Abstract
Listeria rhombencephalitis (LRE) is a rare encephalitis of the hindbrain that can present with a variety of neurological symptoms. It is a diagnostic challenge, but prompt antimicrobial therapy is important to prevent high rates of mortality and morbidity. We report two cases of LRE, with several contrasting clinical features and different disease courses. Despite being rare, it is important to consider listeria in patients with possible meningoencephalitis, even if cultures are negative. Empirical treatment of meningoencephalitis should provide coverage for listeria, especially if the patient is at risk of listeriosis or there is a potential history of listeria exposure.
Keywords: Listeria monocytogenes, Rhombencephalitis, Encephalitis, Hindbrain, Meningoencephalitis
Introduction
Rhombencephalitis is a rare form of encephalitis of the hindbrain, of which Listeria monocytogenes is one of the most common infectious etiologies. It normally presents with a prodromal flu-like illness followed by a phase with neurological manifestations [1], which may include cranial nerve pathology, cerebellar ataxia and long-tract motor and sensory features [2]. Rapid diagnosis and adequate antibacterial treatment are important to reduce mortality and morbidity; however, it can be a diagnostic challenge due to the multitude of possible differential diagnoses and also because the exact etiology can be difficult to determine, despite advances in microbiology and molecular techniques. The presentation, laboratory findings and course of the disease are also highly variable. Here we report two cases with several contrasting features that we managed at a district general hospital in the United Kingdom.
Cases
Case 1
A 79 year old male with a background of localized prostate cancer (on hormonal therapy), ischemic heart disease and hypertension was admitted with a fall and mild confusion. He complained of a mild headache. He was apyrexial and was leaning to the left side but did not have any focal neurological deficits on initial examination. Blood tests revealed lymphopenia (680 cells/microL) and hyponatremia (124 mEq/L). Inflammatory markers were normal. Human immunodeficiency virus antibody and Lyme IgM/G were negative. Unenhanced computerized tomography (CT) of the brain was normal.
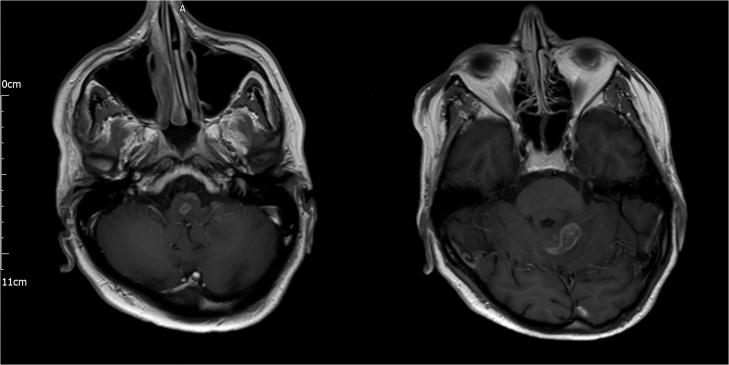
Later on the day of admission, he became less responsive (Glasgow Coma Score 12/15) and was started on intravenous ceftriaxone and acyclovir for possible encephalitis. He was transferred to the intensive care unit for observation but his condition continued to deteriorate and, two days later, he required intubation and ventilation for increasing oxygen requirements and a poor cough. Blood cultures then returned as positive for L. monocytogenes and his antimicrobial therapy was changed to intravenous amoxicillin 2 g 4-hourly and oral co-trimoxazole 960 mg 6-hourly (administered via nasogastric tube). Lumbar puncture was performed and cerebrospinal fluid (CSF) analysis revealed high protein 115 mg/dL, normal glucose 54 mg/dL and white cell count of 63 cells/microL (90% polymorphs). Culture was negative after 48 h incubation. Magnetic resonance imaging (MRI) of the brain revealed multiple ring-enhancing lesions in the brainstem and cerebellum (Fig. 1), characteristic of listeria rhombencephalitis (LRE).
Fig. 1.
Cranial magnetic resonance T1-weighted sequence showing ring-enhancing lesions in the brainstem (left) and left cerebellar hemisphere (right).
He failed extubation two days later and required a tracheostomy and underwent a slow respiratory wean. Repeat neurological exam revealed horizontal and vertical nystagmus and bilateral dysmetria. Power was reduced (MRC grade 3/5) in his right upper and lower limbs and there were brisk reflexes on the right side. There were no cranial nerve deficits. He was transferred to a medical ward where he made slow neurological improvement and his hyponatremia gradually improved. Repeat MRI was performed one month post-admission and revealed improvement in edema around the posterior fossa and brainstem lesions but some hemosiderin deposition suggestive of some hemorrhagic transformation. He completed six weeks of intravenous amoxicillin and oral co-trimoxazole (only given intravenously when there were problems with the patient’s nasogastric tube or concerns about safe swallow) and was then discharged with a further six week course of oral co-trimoxazole monotherapy. He was reviewed in clinic two months post-admission and had residual horizontal nystagmus and left-sided dysmetria.
Case 2
A 66 year old female with a history of mild chronic obstructive pulmonary disease (on inhaled therapy only) and hypertension presented with a two day history of gradually worsening diplopia and unsteadiness. She had been feeling generally unwell for the preceding 10 days, with a mild frontal headache and lethargy. She was apyrexial with a normal mental state and no signs of meningism. She had an ataxic gait, right-sided gaze-evoked nystagmus and dysmetria. Cranial nerve examination was unremarkable. Peripheral nervous system examination revealed brisk right lower limb reflexes with an upgoing Babinski reflex.
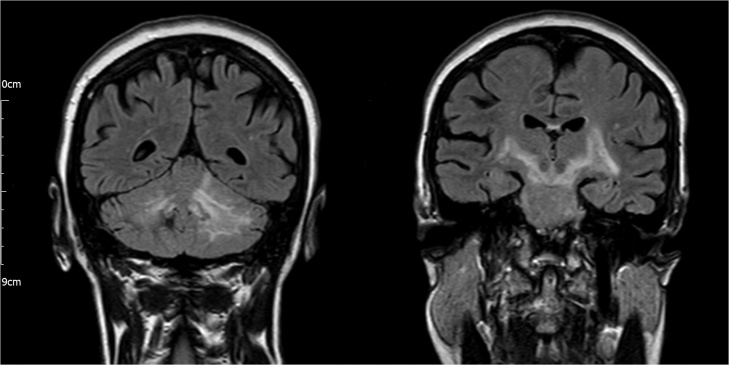
Bloods tests revealed neutrophilia (14,400 cells/microL). All bloods cultures were negative. Human immunodeficiency virus antibody, Lyme IgM/G, anti-nuclear and anti-neuronal antibodies were negative. Unenhanced CT of the brain was normal. Cerebrospinal fluid analysis revealed high protein 167 mg/dL, normal glucose 64.8 mg/dL and high white cell count 195 cells/microL (95% polymorphs). Culture was negative after 48 h incubation. Cerebrospinal fluid polymerase chain reaction (PCR) was negative for Herpes simplex, Epstein-Barr and varicella zoster viruses, L. monocytogenes, Streptococcus pneumoniae, Escherichia coli, bacterial 16 s rRNA and fungal 18 s rRNA. She was initially treated with intravenous ceftriaxone and acyclovir for a possible diagnosis of meningoencephalitis. Magnetic resonance imaging was performed on day two post-admission and T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences revealed hyperintensity in the cerebellum, with extension into the brainstem and internal capsule bilaterally (Fig. 2). Hemosiderin deposition was seen in the right cerebellum suggesting previous hemorrhage. She was diagnosed with rhombencephalitis.
Fig. 2.
Cranial magnetic resonance fluid-attenuated inversion recovery sequence showing hyperintensity in the cerebellum (left) with extension into the brainstem and internal capsule (right).
Despite a negative CSF PCR, CSF culture and blood cultures, a trial-of-treatment was undertaken for a presumptive diagnosis of L. monocytogenes rhombencephalitis, the cause most consistent with the clinical, hematological, radiological and CSF findings. After six weeks of intravenous amoxicillin 2 g 4-hourly and intravenous co-trimoxazole 960 mg 6-hourly, repeat MRI showed almost complete resolution, in keeping with the complete resolution of her neurological deficits. She was discharged with six weeks oral co-trimoxazole.
Discussion
Rhombencephalitis is a form of encephalitis that affects the hindbrain. Diagnosis is difficult because it may present with a variety of neurological features [2]. This was observed in the described cases, with the second patient presenting with both cerebellar and long tract motor features and the first patient having no focal neurological findings at presentation but rapidly developing respiratory failure − a complication that occurs in 41% of patients [3]. Various etiologies may be implicated in the pathogenesis of rhombencephalitis. Non-infectious causes are most common and include multiple sclerosis, Behçet’s disease and paraneoplastic syndromes. Frequently reported infectious causes include L. monocytogenes, Epstein-Barr virus, tuberculosis and Streptococcus pneumoniae [3], [4].
L. monocytogenes is one of the most common infectious causes of rhombencephalitis. In a Spanish case series of 97 patients with rhombencephalitis, L. monocytogenes accounted for nine cases (47% of the 19 cases with an identified infectious etiology) [4]. L. monocytogenes is a gram-positive facultative intracellular bacterium, for which soil appears to be the chief environmental reservoir and flowing bodies of water are thought to facilitate spread [5]. Ready-to-eat foods, soft cheeses, undercooked or inadequately reheated meats and delicatessen meats are among the most common modes of transmission to humans [6]. On retrospective questioning, the first described patient had not been exposed to any risk foods; however, the second patient did remember eating a large quantity of brie two weeks prior to admission. After exposure, extremes of age, pregnancy, immunosuppression, and comorbidities such as malignancy or diabetes are major risk factors for developing listeriosis [7]. These groups are at risk due to the critical role of T-cell mediated immunity, particularly CD8+ T-cells, in the protection against L. monocytogenes [8], [9], [10]. A depression in cell-mediated immunity (CMI) occurs in pregnancy to allow fetal retention [11] and the CMI of newborns is immature. Aging also causes a depression in CMI, due to a reduction in bone marrow progenitor cells, thymic involution and a reduction in the function of mature lymphocytes [12]. Both of the described patients were older than 65 years of age but were otherwise well without other major risk factors, and this is in line with other reports that suggest LRE usually occurs in healthy non-pregnant individuals, in contrast to other forms of listeriosis [3], [4], [13], [14].
Confirmation of L. monocytogenes as the etiological agent in rhombencephalitis can be problematic because blood and CSF cultures are only positive in 61% and 11–41% of cases respectively [3]. Culture-negative cases of LRE may be diagnosed by PCR, or suspected, based upon the combination of the clinical presentation, MRI and CSF findings and response to treatment [15], [16]. Blood cultures were positive in the first case, which led to the diagnosis being made and effective antimicrobials being commenced. In contrast, all cultures were negative in the second case and, hence, the diagnosis was only considered after MRI. Indeed, gadolinium-enhanced MRI usually shows abnormalities which aid in diagnosis [3], [4], [13] and should be performed in patients with brainstem or cerebellar signs and a non-diagnostic CT scan. Characteristic MRI changes include T2 and FLAIR hyperintensity in the brainstem and cerebellum [17], which was seen in the second case, and ring enhancing lesions, principally in LRE [1], [13], which were seen in the first case. Computed tomography is less useful but may show hypodense areas in the brainstem [3], [7].
Cerebrospinal fluid findings may be non-specific but often include a raised protein, a normal or low glucose and a lymphocytosis, although a CSF neutrophilia may also be seen [3], [7]. Both of our patients had a CSF neutrophilia. Cerebrospinal fluid PCR is now recognized as an important diagnostic tool in the diagnosis of many neurological infections [18], [19], including LRE [15]. However, although PCR is thought to have greater sensitivity than CSF culture, PCR may still be negative in LRE [16]. In both of the described cases, like the CSF cultures, L. monocytogenes PCRs were negative. In the first case, pre-treatment may have been a confounding factor [16] but, in the second case, lumbar puncture was promptly performed. It has been hypothesized that bacteria may be localized to the brain parenchyma in rhombencephalitis or cerebritis without any coexistent meningitis [7] and, hence, may be undetectable in the CSF [16]. In the second case, we suspected that L. monocytogenes was the cause owing to the absence of systemic features suggesting autoimmune disease or malignancy, the MRI appearance, and the CSF findings strongly suggesting bacterial infection and, after a trial of treatment, an excellent treatment response was observed.
Management of rhombencephalis includes treating the underlying cause in addition to supportive management. Amoxicillin or ampicillin is generally considered as the most effective antimicrobial for LRE [3], [20], [21] and is usually included as part of the empirical treatment for central nervous system infections if patients are at higher risk of listeriosis. This includes patients over 65 years of age and, arguably, both of described patients should have been commenced on this empirically once the diagnosis of infectious encephalitis was considered. Resistance of L. monocytogenes to antimicrobials is of increasing concern [22], [23] and, hence, antimicrobial combinations are usually employed to provide synergy. Both gentamicin and co-trimoxazole have been used [3], [21], [24]; however, in a cohort of 22 adult patients with severe L. monocytogenes meningoencephalitis, failure of the ‘gold standard' regimen (amoxicillin and gentamicin) was significantly higher (57%) than the group who were treated with amoxicillin and co-trimoxazole (6.7%; P < 0.05) [25]. Another advantage of using co-trimoxazole is its high oral bioavailability and, indeed, there are several case reports indicating that oral co-trimoxazole is effective in listeria meningitis [26], [27]. We used oral co-trimoxazole for the majority of the treatment of the first patient and as follow-up treatment for the second patient. Overall mortality has been quoted as 51%, although this may be reduced to less than 30% if treatment is initiated early [3], which is comparable to the 20–30% mortality of listeriosis in general [28]. Morbidity is also a significant concern because, like the first patient, 61% of patients have neurological sequelae [3].
Conclusions
Listeria rhombencephalitis is a severe infection with high mortality and morbidity. Early diagnosis and prompt commencement of appropriate antimicrobial therapy are essential to improve outcomes. Hence, it is important to consider including amoxicillin as part of the empirical therapy for cases of suspected meningoencephalitis, especially if the patient is at risk of listeriosis or there is a potential history of listeria exposure. Once treatment for LRE is commenced, repeat MRI is useful for follow up and for confirmation of the diagnosis if L. monocytogenes is suspected but unconfirmed by microbiological tests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that they have no competing interests.
Contributor Information
Christopher Thomas Mansbridge, Email: cmansbridge@doctors.org.uk.
Irina Grecu, Email: irina.grecu@hhft.nhs.uk.
Jimmy SW Li Voon Chong, Email: jimmy.chong@hhft.nhs.uk.
Clive Vandervelde, Email: clive.vandervelde@hhft.nhs.uk.
Kordo Saeed, Email: kordo.saeed@hhft.nhs.uk.
References
- 1.Kayaaslan B.U., Akinci E., Bilen S., Gozel M.G., Erdem D., Cevik M.A. Listerial rhombencephalitis in an immunocompetent young adult. Int J Infect Dis. 2009;13:e65–67. doi: 10.1016/j.ijid.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Clauss H.E., Lorber B. Central nervous system infection with Listeria monocytogenes. Curr Infect Dis Rep. 2008;10:300–306. doi: 10.1007/s11908-008-0049-0. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong R.W., Fung P.C. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis. 1993;16:689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 4.Moragas M., Martinez-Yelamos S., Majos C., Fernandez-Viladrich P., Rubio F., Arbizu T. Rhombencephalitis: a series of 97 patients. Medicine (Baltimore) 2011;90:256–261. doi: 10.1097/MD.0b013e318224b5af. [DOI] [PubMed] [Google Scholar]
- 5.Linke K., Ruckerl I., Brugger K., Karpiskova R., Walland J., Muri-Klinger S. Reservoirs of listeria species in three environmental ecosystems. Appl Environ Microbiol. 2014;80:5583–5592. doi: 10.1128/AEM.01018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allerberger F., Wagner M. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect. 2010;16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 7.Mylonakis E., Hohmann E.L., Calderwood S.B. Central nervous system infection with Listeria monocytogenes: 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998;77:313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Cossart P., Bierne H. The use of host cell machinery in the pathogenesis of Listeria monocytogenes. Curr Opin Immunol. 2001;13:96–103. doi: 10.1016/s0952-7915(00)00188-6. [DOI] [PubMed] [Google Scholar]
- 9.Zenewicz L.A., Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 2007;9:1208–1215. doi: 10.10110/2/076/j.micinf.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen E.R., Glass A.A., Clark W.R., Wing E.J., Miller J.F., Gregory S.H. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect Immun. 1998;66:4143–4150. doi: 10.1128/iai.66.9.4143-4150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg E.D. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984;6:814–831. doi: 10.1093/clinids/6.6.814. [DOI] [PubMed] [Google Scholar]
- 12.Montecino-Rodriguez E., Berent-Maoz B., Dorshkind K. Causes consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jubelt B., Mihai C., Li T.M., Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep. 2011;11:543–552. doi: 10.1007/s11910-011-0228-5. [DOI] [PubMed] [Google Scholar]
- 14.Antal E.A., Dietrichs E., Loberg E.M., Melby K.K., Maehlen J. Brain stem encephalitis in listeriosis. Scand J Infect Dis. 2005;37:190–194. doi: 10.1080/00365540410020938. [DOI] [PubMed] [Google Scholar]
- 15.O'Callaghan M., Mok T., Lefter S., Harrington H. Clues to diagnosing culture negative Listeria rhombencephalitis. BMJ Case Rep. 2012:2012. doi: 10.1136/bcr-2012-006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Monnier A., Abachin E., Beretti J.L., Berche P., Kayal S. Diagnosis of Listeria monocytogenes meningoencephalitis by real-time PCR for the hly gene. J Clin Microbiol. 2011;49:3917–3923. doi: 10.1128/JCM.01072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos L.G., Trindade R.A., Faistauer A. Rhombencephalitis: pictorial essay. Radiol Bras. 2016;49:329–336. doi: 10.1590/0100-3984.2015.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corless C.E., Guiver M., Borrow R., Edwards-Jones V., Fox A.J., Kaczmarski E.B. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–1558. doi: 10.1128/JCM.39.4.1553-1558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espy M.J., Uhl J.R., Sloan L.M., Buckwalter S.P., Jones M.F., Vetter E.A. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollock S.S., Pollock T.M., Harrison M.J. Infection of the central nervous system by Listeria monocytogenes: a review of 54 adult and juvenile cases. Q J Med. 1984;53:331–340. [PubMed] [Google Scholar]
- 21.Hof H., Nichterlein T., Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345–357. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morvan A., Moubareck C., Leclercq A., Herve-Bazin M., Bremont S., Lecuit M. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob Agents Chemother. 2010;54:2728–2731. doi: 10.1128/AAC.01557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan V., Nam H.M., Nguyen L.T., Tamilselvam B., Murinda S.E., Oliver S.P. Prevalence of antimicrobial resistance genes in Listeria monocytogenes isolated from dairy farms. Foodborne Pathog Dis. 2005;2:201–211. doi: 10.1089/fpd.2005.2.201. [DOI] [PubMed] [Google Scholar]
- 24.Alper G., Knepper L., Kanal E. MR findings in listerial rhombencephalitis. AJNR Am J Neuroradiol. 1996;17:593–596. [PMC free article] [PubMed] [Google Scholar]
- 25.Merle-Melet M., Dossou-Gbete L., Maurer P., Meyer P., Lozniewski A., Kuntzburger O. Is amoxicillin-cotrimoxazole the most appropriate antibiotic regimen for listeria meningoencephalitis? Review of 22 cases and the literature. J Infect. 1996;33:79–85. doi: 10.1016/s0163-4453(96)92929-1. [DOI] [PubMed] [Google Scholar]
- 26.Grant M.H., Ravreby H., Lorber B. Cure of Listeria monocytogenes meningitis after early transition to oral therapy. Antimicrob Agents Chemother. 2010;54:2276–2277. doi: 10.1128/AAC.01815-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunther G., Philipson A. Oral trimethoprim as follow-up treatment of meningitis caused by Listeria monocytogenes. Rev Infect Dis. 1988;10:53–55. doi: 10.1093/clinids/10.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Watson R. Listeriosis remains a cause for concern in Europe. BMJ. 2009;338:b319. doi: 10.1136/bmj.b319. [DOI] [PubMed] [Google Scholar]