Abstract
Early onset effects of methylmercury (MeHg) on recombinant α1β2γ2S or α6β2γ2S subunit-containing GABAA receptors were examined. These are two of the most prevalent receptor types found in cerebellum–a consistent target of MeHg-induced neurotoxicity. Heterologously expressed receptors were used in order to: 1) isolate receptor-mediated events from extraneous effects of MeHg due to stimulation of the receptor secondary to increased release of GABA seen with MeHg in neurons in situ and 2) limit the phenotypes of GABAA receptors present at one time. Initial changes in IGABA in Xenopus laevis oocytes expressing either α1β2γ2S or α6β2γ2S receptors were compared during continuous bath application of MeHg. A time-dependent increase in IGABA mediated by both receptor subtypes occurred following the first 25–30 min of MeHg (5 μM) exposure. In α6β2γ2S containing receptors, the MeHg-induced increase in IGABA was less pronounced compared to that mediated by α1β2γ2S containing receptors, although the pattern of effects was generally similar. Washing with MeHg-free solution reversed the increase in current amplitude. Application of bicuculline at the time of peak potentiation of IGABA rapidly and completely reversed the MeHg-induced currents. Therefore these MeHg-increased inward currents are mediated specifically by the two subtypes of GABAA receptors and appear to entail direct actions of MeHg on the receptor. However bicuculline did not affect stimulation by MeHg of oocyte endogenous Cl−-mediated current, which presumably results from increased [Ca2+]i. Thus, MeHg initially potentiates IGABA in oocytes expressing either α1β2γ2S or α6β2γ2S receptors prior to its more defined later effects, suggesting that MeHg may initially interact directly with GABAA receptors in a reversible manner to cause this potentiation.
Keywords: Methylmercury, GABAA receptor, α1 and α6 subunit, heterologous expression
INTRODUCTION
Methylmercury (MeHg) is a potent environmental neurotoxicant that preferentially affects the somatosensory, visual and auditory cortices and the cerebellum (See review by Ekino et al., 2007.) Each of these regions contains large numbers of small diameter granular cells, which are especially sensitive to MeHg. In the cerebellar cortex, for example, the granule cells are much more sensitive to MeHg exposure than are their neighboring Purkinje cells (Chang, 1988; Leyshon-Sorland and Morgan, 1991; Patel and Reynolds, 2013). This relative sensitivity can be recaptured in vitro- both in freshly isolated brain slices (Yuan and Atchison, 2003; 2007) and organotypic slice culture (Bradford et al., 2016) as well as in single cells in primary culture (Edwards et al., 2005).
Among the numerous differences between cerebellar granule and Purkinje cells are those of GABAA receptors. The two cell types express GABAA receptors with different subunit compositions (Fritschy et al., 1992; Laurie et al., 1992; Thompson et al., 1992; Thompson and Stephenson, 1994; Gao and Fritschy, 1995; Wisden et al., 1996; Mäkelä et al., 1999). This is important because the pharmacological and electrophysiological properties of GABAA receptors vary markedly based on the composition of GABAA receptor subunits and subtypes (Smith, 2001; Trincavelli et al., 2012; Nikas et al., 2015). Thus subtype-specific effects of MeHg on GABAA receptors could contribute to specific cell cytotoxicity. Mature granule cells express α1 or α6 subunits alone or in combination, whereas Purkinje cells express only the α1 subunit (Lüddens et al., 1990; Varecka et al., 1994; Nusser et al., 1995; Wisden et al., 1996; Siegel, 1998; Fritschy and Panzanelli, 2006). Furthermore, diversity is provided to granule cells by substitution in some receptors of a δ for a γ subunit. However this co-expression is strictly dependent on the presence of α6 subunits (Quirk et al., 1994; Jones et al., 1997; Nusser et al., 1999; Tretter et al., 2001). Differential expression of α1 or α6 subunits confers unique pharmacological and biophysical properties on recombinant GABAA receptors (Whiting et al., 1999, Olsen and Sieghart, 2008, 2009; Brickley and Mody, 2012). This difference could be important to granule cell vulnerability, because in granule cells a tonic GABA-mediated conductance regulates granule cell excitability (Brickley et al., 1996; Mody 2001; Semyanov et al., 2004; Brickley and Mody, 2012; Lee and Maguire, 2014). Thus, preferential block of the GABAA receptors responsible for this conductance could cause the granule cell to become more excitable, leading to membrane depolarization and subsequent increase of [Ca2+]i. Both of these latter effects occur in granule cells in response to MeHg (Marty and Atchison, 1997, 1998; Yuan and Atchison, 2003, 2007; Limke et al., 2003; Yuan and Atchison, 2016)
Early studies suggested that GABAA receptors could be a sensitive target to MeHg. Following administration of MeHg to neonatal rats, morphological examination of the visual cortex indicated that aspinous or sparsely-spinous GABAergic interneurons in layer IV had degenerated selectively (O’Kusky, 1985; O’Kusky and McGeer, 1985, 1989; O’Kusky et al., 1988). MeHg also affects GABAA receptors in several cell types in culture (Arakawa et al., 1991; Komulainen et al., 1995; Fonfría et al., 2001; Herden et al., 2007; Suñol et al., 2008). GABAergic neurons are more sensitive to effects of MeHg than are glutamatergic neurons in hippocampal (Yuan and Atchison, 1995; 1997) and cerebellar slices (Yuan and Atchison, 2003). In the latter, granule cell GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) are additionally more sensitive to MeHg-induced block than are those of Purkinje cells (Yuan and Atchison, 2003). Furthermore, among a series of ion channels examined, GABAA receptor-mediated Cl− channels were the most sensitive to MeHg (Yuan et al., 2005; Yuan and Atchison, 2005). Thus, the relative sensitivity of GABAergic systems to MeHg could play a role in the MeHg-induced differential sensitivity of cerebellar granule and Purkinje cells.
The effects of MeHg on GABAergic function at intact CNS synapses are complex; they involve both a transient stimulation of inhibitory postsynaptic currents (IPSCs) amplitude, followed by reduction to their complete block (Yuan and Atchison, 2003, 2005, 2007). While MeHg exposure ultimately decreases IGABA to complete block, an early effect appears to be transient increase in IGABA amplitude. However, this effect is difficult to isolate at intact synapses due to the multiplicity of MeHg-induced effects that are time-dependent. These include a pronounced stimulation of spontaneous IPSC (sIPSC) frequency, so both pre- and postsynaptic effects contribute to actions of MeHg on cerebellar inhibitory circuits. Whereas in granule cells, GABAergic currents are blocked with a faster time course than those of Purkinje cells, the transient stimulation of IPSC amplitude was lesser in magnitude and frequency; it occurred in less than 50% of the granule cells examined compared to that which occurred in all Purkinje cells (Yuan and Atchison, 2003). This may reflect cell-specific subtype differences in combinations of GABAA receptors. However, studies of selective effects of MeHg on different GABAA receptor subtypes in culture cells or slices can be hindered by uncertainty about the receptor phenotype because granule cells contain a mixture of GABAA receptor subunits that is both developmentally and spatially regulated (Zheng et al., 1993; Thompson and Stephenson, 1994; Varecka et al., 1994; Carlson et al., 1998; Takayama and Inoue, 2004).
Consequently, in this study, we focused specifically on the initial effect of MeHg on GABA-evoked currents in Xenopus laevis oocytes expressing either subtype of GABAA receptor in isolation. We sought to determine if MeHg has an initial stimulatory effect on the GABA-induced currents in Xenopus oocytes expressing heterologously α1- or α6- subunit-containing GABAA receptors as it does on native cerebellar neurons in slices, and if the two subtypes of GABAA receptors respond to MeHg differently. Consistent with results seen in native cerebellar neurons in slices, MeHg caused an initial and reversible potentiation of GABA-evoked currents in both subtypes of receptors expressed in oocytes. However unlike the situation in slices, in which intact synapses can contribute enhanced GABA release and thereby confound the source of the facilitated response, there was no endogenous source of GABA for the isolated oocyte. Thus a stimulated response caused by MeHg would reflect a direct action on the receptor/channel complex.
MATERIALS AND METHODS
Solutions and Chemicals
Methylmercuric chloride (MeHg) (ICN Biomedical Inc., Costa Mesa, CA, USA) was applied continuously by oocyte perfusion. A stock solution (10 mM) was prepared in deionized water. On the day of experiments, MeHg solutions (5 μM) were constituted in ND 96 extracellular solution consisting of (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid, Sigma-Aldrich Chemicals, St. Louis, MO, USA) titrated to pH 7.4 with NaOH, supplemented with 2.5 mM Na pyruvate (Sigma) and 50 μg/ml gentamicin (Sigma). Consistent with our previous observations obtained from acutely isolated brain slices of rat (Yuan and Atchison, 1993, 2003), preliminary experiments revealed that MeHg at different concentrations (1 – 10 μM) produced a similar pattern of effects on GABA currents but varying with different time courses inversely related to the concentration employed (data not shown). Consequently, only a single concentration of MeHg (5 μM) was used for the data depicted. This is within the range of concentrations (~19.5 μM) found in the blood of patients poisoned with MeHg in Iraq in the 1970’s (Bakir et al., 1973). It is also at the low range of concentrations used in cerebellar slice and at which stimulatory effects of MeHg were observed previously (Yuan and Atchison, 2003, 2005, 2007).
GABA, bicuculline, niflumic acid, type IV collagenase, HEPES, deoxyribonuclease (DNase I), and ethylene glycol-bis(β-aminoethyl ether)-N, N, N′, N′,-tetraacetic acid (EGTA), trypsin, poly-L-lysine were all purchased from Sigma Chemical Co. (St. Louis, MO). Qiagen kits, used for plasmid purification, were purchased from Qiagen Inc. (Valencia, CA) and Fugene 6 was purchased from Roche Molecular Biochemicals (Indianapolis, IN).
Preparation of cRNA
The plasmid cDNAs from rat for α1, β2 and γ2S GABAA receptor subunits were generously provided by Dr. Cynthia Czjkowski, University of Wisconsin-Madison, while that for α6 was generously provided by Dr. Bill Wisden (University of Heidelberg, Heidelberg, Germany). Plasmids containing cDNAs for the GABAA subunits were linearized after the poly (A) signal sequences. The linear plasmid DNA was ‘agarose’ gel purified and used for enzymatic cRNA synthesis using a mMessage mMachine T7 kit (Ambion, Austin, TX). Linearized plasmid DNA (1 mg) was mixed with reaction buffer, NTP/Cap and enzyme mix from the kit and incubated for 2 hr at 37°C. RNAase-free DNaseI was then added and incubation continued for another 15 min. The resulting capped, DNA-free cRNA was used to add poly-A tails with Poly (A) Tailing Kit (Ambion) by adding water, MnCl2, ATP, E-PAP (E. coli Poly (A) polymerase) and E-PAP buffer and then incubating for 1 hr at 37°C. The products of the capped cRNA synthesis reaction and the poly (A) tailing reactions were compared using mobility shift and denaturing agarose gel electrophoresis to verify addition of the poly (A) tails. The final products were purified to remove unincorporated nucleotides and other reaction components by gel filtration using a MEGAclear kit also from Ambion. Purified cRNA was quantitated using UV spectrophotometery on a Nanodrop spectrophotometer (Nanodrop, Wilmington, DE), and stored frozen at −80°C until use.
Xenopus oocyte preparation and electrophysiological recordings
All animal procedures complied with the National Institutes of Health of the USA guidelines on animal care and were approved by Michigan State University Institutional Animal Care and Use Committee. Clusters of Xenopus laevis oocytes were removed surgically from adult female frogs (Xenopus One, Ann Arbor, MI) under tricaine (Sigma-Aldrich) anesthesia (0.17% w/v) and were incubated in ND96 solution. Defolliculated oocytes were obtained by incubating oocytes for 30 – 120 min in Ca2+-free ND96 medium containing 0.6 mg/ml type IV collagenase; any remaining follicular layers were manually removed using fine forceps. Only stage IV–V oocytes were collected for further use. The GABAA receptors expressed in oocytes in the present study were either α1β2γ2S or α6β2γ2S subtype, because the former is the most common subtype of GABAA receptor in the brain including the cerebellum, whereas the latter is found specifically in cerebellar granule cells (Benke et al., 1991, 1994; Laurie et al., 1992). Each oocyte received a 50 nl injection of a mixture of α1 or α6, β2 and γ2S in the proportion of 1:1:10 (Boileau et al., 2003). After cRNA injection, occytes were incubated in ND96 at 19°C for at least 2 days before electrophysiological experiments.
Electrophysiological recordings were made within 3 to 7 days after cRNA injection. For each experiment, oocytes from two or more frogs were used. Whole oocyte recordings of GABA-evoked currents were made using two-microelectrode voltage-clamp recording. Microelectrodes for voltage-sensing and current-passing were fabricated from thick-wall borosilicate glass (o.d. = 1.0 mm, i.d. = 0.5 mm) (WPI, Inc., Sarasota, FL) and had an impedance of 0.5 – 2.0 MΩ when filled with 3 M KCl. Signals from the current-passing electrode were amplified using an OC-725C amplifier (Warner Instruments Corp., Hamden, CT). Data were acquired using a Digidata 1200 interface and pClamp 9.0 software (Molecular Devices, Sunnyvale, CA). Currents were filtered at 20 – 100 Hz and digitized at 50 – 200 Hz, respectively, for off-line analysis. To do this, two computer recording systems were used: one was devoted specifically to episode recordings of GABA-evoked currents at a sampling rate of 200 Hz; the other was tasked for continuous recording of baseline currents at a sampling rate of 50 Hz. All recordings were made at a holding potential of −60 mV and at room temperature of ~22 °C. Oocytes were superfused at a constant rate ~4 ml/min. GABAA receptor-mediated currents were evoked by sequential 15-sec pulse applications of 0.2–1000 μM GABA in bath solution. A standard stimulus protocol was used in most experiments. It consisted of a 1.0 sec ramp protocol with voltage changing from −140 mV to +60 mV at a rate of 1 mV/5 ms, followed by a 15-sec pulse application of GABA to evoke IGABA. In some cases, mostly α1β2γ2S receptors, a second identical voltage ramp was applied at the end of the 15-sec GABA application (Figure 1). The ramp protocols were used to monitor changes in voltage-dependent responses, particularly those mediated by endogenous voltage-gated Cl− channels before and after GABA application. A 5-min interval between two consecutive GABA applications was given to allow receptors to recover from deactivation/desensitization and currents to return to baseline. This interval was adequate to reverse desensitization associated with GABAA receptors (data not shown). When MeHg and other receptor antagonists or inhibitors were applied, they were perfused continuously with ND96 recording solution controlled by a programmable six channel valve perfusion system (VC-6, Warner Instrument, Hamden, CT). The composition of ND96 recording solution was similar to that used for incubation, but without Na pyruvate and gentamicin, and the concentration of HEPES was increased from 5 mM to 10 mM.
Figure 1.
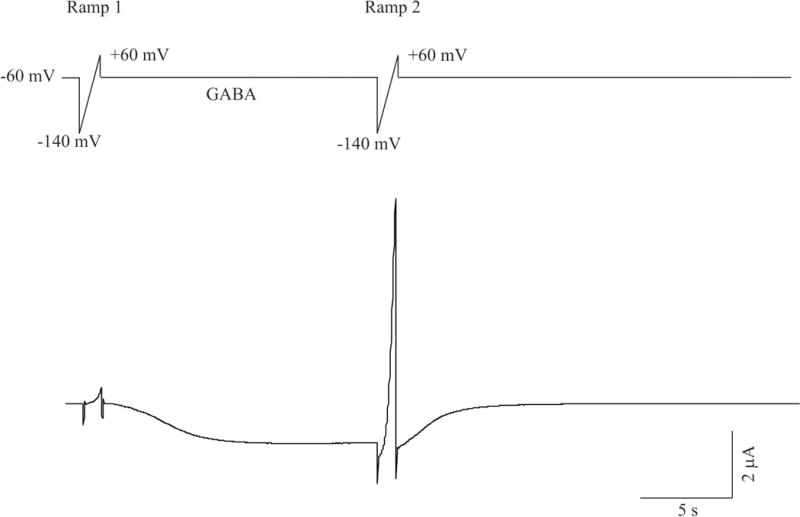
Current recording protocols used for two-electrode voltage clamp recordings in Xenopus oocytes. Top: A given oocyte is voltage clamped at a holding potential of −60 mV and is activated first by a voltage ramp (Ramp 1, 1 sec duration, voltage changes at a rate of 1mV/5 ms from −140 mV to 60 mV), followed by a 15 sec pulse application of 1 μM GABA. Immediately following GABA application, a second ramp (Ramp 2) with identical features as the first one is applied. Bottom: A representative current trace evoked by this protocol in an oocyte expressing recombinant α1β2γ2S GABAA receptors.
Data analysis
Data were collected prior to and during application of MeHg and analyzed statistically using one-way analysis of variance (ANOVA) or Student’s paired t test. Dunnett’s procedure was used for post hoc comparison. Values were considered statistically significant at P < 0.05. Each experiment were repeated at least three times in oocytes from different frogs. Values are expressed as mean ± SEM of individual experiments.
RESULTS
Differential sensitivity of α1β2γ2S or α6β2γ2S receptors to GABA and niflumic acid
Among GABAA receptors the α subunit subfamily, especially the α1 or α6 subunits have the most divergent properties (Pritchett et al., 1989; Draguhn et al., 1990; Korpi et al., 1995; Tia et al., 1996; Saxena and Macdonald, 1996; Fisher et al., 1997; Fisher and Macdonald, 1998; Zhu et al., 1998; Sigel and Baur, 2000; Smith, 2001; Fisher, 2004). In both native and recombinant GABAA receptors, presence of the α6 subunit confers distinct pharmacological properties including higher sensitivity to receptor agonists and insensitivity to desensitization. To ensure that the α1β2γ2S or α6β2γ2S subtype receptors expressed in our oocyte expression system retain these properties, we compared the sensitivity of the two receptors subtypes to GABA. As shown in Figure 2, sequential pulse application of 0.2 – 1000 μM GABA for 15 sec to oocytes expressing α1β2γ2S or α6β2γ2S subtypes induced a concentration-dependent increase in GABA-evoked inward current (IGABA) in both receptor subtypes at a holding potential of −60 mV. However, IGABA evoked by the same agonist concentrations in α6β2γ2S-containing receptors was much larger than that in α1β2γ2S subtype. The averaged EC50 values for α1β2γ2S and α6β2γ2S receptor were 9.2 μM (n = 5) and 2.0 μM (n = 6), respectively (P<0.05). Thus, these data are consistent with the general concept that α6 subunit-containing GABAA receptors have a relatively higher affinity for GABA than do α1 subunit-containing receptors (Saxena and Macdonald, 1996). For this reason, in the subsequent experiments, the whole cell IGABA in oocytes expressing α1β2γ2S receptors was evoked by 5 μM GABA, whereas that in oocytes expressing α6β2γ2S receptors was evoked by 1 μM GABA, about half of the EC50 value for either subtype, respectively. In addition, data shown in Figure 2 suggest that the 5 min interval between two GABA pulse applications was sufficient to allow IGABA to return to baseline level.
Figure 2.
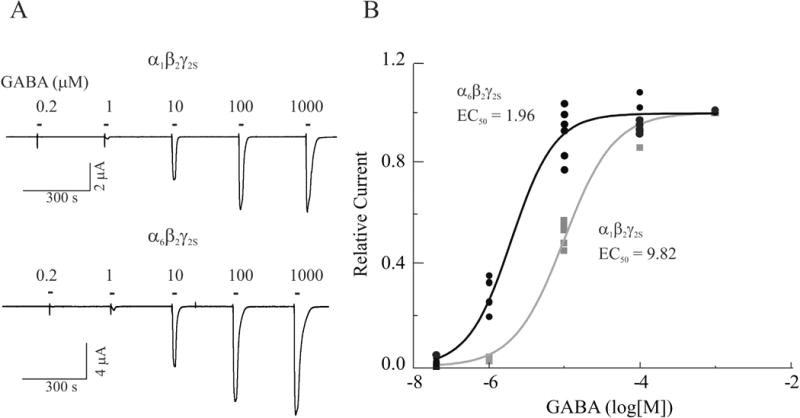
Differential sensitivity of recombinant α1β2γ2S and α6β2γ2S receptors to GABA. A, Whole cell currents recorded from representative oocytes expressing α1β2γ2S (Top) and α6β2γ2S (Bottom) in response to 0.2–1000 μM GABA. B, Concentration-dependent response curves were generated by nonlinear regression fit of normalized GABA-evoked currents recorded from oocytes expressing α1β2γ2S (Black) and α6β2γ2S (Grey) receptors. The peak current evoked by each concentration of GABA is normalized to that evoked by 0.2 μM GABA. Each value is a representative example of 5 – 6 individual experiments.
α1 or α6 subunit-containing receptors expressed in oocytes also respond differentially to niflumic acid (NA), a nonsteroidal anti-inflammatory drug and anion channel blocker (Sinkkonen et al., 2003). We compared sensitivity of α1 and α6- containing receptors to NA (500 μM). IGABA mediated by α1β2γ2S subtype, was potentiated whereas that mediated by α6β2γ2S subtype was inhibited by niflumic acid (Figure 3A). Both the potentiation and inhibition of IGABA are statistically significant (Figure 3B, p < 0.05, n = 3 – 4). Thus, these results again suggest that the recombinant α1β2γ2S and α6β2γ2S receptor subtypes expressed in our oocyte expression system retain, at least in part, those pharmacological properties that are typical of α1 or α6 subunit-containing native GABAA receptors. Therefore, no further pharmacological characterization of the two receptor subtypes was carried out.
Figure 3.
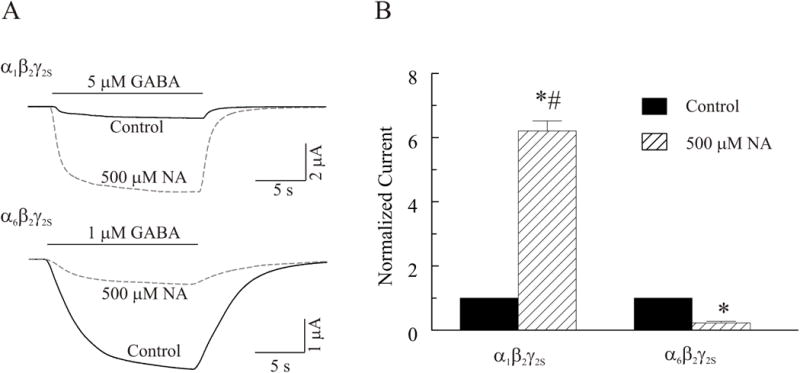
Differential sensitivity of recombinant α1β2γ2S and α6β2γ2S GABAA receptors to niflumic acid (NA). A, Whole cell currents were recorded from oocytes expressing α1β2γ2S (Top) or α6β2γ2S (Bottom) in response to 5 or 1 μM GABA, respectively, at a holding potential of −60 mV in the absence and presence of 500 μM NA. B, Comparison of effects of NA on IGABA on the two subtype receptors (n = 3 – 4). The asterisk (*) indicates a significant difference between control and NA treatment (p≤0.05). The number sign (#) indicates a significant difference between α1β2γ2S and α6β2γ2S containing receptors (p≤0.05).
MeHg potentiates IGABA mediated by α1β2γ2S and α6β2γ2S receptors in a similar pattern
Initial preliminary studies utilized 1, 5, or 10 μM MeHg to examine its early onset effects on IGABA. Consistent with our previous observations in brain slices (Yuan and Atchison, 1993; 1999; 1997; 2003, 2007), 1 – 10 μM MeHg produced a similar pattern but with very different time-courses of effects on IGABA expressed in oocytes. At 1 μM MeHg, it took much longer time to produce an effect similar to that induced by 5 μM MeHg, whereas 10 μM MeHg produced an effect that was too rapid to allow for other subsequent modulations. Considering the potential current run-down of GABAA receptors expressed in oocytes and the lack of a clearly defined concentration-dependent effect of MeHg on IGABA (data not shown), all data presented in this and subsequent figures were collected from oocytes treated with 5 μM MeHg. As the primary purpose of the present study was to determine if MeHg initially stimulated GABA-evoked currents mediated by the two recombinant GABAA receptors, we limited the MeHg exposure duration to 30 min, or the point at which a MeHg-induced peak stimulation was achieved. Also, after ~40–50 min, continuous oocyte recordings in the presence of MeHg became unstable. Figure 4A shows two representative recordings of effects of MeHg on IGABA mediated by α1β2γ2S and α6β2γ2S, respectively. In both cases, exposure of oocytes to 5 μM MeHg for 25 min caused an increase in GABA-evoked currents. IGABA recorded from both receptor subtypes appears to decay faster in the presence of MeHg, suggesting that MeHg may affect the deactivation or/and desensitization process of the two subtypes of receptors. However, detailed kinetic analysis using ultrafast-step application of GABAA receptor agonist is needed to substantiate further this effect and was beyond the scope of the study. Figure 4B summarizes the peak increases in IGABA mediated by α1β2γ2S and α6β2γ2S receptors, respectively, during the first 30 min exposure to 5 μM MeHg. Clearly, MeHg caused a significant increase in IGABA mediated by both α1β2γ2S and α6β2γ2S receptors compared with their own controls. The peak increases in IGABA mediated by α1β2γ2S and α6β2γ2S receptors are 215 ± 16% (n = 7) and 115± 5% (n = 7) of their own control, respectively (P<0.05). MeHg appears to potentiate the IGABA mediated by α1β2γ2S more strongly than it does that mediated by the α6β2γ2S receptor subtype (p < 0.05), although the pattern of effects of MeHg on both receptor subtypes is generally similar.
Figure 4.
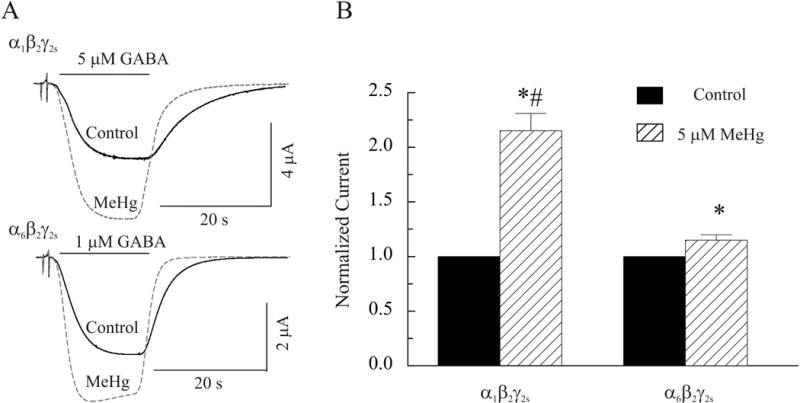
MeHg potentiates GABA-evoked currents mediated by either α1β2γ2S or α6β2γ2S receptors. A, Whole cell currents were recorded from oocytes expressing α1β2γ2S (Top) or α6β2γ2S (Bottom) in response to 5 or 1 μM GABA, respectively, at a holding potential of −60 mV in the absence and presence of 5 μM MeHg. B, Comparison of effects of MeHg on IGABA amplitude of the two receptor subtypes (n = 7). The asterisk (*) indicates a significant difference between control and MeHg treatment (p≤0.05). The number sign (#) indicates a significant difference between α1β2γ2S and α6β2γ2S receptors (p≤0.05).
The time course of 5 μM MeHg-induced potentiation of IGABA in α1β2γ2S and α6β2γ2S receptors is shown in Figures 5 and 6, respectively. In Figure 5, the top three current traces from a representative experiment depict IGABA mediated by α1β2γ2S receptors that were collected before, at peak increase during MeHg exposure, and after 10 min of MeHg-washout. The bottom curve is the averaged time course of effects of MeHg on IGABA in oocytes expressing α1β2γ2S receptors (n = 7). Application of MeHg began (0 min) after the baseline had remained stable for at least 10 min, and continued for 30 min. MeHg was then washed out with MeHg-free ND96 solution for another 10 min. There was a time-dependent increase of IGABA in oocytes expressing α1β2γ2S receptors. It reached a maximum at 30 min. The increase might not be the real peak effect of MeHg because of the limited MeHg exposure duration. This increase could possibly continue if longer exposure time is allowed, which is actually demonstrated later in an experiment shown in the inset. However, because recordings under our experimental conditions usually became unstable after 40 – 50 min, most experiments were terminated after washing for 10 min. Therefore, only a partial recovery is shown in this figure. In two individual recordings in which longer duration of MeHg exposure and washing were made successfully, complete recovery of MeHg-induced increase in IGABA was attained (The inset shows one of them.). A similar pattern of time-course of effects of MeHg on IGABA was seen in oocytes expressing α6β2γ2S receptors (Figure 6). In these experiments, oocytes were exposed to MeHg for 25 min because enhancement of IGABA usually reached the peak after 20 min of exposure; thus wash out began at 25 min and lasted for 10 min. Again, the continuous recordings showed that the effect of MeHg on IGABA mediated by α6β2γ2S receptors was less pronounced as compared to that mediated by α1β2γ2S receptors. In fact, two of the seven α6-expressing oocytes examined had no detectable increase in IGABA. However, when it occurred, the MeHg-induced peak increase in IGABA mediated by α6β2γ2S receptors had a more rapid onset than in those cells with α1-containing receptors. Thus although the general pattern of effect of MeHg on the two recombinant receptors is similar, the time course of MeHg effects differed substantially. The inset in Figure 6 demonstrates a representative recording that was sufficiently stable to permit one to observe a complete recovery of MeHg-induced effect on IGABA mediated by α6-containing receptors. Overall, these data suggest that potentiation by MeHg of IGABA occurred with both subtypes of recombinant GABAA receptors is MeHg exposure time-dependent, and at least partially reversible.
Figure 5.
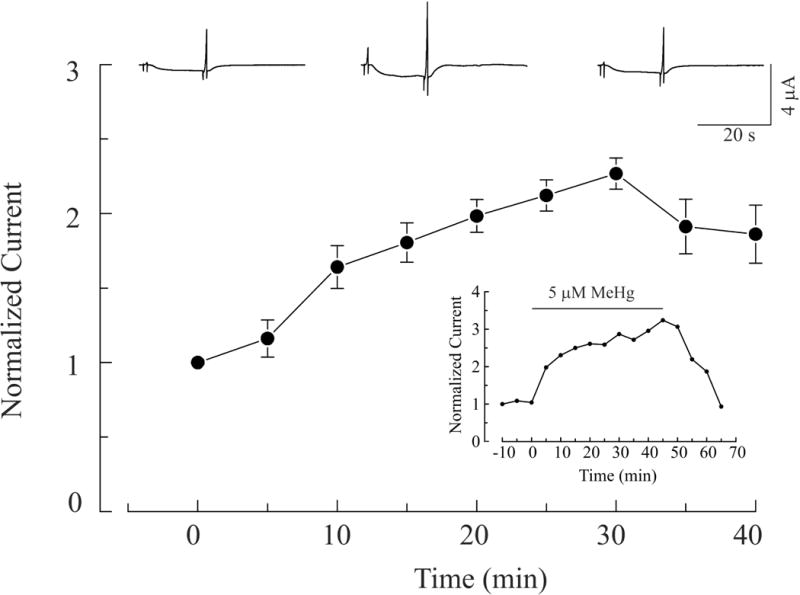
MeHg-induced potentiation of IGABA in oocytes expression α1β2γ2S receptors is time-dependent and reversible. Top: whole cell currents were recorded before, at 30 min after MeHg exposure, and following a 10 minute wash respectively. Bottom: Time-course of MeHg-induced potentiation of GABA currents mediated by α1β2γ2S receptors. Application of 5 μM MeHg began at 0 min, continued for 30 min, was subsequently stopped, and the oocyte washed with MeHg-free solution. Values are mean ± SEM (n = 7).
Figure 6.
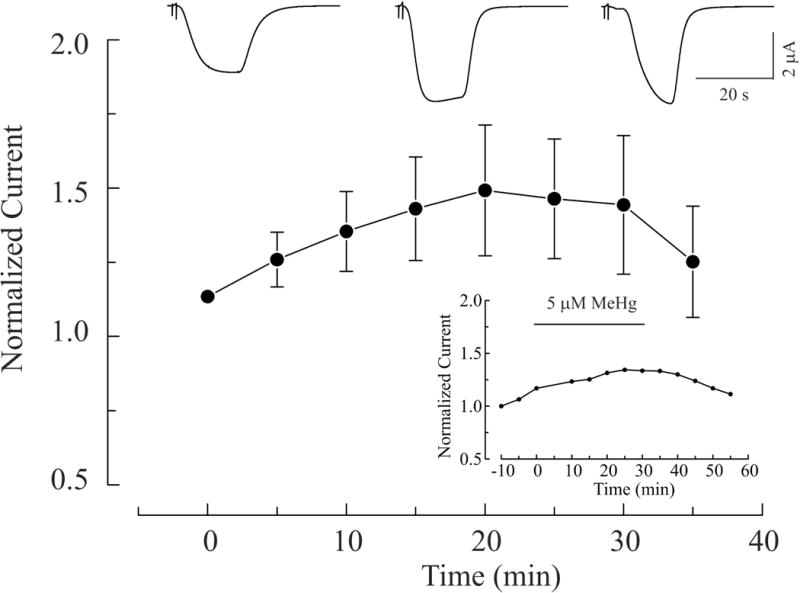
MeHg-induced potentiation of IGABA in oocytes expressing α6β2γ2S receptors is time-dependent and reversible. Top: whole cell currents recorded before, at 20 min after MeHg exposure and following a 10 min wash with MeHg-free solution respectively. Bottom: Time-course of MeHg-induced potentiation of GABA currents mediated by α6β2γ2S receptors. Application of 5 μM MeHg began at 0 min, continued for 30 min, was subsequently stopped, and the oocyte washed with MeHg-free solution. Values are mean ± SEM (n = 7).
MeHg-increased IGABA was sensitive to block by GABAA receptor antagonist
We next sought to ascertain whether the action of MeHg on these receptors could be reversed by a GABAA receptor antagonist, that is, if the MeHg-induced stimulatory effect was mediated directly by access to the receptor. Bicuculline (20 μM) was applied at the time when MeHg caused peak potentiation of IGABA in oocytes expressing either α1 or α6-containing receptors (Figure 7). Bicuculline rapidly and completely (100%) blocked GABA-evoked inward currents in oocytes expressing either subunit-containing receptors. As expected, the bicuculline effect was completely reversible when washing with bicuculline-free, but MeHg-containing solution. Thus the inward currents were indeed mediated specifically by these two subtypes of GABAA receptors and appear to be due to a direct and reversible action of MeHg at the receptor.
Figure 7.
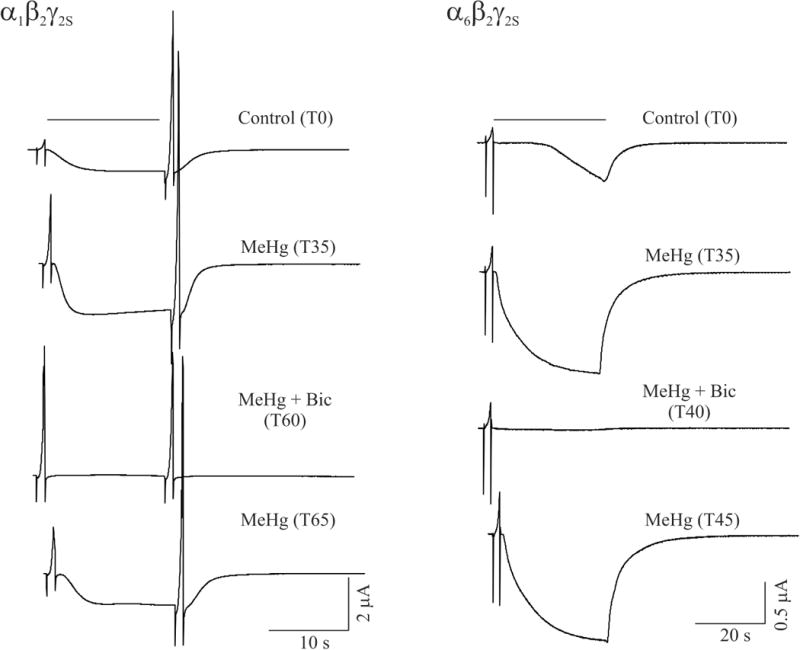
MeHg-induced potentiation of IGABA mediated by both subtype receptors is sensitive to block by the GABAA receptor antagonist bicuculline. Left, whole cell currents were recorded from a representative oocyte expressing recombinant α1β2γ2S containing receptors ar a holding potential of −60 mV. Currents were recorded before (control), at 35 min (T35) in the presence of 5 μM MeHg, at 60 min (T60) in the presence of both 5 μM MeHg and 20 μM bicuculline and 65 min again in the presence of MeHg alone. Right, whole cell currents were recorded from a representative oocyte expressing recombinant α6β2γ2S receptors under similar conditions as for α1β2γ2S receptors, but with different time points.
As shown in Figures 1, 5 and 7 for oocytes expressing α1β2γ2S receptors, the second voltage ramp immediately following GABA application induced a much larger outward current than that induced by one immediately prior to GABA application. MeHg also potentiated both sets of outward currents. Bicuculline did not affect the outward currents evoked by the first voltage ramp, but reduced those evoked by second voltage ramp to a level equal to that of the first voltage ramp. This suggests that GABA-evoked currents may contribute to or facilitate an increase in the outward currents evoked by the second voltage ramp, whereas the MeHg-induced increase in the residual outward currents evoked by the first and second voltage ramps in the presence of bicuculline is not mediated by GABAA receptors.
To test whether the MeHg-increased, bicuculline-insensitive outward currents were due to MeHg-induced stimulation of the endogenous Ca2+-activated Cl− channels in oocytes (Philips et al., 2003), we next examined the effect of MeHg on whole-cell currents in oocytes injected with water only. Figure 8 representatively shows exposure of water-injected oocytes to MeHg induced a significant increase in voltage-dependent outward currents in a time-dependent manner (Figure 8A–D). In this case, the outward current was increased from 0.22 μA to 2.56 μA at a membrane potential of +60 mV after 25 min MeHg exposure (Figure 8D and 8F). As expected, the MeHg-increased voltage-dependent outward currents were blocked completely by 300 μM niflumic acid (NA), a Ca2+-activated Cl− channel blocker (Figure 8E and 8F). These results suggest that the MeHg-increased, bicuculline-insensitive outward currents are mediated, most likely, by the endogenous Ca2+-activated Cl− channels.
Figure 8.
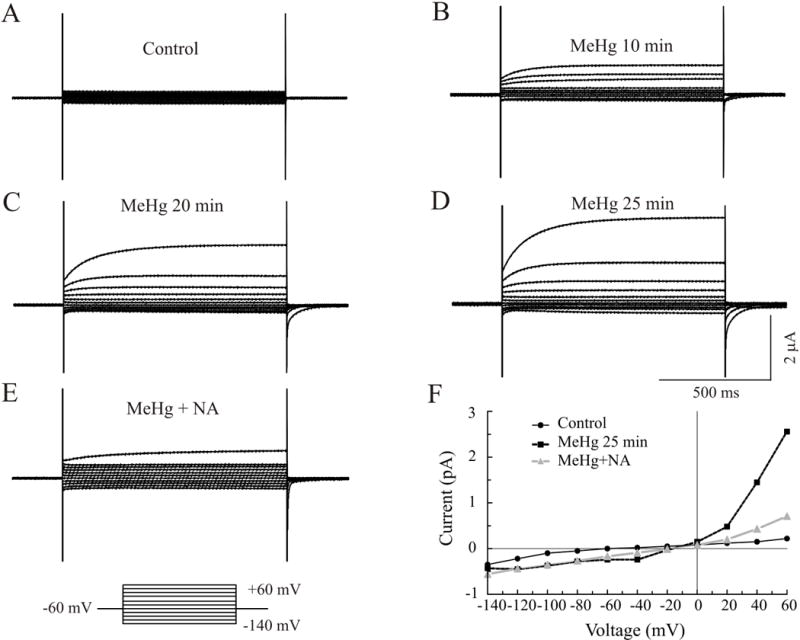
MeHg caused an increase in outward currents in the water-injected oocytes. A family of whole cell currents was recorded from a water-injected oocyte at a holding potential of −60 mV before (A) and after exposure to MeHg for 10 (B), 20 (C) and 25 min (D). Treatment with niflumic acid (NA, 300 μM) (B) for 5 min, a Ca2+-activated chloride channel blocker, caused a significant decrease in MeHg-induced outward currents (E). Effect of MeHg and NA on whole cell current-voltage relationships are shown in F. Clearly, MeHg increased both inward and outward currents, but primarily so outward currents. Each trace is a representative depiction of 5 individual experiments.
DISCUSSION
The primary objective of the present study was to examine the early onset effects of MeHg on two types of GABAA receptors expressed abundantly in the cerebellum. In native cerebellar neurons in cerebellar slices, MeHg causes an initial stimulatory effect on presumptive α1β2γ2S or α6β2γ2S GABAA receptors. This effect precedes the more commonly-described, and apparently irreversible, block of IGABA, and appears to result from a transient increased sensitivity of the receptor. However, in slices, MeHg also dramatically increases the frequency of occurrence of GABA-mediated IPSCs (Yuan and Atchison, 2003, 2007), suggesting an involvement of presynaptic action. This presynaptic effect certainly complicated attempts to resolve postsynaptic effects in isolation. Thus a recombinant system was used in this study to examine this effect in isolation. In HEK293 cells, expressing either phenotype, the stimulatory effect could not be reproduced reliably for unknown reasons (Herden et al., 2007). Thus we re-examined the effect in the well described oocyte expression system. MeHg potentiated IGABA mediated by both subtypes of recombinant GABAA receptors expressed in Xenopus oocytes. This potentiation is reversible and is sensitive to block by the specific GABAA receptor antagonist bicuculline.
α1 Subunit-containing GABAA receptors display lower affinity to GABA than do α6 subunit-containing receptors (Saxena and Macdonald, 1996; Fisher et al., 1997; Sinkkonen et al., 2003; Fisher, 2004). Differential sensitivity of recombinant α1β2γ2S or α6β2γ2S receptors expressed in our oocyte expression system to GABA also occurred, with EC50 values similar to those reported for the α1 or α6 subunit-containing receptors expressed in L929 cells (Saxena and Macdonald, 1996; Fisher et al., 1997), oocytes (Sinkkonen et al., 2003) and HEK293 cells (Fisher, 2004). We also obtained a similar pattern of responses of α1β2γ2S or α6β2γ2S receptors to NA as that reported by Sinkkonen et al. (2003) for positive and negative modulation of α1 and α6 subunit-containing GABAA receptors, respectively. These data clearly demonstrate that α1β2γ2S or α6β2γ2S receptors expressed in our oocyte expression system were indeed two different subtypes of GABAA receptors. Thus, any difference between the two subtypes of receptors displayed in our experiments should be related to the unique pharmacological and electrophysiological properties of α1 and α6 subunits.
In acutely isolated cerebellar slices, we have consistently shown that acute bath application of MeHg causes an increase prior to subsequent suppression of both frequency and amplitude of GABAA receptor-mediated sIPSCs in both Purkinje and granule cells (Yuan and Atchison, 2003). The initial increases in sIPSCs in Purkinje cells appear to be more prominent than those in granule cells (Yuan and Atchison, 2003). In fact, almost half of the granule cells examined in cerebellar slices had no initial increase in either sIPSC frequency or amplitude. Consistently, MeHg-induced potentiation of IGABA also appears to be more prominent in oocytes expressing α1β2γ2S receptors than those α6β2γ2S receptors, and two of seven recordings from oocyte expressing α6β2γ2S receptors exhibited no initial increase in IGABA. If the response is subunit dependent, then cells expressing the sensitive subunit would be expected to express the response whereas those with fewer of the responsive receptors would not, or would only do at a reduced frequency. This makes sense because native Purkinje cells only express α1 subunit-containing GABAA receptors, whereas granule cells express GABAA receptors containing α1, α6 or both α1 and α6 subunits. In addition, the relatively rapid time course to peak stimulation of IGABA mediated by α6β2γ2S receptors compared to that mediated by α1β2γ2S receptors also appears to be consistent with the different time courses of effects of MeHg on sIPSCs in granule and Purkinje cells (Yuan and Atchison, 2003). Interestingly, the reversibility of MeHg-induced initial potentiation of IGABA in both subtypes of receptors also appeared to be consistent with our observations in native cerebellar neurons (Yuan and Atchison, 2003). In cerebellar slices, MeHg-induced block of sIPSCs in granule cells occurred much more rapidly than it did those in Purkinje cells. Because of the relatively short time course of onset of block, sIPSCs in most granule cells could be recovered completely to the pretreatment control level by washing cells with D-penicillamine, a MeHg chelator. In contrast, it took about 3-fold longer for the same concentrations of MeHg to block sIPSCs in Purkinje cells than it did in granule cells. Once blocked, sIPSCs in Purkinje cells could not be restored by washing the cells with D-penicillamine. This irreversibility of sIPSCs in Purkinje cells by washing appeared to be due to MeHg-induced nonspecific effects such as irreversible membrane damage resulting from the longer time of MeHg exposure that is typically needed to cause complete block of sIPSCs in Purkinje cells. So, one should not be surprised that MeHg-induced potentiation of IGABA in oocytes expressing either α1β2γ2S or α6β2γ2S receptors was reversible in the present study, because the oocytes were only exposed to MeHg for relatively brief durations and washed before the reduction of IGABA or nonspecific MeHg effects occurred. Thus, these data suggest that α1β2γ2S and α6β2γ2S receptors do respond to MeHg somewhat differently in terms of the peak stimulation and time course of effects, though the pattern is similar. This is generally consistent with the observations obtained in native cerebellar granule and Purkinje cells in slices (Yuan and Atchison, 2003).
Whether or not these differential responses of α1β2γ2S and α6β2γ2S receptors to MeHg play any role in differential sensitivity of granule and Purkinje cells remains to be determined. Increases in sIPSC frequency are usually related to increased transmitter release from presynaptic terminals, whereas increased sIPSC amplitude could be due to either pre- or postsynaptic mechanisms. In the present study, GABA was applied directly to GABAA receptors (equivalent to postsynaptic receptors), thereby bypassing any presynaptic actions. Therefore, potentiation by MeHg of IGABA in oocytes expressing the two subtypes of GABAA receptors clearly indicates that it results from a direct postsynaptic action of MeHg on GABAA receptors. Then the question is how MeHg causes this initial increase or potentiation of IGABA. One possibility is that MeHg may directly interact with the GABA receptor complex and modulate the benzodiazepine or barbiturate modulation sites to cause this initial potentiation since MeHg increases the total number of benzodiazepine binding sites of GABAA receptors in the retina and other brain areas including the cerebellum (Corda et al., 1981; Concas et al., 1983; Komulainen et al., 1995; Fonfría et al., 2001). If this is the case, α6-containing receptors would be less responsive because the α1 subunit confers greater benzodiazepine sensitivity (Smith, 2001; Trincavelli et al., 2012). Alternatively, MeHg may interact with protein kinases such as the cyclic AMP-dependent protein kinase (PKA) or Ca2+-phospholipid-dependent protein kinase C (PKC) to affect phosphorylation of GABAA receptors and their functions. HgCl2-induced potentiation of GABAA receptor-mediated current in rat dorsal root ganglion cells involves changes in phosphorylation (Huang and Narahashi, 1997). However, whether or not these effects indeed underlie mechanisms by which MeHg causes this potentiation remain to be determined.
Although MeHg affects function of multiple ion channels, the sensitivity of MeHg-induced potentiation of IGABA to bicuculline confirms that these effects of MeHg are mediated specifically by these two subtypes of GABAA receptors. In contrast, those bicuculline-insensitive, voltage ramp-activated outward currents also affected by MeHg are not mediated by GABAA receptors. Our recordings from oocytes injected with water only suggest that the MeHg-increased, bicuculline-insensitive outward currents are probably mediated by the endogenous Ca2+-activated Cl− channels since the Ca2+-activated Cl− channel blocker NA could block the MeHg-induced increase of these outward currents. In addition, 4′, 4′-diisothiocyanostibene-2,2′-disulfonic acid (DIDS) or pre-injection of Ca2+ chelator BAPTA could also block MeHg-induced increase of these outward currents (Unpublished observations).
The question is why the second voltage ramp caused a larger outward current compared with the first voltage ramp. One possible explanation is that the GABA-evoked, GABAA receptor-mediated inward currents (efflux of Cl−) increases the driving force for influx of Cl− (outward currents) mediated by voltage-gated Cl− channels such as Ca2+-activated Cl− channels following GABA applications. When MeHg increases the GABAA receptor-mediated inward current, it will further enhance the driving force for influx of Cl−, leading to a even bigger outward current evoked by the second voltage ramp.
In conclusion, consistent with our previous observations from native cerebellar neurons in slices, MeHg initially potentiates IGABA in oocytes expressing either α1β2γ2S or α6β2γ2S receptors. This effect is reversible and is directed specifically at the receptor level, whereas the subsequent inhibition of receptor function involves irreversible effects of MeHg. The pattern of effects of MeHg on the two subtypes of GABAA receptor is generally similar, but slightly different in terms of time-courses and potency of MeHg effect. Whether or not these differences contribute to differential effects of MeHg on cerebellar granule and Purkinje cells remains to be determined.
Acknowledgments
The authors acknowledge the generous gift of GABAA receptor subunit DNA from Dr. Cynthia Czjkowski, University of Wisconsin-Madison (α1, β2, γ2s) and Dr. Bill Wisden, University of Heidelberg, Heidelberg, Germany (α6). The word processing assistance of Erin E. Koglin and Tara S. Oeschger and artwork assistance of Jessica M. Hauptman is especially appreciated.
FUNDING
This work was supported by the National Institutes of Health grants [R01ES03299, R01ES11662 and R01ES024064]
References
- Arakawa O, Nakahiro M, Narahashi T. Mercury modulation of GABA-activated chloride channel and non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991;551:58–63. doi: 10.1016/0006-8993(91)90913-g. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Benke D, Cicin-Sain A, Mertens S, Mohler H. Immunochemical identification of the α1- and α3-subunits of the GABAA-receptor in rat brain. J Recept Res. 1991;11:407–424. doi: 10.3109/10799899109066418. [DOI] [PubMed] [Google Scholar]
- Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. Distribution, prevalence, and drug binding profile of γ-aminobutyric acid type A receptor subtypes differing in the β-subunit variant. J Biol Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of γ2s subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Bradford AB, Mancini JD, Atchison WD. Methylmercury-dependent increases in fluo4 fluorescence in neonatal rat cerebellar slices depend on granule cell migrational stage and GABAA receptor modulation. J Pharmacol Exp Ther. 2016;356:2–12. doi: 10.1124/jpet.115.226761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Carlson BX, Elster L, Schousboe A. Pharmacological and functional implications of developmentally-regulated changes in GABAA receptor subunit expression in the cerebellum. Eur J Pharmacol. 1998;352:1–14. doi: 10.1016/s0014-2999(98)00355-0. [DOI] [PubMed] [Google Scholar]
- Chang LW. In: Mercury. Spencer PS, Schaumberg HH, editors. Williams & Wilkins; Baltimore/London: 1988. pp. 508–526. [Google Scholar]
- Concas A, Corda MG, Salis M, Mulas ML, Milia A, Corongiu FP, Biggio G. Biochemical changes in the rat cerebellar cortex elicited by chronic treatment with methyl mercury. Toxicol Lett. 1983;18:27–33. doi: 10.1016/0378-4274(83)90066-8. [DOI] [PubMed] [Google Scholar]
- Corda MG, Concas A, Rossetti Z, Guarneri P, Corongiu FP, Biggio G. Methyl mercury enhances [3H]diazepam binding in different areas of the rat brain. Brain Res. 1981;229:264–269. doi: 10.1016/0006-8993(81)90769-1. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Marty MS, Atchison WD. Comparative sensitivity of rat cerebellar neurons to dysregulation of divalent cation homeostasis and cytotoxicity caused by methylmercury. Toxicol Appl Pharmacol. 2005;208:222–232. doi: 10.1016/j.taap.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Multiple actions of propofol on αβγ and αβδ GABAA receptors. Mol Pharmacol. 2004;66:1517–1524. doi: 10.1124/mol.104.003426. [DOI] [PubMed] [Google Scholar]
- Fisher JL. The α1 and α6 subunit subtypes of the mammalian GABAA receptor confer distinct channel gating kinetics. J Physiol (Lond) 2004;561:433–448. doi: 10.1113/jphysiol.2003.051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. The role of an α subtype M2-M3 His in regulating inhibition of GABAA receptor current by zinc and other divalent cations. J Neurosci. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Zhang J, Macdonald RL. The role of α1 and α6 subtype amino-terminal domains in allosteric regulation of γ-aminobutyric acida receptors. Mol Pharmacol. 1997;52:714–724. doi: 10.1124/mol.52.4.714. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P. Molecular and synaptic organization of GABAA receptors in the cerebellum: Effects of targeted subunit gene deletions. Cerebellum. 2006;5:275–285. doi: 10.1080/14734220600962805. [DOI] [PubMed] [Google Scholar]
- Fonfría E, Rodríguez-Farré E, Suñol C. Mercury interaction with the GABAA receptor modulates the benzodiazepine binding site in primary cultures of mouse cerebellar granule cells. Neuropharmacology. 2001;41:819–833. doi: 10.1016/s0028-3908(01)00130-7. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Cerebellar granule cells in vitro recapitulate the in vivo pattern of GABAA-receptor subunit expression. Brain Res Dev Brain Res. 1995;88:1–16. doi: 10.1016/0165-3806(95)00062-i. [DOI] [PubMed] [Google Scholar]
- Herden CJ, Pardo NE, Hajela RK, Yuan Y, Atchison WD. Differential effects of methylmercury on γ-aminobutyric acid type A receptor currents in rat cerebellar granule and cerebral cortical neurons in culture. J Pharmacol Exp Ther. 2007;324:517–528. doi: 10.1124/jpet.107.123976. [DOI] [PubMed] [Google Scholar]
- Huang CS, Narahashi T. The role of phosphorylation in the activity and mercury modulation of GABA-induced currents in rat neurons. Neuropharmacology. 1997;36:1631–1640. doi: 10.1016/s0028-3908(97)00172-x. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Area and lamina-specific expression of GABAA receptor subunit mRNAs in monkey cerebral cortex. Can J Physiol Pharmacol. 1997;75:452–469. [PubMed] [Google Scholar]
- Komulainen H, Keränen A, Saano V. Methylmercury modulates GABAA receptor complex differentially in rat cortical and cerebellar membranes in vitro. Neurochem Res. 1995;20:659–662. doi: 10.1007/BF01705532. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Lüddens H. Selective antagonist for the cerebellar granule cell-specific GABAA receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits. 2014;8:3.1–3.27. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyshon-Sorland K, Morgan AJ. An integrated study of the morphological and gross-elemental consequences of methyl mercury intoxication in rats, with particular attention on the cerebellum. Scanning Microsc. 1991;5:895–904. [PubMed] [Google Scholar]
- Limke TL, Otéro-Montañez JK, Atchison WD. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J Pharmacol Exp Ther. 2003;304:949–958. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Lüddens H, Killisch I, Seeburg PH. More than one alpha variant may exist in a GABAA/benzodiazepine receptor complex. J Recept Res. 1991;11:535–551. doi: 10.3109/10799899109066426. [DOI] [PubMed] [Google Scholar]
- Mäkelä R, Wisden W, Korpi ER. Loreclezole and La3+ differentiate cerebellar granule cell GABAA receptor subtypes. Eur J Pharmacol. 1999;367:101–105. doi: 10.1016/s0014-2999(98)00944-3. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol Appl Pharmacol. 1997;147:319–330. doi: 10.1006/taap.1997.8262. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Elevations of intracellular Ca2+ as a probable contributor to decreased viability in cerebellar granule cells following acute exposure to methylmercury. Toxicol Appl Pharmacol. 1998;150:98–105. doi: 10.1006/taap.1998.8383. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Nikas P, Gatta E, Cupello A, Di Braccio M, Grossi G, Pellistri F, Robello M. Study of the interaction of 1,4- and 1,5-benzodiazepines with GABAA receptors of rat cerebellum granule cells in culture. J Mol Neurosci. 2015;56:768–772. doi: 10.1007/s12031-015-0495-8. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi PJ. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;4:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. Eur J Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- O’Kusky J. Synaptic degeneration in rat visual cortex after neonatal administration of methylmercury. Exp Neurol. 1985;89:32–47. doi: 10.1016/0014-4886(85)90263-8. [DOI] [PubMed] [Google Scholar]
- O’Kusky JR, Boyes BE, McGeer EG. Methylmercury-induced movement and postural disorders in developing rat: regional analysis of brain catecholamines and indoleamines. Brain Res. 1988;439:138–146. doi: 10.1016/0006-8993(88)91470-9. [DOI] [PubMed] [Google Scholar]
- O’Kusky JR, McGeer EG. Methylmercury poisoning of the developing nervous system in the rat: decreased activity of glutamic acid decarboxylase in cerebral cortex and neostriatum. Brain Res. 1985;353:299–306. doi: 10.1016/0165-3806(85)90219-6. [DOI] [PubMed] [Google Scholar]
- O’Kusky JR, McGeer EG. Methylmercury-induced movement and postural disorders in developing rat: high-affinity uptake of choline, glutamate, and γ-aminobutyric acid in the cerebral cortex and caudate-putamen. J Neurochem. 1989;53:999–1006. doi: 10.1111/j.1471-4159.1989.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel E, Reynolds M. Methylmercury impairs motor function in early development and induces oxidative stress in cerebellar granule cells. Toxicol Lett. 2013;222:265–272. doi: 10.1016/j.toxlet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Philip S, Gersten D, Yuan Y, Atchison WD. Program No. 368.14. 2003 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2003. Inwardly rectifying potassium channels expressed in Xenopus laevis oocytes are resistant to methyl mercury. 2003.Online. [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Quirk K, Gillard NP, Ragan CI, Whiting PJ, McKernan RM. Model of subunit composition of γ-aminobutyric acid A receptor subtypes expressed in rat cerebellum with respect to their α and γ/δ subunits. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullman DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Siegel RE. Developmental expression of cerebellar GABAA-receptor subunit mRNAs. Nature versus nurture. Perspect Dev Neurobiol. 1998;5:207–217. [PubMed] [Google Scholar]
- Sigel RE, Baur R. Electrophysiological evidence for the coexistence of α1 and α6 subunits in a single functional GABAA receptor. J Neurochem. 2000;74:2590–2596. doi: 10.1046/j.1471-4159.2000.0742590.x. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Manskikamäki S, Möykkynen T, Lüdden H, Uusi-Oukari M, Korpi ER. Receptor subtype-dependent positive and negative modulation of GABAA receptor function by niflumic acid, a nonsteroidal anti-inflammatory drug. Mol Pharmacol. 2003;84:753–763. doi: 10.1124/mol.64.3.753. [DOI] [PubMed] [Google Scholar]
- Smith TA. Type A gamma-aminobutyric acid GABAA receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58:111–121. [PubMed] [Google Scholar]
- Suñol C, Babot Z, Fonfría E, Galofré M, García D, Herrera N, Iraola S, Vendrell I. Studies with neuronal cells: from basic studies of mechanisms of neurotoxicity to the prediction of chemical toxicity. Toxicol In Vitro. 2008;22:1350–1355. doi: 10.1016/j.tiv.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Takayama C, Inoue Y. Morphological development and maturation of the GABAergic synapses in the mouse cerebellar granular layer. Brain Res Dev Brain Res. 2004;150:177–190. doi: 10.1016/j.devbrainres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Bodewitz G, Stephenson FA, Turner JD. Mapping of GABAA receptor α5 and α6 subunit-like immunoreactivity in rat brain. Neurosci Lett. 1992;144:53–56. doi: 10.1016/0304-3940(92)90714-i. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Stephenson FA. GABAA receptor subtypes expressed in cerebellar granule cells: a developmental study. J Neurochem. 1994;62:2037–2044. doi: 10.1046/j.1471-4159.1994.62052037.x. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Höger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA receptor δ subunit gene leads to an up-regulation of γ2 subunit-containing receptors in cerebellar granule cells. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- Trincavelli ML, Da Pozzo E, Daniele S, Martini C. The GABAA-BZR complex as target for the development of anxiolytic drugs. Curr Top Med Chem. 2012;12:254–269. doi: 10.2174/1568026799078787. [DOI] [PubMed] [Google Scholar]
- Varecka L, Wu CH, Rotter A, Frostholm AJ. GABAA/benzodiazepine receptor α6 subunit mRNA in granule cells of the cerebellar cortex and cochlear nuclei: expression in developing and mutant mice. Comp Neurol. 1994;339:341–352. doi: 10.1002/cne.903390304. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdellès B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABAA receptor gene family. Ann NY Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Disruption of by methylmercury of membrane excitability and synaptic transmission of CA1 neurons in hippocampal slices of the rat. Toxicol Appl Pharmacol. 1993;120:203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury acts at multiple sites to block hippocampal synaptic transmission. J Pharmacol Exp Ther. 1995;275:1308–1316. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Action of methylmercury on GABAA receptor-mediated inhibitory synaptic transmission is primarily responsible for its early stimulatory effects on hippocampal CA1 excitatory synaptic transmission. J Pharmacol Exp Ther. 1997;282:64–73. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Comparative effects of methylmercury on parallel-fiber and climbing-fiber response of rat cerebellar slices. J Pharmacol Exp Ther. 1999;288:1015–1025. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury differentially affects GABAA receptor-mediated spontaneous IPSCs in Purkinje and granule cells of rat cerebellar slices. J Physiol (Lond) 2003;550:191–204. doi: 10.1113/jphysiol.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury induces a spontaneous, transient slow inward chloride current in Purkinje cells of rat cerebellar slices. J Pharmacol Exp Ther. 2005;2313:751–764. doi: 10.1124/jpet.104.080721. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury-induced increase of intracellular Ca2+ increases spontaneous synaptic current frequency in rat cerebellar slices. Mol Pharmacol. 2007;71:1109–1121. doi: 10.1124/mol.106.031286. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Multiple sources of Ca2+ contribute to methylmercury-induced increased frequency of spontaneous inhibitory synaptic responses in cerebellar slices of rat. Toxicol Sci. 2016;150:117–130. doi: 10.1093/toxsci/kfv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Otéro-Montañez JK, Yao A, Herden CJ, Sirois JE, Atchison WD. Inwardly rectifying and voltage-gated outward potassium channels exhibit low sensitivity to methylmercury. NeuroToxicology. 2005;26:439–454. doi: 10.1016/j.neuro.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Zheng T, Santi MR, Bovolin P, Marlier LN, Grayson DR. Developmental expression of the α6 GABAA receptor subunit mRNA occurs only after cerebellar granule cell migration. Brain Res Dev Brain Res. 1993;75:91–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Corsi L, Vicini S. Lanthanum-mediated modification of GABAA receptor deactivation, desensitization and inhibitory synaptic currents in rat cerebellar neurons. J Physiol (Lond) 1998;511:647–661. doi: 10.1111/j.1469-7793.1998.647bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


