Abstract
We encountered a case of a 73-year-old man with acute myeloid leukemia who developed Trichosporon asahii systemic infection while on itraconazole prophylaxis during severe neutropenia. Cryptococcal antigen was useful for diagnosis. Although itraconazole was ineffective in protecting against trichosporonosis, treatment was successful with voriconazole following liposomal amphotericin B.
Keywords: Trichosporon asahii, Itraconazole, Liposomal amphotericin B, Voriconazole, Cryptococcal antigen
1. Introduction
Trichosporon asahii is well recognized as a pathogen causing summer-type hypersensitivity pneumonitis in Japan [1]. Trichosporonosis, including T. asahii infection, is uncommon, but frequently causes devastating disseminated disease in patients with neutropenia [2]. The approximate mortality rate was reportedly very high in patients with hematological malignancies [2], [3]. Although the standard therapy remains to be established, some reports recommend azoles as optimal therapy for Trichosporon infection [4], [5]. While it is well-known that breakthrough trichosporonosis develops in patients with hematologic malignancies receiving echinocandin [6], [7], we encountered a patient with breakthrough T. asahii fungemia while taking itraconazole (ITCZ) as prophylaxis. Since only sporadic cases of breakthrough trichosporonosis have been reported in patients receiving azoles [8], [9], [10], we herein describe the clinical course of our case.
2. Case
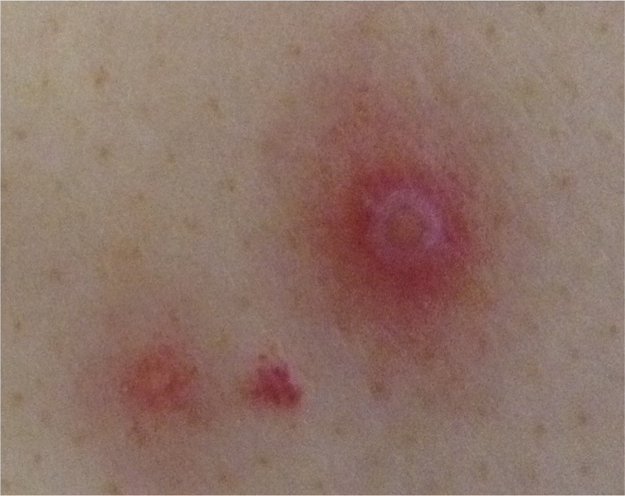
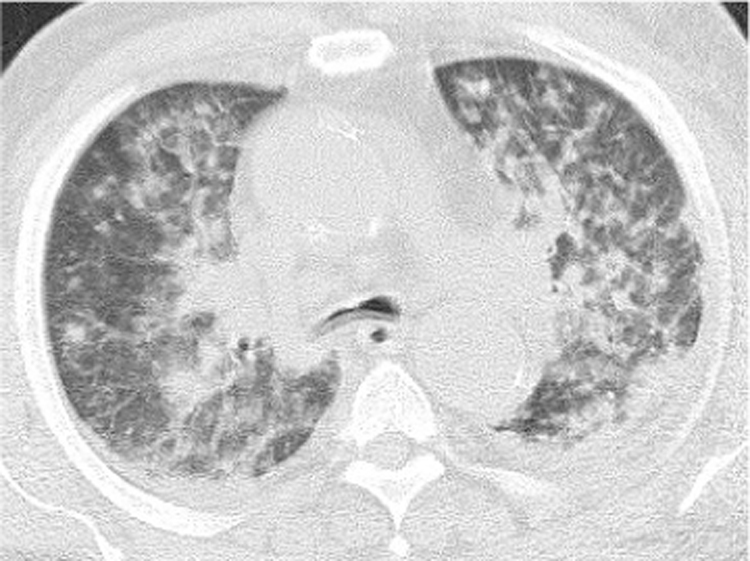
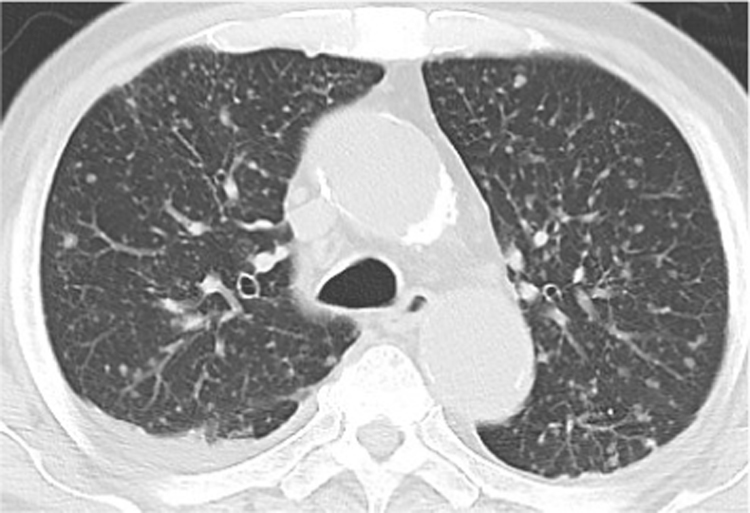
A 73-year-old patient with acute myeloid leukemia (AML) complained of oral aphthae and fever, and was admitted on day -6, prior to receiving induction chemotherapy (day 0), including idarubicin and cytarabine. Blood tests showed an elevated blast cell count (197 × 109 /L) and no neutrophils. Meropenem was administered on day -4 upon the diagnosis of extended-spectrum β-lactamase producing Escherichia coli infection. Oral ITCZ solution (200 mg/day) was also prescribed on day -5 as prophylaxis for fungal infection during the period of neutropenia. Although he defervesced on day 2, he developed a fever again on day 7 which gradually worsened, accompanied by multiple nodules in both lungs on day 13 and multiple skin rashes on day 14 (Fig. 1). Various antibiotics were ineffective in treating these symptoms and signs. Blood tests yielded positive results for (1-3)-β-D-glucan and negative results for Aspergillus galactomannan. In addition, oral and fecal cultures yielded positive results for Candida glabrata. These findings suggested that C. glabrata entered through oral or intestinal ulcers and caused fungemia and septic emboli in both lungs and the skin; thus, the oral ITCZ solution was switched to liposomal amphotericin B (L-AMB; 5 mg/kg) on day 13. He was intubated on day 18 because of severe hypoxia due to diffuse septic emboli (Fig. 2). On day 20, blood tests showed recovery of neutrophils, positive results for Cryptococcus neoformans antigen and negative results for Candida antigen, suggesting trichosporonosis because Trichosporon antigen reportedly could cross-react with Cryptococcal antigen [11]. Trichosporon species was identified in blood culture (amphotericin B, 1 μg/mL; ITCZ, 0.5 μg/mL; voriconazole (VRCZ), 0.06 μg/mL) on day 25 and in skin culture on day 43. In addition, positive results for T. asahii antibody were obtained on day 29. Based on these findings, T. asahii fungemia was diagnosed and L-AMB was switched to VRCZ (4 mg/kg) on day 26. VRCZ improved his respiratory condition and skin lesions. Bone marrow examination showed complete remission hematologically on day 56. We decided to continue administering VRCZ because chest computed tomography scan showed bilateral emboli despite improved oxygen saturation on day 90 (Fig. 3). He was transferred to a rehabilitation hospital on day 102.
Fig. 1.
Pustule with red macule. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Computed tomography scan image of lungs on day 18. Multiple nodules and consolidation are observed diffusely in both lungs.
Fig. 3.
Computed tomography scan image of lungs on day 90. Multiple residual nodules are observed diffusely in both lungs.
3. Discussion
The present case, which involved breakthrough trichosporonosis while on ITCZ prophylaxis, was successfully treated with VRCZ following L-AMB. Trichosporon species is well-known as a cause of systemic breakthrough infection in patients on echinocandin prophylaxis, because they show low susceptibility to echinocandin in vitro [6]. Recently, the European Confederation of Medical Mycology and the European Society for Clinical Microbiology and Infectious Diseases published a joint clinical guideline for the diagnosis and management of rare invasive yeast infections, which recommends VRCZ as an optimal option and fluconazole as a suboptimal option for treating trichosporonosis [5]. In addition, ITCZ is also reportedly effective [12], [13]. Based on these reports, azoles are considered as optimal treatments for trichosporonosis. On the other hand, some reports and the present case showed breakthrough Trichosporon infection in patients with neutropenia receiving azoles [8], [9], [10]. Our case received L-AMB, to which Trichosporon species have been reported to show low susceptibility [5]. However, his condition improved with increasing neutrophil count before VRCZ administration. This finding and a previous report [6] suggested that improvement of trichosporonosis depends more on neutrophil recovery compared to antifungal treatment, suggesting that prophylaxis with antifungal agents, even azoles, may not provide complete protection against trichosporonosis in patients with severe neutropenia. Therefore, trichosporonosis should be considered in the differential diagnosis of refractory fever in patients with severe neutropenia regardless of prophylaxis medication, including echinocandin and azoles.
In the present case C. glabrata, which is commonly resistant to VRCZ, was identified in fecal and sputum cultures, while cryptococcal antigen, which cross-reacts with Trichosporon antigen [11], showed positive results. Cryptococcosis commonly develops in patients who have cell-mediated immunodeficiency or receive immunosuppressants, including steroids [14]. Cryptococcosis is uncommon in patients with AML because such patients basically exhibit immunocomprised status due to neutropenia [14]. Positive cryptococcal antigen might show the probability of developing trichosporonosis more in patients with AML than in patients who have cell-mediated immunodeficiency. Therefore, we could not decide which pathogen (C. glabrata or Trichosporon) caused the infection due to limited information, including only positive (1-3)-β-D-glucan and the presence of septic emboli. Indeed, C. glabrata was found in fecal and sputum cultures, and C. glabrata infection is more common than trichosporonosis. Thus, we decided to continue the administration of L-AMB until we got accurate results of blood culture. The cryptococcal antigen test may lead to early diagnosis of trichosporonosis in patients with AML and may be useful for deciding optimal treatment. However, little is known about the usability of cryptococcal antigen test in patients with AML during neutropenia. Further studies are needed in order to address this issue.
We conclude that trichosporonosis should be recognized as a breakthrough infection under ITCZ prophylaxis during neutropenia. Our case suggested that the cryptococcal antigen test may lead to early diagnosis of trichosporonosis in patients with AML during refractory febrile neutropenia.
Acknowledgements
We appreciate all staff of the Hematology ward in Tachikawa hospital.
Acknowledgments
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Sugita T., Ichikawa T., Matsukura M., Sueda M., Takashima M., Ikeda R. Genetic diversity and biochemical characteristics of Trichosporon asahii isolated from clinical specimens, houses of patients with summer-type-hypersensitivity pneumonitis, and environmental materials. J. Clin. Microbiol. 2001;39(7):2405–2411. doi: 10.1128/JCM.39.7.2405-2411.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girmenia C., Pagano L., Martino B., D'Antonio D., Fanci R., Specchia G. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 2005;43(4):1818–1828. doi: 10.1128/JCM.43.4.1818-1828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo A.L., Padovan A.C., Chaves G.M. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin. Microbiol. Rev. 2011;24(4):682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki K., Nakase K., Kyo T., Kohara T., Sugawara Y., Shibazaki T. Fatal Trichosporon fungemia in patients with hematologic malignancies. Eur. J. Haematol. 2010;84(5):441–447. doi: 10.1111/j.1600-0609.2010.01410.x. [DOI] [PubMed] [Google Scholar]
- 5.Arendrup M.C., Boekhout T., Akova M., Meis J.F., Cornely O.A., Lortholary O. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014;20(Suppl 3):S76–S98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 6.Matsue K., Uryu H., Koseki M., Asada N., Takeuchi M. Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin. Infect. Dis. 2006;42(6):753–757. doi: 10.1086/500323. [DOI] [PubMed] [Google Scholar]
- 7.Akagi T., Yamaguti K., Kawamura T., Nakumura T., Kubo K., Takemori H. Breakthrough trichosporonosis in patients with acute myeloid leukemia receiving micafungin. Leuk. Lymphoma. 2006;47(6):1182–1183. doi: 10.1080/10428190500272499. [DOI] [PubMed] [Google Scholar]
- 8.Rieger C., Geiger S., Herold T., Nickenig C., Ostermann H. Breakthrough infection of Trichosporon asahii during posaconazole treatment in a patient with acute myeloid leukaemia. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26(11):843–845. doi: 10.1007/s10096-007-0366-5. [DOI] [PubMed] [Google Scholar]
- 9.Hosoki K., Iwamoto S., Kumamoto T., Azuma E., Komada Y. Early detection of breakthrough trichosporonosis by serum PCR in a cord blood transplant recipient being prophylactically treated with voriconazole. J. Pediatr. Hematol. Oncol. 2008;30(12):917–919. doi: 10.1097/MPH.0b013e3181864aa7. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Chen F., Wang Y., Yang L.Y., Miao M., Han Y. Use of combination therapy to successfully treat breakthrough Trichosporon asahii infection in an acute leukemia patient receiving voriconazole. Med. Mycol. Case Rep. 2014;6:55–57. doi: 10.1016/j.mmcr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyman C.A., Devi S.J., Nathanson J., Frasch C.E., Pizzo P.A., Walsh T.J. Detection and quantitation of the glucuronoxylomannan-like polysaccharide antigen from clinical and nonclinical isolates of Trichosporon beigelii and implications for pathogenicity. J. Clin. Microbiol. 1995;33(1):126–130. doi: 10.1128/jcm.33.1.126-130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canales M.A., Sevilla J., Ojeda Gutierrez E., Hernandez Navarro F. Successful treatment of Trichosporon beigelii pneumonia with itraconazole. Clin. Infect. Dis. 1998;26(4):999–1000. doi: 10.1086/517648. [DOI] [PubMed] [Google Scholar]
- 13.Meyer M.H., Letscher-Bru V., Waller J., Lutz P., Marcellin L., Herbrecht R. Chronic disseminated Trichosporon asahii infection in a leukemic child. Clin. Infect. Dis. 2002;35(2):e22–e25. doi: 10.1086/340983. [DOI] [PubMed] [Google Scholar]
- 14.Kontoyiannis D.P., Peitsch W.K., Reddy B.T., Whimbey E.E., Han X.Y., Bodey G.P. Cryptococcosis in patients with cancer. Clin. Infect. Dis. 2001;32(11):E145–E150. doi: 10.1086/320524. [DOI] [PubMed] [Google Scholar]