Abstract
Introduction
Amyloid-related decline in semantic memory was recently shown to be observable in the preclinical period of Alzheimer's disease. Cognitive composites designed to be sensitive to cognitive change in preclinical Alzheimer's disease (e.g., preclinical Alzheimer's cognitive composite [PACC]) and currently used in secondary prevention trials do not currently integrate measures of semantic processing. Our objective was to determine whether a standard semantic measure (i.e., category fluency [CAT] to animals, fruits, and vegetables) adds independent information above and beyond Aβ-related decline captured by the PACC.
Methods
Clinically normal older adults from the Harvard Aging Brain Study were identified at baseline as Aβ+ (n = 70) or Aβ− (n = 209) using Pittsburgh compound B–positron emission tomography imaging and followed annually with neuropsychological testing for 3.87 ± 1.09 years. The relationships between PACC, CAT, and variations of the PACC including/excluding CAT were examined using linear mixed models controlling for age, sex, and education. We additionally examined decline on CAT by further grouping Aβ+ participants into preclinical stage 1 and stage 2 on the basis of neurodegeneration markers.
Results
CAT explained unique variance in amyloid-related decline, with Aβ+'s continuing to decline relative to Aβ−'s in CAT even after controlling for overall PACC decline. In addition, removal of CAT from the PACC resulted in a longitudinal Aβ+/− effect size reduction of 20% at 3-year follow-up and 12% at 5-year follow-up. Finally, both stage 1 and stage 2 participants declined on CAT in comparison with stage 0, suggesting CAT declines early within the preclinical trajectory.
Conclusion
Addition of CAT to the PACC provides unique information about early cognitive decline not currently captured by the episodic memory, executive function, and global cognition components and may therefore improve detection of early Aβ-related cognitive decline.
Keywords: Alzheimer's disease, Preclinical, Neuropsychological test, Semantic, Verbal fluency
Highlights
-
•
Semantic fluency decline occurs early in the preclinical Alzheimer's disease trajectory.
-
•
Adding semantic fluency to the PACC provides unique information about Aβ-related decline.
-
•
Inclusion of more than one semantic category is preferable for maximizing Aβ group differentiation.
1. Introduction
Alzheimer's disease (AD) has an extended preclinical phase whereby changes in the brain, including accumulation of amyloid β (Aβ) plaques and neurofibrillary tau tangles, are occurring many years before the clinical diagnosis of AD dementia [1], [2]. Current clinical trials are targeting this preclinical period [3], [4] by recruiting individuals with elevated amyloid with the hope of applying disease-modifying therapies at a point before widespread pathological brain changes. Development of cognitive composites that can track the earliest cognitive changes associated with underlying AD pathology is vital for assessing the efficacy of these treatments [4], [5], [6].
Current cognitive composites focus on measures of episodic memory, executive function, and global cognition [7], [8]. For example, the preclinical Alzheimer's cognitive composite (PACC) was developed [7] using data from three observational cohort studies (AIBL, ADNI, and ADCS Prevention Instrument Study) and includes (1) the Mini–Mental State Examination (MMSE) [9], (2) Logical Memory Delayed Story Recall [10], (3) the Digit-Symbol Substitution Test [11], and (4) recall from the Free and Cued Selective Reminding Test (FCSRT) [12]. The PACC was recently shown to capture decline in Aβ+ versus Aβ− clinically normal older adults within an independent study sample, The Harvard Aging Brain Study [13].
Semantic memory decline, despite serving as a prototypical cognitive feature associated with plaque and tangle pathology [14], has not yet been included in these composites. This may be in part because semantic memory remains understudied and underutilized compared with episodic memory and is not considered an early cognitive change in the AD trajectory [15]. Interestingly, a measure of semantic memory was the first indicator of cognitive decline 12 years before diagnosis of AD dementia in the large PAQUID cohort [16]. Furthermore, two measures of semantic memory (i.e., category fluency [CAT] and the Boston Naming Test) were recently shown to be among a total of seven test outcomes identified as the most statistically sensitive measures of progression from normal cognition to a clinical stage of AD [17]. In addition, we recently identified decline in semantic fluency among Aβ+ clinically normal individuals, even after controlling for phonemic fluency [18]. Thus, recent evidence across multiple research groups has converged to suggest that semantic memory decline is occurring earlier in the AD trajectory than previously suspected [16], [18], [19], [20].
Given this background, our goal was to determine whether inclusion of a standard semantic memory measure added unique amyloid-related cognitive signal not captured by the PACC. We hypothesized that semantic memory would provide relevant information above and beyond the current PACC if the following criteria were met: (1) CAT would explain some portion of amyloid-related decline even when controlling for PACC decline; (2) inclusion of CAT in the PACC would increase the difference in longitudinal decline across Aβ groups; and (3) CAT showed evidence of early changes within individuals classified as preclinical stage 1 (positive for amyloidosis but negative for neurodegeneration [ND]) [21].
2. Methods
2.1. Sample characteristics
Harvard Aging Brain Study participants (n = 279) were recruited from the community. They were deemed clinically normal at baseline by (1) a global Clinical Dementia Rating score of 0; (2) performance above education-adjusted cutoffs on Logical Memory Story A Delayed Recall [1]; and (3) normal performance on the MMSE [22], [23]. The sample is 82% Caucasian, 15% African-American, 2% Asian-American, and 1% other. Review of medical history and physical and neurological examinations were completed to rule out major neurologic disorder. The study was conducted at Massachusetts General Hospital using protocols and informed consent procedures approved by the Partners Human Research Committee.
2.2. Magnetic resonance imaging data acquisition and analysis
Magnetic resonance imaging was completed at the MGH Martinos Center on the Siemens TIM Trio 3T system with a 12-channel head coil. Structural T1-weighted volumetric magnetization-prepared, rapid acquisition gradient echo scans (repetition time/echo time/inversion time = 6400/2.8/900 ms, flip angle = 8°, 1 × 1 × 1.2 mm resolution) were used to extract hippocampus volume with FreeSurfer v5.1 [24]. Total bilateral hippocampus volume was adjusted for estimated total intracranial volume [25].
2.3. PET data acquisition and analysis
Positron emission tomography (PET) scanning was used to measure fibrillar amyloid binding using Pittsburgh compound B (PIB) [26], [27] and glucose metabolism using fludeoxyglucose-18F (FDG). Scans were completed at the MGH PET facility using the Siemens ECAT EXACT HR+ PET scanner (three-dimensional mode; 63 image planes; 15.2 cm axial field of view; 5.6 mm transaxial resolution; and 2.4 mm slice interval).
Ten-minute transmission scans for attenuation correction were collected before emission data. For PIB, 8.5–15 mCi was injected, and 60 minutes of dynamic data were acquired in 69 frames (12 × 15 seconds, 57 × 60 seconds). For FDG, 5.0–10.0 mCi was injected, and images were acquired across 6 × 5-minute frames 45 minutes after injection.
PET preprocessing was performed using SPM8. PIB images were realigned, and the first 8 minutes were averaged and used for normalization to the Montreal Neurological Institute FDG template. Distribution volume ratio images were created with Logan plotting (40-60 minutes, gray matter cerebellar reference). PIB signal from a global cortical aggregate was extracted for each participant [25]. FDG-PET data were realigned, summed, and normalized to the Montreal Neurological Institute FDG template. FDG was extracted from a meta-region of interest reflecting AD-vulnerable cortical regions and normalized using a pons/vermis reference region [28].
2.4. Classification into Aβ+/− groups and preclinical stages using Aβ and ND status
A gaussian mixture modeling approach was used to classify participants as Aβ+ or Aβ− (cutoff value = 1.20) [29]. As previously described in detail [25], we used the presence of either hippocampal atrophy or FDG hypometabolism in the meta-region of interest to define the presence/absence of ND. Based on combined Aβ and ND status, participants were classified as stage 0 (Aβ−/ND−), stage 1 (Aβ+/ND−), and stage 2 (Aβ+/ND+) [30]. This subset consisted of fewer participants (n = 178) because only those with data from all three imaging modalities were selected. In addition, we did not include an Aβ−/ND+ group in this analysis given our goal to examine amyloid-related cognitive decline.
2.5. Neuropsychological tasks
Participants completed annual neuropsychological testing including the PACC and CAT [31] for a minimum of 1 year after baseline and for a maximum of 5 years of follow-up. The PACC z-score [7], [13] is calculated as mean performance across four measures including the MMSE (0–30) [9], the WMS-R Logical Memory Delayed Recall (LMDR; 0–25) [10], the Digit-Symbol Coding Test (DSC; 0–93) [11], and the Free and Cued Selective Reminding Test–Free + Total Recall (FCSRT96; 0–96) [12]. The PACC was originally designed to be computed as a sum across z-scores [7]; however, we averaged the z-scores to facilitate comparisons between different PACC versions in line with previous methods employed [13]. Alternate forms were administered for the FCSRT (A-B-C-A-B-C), whereas same forms were administered for all other measures. For the purpose of our analyses, the PACC5 was computed by including CAT as a fifth variable in the PACC. CAT included three 1-minute trials for generation of items belonging in the categories of animals, fruits, and vegetables. The number of correct words produced during each trial was summed resulting in the CAT score. Comparable results were obtained when averaging standardized scores of each category separately.
2.6. Statistical analyses
Statistical analyses were completed using R v3.3. Cognitive variables (MMSE, LMDR, DSC, FCSRT96, and CAT) were z-transformed using the baseline sample's mean and standard deviation. The PACC was computed by taking the mean of the z-scores across the four tests as previously described [13]. Linear mixed models (LMM) were used to assess the association between baseline Aβ status and change in cognition. In the first model, CAT was the dependent variable. Effects of baseline Aβ, age, sex and education, as well as their interactions with time were modeled as covariates. The PACC was added as a time-varying covariate to determine whether Aβ was associated with CAT over time after controlling for PACC performance over time. In addition, we included a term for the interaction between time-varying PACC and Aβ group, to establish whether Aβ group remains significantly associated with change in CAT. The model included a random intercept and slope for each participant.
We computed the PACC5 and examined the effect sizes reflecting the Aβ group difference in longitudinal cognitive trajectories by dividing the “LMM β” by its associated error term for the Aβ × time term. We then serially subtracted one component of the PACC5 individually to determine which cognitive measure resulted in a minimization of the Aβ group difference. We computed the percent decrease in effect size from the PACC5 for each iteration of the four-component PACC to assess the contribution of CAT in comparison with other PACC components. We also examined each category from CAT separately and average performance for two categories combined (e.g., animals + vegetables).
Analyses were completed using (1) the entire follow-up period (using all available data), referred to as the 5-year follow-up period and (2) the short (3-year) follow-up (excluding all follow-up data after 3 years of follow-up). Both samples contained the same 279 participants; however, mean follow-up for the short period was 2.87 years with a minimum of 1 year and maximum of 3 year follow-up. The 5-year follow-up group had a mean follow-up of 3.87 years with a range of 1 to 5 years.
Finally, we examined longitudinal cognitive changes on the individual components of the PACC by preclinical stages (0, 1, and 2) using LMMs [25] to determine whether CAT, in addition to individual PACC components, was changing in only those who were Aβ+/ND+ or whether decline was observable in Aβ+/ND−. Decline in stage 1 and stage 2 may represent a cognitive test sensitive earlier within the preclinical period compared with a test not exhibiting signal until both amyloidosis and ND are present (i.e., stage 2). We examined all pairwise contrasts (stage 0 vs. stage 1; stage 0 vs. stage 2; and stage 1 vs. stage 2) and quantified the relative magnitude of decline between groups using an effect size (β estimate/standard error) for each contrast.
3. Results
There were no differences in performance between Aβ+/− groups on CAT or any individual PACC components at study outset (Table 1). Aβ+ participants were older compared with Aβ− participants. At baseline, participants produced more animal names in comparison with vegetable or fruit names (Table 1). The “n” for Aβ+'s contributing to each year of follow-up after baseline was 70/69/69/48/28, and for the Aβ−'s, it was 209/197/193/129/75.
Table 1.
Descriptive characteristics of Harvard Aging Brain Study cohort, by baseline Aβ+/− status
| All | Aβ+ | Aβ− | |
|---|---|---|---|
| N (%) | 279 | 70 (25%) | 209 (74.9%) |
| Age (years)∗ | 73.42 ± 6.01 | 74.99 ± 5.74 | 72.88 ± 6.02 |
| APOE ε4+ (%)∗ | 29 | 61 | 18 |
| Female (%) | 59 | 61 | 59 |
| Education | 15.85 ± 3.04 | 16.25 ± 2.94 | 15.71 ± 3.08 |
| MMSE | 29.00 ± 1.10 | 28.83 ± 1.06 | 29.06 ± 1.11 |
| LMDR | 13.73 ± 3.27 | 14.01 ± 3.14 | 13.62 ± 3.32 |
| DSC | 47.23 ± 10.69 | 46.93 ± 10.03 | 47.34 ± 10.93 |
| FCSRT96 | 80.91 ± 5.87 | 80.73 ± 5.95 | 80.96 ± 5.86 |
| CAT | 44.42 ± 9.99 | 44.93 ± 9.97 | 44.24 ± 10.02 |
| Animals | 17.85 ± 5.07 | 18.19 ± 4.50 | 17.73 ± 5.25 |
| Vegetables | 13.37 ± 3.53 | 13.60 ± 3.42 | 13.29 ± 3.57 |
| Fruits | 13.21 ± 3.50 | 13.67 ± 3.64 | 13.05 ± 3.44 |
Abbreviations: CAT, category fluency; DSC, Digit-Symbol Coding Test; FCSRT96, Free and Cued Selective Reminding Test; MMSE, Mini–Mental State Examination; LMDR, Logical Memory Delayed Recall.
NOTE. χ2 tests were used to compare sex distributions, and t-tests were used to compare continuous variables. Mean and standard deviations are reported unless otherwise stated.
Variables with significant differences between Aβ groups (P < .05).
3.1. Correlations among cognitive variables at baseline and longitudinally
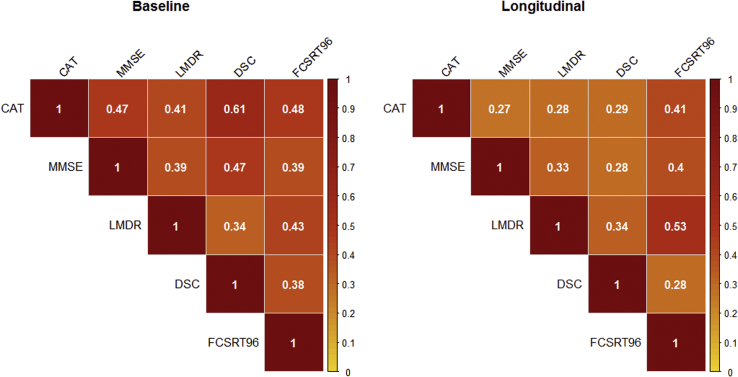
At baseline, CAT shared 22% of the variance in MMSE, 17% of the variance in LMDR, 37% of the variance in DSC, and 23% of the variance in FCSRT96 (Fig. 1). Longitudinally, the relationship between change in CAT and change in each PACC component was smaller overall, accounting for 7% of the variance in MMSE, 8% of the variance in LMDR, 8% of the variance in DSC, and 17% of the variance in FCSRT96. The relatively low correlation between CAT and other PACC components, especially when examined longitudinally, suggests that this metric may provide complementary information regarding Aβ-related decline among clinically normal participants (Fig. 1).
Fig. 1.
Correlation matrix of individual tests from the PACC, category fluency. Cross-sectional relationships (Pearson's r) are represented in left panel, and correlations among slopes are represented in the right panel. Abbreviations: CAT, category fluency, MMSE, Mini–Mental State Examination, LMDR, Logical Memory Delayed Recall; DSC, Digit-Symbol Coding Test, FCSRT96, Free and Cued Selective Reminding Test–Free + Total.
3.2. Longitudinal decline in CAT in relation to Aβ status and PACC
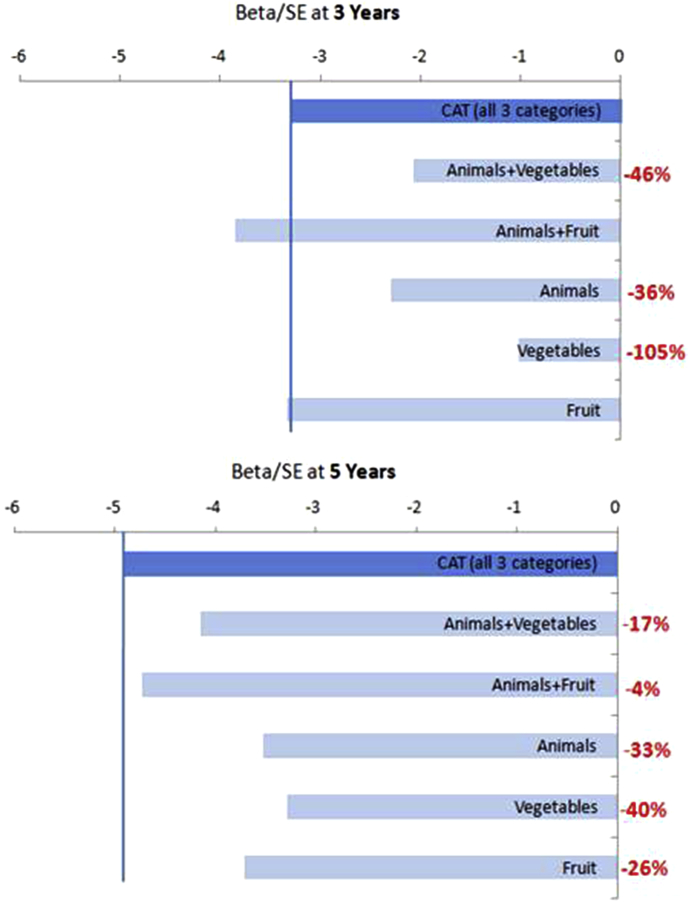
Aβ+'s exhibited greater decline over time compared with Aβ−'s on CAT (t(1082) = −4.79, P < .001), and this association remained significant after adding PACC as a covariate (Table 2). Furthermore, the time by Aβ group term remained significant despite inclusion of a term for the interaction between time-varying PACC and Aβ group highlighting that even after controlling for PACC decline specifically within the Aβ+ group, there was a significant effect of Aβ group on CAT. Likewise, Aβ+'s exhibited greater decline over time compared with Aβ−'s on the PACC (t(1082) = −4.21, P < .001) as has been previously reported [13], and this association remained significant after controlling for CAT. While Aβ+'s declined on all individual categories (i.e., animals, vegetables, and fruit), the amyloid effect size was between 26% and 40% larger when combining all three trials into CAT (Fig. 3) when using 5 years of follow-up. During the shorter follow-up of 3 years, the comparison between individual categories was more complex. Specifically, fruit independently exhibited an effect size comparable to combining across all three categories, whereas vegetable alone was not statistically significant (Fig. 3).
Table 2.
Linear Mixed Model Assessing the Interaction Between Aβ Status and Time on the PACC and CAT
| Model 1: CAT, covary PACC |
||||
|---|---|---|---|---|
| b | SE | t | P | |
| Intercept | 0.06 | 0.07 | 0.83 | .408 |
| Time | −0.07 | 0.20 | −4.76 | <.001 |
| Aβ+ | 0.11 | 0.11 | 0.97 | .332 |
| Age | −0.03 | 0.01 | −3.44 | <.001 |
| Education | 0.10 | 0.02 | 6.38 | <.001 |
| Sex (male) | −0.32 | 0.10 | −3.36 | <.001 |
| PACC | 0.28 | 0.04 | 6.56 | <.001 |
| Aβ+ × PACC | 0.03 | 0.07 | 0.42 | .672 |
| Aβ+ × time | −0.09 | 0.02 | −3.74 | <.001 |
| Age × time | −0.00 | 0.00 | −1.54 | .123 |
| Education × time | 0.00 | 0.00 | −0.25 | .805 |
| Sex × time | 0.02 | 0.02 | 1.01 | .322 |
Abbreviations: CAT, category fluency; PACC, preclinical Alzheimer's cognitive composite; SE, standard error.
NOTE. Age is centered at 75 years, and education is centered at 16 years.
Fig. 3.
Annualized effect sizes (β/SE) reflecting Aβ+/− group difference for variations of CAT. Only Aβ+ × time effects are shown. Models included maximal follow-up time (bottom) and 3-year follow-up (top). All models with 5-year follow-up are significant (P < .01). With 3-year follow-up, the Aβ × time interaction for vegetables is no longer significant (P = .310) and for animals + vegetables is significant at P < .05. In red is the percentage decrease in effect when comparing individual or combined categories to three categories (top bar). Abbreviations: CAT, category fluency; SE, standard error.
3.3. Comparing different versions of the PACC
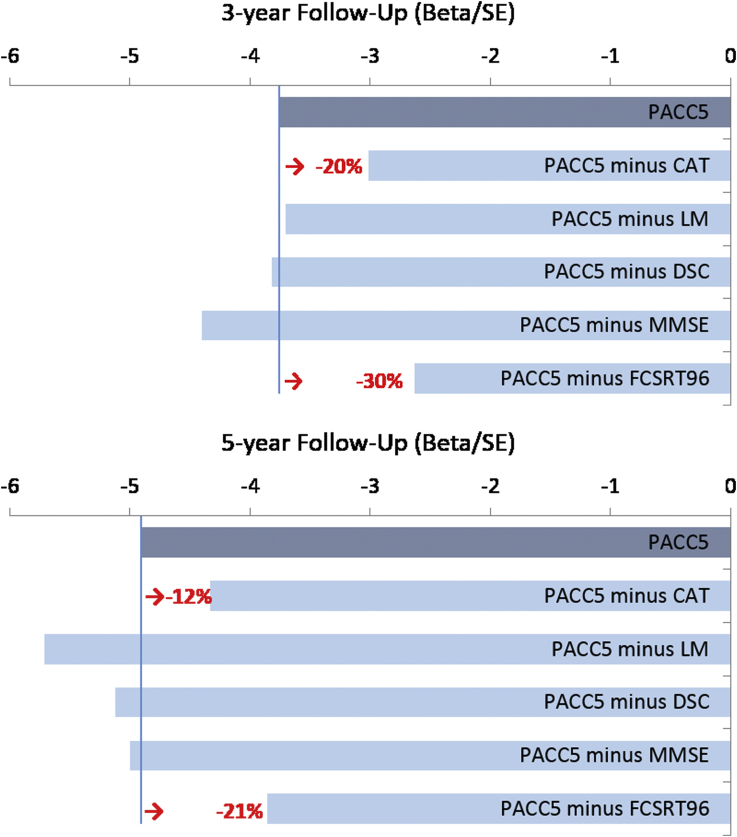
Estimated effects (z-score change per year) across multiple variations of the PACC were consistent across models assessing longer (5-year) and shorter (3-year) follow-up (Fig. 2, Supplementary Table 1) and ranged between −0.06 to −0.12 (Fig. 2). Removal of CAT from the PACC results in a 20% reduction in effect size at year 3 and a 12% reduction at year 5. The only other outcome associated with a reduction in effect size was FCSRT, where its removal resulted in a 30% reduction in effect at 3 years and a 21% reduction at 5 years. An LMM assessing Aβ-related decline in FCSRT96 controlling for CAT, as well as an LMM assessing Aβ-related decline in CAT controlling for FCSRT96, confirmed independent Aβ-related signal in both FCSRT96 and CAT (Supplementary Table 2).
Fig. 2.
Annualized effect sizes reflecting Aβ+/− group difference for variations of the preclinical Alzheimer's cognitive composite (PACC) after 3-year (top) and 5-year (bottom) follow-up. All models are statistically significant (P < .01). In red is the percentage decrease in effect size comparing a four-component PACC to the PACC5 where applicable. PACC5 includes CAT, LMDR, DSC, MMSE, and FCSRT96. Abbreviations: CAT, category fluency; DSC, Digit-Symbol Coding Test; FCSRT96, Free and Cued Selective Reminding Test; LMDR, Logical Memory Delayed Recall; MMSE, Mini–Mental State Examination.
3.4. Category fluency decline by preclinical stage
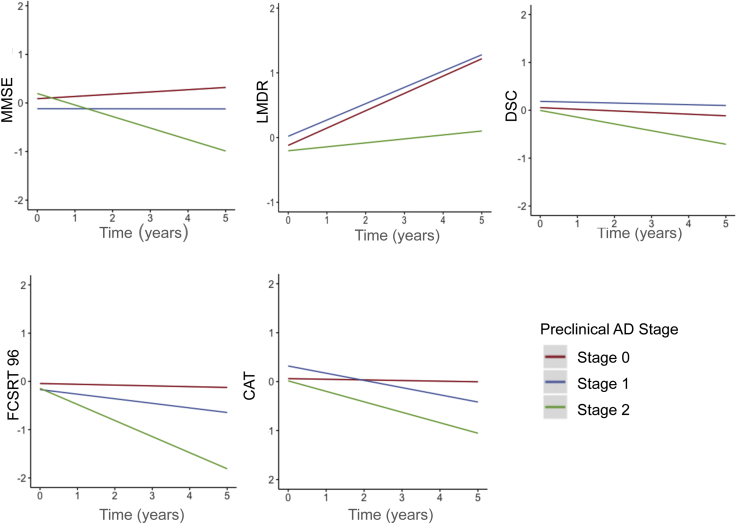
Demographic characteristics for the sample by preclinical stage are available in Supplementary Table 3. Stage 2 participants declined on all individual components of the PACC, including CAT in comparison with stage 0 participants (Supplementary Table 4, Fig. 4). Interestingly, the only difference in longitudinal performance between stage 0 and stage 1 was observed in CAT. There was a nonsignificant trend for decline in stage 0 versus stage 1 for FCSRT96 performance (P = .178); however, stage 1 participants performed similarly to stage 0 participants for MMSE, LMDR, and DSC.
Fig. 4.
Modeled slopes for cognitive decline on individual components of the PACC-5 by biomarker-defined preclinical stage 0 (Aβ-/ND-), 1(Aβ+/ND-), and 2 (Aβ+/ND+). Abbreviations: CAT, category fluency; DSC, Digit-Symbol Coding Test, FCSRT96, Free and Cued Selective Reminding Test; LMDR, Logical Memory Delayed Recall; MMSE, Mini–Mental State Examination.
4. Discussion
Our results suggest that a standard semantic memory measure (i.e., CAT to animals, fruits, and vegetables) adds independent information about amyloid-related cognitive decline not currently captured in the PACC. More specifically, we showed that CAT continued to explain some portion of amyloid-related cognitive decline even when controlling for overall PACC performance. Second, we showed that removal of CAT from the PACC resulted in a 20% reduction in amyloid-related decline at 3 years of follow-up and a 12% reduction at 5 years of follow-up. Finally, we showed that CAT is a measure that declines early within the preclinical trajectory, showing decline even among the stage 1 group.
These results add to a growing body of work indicating that semantic memory decline is observable in the preclinical stages of AD [18], [32], [33]. These findings also provide new information about the natural history of Aβ-related semantic fluency decline. More specifically, the greater effect size drop at 3- versus 5-year follow-up when removing CAT from the PACC suggests that CAT declines are observable early within the preclinical period [13]. Furthermore, our finding that CAT was the only PACC component that differentiated stage 0 versus stage 1 suggests that CAT may be particularly useful in secondary prevention trials targeting individuals at the beginning stages of the preclinical AD trajectory (i.e., stage 1 vs. stage 2/3). However, in addition to this early signal, CAT continues to add information regarding Aβ-related cognitive decline over the longer 5-year follow-up period.
Both CAT and the FCSRT were the greatest contributors to the PACC regardless of follow-up time. While the FCSRT falls under the umbrella of episodic or associative memory tests, a semantic element is involved. Specifically, the FCSRT is distinct from most list-learning paradigms in that this test includes a semantic cueing component to facilitate learning and recall. As such, the FCSRT may be more reliant on the integrity of semantic networks versus traditional episodic memory measures such as story learning and recall (i.e., Logical Memory). In line with this, the FCSRT showed similar correlation strengths with CAT (baseline r = 0.48 and longitudinal r = 0.41) as it did with LMDR (baseline r = 0.43 and longitudinal r = 0.53), highlighting that the FCSRT incorporates both semantic processing and memory domains. Importantly, despite similarities across the FCSRT and CAT, each measure was independently related to amyloidosis, highlighting the relevant signal encapsulated in each test.
Our finding of sequentially larger effect sizes when examining three categories versus individual or two categories indicates that inclusion of more than one category is preferable for maximizing Aβ+/− group differentiation over time. The greater variability in effect sizes between different categories at 3- versus 5-year follow-up implies that use of individual categories (e.g., vegetables rather than animals) may have important implications for (1) whether an Aβ effect is observed and (2) the magnitude of the Aβ effect, particularly in shorter studies with unimpaired individuals. More specifically, vegetable fluency was nonsignificant (β/SE = −1.03, P = .310), whereas fruit fluency (β/SE = −3.33, P = .001) exhibited a longitudinal Aβ effect comparable to that observed in the three-component CAT (β/SE = −3.30, P = .001). Differential Aβ effects combined with global differences in performance by category (e.g., participants reliably produce more animals compared with vegetables) suggest they may be measuring different aspects of semantic memory. For example, in a study of primarily MCI participants, vegetable fluency performance corresponded to atrophy in a more diffuse set of cortical regions compared with animal fluency concluding that different categories may be dependent on the cortical integrity of different regions [34]. If only two categories can be used, animals and fruits outperform animals and vegetables.
While we chose CAT as our metric of global semantic decline, there are varying semantic measures, which may also exhibit sensitivity at a preclinical stage, such as measures of confrontation naming. However, on measures such as the Boston Naming Test, performance is often strongly linked with educational level, ceiling effects are present in normal older adults, and cultural specificity limits the production of valid alternate forms in global trials [35]. The lack of ceiling effects and normal distribution of scores produced by CAT are test properties, which facilitate its incorporation into a composite score.
A caveat to consider in applying our findings to clinical trial design is the differences in statistical models employed. While our analyses centered on LMMs, future work may be necessary to examine PACC iterations with statistical models used in clinical trials such as mixed effect model of repeat measurement. In addition, our sample is American, English-speaking, and 82% Caucasian, and future work is required to determine the generalizability of the PACC5 to more ethnically, educationally, and culturally diverse communities. Another potential limitation of the current work is the diminishing returns of further optimizing a well-functioning composite. Multiple reports have shown efficacy of the PACC to detection of amyloid-related cognitive decline. These findings persist despite variations on the cohorts employed [13], measures used [8], and test weightings adjusted [36]. These converging findings suggest that the use of a cognitive composite that incorporates multiple domains is effective in detecting amyloid-related cognitive decline within the preclinical period. However, our finding that semantic memory provides unique information about amyloid-related cognitive decline not currently captured in measures of memory, executive functions, and global cognition suggests that future prevention trial designs should consider including category generation in their cognitive composites.
Research in Context.
-
1.
Systematic review: Review of the literature reveals that semantic memory is not included in multidomain composites designed to detect cognitive decline in the preclinical stages of Alzheimer's disease.
-
2.
Interpretation: The addition of category fluency to the preclinical Alzheimer's cognitive composite provides unique information about early cognitive decline not currently captured by the episodic memory, executive function, and global cognition components and may therefore improve detection of early Aβ-related decline. Future prevention trial designs should consider including category generation in their cognitive composites.
-
3.
Future directions: As more longitudinal cognitive data of normal older adults with biomarker abnormalities become available, a better understanding of the nature of cognitive decline in preclinical Alzheimer's disease will emerge. This will allow us to optimize outcomes in clinical trials targeting this preclinical population.
Acknowledgments
K.V.P. was supported by NIA grant K23 AG053422-01 and the Alzheimer's Association. D.M.R. received research support from the NIH grants P01 AG036694, R01 MH090291, U01 AG024904, R01 AG027435, R01 AG037497, and P50 AG005134, Fidelity Investigator-Initiated grant, and Alzheimer Association grant SGCOG-13-282201.D. Author I.O. receives research support from NIH grant P01 AG036694. R.A.S. receives research support for NIH grants U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, and P01 AG036694, Alzheimer's Association, Fidelity Biosciences, Harvard NeuroDiscovery Center, Janssen Pharmaceuticals, and Eli Lilly and Co. E.C.M. received funding from NIH grant K01 AG051718.
The authors would like to thank all of the participants in the Harvard Aging Brain Study for their dedication to the project. We would like to also thank our co-investigators: Keith A. Johnson, Gad A. Marshall, Rebecca E. Amariglio, Yakeel Quiroz, Nancy Donovan, Jennifer Gatchel, Rachel Buckley, Aaron P. Schultz, Jonathan Jackson, Jasmeer Chhatwal, Hyun-Sik Yang, Patrizia Vannini, Bernard Hanseeuw, as well as research assistants and affiliated staff: Dylan Kirn, Michael Properzi, Emily Kilpatrick, Sarah Aghjayan, Paige Sparks, Maria Dekhtyar, Chris Lee, Victoria Jonas, Martha Muniz, Nick Andrea, Fred Uquillas, and Molly Lapoint. K.V.P. has served as a consultant for Biogen Idec. D.M.R. has served as a consultant for Eli Lilly, Biogen Idec, Lundbeck Pharmaceuticals, and serves as a member of the Scientific Advisory Board for Neurotrack. I.O. has no disclosures to report. R.A.S. has research support from Eli Lilly, Avid, and Janssen. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen Alzheimer Immunotherapy. These relationships are not related to the content in the manuscript. E.C.M. has served as a consultant for Janssen, Eli Lilly, and Biogen Idec.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2017.10.004.
Supplementary data
References
- 1.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price J.L., Morris J.C. Tangles and plaques in nondemented aging and “ preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M.C., Salmon D.P. The A4 Study: stopping AD Before Symptoms Begin? Sci Transl Med. 2014;6:228. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling R.A., Jack C.R., Jr., Aisen P.S. Testing the Right Target and Right Drug at the Right Stage. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002609. 111cm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rentz D.M., Rodriguez M.A.P., Amariglio R.E., Stern Y., Sperling R., Ferris S. Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer's disease: a selective review. Alzheimers Res Ther. 2013;5:1–20. doi: 10.1186/alzrt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U. Food, D. Administration . 2013. Guidance for Industry. Alzheimer's Disease: Developing Drugs for the Treatment of Early Stage Disease. Draft Guidance. Federal Register: Center for Drug Evaluation and Research. Available at: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338287.pdf. Accessed November 21, 2017. [Google Scholar]
- 7.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. AIBL, ADNI, ADCS, the preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim Y.Y., Snyder P.J., Pietrzak R.H., Ukiqi A., Villemagne V.L., Ames D. Sensitivity of composite scores to amyloid burden in preclinical Alzheimer's disease: Introducing the Z-scores of Attention, Verbal fluency, and Episodic memory for Nondemented older adults composite score. Alzheimers Dement (Amst) 2016;2:19–26. doi: 10.1016/j.dadm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler D., Stone C.P. Psychological Corporation; San Antonio, TX: 1987. Wechsler Memory Scale-revised. [Google Scholar]
- 11.Wechsler D. Psychological Corporation; San Antonio, TX: 1981. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- 12.Grober E., Ocepek-Welikson K., Teresi J.A. The free and cued selective reminding test: evidence of psychometric adequacy. Psychol Sci Q. 2009;51:266–282. [Google Scholar]
- 13.Mormino E.C., Papp K.V., Rentz D.M., Donohue M.C., Amariglio R., Quiroz Y.T. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated β-amyloid. Alzheimers Dement. 2017;13:1004–1012. doi: 10.1016/j.jalz.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butters N., Granholm E., Salmon D.P., Grant I., Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 1987;9:479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- 15.Joubert S., Brambati S.M., Ansado J., Barbeau E.J., Felician O., Didic M. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer's disease. Neuropsychologia. 2010;48:978–988. doi: 10.1016/j.neuropsychologia.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Pérès K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 17.Langbaum J.B., Hendrix S.B., Ayutyanont N., Chen K., Fleisher A.S., Shah R.C. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:666–674. doi: 10.1016/j.jalz.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papp K.V., Mormino E.C., Amariglio R.E., Munro C., Dagley A., Schultz A.P. Biomarker validation of a decline in semantic processing in preclinical Alzheimer's disease. Neuropsychology. 2016;30:624–630. doi: 10.1037/neu0000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snitz B.E., Weissfeld L.A., Lopez O.L., Kuller L.H., Saxton J., Singhabahu D. Cognitive trajectories associated with b-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80:1378–1384. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen V.M., Sunderland T., Levy J., Harwell A., McGee L., Hammond C. Apolipoprotein E and category fluency: evidence for reduced semantic access in healthy normal controls at risk for developing Alzheimer's disease. Neuropsychologia. 2005;43:647–658. doi: 10.1016/j.neuropsychologia.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mungas D., Marshall S.C., Weldon M., Haan M., Reed B.R. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46:700–706. doi: 10.1212/wnl.46.3.700. [DOI] [PubMed] [Google Scholar]
- 23.Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 24.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 25.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Amariglio R.E., Rentz D.M. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathis C.A., Wang Y., Holt D.P., Huang G.F., Debnath M.L., Klunk W.E. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 27.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging Brain Amyloid in Alzheimer's Disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 28.Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L. A.s.D.N. Initiative, Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mormino E., Betensky R.A., Hedden T., Schultz A.P., Ward A., Huijbers W. Amyloid and APOE e4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack C.R., Jr., Knopman D.S., Weigand S.D., Wiste H.J., Vemuri P., Lowe V. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monsch A.U., Bondi M.W., Butters N., Salmon D.P., Katzman R., Thal D.R. Comparisons of Verbal Fluency Tasks in the Detection of Dementia of the Alzheimer Type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 32.Rao S.M., Bonner-Jackson A., Nielson K.A., Seidenberg M., Smith J.C., Woodard J.L. Genetic risk for Alzheimer's disease alters the five-year trajectory of semantic memory activation in cognitively intact elders. Neuroimage. 2015;111:136–146. doi: 10.1016/j.neuroimage.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venneri A., Mitolo M., De Marco M. Paradigm shift: semantic memory decline as a biomarker of preclinical Alzheimer's disease. Biomark Med. 2016;10:5–8. doi: 10.2217/bmm.15.53. [DOI] [PubMed] [Google Scholar]
- 34.Eastman J.A., Hwang K.S., Lazaris A., Chow N., Ramirez L., Babakchanian S. Cortical thickness and semantic fluency in Alzheimer's disease and mild cognitive impairment. Am J Alzheimers Dis (Columbia) 2013;1:81–92. doi: 10.7726/ajad.2013.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lansing A.E., Ivnik R.J., Cullum C.M., Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol. 1999;14:481–487. [PubMed] [Google Scholar]
- 36.Donohue M.C., Sun C.K., Raman R., Insel P.S., Aisen P.S. Cross-validation of optimized composites for preclinical Alzheimer. Alzheimers Dement (N Y) 2017;3:123–129. doi: 10.1016/j.trci.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.