Abstract
Citrus Huanglongbing (HLB), a highly devastating citrus disease, is associated with ‘Candidatus Liberibacter asiacitus’ (CLas), a member of phloem-inhabiting α-proteobacteria. HLB can affect all cultivated citrus and no cure is currently available. Previous studies showed that Kaffir lime (Citrus hystrix), primarily grown in South Asia and Southeast Asia, was tolerant to HLB but the molecular mechanism remains unknown. In this study, gene expression profiling experiments were performed on HLB-tolerant C. hystrix and HLB-susceptible C. sinensis three months after inoculation with CLas using RNA-seq data. Differentially expressed genes (DEGs) in the two citrus cultivars were mainly involved in diverse cellular functions including carbohydrate metabolism, photosynthesis, cell wall metabolism, secondary metabolism, hormone metabolism and oxidation/reduction processes. Notably, starch synthesis and photosynthesis process were not disturbed in CLas-infected C. hystrix. Most of the DEGs involved in cell wall metabolism and secondary metabolism were up-regulated in C. hystrix. In addition, the activation of peroxidases, Cu/Zn-SOD and POD4, may also enhance the tolerance of C. hystrix to CLas. This study provides an insight into the host response of HLB-tolerant citrus cultivar to CLas. C. hystrix is potentially useful for HLB-tolerant/resistant citrus breeding in the future.
Introduction
Citrus is an important economic crop in tropical and sub-tropical regions worldwide. Global citrus production in 2014 exceeded 121 million metric tons (http://www.fao.org/economic/est/est-commodities/citrus/en/), ranked the top among all the fruit crops. Citrus Huanglongbing (HLB, yellow shoot disease, also known as citrus greening disease) is one of the most destructive diseases in citrus production [1]. The disease is associated with three species of non-culturable, phloem-limited, α-Proteobacteria: ‘Candidatus Liberibacter asiaticus’, ‘Ca. L. africanus’, and ‘Ca. L. americanus’ [2, 3]. Among the three species, ‘Ca. L. asiaticus’ (CLas) is the most prevalent in major citrus-producing regions, e.g. China, Brazil and the United States.
No effective cure is currently available for HLB-infected citrus plants. Integrated management practices including the use of pathogen-free nursery stocks, control of insect vectors and removal of infected trees are the commonly recommended strategies. Although all commercial citrus cultivars are susceptible to HLB, a few HLB resistance or tolerance citrus and citrus relativeshave been reported [4–6]. Kaffir lime (C. hystrix), primarily grown in South and Southeast Asia, has been used for cooking and traditional medicine. Extracts of Kaffir lime leaves, rich in terpenoids and phenolic contents, could inhibit the proliferation of cancer cells and biofilm formation of bacteria [7, 8]. C. hystrix was experimentally validated as HLB-tolerance citrus cultivar in Malaysia [4, 9]. Similar result was also obtained in our laboratory based on three-year biological indexing and qPCR monitoring (unpublished data).
To better understand the HLB resistance and tolerance mechanisms in citrus, substantial research efforts have been made to study citrus-CLas interaction through microarray and high-throughput sequencing technology [10–16]. Gene expression profiles of different citrus leaves, fruits and roots from susceptible citrus cultivars infected with CLas showed that genes involved in sugar and starch metabolism, photosynthesis, cell wall metabolism, stress response and hormone signaling were significantly altered [10–16]. Comparative transcriptional and transcriptome studies between HLB tolerant and susceptible citrus cultivars were performed to identify genes of HLB tolerance to HLB (17–19). The HLB tolerance breeding line US-897 (C. reticulata×P. trifoliata) might be associated with the constitutively higher expression of defense-related genes, compared with the susceptible ‘Cleopatra’ mandarin (C. reticulata) [17]. Comparative transcriptional analysis of tolerant rough lemon (C. jambhiri) and susceptible sweet orange (C. sinensis) in response to CLas infection revealed more differentially expressed genes in HLB-infected rough lemon than those in sweet orange at early stages but substantially fewer at late time points. Genes coding for cell wall proteins, β-1, 3-glucanases, and GASA1, were identified as potential HLB tolerant citrus improvements program [18]. Recently, expression differences in secondary metabolites, pathogenesis related genes, transcription factors, hormone signaling pathways and receptor-like kinases were found from ‘Jackson’ grapefruit-like-hybrid and susceptible ‘Marsh’ grapefruit (C. paradisi) [19].
In light of the successful examples above, this study was to study transcriptical difference between highly susceptible sweet orange trees and tolerant kaffir lime trees using RNA-seq approach. The identification of differentially expressed genes in the two citrus cultivars at an early stage of HLB infection provides new information for future HLB management.
Materials and methods
Plant materials
The disease free budwoods of tolerant kaffir lime (C. hystrix) and susceptible pineapple sweet orange (C. sinensis) collected from virus-free citrus repository of National Citrus Virus Exclusion Center (NCVEC) in Southwest University were grafted on two-year-old Carrizo citrange rootstocks (C. sinensis×P. trifoliata), respectively. The budwood used for inoculation was Guanximiyou pummelo (C. grandis) solely infected with CLas strain GZBJT maintained in greenhouse in NCVEC. Three replicate trees per genotype were infected with CLas through bud grafting, with three CLas-free bud grafted trees as the control.
DNA extraction and ‘Ca. L. asiaticus’ detection
Fully expanded mature leaves were collected from diseased and mock-inoculated trees every twenty days for DNA extraction described previously [20]. Both conventional PCR using primer set OI1/OI2c and TaqMan qPCR assays with an iCycler IQ5 (BioRad, Hercules, CA) were used to detect CLas [2, 21].
RNA extraction and RNA-sequencing
Once infection had been confirmed by PCR and qPCR, total RNA were extracted from fully expanded young leaves with mortar and pestle in liquid nitrogen. RNA extraction process was performed according to TRIzol protocol (Invitrogen, Carlsbad, CA). The concentration of total RNA was determined by Nanodrop 2000 Spectrophotometer, and the RNA integrity number (RIN) was evaluated by Agilent 2100 Bioanalyzer. Three RNA replicates of each treatment were pooled in equal amount, and sent to Beijing Genomics Institute (BGI)-Wuhan (China) for RNA sequencing.
Data analysis
After cleaning the raw reads, we mapped the clean reads to Citrus sinensis genome (http://www.ncbi.nlm.nih.gov/genome/ 10702) using Bowtie 2 [22] and then calculated gene expression level with RSEM [23]. Differentially expressed genes (DEGs) were screened based on PossionDis with a cutoff threshold FDR of 0.001 and log2 fold change (FC) of ≥ 1.00 or ≤ -1.00.
Functional enrichment analysis was carried out using the GO database (http://www.geneontology.org/). The DEGs were also analyzed by PageMan [24] embedded in MapMan [25]. A Wilcoxon test was applied and a statistics-based overview of changed pathways from global gene expression alterations was provided. The same data sets were also imported into MapMan to map the differentially expressed genes into specific pathways.
RT-qPCR validation
To verify the results of RNA-seq, 16 DEGs involved in carbohydrate metabolism, photosynthesis, cell wall metabolism, secondary metabolism, hormone metabolism and Pathogenesis related (PR)-proteins were selected for RT-qPCR analysis. The same batch RNA samples used in RNA-seq analysis were used for RT-qPCR. The first strand cDNA was synthesized using HiScript® Reverse Transcriptase (Vazyme, Nanjing, China) and 6N random primers according to the manufacture’s protocol. RT-qPCR was performed on an iCycler IQ5 instrument with SYBR® Premix Ex Taq™ II (TakaRa, Dalian, China). The cycling conditions included incubation for 30 s at 95°C followed by 40 cycles of amplification (95°C for 5 s and 60°C for 25 s). GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) was selected as the internal reference gene [26] and the relative expression values were calculated with Ct method (2-ΔΔCt). The primers used in RT-qPCR are listed in S1 Table.
Results and discussion
Identification of differentially expressed genes
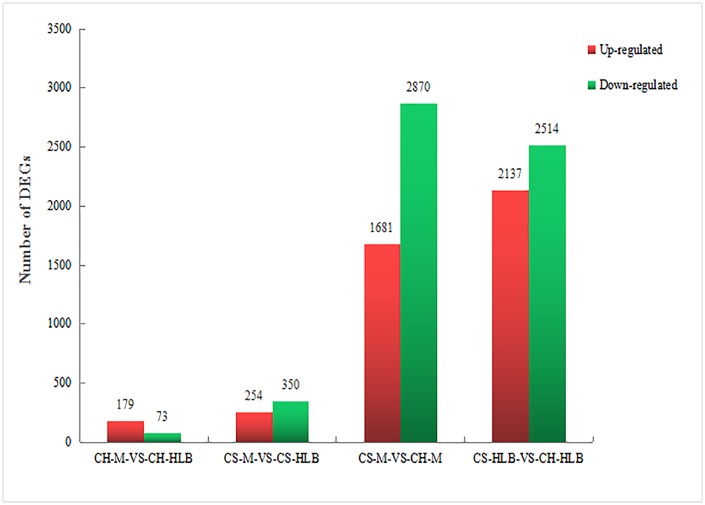
Around 44 million clean reads were obtained from infected and control plants and mapped to Citrus sinensis genome [27], with approximately 62% success for C. hystrix, and 77%-78% for C. sinensis libraries (S2 Table). Dramatic differences between the transcriptome profiles of C. hystrix and C. sinensis were observed in response to CLas infection. Overall, the number of differentially expressed genes (DEGs) was greater in susceptible sweet orange than in tolerant kaffir lime when compared to their respective healthy control. Among these DEGs, 179 genes were up-regulated and 73 were down-regulated in C. hystrix, while 254 genes were up-regulated and 350 were down-regulated in C. sinensis (Fig 1). Due to the differences in genetic background between the two citrus cultivars, the DEG number of infected/healthy C. hystrix in relation to infected/healthy C. sinensis was much greater than the number of the two infected cultivars in relation to their mock-inoculated healthy controls (Fig 1).
Fig 1. Statistic of differentially expressd genes (DEGs) of different citrus cultivars in response to CLas.
CH, Citrus hystrix; CS, C. sinensis; M, Mock/healthy; HLB, Huanglongbing; CH-M-VS-CH-HLB, DEGs in HLB-infected C. hystrix compared with healthy control; CS-M-VS-CS-HLB, DEGs in HLB-infected C. sinensis compared with healthy control; CS-M-VS-CH-M, DEGs between healthy C. hystrix and healthy C. sinensis; CS-HLB-VS-CH-HLB, DEGs between HLB-infected C. hystrix and HLB-infected C. sinensis.
Gene ontology classification of DEGs
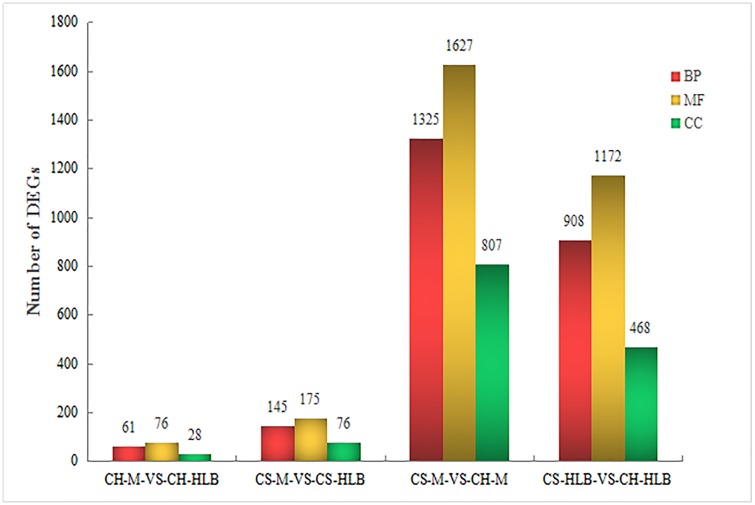
The DEGs were enriched into different function categories through Gene ontology (GO) enrichment analysis (Fig 2). Significantly enriched GO terms were extracted using TopGO [28]. For DEGs in HLB tolerant C. hystrix, the most significantly enriched GO terms in biological process were related to ‘threonine metabolic process’ (GO:0006566), ‘negative regulation of peptidase activity’ (GO:0010466), ‘regulation of proteolysis’ (GO:0045861), and ‘regulation of peptidase activity’ (GO:0052547). The most significant GO terms in molecular function included ‘terpene synthase activity’ (GO: 0010333), and ‘carbon-oxygen lyase activity, acting on phosphates’ (GO:0016838). No GO term in cellular component was enriched in DEGs in C. hystrix (S2 Table). For DEGs in susceptible C. sinensis, only two GO terms in biological process, ‘reactive oxygen species metabolic process’ (GO: 0072593) and ‘cellular protein complex assembly’ (GO: 0043623) were enriched. The most significant GO terms in molecular function were related to ‘transferase activity’ (GO: 0016758), ‘cellulose synthase activity’ (GO: 0016759), and ‘glucuronosyltransferase activity’ (GO: 0015020). Only three GO terms in cellular component were enriched in DEGs in C. sinensis and the most significant one was ‘external encapsulating structure’ (GO: 0030312) (S3 Table).
Fig 2. Gene ontology classification of differentially expressed genes in different citrus hosts in response to ‘Candidatus Liberibacter asiaticus’.
BP, Biological Process; CC, Cell Component; MF, Molecular Function.
DEGs within two infected cultivars were mainly enriched in nine biological process GO terms, including ‘drug transport’ (GO: 0015893), ‘response to drug’ (GO: 0042493) and ‘response to chemical’ (GO:0042221). Besides, nine molecular function GO terms were enriched, such as ‘catalytic activity’ (GO: 0003824), ‘oxidoreductase activity’ (GO: 0016491), ‘transferase activity’ (GO: 0016758, GO: 0016757), whereas only one cellular component GO term, ‘intrinsic component of membrane’ (GO: 0031224), was enriched in DEGs in the two cultivars in response to CLas (S4 Table).
Gene pathway enrichment analysis of CLas-modulated host pathways
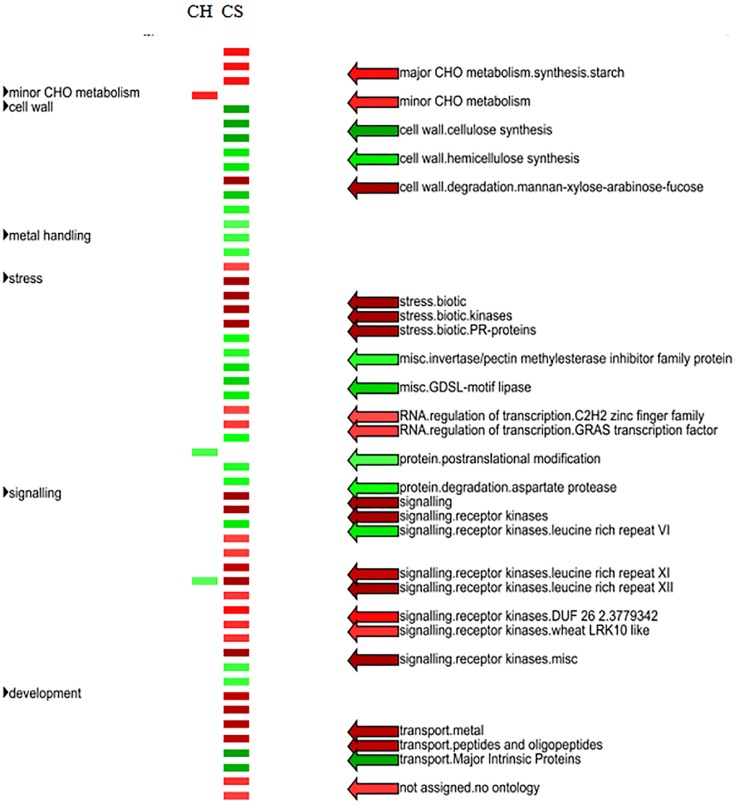
PageMan analysis showed that only three pathways significantly enriched were associated with the DEGs identified in C. hystrix (Fig 3). Pathways related to major carbohydrates (CHO) metabolism was up-regulated, while pathways related to posttranslational modification and LRR XII receptor kinases were down-regulated in tolerant citrus plants. Instead, much more pathways were significantly enriched in C. sinensis (Fig 3). The up-regulated pathways were starch synthesis, cell wall degradation, stress biotic, regulation of transcription, receptor kinases signaling, and metal transport in susceptible citrus plants. The down-regulated pathways included cellulose synthesis, hemicellulose synthesis, invertase/pectin methylesterase inhibitor family protein, GDSL motif lipase, and aspartate protease mediated protein degradation.
Fig 3. Comparative PageMan display of perturbed pathways in CLas-affected C. hystrix and C. sinensis.
The log2 fold change of gene expression (mock-inoculated controls versus CLas-inoculated plants) was input into PageMan and subjected to a Wilcoxon test. Results were shown as a false-color heat-map-like display. Significantly up-regulated pathways are colored in red, while those colored in green are significantly down-regulated. Pathways without significant changes are white. Names of pathways are indicated on the right panel. CH, CH-M-VS-CH-HLB; CS, CS-M-VS-CS-HLB.
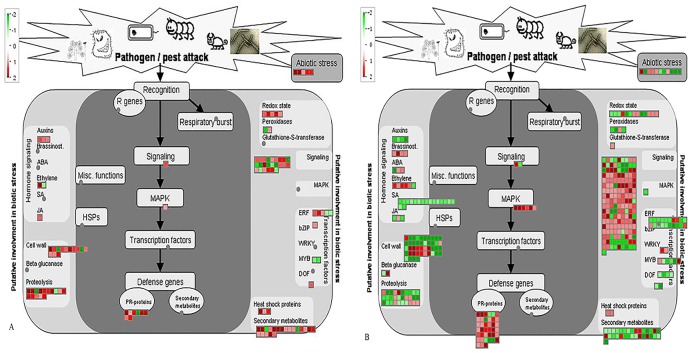
MapMan software was applied to display and analyze the functional classes those were significantly different in CLas-affected C. hystrix and C. sinensis. The results showed that DEGs were mainly involved in diverse cellular functions including carbohydrate metabolism, photosynthesis, cell wall metabolism, secondary metabolism, hormone metabolism, PR proteins and oxidation/reduction processes (Figs 4 and 5, S5 Table).
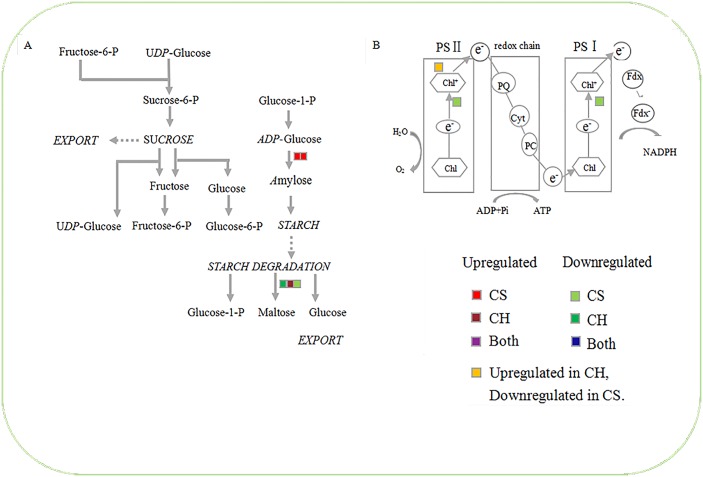
Fig 4. Differentially expressed genes involved in starch metabolism (A) and photosynthesis (B).
Colored squares indicate up- or down-regulated genes with log2 fold change (FC) ≥ 1.00 or ≤ -1.00.
Fig 5. MapMan analysis of differentially expressed genes in CLas-affected Citrus hystrix (A) and CLas-affected C. sinensis (B) involved in stress responses.
Red squares represent genes those were significantly up-regulated; green squares represent genes those were significantly down-regulated.
Carbohydrate metabolism and photosynthesis process
Cytopathology in different stage of HLB infection revealed swelling of the middle lamella between cell walls surrounding sieve elements in non-symptomatic citrus new flushes at the early infection stage and then necrosis of sieve elements and companion cells and phloem plugging by the callose-like material and excessive starch at the later infection stage [29–31]. It suggests that the disturbance of starch metabolism and the inhibition of the transport of photosynthate may contribute to HLB development in citrus hosts. Microarray or RNA-seq analysis also proved that carbohydrate metabolic process was significantly changed and photosynthesis was repressed in HLB-affected citrus [15, 17, 18, 30, 32]. From these studies, the key starch synthesis genes were generally up-regulated and the starch degrading genes were down-regulated in affected susceptible citrus. Recently, proteomic analysis suggested no clear correlation was observed from starch pathway regulation between moderately tolerant cultivar ‘Volkameriana’ and susceptible navel orange [33].
In this study, several genes involved in carbohydrate metabolism were regulated in the early stage of infection with CLas (Fig 4, S5 Table). The starch synthesis was induced while the starch degradation was depressed in C. sinensis. Two glycogen synthase (starch synthase, glgA) geneswere significantly up-regulated, whereas beta-amylase (BAM) gene was down-regulated in C. sinensis (Fig 4, S5 Table). However, the expression levels of these genes were not significantly changed in CLas-affected C. hystrix. Instead, one gene encoding alpha-amylase (AAM), associated with starch degradation, was slightly up-regulated in C. hystrix (Fig 4, S5 Table).
Genes encoding light-harvesting complex II chlorophyll a/b binding protein 1, Photosystem II psbP domain-containing protein 1 and photosystem I subunit O, which involved in light action of photosynthesis, were all down-regulated in C. sinensis (Fig 4, S5 Table). The three genes were not significantly changed in C. hystrix, suggesting the photosynthesis process may not be depressed in tolerant C. hystrix.
Taken together these findings showed that the repression of key proteins involved in photosynthetic light reactions and up-regulation of starch-related pathways in C. sinensis was in agreement with previous studies. Notably, no significant change in starch synthesis and photosynthesis process may play important roles in tolerance of C. hystrix. Anatomical evidences from different infection stage of kaffir limeare required to prove the transcriptome data.
Genes involved in cell wall and cell organization
The plant cell wall is comparable to an exoskeleton surrounding the plant cell and providing both structural support and protection from biotic as well as abiotic stresses [34]. It is well established that the plant susceptibility to pathogens depends on the cell wall composition and structure, which determines its recalcitrance to degradation by cell wall modifying enzymes (CWMEs) produced by pathogens [35]. Plant cell walls are composed of layers of cellulose microfibers embedded in a matrix of pectin and hemicellulose, plus some structural proteins [36–38]. There were 44 and 13 genes found related to cell wall metabolism among the DEGs in HLB-susceptible and tolerant cultivars, respectively (Fig 5, S5 Table). Overall, most genes involved in cellulose synthesis, cell wall proteins and cell wall modification were depressed in diseased C. sinensis. For instance, several genes encoding expansin, which are related to cell wall breakdown, including EXLA1, EXPA5 and EXLB1, were down-regulated in C. sinensis, and EXLA1 was also down-regulated in C. hystrix. Expansin related genes were reported to exhibit high expressions in susceptible ‘Marsh’ grapefruit [19]. Down-regulation of these genes in both C. hystrix and C. sinensis indicates a host defense response to CLas infection. A group of genes encoding xyloglucan endotransglucosylase/hydrolase proteins (XTH22, XTH23), associated with cell wall degradation, were slightly up-expressed in CLas-affected C. sinensis, whereas no significant difference was observed in CLas-affected C. hystrix. Furthermore, DEGs involved in cellulose synthesis, such as cellulose synthase A (CESA), cellulose synthase-like protein A2 (CSLA2), cellulose synthase-like protein A9 (CSLA9) and cellulose synthase-like protein C5 (CSLC5) were all down-regulated in CLas-affected C. sinensis, while CSLA2 and CSLA9 were up-regulated in CLas-affected C. hystrix. Thus, kaffir lime responded to CLas by activating enzymes that would work together to strengthen the cell wall, which may contribute to the reinforcement of physical barriers to restrict the invasion of CLas.
Secondary metabolism
Secondary metabolism plays important roles in plant defenses. Previous studies revealed that most genes involved in secondary metabolites, including terpenoid, flavonoid and phenylpropanoid biosynthesis pathways, were highly induced in CLas-affected leaves [15, 17, 19, 39], whereas most of these genes were down-regulated in roots following HLB infection [16]. Here, most DEGs involved in secondary metabolism pathway were activated in C. hystrix, but were suppressed in C. sinensis. These DEGs were categorized into the biological processes of terpene biosynthesis, flavonoids biosynthesis and phenylpropanoids biosynthesis (Fig 5, S5 Table). Terpenoids are a structurally diverse group of natural products, which function as plant antioxidants, insect attractants or repellents [40, 41]. Flavonoids are anti-fungi substances and antioxidants [42]. Phenylpropanoids serve as structural polymers including lignins, provide protection from pests and UV light and attract pollinators as pigments [43]. Two genes encoding germacrene D synthase-like (GDSL) were up-regulated in both two cultivars in response to CLas infection. Two genes encoding gamma-terpinene synthase (TPS2) were only up-regulated in HLB tolerant citrus trees. The expression level of one gene encoding (R)-limonene synthase 1 was increased in C. hystrix, while the other two genes related to this enzyme were suppressed in C. sinensis. GDSL, TPS2 and (R)-limonene synthase were involved in terpene biosynthesis. One gene encoding 2-oxoglutarate and Fe (II)-dependent (2OG-Fe (II)) oxygenases, which is associated with flavonoids biosynthesis, was strongly up-regulated in C. hystrix, whereas it was down-regulated in C. sinensis. Genes involved in phenylpropanoids biosynthesis such as isoflavone reductase (IRL) and cinnamoyl-CoA reductase 1 (CCR), were induced in C. hystrix, while phenylalanine ammonia-lyase (PAL) and 4-coumarate—CoA ligase 1 (4CL1), which also play an important role in phenylpropanoids biosynthesis, were suppressed in C. sinensis.
The tolerant cultivars contain relatively higher specific volatile compounds such as monoterpenes and aldehydes than susceptible cultivars [44]. It should be noted that Kaffir lime leaves contain various classes of phytochemical substances including terpenoids and polyphenolic which are known for their antimicrobial activities [7, 8]. The up-regulation of genes involved in secondary metabolism pathways, and high phytochemical substances containing in kaffir lime leaves may contribute to its tolerance to CLas.
Hormone metabolism
Phytohormones play critical roles in helping the plants to adapt to adverse environmental conditions. The elaborate hormone signaling networks and their ability of crosstalk make them ideal candidates for mediating defense responses [45]. There were 35 hormone signaling genes in identified DEGs (Fig 5, S5 Table). Auxin was found to promote the expression of expansins in plants [46–48], which contribute to breakage of plant cell walls. Of the seven auxin-related genes, three genes related to crocetin glucosyltransferase were up-regulated in C. hystrix, two auxin-responsive proteins, SAUR72 and IAA1, were down-regulated in C.sinensis. SAUR was proved to act as a negative regulator of auxin synthesis in rice and may promote resistance to pathogens [49]. Transcription analysis showed that three SAUR-like genes were up-expressed in HLB tolerant ‘Jackson’ grapefruit, which may also contribute to the down expression of expansin [19]. A gene encoding the senescence-related gene (SRG1), involved in ethylene metabolism, was highly induced in response to CLas in C. hystrix. The expression of ethylene-responsive transcription factor ERF07 was up-regulated in both cultivars, while ERF107 was down-regulated. Ethylene-responsive transcription factor and EIN3-binding F-box protein 1 were only up-regulated in C. sinensis. Notably, five genes involved in salicylic acid metabolism were suppressed in C. sinensis but not in C. hystrix. JA levels in response to pathogen infection clearly highlight its involvement in plant defense responses. The key enzyme involved in jasmonate synthesis, lipoxygenase 2 (LOX2) [50], were up-regulated in both cultivars. JA-responsive gene expression for defense response is mainly mediated by a transcription factor JASMONATE INSENSITIVE 1/MYC2 (JIN1/MYC2) [51]. Up-regulation of transcription factor MYC2 was only observed in C. hystrix. These results showed that JA signal transduction may be activated by infection with CLas, especially in C. hystrix.
Pathogenesis-related (PR) genes
The pathogenesis-related (PR) proteins of plants are a group of host-encoded, inducible proteins whose synthesis is often associated with certain forms of resistance to pathogens and stresses [52]. Previous studies showed that most genes related to PR-proteins were induced by CLas infection, including receptor-like protein kinase, TIR-NBS-LRR (Toll interleukin-1 receptor nucleotide-binding site leucine-rich repeat) class and NB-ARC (nucleotide binding-adaptor shared by APAF-1, certain R gene products and CED4) domain proteins, and Kunitz family trypsin and protease inhibitor protein [11, 18, 32, 39].
In this work, more genes related to PR genes were induced in C. sinensis than in C. hystrix (Fig 5, S5 Table). Several genes related to kinases involved in biotic stress, including LRR receptor-like serine/threonine-protein kinase GSO1, LRR receptor-like serine/threonine-protein kinase ERL2, LRR receptor-like protein kinase At1g35710, and receptor-like protein 12, were slightly up-expressed in C. sinensis. A gene encoding leucine-rich repeat receptor-like serine/threonine-protein kinase (At2g24130) was up-regulated more than 8-fold in C. hystrix, but showed no significant difference in C. sinensis. Notably, two genes encoding miraculin, a Kunitz family trypsin and protease inhibitor protein which are associated with plant defenses, were only up-regulated in C. hystrix (S5 Table). In addition, some genes related to disease resistance proteins, such as RPS4 and RPM1, exhibited higher expression level in C. hystrix. RPS4 and RPM1 act as R genes to participate in the effector-triggered immunity (ETI) process, which play an important role in plant defense response [53, 54].
Oxidation/Reduction processes
14 genes involved in oxidation/reduction processes were differentially regulated between the HLB-susceptible and tolerant cultivars. It should be noted that most of them were up-regulated in tolerant C. hystrix and down-regulated in C. sinensis. Up-regulation of several glutaredoxin genes such as GRXC6, GRXC9 and GRXS9, were only identified in C. hystrix. These proteins have activity of glutathione-disulfide bond oxidordeuctase, which can reduce the amount of micromolecular disulfide and proteins. A gene encoding thioredoxin M3, a thiol-disulfide bond oxidordeuctase which required for maintaining permeability of plant meristem and regulation of callose deposition in Arabidopsis, was inhibited in C. sinensis. Besides, a group of superoxide dismutase (SOD) and peroxidase (POD) genes were differentially expressed in both cultivars. Of them, Cu/Zn-SOD and POD4 were up-regulated in C. hystrix, whereas they were down-regulated in C. sinensis (S5 Table). Proteins involved in oxidation/reduction processes, including peroxidases and glutathione-S-transferases, are usually associated with the prevention of oxidative stress, which are induced by reactive oxygen species (ROS). Proteomic studies revealed that a higher activation of glutathione-S-transferases, which include several isozymes that help detoxify xenobiotic compounds, was observed in tolerant cultivar ‘Volkameriana’ [33]. Therefore, activation of peroxidases, Cu/Zn-SOD and POD4, may also enhance the tolerance of C. hystrix to CLas.
RT-qPCR validation
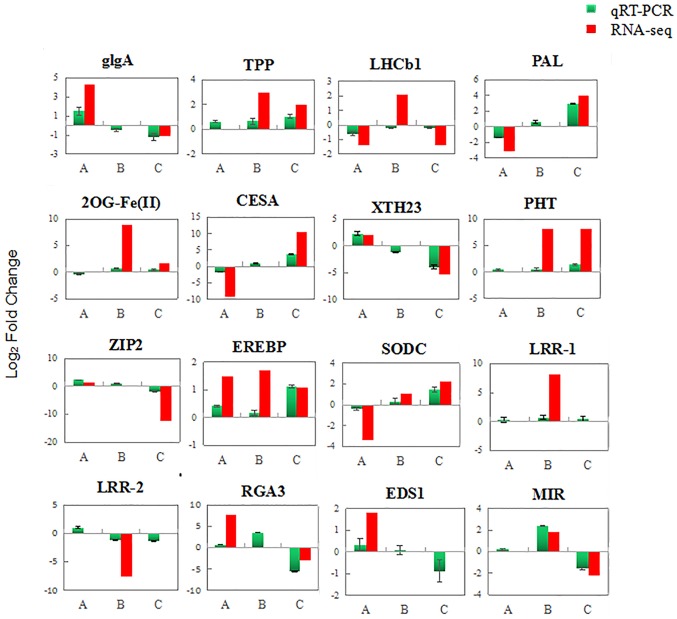
To validate the accuracy of the RNA-seq data, 16 genes classified in different function groups were tested by RT-qPCR. The expression profiles of 15 genes were consistent with the RNA-seq data, demonstrating the reliability of RNA-seq analysis. The RT-qPCR result of LHCb, encoding light-harvesting complex II chlorophyll a/b binding protein 1, did not agree with those obtained from the RNA-seq in C. hystrix. In addition, several genes showed no difference in RNA-seq data, but differentially expressed by RT-qPCR analysis. For example, glgA and XTH23 showed lower expression levels, PAL and RGA3 showed higher expression levels in CLas-infected C. hystrix based on qRT-PCR analysis (Fig 6).
Fig 6. RT-qPCR and RNA-seq profiles of 16 selected differentially expressed genes (DEGs).
A. DEGs between healthy and HLB-infected Citrus sinensis; B, DEGs between healthy and HLB-infected C. hystrix; C, DEGs between HLB-infected C. hystrix and HLB-infected C. sinensis. glgA, starch synthase; TPP, trehalose 6-phosphate phosphatase; LHCb1, light-harvesting complex II chlorophyll a/b binding protein 1; PAL, phenylalanine ammonia-lyase-like; 2OG-Fe(II), 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase; CESA, cellulose synthase A; XTH23, xyloglucan endotransglucosylase/hydrolase protein 23; PHT, phosphate transporter; ZIP2, zinc transporter 2; EREBP, ethylene-responsive transcription factor ERF017; SODC, superoxide dismutase [Cu-Zn]; LRR-1, LRR receptor-like serine/threonine-protein kinase; LRR-2, LRR-like serine/threonine-protein kinase BAM2; RGA3, disease resistance protein RGA3, NB-ARC class; EDS1 (enhanced disease susceptibility 1 protein); MIR, miraculin.
Conclusions
Global transcriptome profiles between the HLB tolerant C. hystrix and susceptible C. sinensis showed that specific responses in carbohydrate metabolism, photosynthesis process, cell wall metabolism, secondary metabolism and oxidation/reduction processes may play important roles against CLas attack in C. hystrix. 1) starch synthesis and photosynthesis process were not disturbed in CLas infected C. hystrix. 2) several cellulose synthase and cellulose synthase-like family proteins, which may strengthen the cell wall synthesis, were induced in C. hystrix. 3) most DEGs involved in secondary metabolism pathways were activated in C. hystrix, but were depressed in C. sinensis. 4) the activation of peroxidases, Cu/Zn-SOD and POD4, may also enhance the tolerance of C. hystrix to CLas, acting as detoxification proteins.
Supporting information
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Acknowledgments
We thank Dr. Jianchi Chen from United States Department of Agriculture, Agricultural Research Service, for critical editing of this manuscript. This study was funded by National Natural Sciences Foundation of China (31671992, 31301635), CQ CSTC (cstc2016shms-ztzx80003) and Guangxi Key Laboratory of Citrus Biology (SYS2015K004).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by National Natural Sciences Foundation of China (31671992, 31301635), CQ CSTC (cstc2016shms-ztzx80003) and Guangxi Key Laboratory of Citrus Biology (SYS2015K004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bové JM. Huanglongbing: a destructive newly-emerging, century-old disease of citrus. Journal of Plant Pathology. 2006; 88(1): 7–37. [Google Scholar]
- 2.Jagoueix S, Bové JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. International. Journal of Systematic Bacteriology. 1994; 44(3): 379–386. doi: 10.1099/00207713-44-3-379 [DOI] [PubMed] [Google Scholar]
- 3.Teixeira DC, Saillard C, Eveillard S, Danet JL, da Costa PI, Ayres AJ, et al. ‘Candidatus Liberibacter americanus’, associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. International Journal of Systematic & Evolutionary Microbiology. 2005; 55(pt5): 1857–1862. [DOI] [PubMed] [Google Scholar]
- 4.Shokrollah H, Abdullah TL, Sijam K, Abdullah SNA, Abdullah NAP. Differential reaction of citrus species in Malysia to Huanglongbing (HLB) disease using grafting method. American Journal of Agricultural and Biological Sciences. 2009; 4(1): 32–38. [Google Scholar]
- 5.Folimonova SY, Robertson CJ, Garnsey SM, Gowda S, Dawson WO. Examination of the responses of different cultivars of citrus to huanglongbing (citrus greening) under different conditions. Phytopathology. 2009; 99(12): 1346–1354. doi: 10.1094/PHYTO-99-12-1346 [DOI] [PubMed] [Google Scholar]
- 6.Ramadugu C, Keremane ML, Halbert SE, Duan Y, Roose ML, Stover E, et al. Long-term field evaluation reveals Huanglongbing resistance in citrus relatives. Plant Disease. 2016; 100(9): 1858–1869. [DOI] [PubMed] [Google Scholar]
- 7.Norkaew O, Pitija K, Pripdeevech P, Sookwong P, Wo S. Supercritical fluid extraction and gas chromatographic-mass spectrometric analysis of terpenoids in fresh kaffir lime leaf oil. Chiang Mai Journal of Science. 2012; 40(2): 240–247. [Google Scholar]
- 8.Kooltheat N, Kamuthachad L, Anthapanya M, Samakchan N, Sranujit RP, Potup P, et al. Kaffir lime leaves extract inhibits biofilm formation by streptococcus mutans. Nutrition. 2015; 32(4): 486–490. doi: 10.1016/j.nut.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Shokrollah H, Abdullah TL, Sijam K, Abdullah SNA. Potential use of selected citrus rootstocks and interstocks against HLB disease in Malaysia. Crop Protection. 2011; 30(5): 521–525. [Google Scholar]
- 10.Albrecht U, Bowman KD. Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Science. 2008; 175(3): 291–306. [Google Scholar]
- 11.Aritua V, Achor D, Gmitter FG, Albrigo G, Wang N. Transcriptional and microscopic analyses of citrus stem and root responses to Candidatus Liberibacter asiaticus infection. PLoS One. 2013; 8(9): e73742 doi: 10.1371/journal.pone.0073742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao H, Burns JK. Gene expression in Citrus sinensis fruit tissues harvested from huanglongbing-infected trees: comparison with girdled fruit. Journal of Experimental Botany. 2012; 63(8): 3307–3319. doi: 10.1093/jxb/ers070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinelli F, Uratsu SL, Albrecht U, Reagan RL, Phu ML, Britton M, et al. Transcriptome profiling of citrus fruit response to huanglongbing disease. PLoS One. 2012; 7(5): e38039 doi: 10.1371/journal.pone.0038039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan J, Chen C, Yu Q, Brlansky RH, Li Z, Gmitte FG. Comparative iTRAQ proteome and transcriptome analyses of sweet orange infected by “Candidatus Liberibacter asiaticus”. Physiologia Plantarum. 2011; 143(3): 235–245. doi: 10.1111/j.1399-3054.2011.01502.x [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Li Y, Zheng Z, Dai Z, Tao Y, Deng X. Transcriptional analyses of mandarins seriously infected by ‘Candidatus Liberibacter asiaticus’. PLoS One, 2015; 10(7): e0133652 doi: 10.1371/journal.pone.0133652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Y, Cheng C, Jiang N, Jiang B, Zhang Y, Wu B, et al. Comparative transcriptome and iTRAQ proteome analyses of citrus root responses to Candidatus Liberibacter asiaticus Infection. PLoS One. 2015; 10(6): e0126973 doi: 10.1371/journal.pone.0126973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht U, Bowman KD. Transcriptional response of susceptible and tolerant citrus to infection with Candidatus Liberibacter asiaticus. Plant Science. 2012; 185-186(4): 118–130. [DOI] [PubMed] [Google Scholar]
- 18.Fan J, Chen C, Yu Q, Khalaf A, Achor DS, Brlansky RH, et al. Comparative transcriptional and anatomical analyses of tolerant rough lemon and susceptible sweet orange in response to ‘Candidatus Liberibacter asiaticus’ infection. Molecular plant-microbe interactions. 2012; 25(11): 1396–1407. doi: 10.1094/MPMI-06-12-0150-R [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zhou L, Yu X, Stover E, Luo F, Duan Y. Transcriptome profiling of Huanglongbing (HLB) tolerant and susceptible citrus plants reveals the role of basal resistance in HLB tolerance. Frontier in Plant Science. 2016; 7(74): 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zhou C, Deng X, Su H, Chen J. Molecular characterization of a mosaic locus in the genome of ‘Candidatus Liberibacter asiaticus’. BMC Microbiology. 2012; 12(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Hartung JS, Levy L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. Journal of Microbiological Methods. 2006; 66(1): 104–115. doi: 10.1016/j.mimet.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 22.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012; 9(4): 357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011; 12(1): 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usadel B, Nagel A, Steinhauser D, Gibon Y, Bläsing OE, Redestig H, et al. Pageman: an interactive ontology tool to generate, display, and annotate overview graphs for profiling experiments. BMC Bioinformatics. 2006; 7(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, et al. Mapman: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant Journal. 2004; 37(6): 914 [DOI] [PubMed] [Google Scholar]
- 26.Yan J, Yuan F, Long G. Y, Qin L, Deng Z. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Molecular biology reports. 2012; 39(2): 1831–1838. doi: 10.1007/s11033-011-0925-9 [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Chen L, Ruan X, Chen D, Zhu A, Chen C, et al. The draft genome of sweet orange (Citrus sinensis). Nature Genetics. 2013; 45(1): 59 doi: 10.1038/ng.2472 [DOI] [PubMed] [Google Scholar]
- 28.Alexa A, Rahnenfuhrer J. topGO: enrichment analysis for gene ontology. R Package Version. 2006.
- 29.Folimonova SY, Achor DS. Early events of citrus greening (huanglongbing) disease development at the ultrastructural level. Phytopathology. 2010; 100(100): 949–958. [DOI] [PubMed] [Google Scholar]
- 30.Kim JS, Sagaram US, Burns JK, Li J, Wang N. Response of sweet orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ infection: microscopy and microarray analyses. Phytopathology. 2009; 99(1): 50–57. doi: 10.1094/PHYTO-99-1-0050 [DOI] [PubMed] [Google Scholar]
- 31.Schneider H. Anatomy of greening-diseased sweet orange shoots. Phytopathology. 1968; 58: 1155–1160. [Google Scholar]
- 32.Zhong Y, Cheng C, Jiang B, Jiang N, Zhang Y, Hu M, et al. Digital gene expression analysis of Ponkan mandarin (Citrus reticulata Blanco) in response to Asia citrus psyllid-vectored Huanglongbing infection. International Journal of Molecular Sciences. 2016; 17(7): 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinelli F, Reagan RL, Dolan D, Fileccia V, Dandekar AM. Proteomic analysis highlights the role of detoxification pathways in increased tolerance to Huanglongbing disease. BMC Plant Biology. 2016; 16(1): 167 doi: 10.1186/s12870-016-0858-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamann T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Frontiers in plant science. 2012; 3(4): 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell ALT. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends in Plant Science. 2008; 13(11): 610–617. doi: 10.1016/j.tplants.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 36.Seifert GJ, Blaukopf C. Irritable walls: the plant extracellular matrix and signaling. Plant Physiology. 2010; 153(2): 467–478. doi: 10.1104/pp.110.153940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somerville C. Cellulose synthesis in higher plants. Annual Review of Cell & Developmental Biology. 2006; 22: 53–78. [DOI] [PubMed] [Google Scholar]
- 38.Vogel J. Unique aspects of the grass cell wall. Current Opinion in Plant Biology. 2008; 11(3): 301–307. doi: 10.1016/j.pbi.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 39.Fu S, Shao J, Zhou C, Hartung JS. Transcriptome analysis of sweet orange trees infected with ‘Candidatus Liberibacter asiaticus’ and two strains of Citrus Tristeza Virus. BMC Genomics. 2016; 17(1): 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends in Plant Science. 2001; 6(2): 78–84. [DOI] [PubMed] [Google Scholar]
- 41.Graßmann J. Terpenoids as plant antioxidants. Vitamins & Hormones. 2005; 72: 505–535. [DOI] [PubMed] [Google Scholar]
- 42.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacology & Therapeutics. 2002; 96(2–3): 67–202. [DOI] [PubMed] [Google Scholar]
- 43.Ferrera JL, Austinb CMB, Stewart J, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiology & Biochemistry. 2008; 46(3): 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hijaz F, Nehela Y, Killiny N. Possible role of plant volatiles in tolerance against Huanglongbing in citrus. Plant Signaling & Behavior. 2016; 11(3): e1138193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma V, Ravindran P, Kumar PP. Plant hormone-mediated regulation of stress responses. BMC Plant Biology. 2016; 16(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Downes BP, Steinbaker CR, Crowell DN. Expression and processing of a hormonally regulated beta-expansin from soybean. Plant Physiology. 2001; 126: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catala C, Rose JKC, Bennett AB. Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiology. 2000; 122: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, et al. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate-and jasmonate-independent basal immunity in rice. Plant Cell. 2008; 20(1): 228–240. doi: 10.1105/tpc.107.055657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kant S, Bi YM, Zhu T, Rothstein SJ. SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiology. 2009; 151(2): 691–701. doi: 10.1104/pp.109.143875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 1995; 92(19): 8675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology. 2007; 10(4): 366–71. doi: 10.1016/j.pbi.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 52.Carr JP, Klessig DF. The Pathogenesis-related proteins of plants Genetic Engineering. Springer; US: 1989. [Google Scholar]
- 53.Gassmann W, Hinsch ME, Staskawicz BJ. The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR-NBS-LRR family of disease-resistance genes. Plant Journal for Cell & Molecular Biology. 1999; 20(3): 265–277. [DOI] [PubMed] [Google Scholar]
- 54.Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, et al. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science. 1995; 269(5225): 843–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.