 1.02; P = .94).82 The AHA/ASA guidelines recommend left atrial appendage closure for consideration in high-risk patients with AF deemed unsuitable for anticoagulation who can tolerate the risk of ≥45 days periprocedural anticoagulation, if performed at a center with low rates of complications. Support for this position comes from the results of the WATCHMAN trial, which found noninferiority of the WATCHMAN device versus warfarin in the primary efficacy end point of stroke/SE/cardiovascular death, as well as the lack of comparison with DOACs.55 The AHA/ACC/HRS guidelines make no recommendation regarding this approach.44 The ASC guidelines describe left arterial appendage closure as reasonable in patients with a CHADS2 score of 2 and contraindications to oral anticoagulation.59
Compliance With Guidelines in Latin America
Nonadherence to guidelines is associated with poor outcomes in anticoagulant therapy.83 In the analysis from ROCKET AF, the individual-level time in therapeutic range for patients in the warfarin group was significantly lower in Latin America than in the United States/Canada.84 Concerns regarding the safety of standard anticoagulation may be a factor; 1 Brazilian study of patients in the oral anticoagulation outpatient clinic at a cardiology hospital found that 68.6% were concerned about the bleeding risk associated with oral VKAs.85 Aspirin, meanwhile, may be overused in the region; a literature search found that antiplatelet therapy was prescribed to 63% of patients with AF in Argentina and Mexico.86 Encouraging signs of progress may be seen with greater attention to adherence. An examination of quality indicators at 1 Brazilian primary stroke center found higher use of some appropriate acute interventions versus those seen in the US-based Get with the Guidelines (GWTG) program, such as intravenous thrombolysis given to eligible patients (69.5% vs 42.1% in the GWTG cohort in 2003 and 72.8% in the GWTG 2007 data set).87 Notably, patients in the Brazilian center were more likely to receive anticoagulation for AF if followed by a neurologist during admission. Creation of a prospective stroke registry using a standardized form filled out by the neurology resident in charge during hospitalization was found to improve adherence to measures of acute stroke care quality in a Mexican study.88 Younger physicians may be more attuned to guideline recommendations; a survey of cardiologists in Rio Grande do Sul, Brazil, found that 87.7% of those having graduated less than 25 years ago used a risk score to determine the need for anticoagulation versus 73.2% of those who had graduated over 25 years ago (P = .02).89
Adherence to guidelines can be limited by cost/access issues, which vary across the region.90 In Brazil, patients treated in the public sector typically continue to receive warfarin or another VKA because these costs are supported by the public system. Only 30% of patients have private health insurance and access to newer therapies (eg, DOACs) for stroke prevention. In Brazil and Mexico, local authorities may subsidize more expensive medications, but it takes time to get new therapies included on formularies and differences in access exist between local areas.91
Conclusions
Both AF and stroke are substantial and impactful health problems in Latin America. Although variations in access to health care may play a role, there is ample room for improvement through greater adherence to evidence-based treatment recommendations. The CHA2DS2-VASc and HAS-BLED (and probably ORBIT) scores offer both predictive value and relative simplicity and can be used together to ensure appropriate anticoagulation therapy for patients able to benefit from it. Although more data are needed regarding the best approaches for specific clinical situations, such as emergency bleeding and stroke in patients who are being anticoagulated with DOACs, these agents offer the promise of treatment without routine monitoring that may help toward meeting the goal of all appropriate patients receiving anticoagulation.
Footnotes
Authors’ Note: Professional medical writing and editorial assistance were provided by Rob Coover and Rosemary Perkins at Caudex, funded by Bristol-Myers Squibb Company and Pfizer Inc. All authors contributed equally to the conception of this paper, which was determined via conference call. All authors agreed to the relevant guidelines discussed. Each author had equal opportunity to identify areas of particular focus and identified additional research illustrating epidemiology and/or practice challenges in Latin America. All authors approved the final draft for submission.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.C.-B. reports consulting fees, advisory boards, travel support, and clinical trials participation from Sanofi, Boehringer Ingelheim, Bayer, Pfizer, AstraZeneca, and Servier. G.S.S. reports consulting fees, advisory boards, travel support, and clinical trials participation from Bristol-Myers Squibb, Bayer, Pfizer, and AstraZeneca. S.F.A. reports consulting fees, advisory boards, travel support, and clinical trials participation from Sanofi, Boehringer Ingelheim, Bayer, Pfizer, Bristol-Myers Squibb, AstraZeneca, Merck, and Servier.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing support was provided by Rob Coover and Rosemary Perkins at Caudex, and funded by Bristol-Myers Squibb and Pfizer.
References
- 1. de Carvalho JJ, Alves MB, Viana GA, et al. Stroke epidemiology, patterns of management, and outcomes in Fortaleza, Brazil: a hospital-based multicenter prospective study. Stroke. 2011;42(12):3341–3346. [DOI] [PubMed] [Google Scholar]
- 2. Porcello Marrone LC, Diogo LP, de Oliveira FM, et al. Risk factors among stroke subtypes in Brazil. J Stroke Cerebrovasc Dis. 2013;22(1):32–35. [DOI] [PubMed] [Google Scholar]
- 3. Saposnik G, Del Brutto OH. Stroke in South America: a systematic review of incidence, prevalence, and stroke subtypes. Stroke. 2003;34(9):2103–2107. [DOI] [PubMed] [Google Scholar]
- 4. Marquez-Romero JM, Arauz A, Gongora-Rivera F, Barinagarrementeria F, Cantu C. The burden of stroke in Mexico. Int J Stroke. 2015;10(2):251–252. [DOI] [PubMed] [Google Scholar]
- 5. Cantu-Brito C, Majersik JJ, Sanchez BN, et al. Door-to-door capture of incident and prevalent stroke cases in Durango, Mexico: the brain attack surveillance in Durango study. Stroke. 2011;42(3):601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melcon CM, Melcon MO. Prevalence of stroke in an Argentine community. Neuroepidemiology. 2006;27(2):81–88. [DOI] [PubMed] [Google Scholar]
- 7. Bahit MC, Coppola ML, Riccio PM, et al. First-ever stroke and transient ischemic attack incidence and 30-day case-fatality rates in a population-based study in Argentina. Stroke. 2016;47(6):1640–1642. [DOI] [PubMed] [Google Scholar]
- 8. Lavados PM, Sacks C, Prina L, et al. Incidence, 30-day case-fatality rate, and prognosis of stroke in Iquique, Chile: a 2-year community-based prospective study (PISCIS project). Lancet. 2005;365(9478):2206–2215. [DOI] [PubMed] [Google Scholar]
- 9. Cabral NL, Goncalves AR, Longo AL, et al. Trends in stroke incidence, mortality and case fatality rates in Joinville, Brazil: 1995-2006. J Neurol Neurosurg Psychiatry. 2009;80(7):749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cantu-Brito C, Majersik JJ, Sanchez BN, et al. Hospitalized stroke surveillance in the community of Durango, Mexico: the brain attack surveillance in Durango study. Stroke. 2010;41(5):878–884. [DOI] [PubMed] [Google Scholar]
- 11. Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke. 2014;9(1):6–18. [DOI] [PubMed] [Google Scholar]
- 12. Bejot Y, Osseby GV, Aboa-Eboule C, et al. Dijon’s vanishing lead with regard to low incidence of stroke. Eur J Neurol. 2009;16(3):324–329. [DOI] [PubMed] [Google Scholar]
- 13. Iguchi Y, Kimura K, Sone K, et al. Stroke incidence and usage rate of thrombolysis in a Japanese urban city: the Kurashiki stroke registry. J Stroke Cerebrovasc Dis. 2013;22(4):349–357. [DOI] [PubMed] [Google Scholar]
- 14. Garritano CR, Luz PM, Pires ML, Barbosa MT, Batista KM. Analysis of the mortality trend due to cerebrovascular accident in Brazil in the XXI century. Arq Bras Cardiol. 2012;98(6):519–527. [DOI] [PubMed] [Google Scholar]
- 15. Mansur Ade P, Favarato D, Avakian SD, Ramires JA. Trends in ischemic heart disease and stroke death ratios in Brazilian women and men. Clinics (Sao Paulo). 2010;65(11):1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaup AO, dos Santos BF, Victor ES, et al. Georeferencing deaths from stroke in Sao Paulo: an intra-city stroke belt? Int J Stroke. 2015;10(suppl A100):69–74. [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–454. [DOI] [PubMed] [Google Scholar]
- 18. Cubillos L, Haddad A, Kuznik A, Mould-Quevedo J. Burden of disease from atrial fibrillation in adults from seven countries in Latin America. Int J Gen Med. 2014;7:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimerman LI, Fenelon G, Martelli Filho M, et al. Sociedade Brasileira de Cardiologia. Diretrizes Brasileiras de Fibrilação Atrial. Arq Bras Cardiol. 2009;92(6):1–39. [Google Scholar]
- 20. Cabral NL, Goncalves AR, Longo AL, et al. Incidence of stroke subtypes, prognosis and prevalence of risk factors in Joinville, Brazil: a 2 year community based study. J Neurol Neurosurg Psychiatry. 2009;80(7):755–761. [DOI] [PubMed] [Google Scholar]
- 21. Figueiredo MM, Rodrigues AC, Alves MB, Neto MC, Silva GS. Score for atrial fibrillation detection in acute stroke and transient ischemic attack patients in a Brazilian population: the acute stroke atrial fibrillation scoring system. Clinics (Sao Paulo). 2014;69(4):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–1764. [DOI] [PubMed] [Google Scholar]
- 23. Cantu-Brito C, Ruiz-Sandoval JL, Murillo-Bonilla LM, et al. The first Mexican multicenter register on ischaemic stroke (the PREMIER study): demographics, risk factors and outcome. Int J Stroke. 2011;6(1):93–94. [DOI] [PubMed] [Google Scholar]
- 24. Cantu C, Arauz A, Ruiz-Sandoval JL, et al. Underuse of antithrombotic therapy and clinical outcome in patients with acute ischemic stroke and atrial fibrillation in a Hispanic population. Stroke. 2011;42(3):e346. [Google Scholar]
- 25. Avezum A, Cantu-Brito C, Gonzalez-Zuelgaray J, et al. How can we avoid a stroke crisis in Latin America: working group report: stroke prevention in patients with atrial fibrillation. [¿Cómo reducir los accidentes cerebrovasculares en Latinoamérica? Parte 2.] Insuf Card 2012;7(3):123–127. [Google Scholar]
- 26. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. [DOI] [PubMed] [Google Scholar]
- 27. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 28. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–2738. [DOI] [PubMed] [Google Scholar]
- 29. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e531S–e575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lavados PM, Hennis AJ, Fernandes JG, et al. Stroke epidemiology, prevention, and management strategies at a regional level: Latin America and the Caribbean. Lancet Neurol. 2007;6(4):362–372. [DOI] [PubMed] [Google Scholar]
- 31. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Banerjee A, Lane DA, Torp-Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107(3):584–589. [DOI] [PubMed] [Google Scholar]
- 33. Fornari LS, Calderaro D, Nassar IB, et al. Misuse of antithrombotic therapy in atrial fibrillation patients: frequent, pervasive and persistent. J Thromb Thrombolysis. 2007;23(1):65–71. [DOI] [PubMed] [Google Scholar]
- 34. Durai PJ, Padma V, Vijaya P, Sylaja PN, Murthy JM. Stroke and thrombolysis in developing countries. Int J Stroke. 2007;2(1):17–26. [DOI] [PubMed] [Google Scholar]
- 35. Pujol Lereis V, Ameriso S, Povedano GP, Ameriso SF. Ischemic stroke in patients with atrial fibrillation receiving oral anticoagulation. J Neurol Sci. 2013;334(1-2):139–142. [DOI] [PubMed] [Google Scholar]
- 36. Rosenman MB, Simon TA, Teal E, McGuire P, Nisi D, Jackson JD. Perceived or actual barriers to warfarin use in atrial fibrillation based on electronic medical records. Am J Ther. 2012;19(5):330–337. [DOI] [PubMed] [Google Scholar]
- 37. Rosenman MB, Baker L, Jing Y, et al. Why is warfarin underused for stroke prevention in atrial fibrillation? A detailed review of electronic medical records. Curr Med Res Opin. 2012;28(9):1407–1414. [DOI] [PubMed] [Google Scholar]
- 38. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492–501. [DOI] [PubMed] [Google Scholar]
- 39. van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288(19):2441–2448. [DOI] [PubMed] [Google Scholar]
- 40. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med. 2003;139(12):1018–1033. [DOI] [PubMed] [Google Scholar]
- 41. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–1510. [DOI] [PubMed] [Google Scholar]
- 42. O’Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36(46):3258–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–1507. [DOI] [PubMed] [Google Scholar]
- 44. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. [DOI] [PubMed] [Google Scholar]
- 45. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. [DOI] [PubMed] [Google Scholar]
- 46. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. [DOI] [PubMed] [Google Scholar]
- 47. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. [DOI] [PubMed] [Google Scholar]
- 48. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. [DOI] [PubMed] [Google Scholar]
- 49. Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–1876. [DOI] [PubMed] [Google Scholar]
- 50. Connolly SJ, Wallentin L, Yusuf S. Additional events in the RE-LY trial. N Engl J Med. 2014;371(15):1464–1465. [DOI] [PubMed] [Google Scholar]
- 51. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. [DOI] [PubMed] [Google Scholar]
- 52. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. [DOI] [PubMed] [Google Scholar]
- 53. Culebras A, Messé SR, Chaturvedi S, Kase CS, Gronseth G. Summary of evidence-based guideline update: prevention of stroke in nonvalvular atrial fibrillation: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(8):716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. [DOI] [PubMed] [Google Scholar]
- 55. Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lorga Filho AM, Azmus AD, Soeiro AM, et al. Brazilian guidelines on platelet antiaggregants and anticoagulants in cardiology [in Portuguese]. Arq Bras Cardiol. 2013;101(3 suppl 3):1–95. [DOI] [PubMed] [Google Scholar]
- 57. Castano-Guerra Rde J, Franco-Vergara BC, Baca-Lopez FM, et al. Clinical guideline for diagnosis and treatment of atrial fibrillation. Rev Med Inst Mex Seguro Soc. 2012;50(2):213–231. [PubMed] [Google Scholar]
- 58. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 59. Sociedad Argentina de Cardiologia Area de Consensos y Normas. Consenso de fibrilacion auricular. Rev Argent Cardiol. 2017;83(suppl 1):1–28. [Google Scholar]
- 60. Hijazi Z, Lindback J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37(20):1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Europace. 2012;14(10):1385–1413. [DOI] [PubMed] [Google Scholar]
- 62. Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511–520. [DOI] [PubMed] [Google Scholar]
- 63. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–2424. [DOI] [PubMed] [Google Scholar]
- 64. Sullivan DW, Jr, Gad SC, Laulicht B, Bakhru S, Steiner S. Nonclinical safety assessment of PER977: a small molecule reversal agent for new oral anticoagulants and heparins. Int J Toxicol. 2015;34(4):308–317. [DOI] [PubMed] [Google Scholar]
- 65. Bechtel BF, Nunez TC, Lyon JA, Cotton BA, Barrett TW. Treatments for reversing warfarin anticoagulation in patients with acute intracranial hemorrhage: a structured literature review. Int J Emerg Med. 2011;4(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (9th Edition). Chest. 2012;141(2 suppl):e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schick KS, Fertmann JM, Jauch KW, Hoffmann JN. Prothrombin complex concentrate in surgical patients: retrospective evaluation of vitamin K antagonist reversal and treatment of severe bleeding. Crit Care. 2009;13(6):R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chan NC, Paikin JS, Hirsh J, Lauw MN, Eikelboom JW, Ginsberg JS. New oral anticoagulants for stroke prevention in atrial fibrillation: impact of study design, double counting and unexpected findings on interpretation of study results and conclusions. Thromb Haemost. 2014;111(5):798–807. [DOI] [PubMed] [Google Scholar]
- 69. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. [DOI] [PubMed] [Google Scholar]
- 70. Nielsen PB, Larsen TB, Skjøth F, Gorst-Rasmussen A, Rasmussen LH, Lip GYH. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality and bleeding: a nationwide cohort study. Circulation. 2015;132(6):517–525. [DOI] [PubMed] [Google Scholar]
- 71. Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6):1511–1517. [DOI] [PubMed] [Google Scholar]
- 72. van Nieuwenhuizen KM, van der Worp HB, Algra A, et al. Apixaban versus Antiplatelet drugs or no antithrombotic drugs after anticoagulation-associated intraCerebral HaEmorrhage in patients with Atrial Fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials. 2015;16(1):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124(25):3692–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation. 2012;126(3):343–348. [DOI] [PubMed] [Google Scholar]
- 75. Hankey GJ, Norrving B, Hacke W, Steiner T. Management of acute stroke in patients taking novel oral anticoagulants. Int J Stroke. 2014;9(5):627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berkhemer OA, Majoie CB, Dippel DW. Intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(12):1178–1179. [DOI] [PubMed] [Google Scholar]
- 77. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. [DOI] [PubMed] [Google Scholar]
- 78. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. [DOI] [PubMed] [Google Scholar]
- 79. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. [DOI] [PubMed] [Google Scholar]
- 80. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. [DOI] [PubMed] [Google Scholar]
- 81. Rozeman AD, Wermer MJ, Lycklama ANG, et al. Safety of intra-arterial treatment in acute ischaemic stroke patients on oral anticoagulants. A cohort study and systematic review. Eur J Neurol. 2015;23(2):290–296. [DOI] [PubMed] [Google Scholar]
- 82. Holmes DR, Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65(24):2614–2623. [DOI] [PubMed] [Google Scholar]
- 83. Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. The Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153(6):1006–1012. [DOI] [PubMed] [Google Scholar]
- 84. Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2(1):e000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Esmerio FG, Souza EN, Leiria TL, Lunelli R, Moraes MA. Constant use of oral anticoagulants: implications in the control of their adequate levels. Arq Bras Cardiol. 2009;93(5):549–554. [DOI] [PubMed] [Google Scholar]
- 86. Nguyen TN, Hilmer SN, Cumming RG. Review of epidemiology and management of atrial fibrillation in developing countries. Int J Cardiol. 2013;167(6):2412–2420. [DOI] [PubMed] [Google Scholar]
- 87. de Carvalho FA, Schwamm LH, Kuster GW, Bueno Alves M, Cendoroglo NM, Sampaio SG. Get With the Guidelines stroke performance indicators in a Brazilian tertiary hospital. Cerebrovasc Dis Extra. 2012;2(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Herrera AL, Gongora-Rivera F, Muruet W, et al. Implementation of a stroke registry is associated with an improvement in stroke performance measures in a tertiary hospital in Mexico. J Stroke Cerebrovasc Dis. 2015;24(4):725–730. [DOI] [PubMed] [Google Scholar]
- 89. van der Sand CR, Leiria TL, Kalil RA. Assessment of the adherence of cardiologists to guidelines for the treatment of atrial fibrillation. Arq Bras Cardiol. 2013;101(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Massaro AR, Lip GYH. Stroke prevention in atrial fibrillation: focus on Latin America. Arq Bras Cardiol. 2016;107(6):576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Camm AJ, Pinto FJ, Hankey GJ, Andreotti F, Hobbs FD. Non-vitamin K antagonist oral anticoagulants and atrial fibrillation guidelines in practice: barriers to and strategies for optimal implementation. Europace. 2015;17(7):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]