Abstract
Background
Fibrinolysis shutdown(SD) is an independent risk factor for increased mortality in trauma. High levels of plasminogen activator inhibitor-1(PAI-1) directly binding tissue plasminogen activator(tPA) is a proposed mechanism for SD, however patients with low PAI-1 levels present to the hospital with a rapid TEG(rTEG) LY30 suggestive SD. We therefore hypothesized that two distinct phenotypes of SD exist, one, which is driven by tPA inhibition, while another is due to an inadequate tPA release in response to injury.
Methods
Trauma activations from our level-1 center between 2014 to 2016 with blood collected within an hour of injury were analyzed with r-TEG and a modified TEG assay to quantify fibrinolysis sensitivity using exogenous tPA(t-TEG). Using the existing rTEG thresholds for SD(<0.9%), physiologic(LY30 0.9–2.9%), and hyperfibrinolysis(LY30 >2.9%) patients were stratified into phenotypes. A t-TEG LY30 > 95th percentile of healthy volunteers(n=140) was classified as tPA hypersensitive and used to sub-divide phenotypes. A nested cohort had tPA and PAI-1 activity levels measured in addition to proteomic analysis of additional fibrinolytic regulators.
Results
This study included 398 patients (median NISS 18), tPA-Sen was present in 27% of patients. Shutdown had the highest mortality rate(20%) followed by hyperfibinolysis(16%) and physiologic(9% p=0.020). In the non-tPA hypersensitive cohort, SD had a 5-fold increase in mortality(15%) compared to non-SD patients(3% p=0.003 figure) which remained significant after adjusting for ISS and age (p=0.033). Overall tPA activity (p=0.002) PAI-1 (p<0.001) and tPA/PAI-1 complex levels (p=0.006) differed between the six phenotypes and 54% of fibrinolytic regulator proteins analyzed (n=19) were significantly different.
Conclusion
In conclusion, acute fibrinolysis shutdown is not caused by a single etiology, and is clearly associated with PAI-1 activity. The differential phenotypes require an ongoing investigation to identify the optimal resuscitation strategy for these patients.
Keywords: fibrinolysis, fibrinolysis shutdown, platelet dysfunction, tissue plasminogen activator, trauma induced coagulopathy
Background
Critically injured patients require a delicate balance of clot formation and degradation (fibrinolysis) to prevent excessive bleeding from injured sites, while keeping the microcirculation patent. It has been recognized for decades that coagulation is never intended to reach a physiologic end point(1); ie clot formation and degradation are always occurring to prevent bleeding but keep the vasculature patent. In trauma, the extreme ends of the fibrinolytic system (hyperfibrinolysis and fibrinolysis shutdown) have been identified as predictors of mortality(2). Fibrinolysis shutdown has been documented in a large population of severely injured adult trauma patients(3) and recently has recently been identified as a pathologic coagulation change in pediatric trauma patients(4). Fibrinolysis shutdown is not a new concept, and was identified as the driver of irreversible shock following hemorrhage by Hardaway in 1963(5). Despite the known existence of fibrinolysis shutdown for over half a century, the mechanisms driving this process remain poorly defined.
The development of postoperative fibrinolysis resistance has been described in other surgical specialties and attributed to a dramatic increase in plasminogen activator inhibitor – 1 (PAI-1) several hours after completion of an operation(6, 7). However, the role of elevated PAI-1 driving fibrinolysis shutdown acutely after injury remains to be defined. The largest source of PAI-1 that can be immediately released into circulation is from platelets(8). A prior analysis using a modified TEG to quantify sensitivity to tissue plasminogen activator (t-PA) identified an inverse correlation between platelet function and sensitivity to fibrinobrinolysis (9). Therefore, platelet activation may result in early increased levels of PAI-1 acutely after injury and result in fibrinolysis shutdown.
Alternative explanations for post injury fibrinolysis resistance also exist. While high levels of t-PA have been ascribed to the hyperfibrinolytic phenotype(10, 11), the converse may also be true for fibrinolysis shutdown; ie these patients have low t-PA activity from impaired t-PA generation following injury. This could represent a component of the endotheliopahty of trauma. In a prior animal study, the development of acute fibrinolysis resistance was associated with low systemic t-PA levels without an increase in PAI-1(12). The rapid TEG (r-TEG) cut offs for fibrinolysis shutdown(2) cannot distinguish a t-PA resistant versus inadequate t-PA production mechanism of fibrinolysis shutdown. Therefore, we hypothesized that two distinct phenotypes of SD exist, one, which is driven by t-PA inhibition, while another is due to an inadequate t-PA release in response to injury.
Methods
Patient population
Prospective observation study of adult trauma patients meeting criteria for the highest level of activation at our level 1 trauma center (Denver Health Medical Center/University of Colorado) from 2014 to 2016 that had blood samples obtained within an hour of injury. Trauma criteria activation includes; hypotension (SBP < 70 or SBP < 90 and HR > 108), airway compromise, gunshot wound to neck, chest or abdomen, Glascow Coma Score (GCS) < 8 secondary to trauma mechanism, blood product transfusion requirement. All patients had samples collected under protocols approved by the Colorado Multiple Institutional Review Board for prospective evaluation of coagulation in response to trauma. Patient demographics, injury mechanism, laboratory results, and transfusion requirements were recorded by professional research assistants (PRA) who provide onsite, continuous coverage of the emergency department (ED).
Blood collection
Blood was collected in 3.5-mL tubes containing 3.2% citrate in the prehospital ambulance or upon arrival to the ED. Prehospital or ED healthcare workers drew study patient blood samples concurrently with the first set of blood samples used for in-hospital laboratory analysis. PRAs performed TEG assays within 2 hours of blood draw.
Outcomes
PRA collected all clinical and demographic data on patients from the time they arrived to the emergency department until discharge or death. The patients presenting clinical coagulopathy score was determined by the attending trauma surgeon in the emergency department. This methodology was the score has been previously published(13). Blood transfusion requirements were recorded over the first 24 hours. A massive transfusion was considered 10 or more units of red blood cells within the first 6 hours of injury. Mortality was determined during hospitalization.
Thrombelastography Assays
Viscoelastic assays were completed by a team of trained PRA with extensive experience in multiple types of TEG assays. Citrated blood samples were analyzed using the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles, IL, USA). The following indices were obtained from the tracings of the TEG: reaction time (R-time min.), angle (°), maximum amplitude (MA [mm]), and lysis 30 min after MA (LY30 [%]). Modified assays to quantify sensitivity to fibrinolysis were run in parallel with rapid TEG assay (r-TEG, activated by tissue factor and kaolin). The same TEG analyzer was used for this assay without an activator (native TEG), but prior to re-calcification exogenous t-PA (t-TEG) was added. This t-PA challenge of whole blood has been previously validated to quantify t-PA sensitivity and resistance in vitro to assess the effects of different proteins (14, 15), as well as clinically in trauma patients (9). In brief, 500 microliters of whole blood were pipetted into a customized vial containing lyophilized t-PA (Molecular Innovation, Novi, MI, USA) to a final concentration of 75 ng/mL of t-PA, and mixed by gentle inversion. A 340-μL aliquot of this mixture was then transferred to a 37 °C TEG cup, preloaded with 20 μL of 0.2 mol/L CaCl2.
Platelet Mapping
Whole blood collected in heparin (19 U mL–1) was analyzed with the TEG/platelet Mapping assay (Haemonetics), and was mixed with a solution containing reptilase and FXIIIa and then activated with 2 milimolar ADP. The same research assistants who ran TEGs also ran all platelet mapping assays. The maxiumum clot strength (ADP MA) was used to quantify platelet function.
Fibrinolysis Phenotyping
Patients were stratified into two groups based on the t-TEG LY30. To our knowledge no pre-existing threshold of t-PA hypersensitivity using TEG has previously been defined. Using data on collected on a population of 160 healthy volunteers using the same t-TEG as describe in the prior methods section, we assumed, based off of the distribution of LY30, that a value > 95th percentile would be an abnormal. Our defined threshold for t-PA hypersensitivity (HS) was a t-TEG LY30 > 27% (95th percentile of 160 healthy volunteers), and those patient with a lower t-TEG LY30 were classified as non-hypersensitive (nHS). Within these two cohorts an additional stratification was performed to divide patients into three fibrinolysis phenotypes based on their r-TEG as reported previously(3). The thresholds for each phenotype were; shutdown LY30 <0.9%, physiologic 0.9–2.9%, and hyperfibrinolysis >2.9%. This resulted in a total of six unique fibrinolysis phenotypes.
Nested Patient Population
Within each phenotype, 18 patients (total 108) were selected based on the highest NISS per group to represent comparable injury severity between phenotypes. This was conducted to eliminate the inclusion of non-severely injured trauma patients for protein analysis and reduce the probability of confounding proteomic changes attributable to a generic response to severe injury.
t-PA and PAI-1 Activity and Concentration
PAI-1 and t-PA activity, in addition to quantification of their inactive PAI-1/t-PA complex were measured via ELISA (Molecular Innovations, Novi, MI). Samples were run in duplicate on previously frozen plasma samples blood samples that were matched to whole blood TEG analysis. Activity levels were quantified by IU per ml and complex levels were represented as ng/ml.
Targeted Proteomic Analysis
A targeted proteomic approach with 13C stable isotope labeled internal standards (heavy standards) was used to identify the relative concentrations of regulators and substrates of the fibrinolytic system. Proteins were selected based on their participation or regulation of coagulation and fibrinolysis (displayed in Table 3). These include regulators of PAI-1 activity and degradation (vitronectin, protein C, proteins S), back up inhibitors of t-PA and plasmin [alpha 1-antitrypsin, alpha 2-antiplasmin (α2-AP), alpha-2-macroglobulin(α2M), C-1 esterase inhibitor] fibrin clot modifying proteins [factor XIII, Von Willebrand Factor (vWF), thrombin activatable fibrinolysis inhibitor (TAFI)], alternative plasminogen activator (urokinase), in addition to tissue factor, plasminogen, and fibrinogen. Proteomic analysis was performed on plasma samples in trauma patients in a similar fashion as described previously(15) without protein depletion. In brief, samples were mixed in a 10-kDa cutoff filter unit with 8 M urea in 0.1 M ABC (pH 8.5) and centrifuged. Proteins were reduced by addition of 100 μL of 10 mM dithiothreitol in 8 M urea and 0.1 M ABC (pH 8.5) and incubation for 30 minutes at room temperature. Subsequently, 100 μL of 55 mM iodoacetamide in 8 M urea in 0.1 M ABC (pH 8.5) was added to the samples, and the samples were incubated for 30 minutes at room temperature followed by centrifugation. Protein digestion was carried out with the presence 0.02% of surfactant trypsin enhancer (ProteaseMax, Promega, Madison, WI) surfactant at 37°C overnight. Peptides were recovered by transferring the filter unit to a new collection tube and centrifuged. Samples were then concentrated to approximately 2 μL and reconstituted to 50 μL with 0.1% formic acid. The resultant peptide mixture was analyzed by LC–single reaction monitoring (SRM). Targeted SRM approach was performed using an LC-MS/MS system interfaced with an UPLC system (QTRAP 5500 and Ultimate 3000, respectively, Thermo Fisher Scientific, San Jose, CA). Five micrograms of proteins was separated on an UPLC BEH C18 1.7 μm 1.0 × 150-mm column (ACQUITY, Waters, Milford, MA) kept at constant 50°C. The mobile phases consisted of 0.1% formic acid in double-distilled (18 mΩ) water (A) and 0.1% formic acid in 80% acetonitrile with (B), respectively. Samples were eluted at a flow rate of 150 μL/min using a gradient of 5% to 32% B for 32 minutes followed by a wash step of 5 minutes at 100% B ending with a reequilibration step of 7 minutes at 5%. The mass spectrometer was run in positive ionization mode with the following settings: a source temperature of 200°C, spray voltage of 5300 V, curtain gas of 20 psi, and a source gas of 35 psi (nitrogen gas). Multiple SRM transitions were monitored using unit resolution in both Q1 and Q3 quadrupoles to maximize specificity. SRM assay optimization was performed with the aid of computer software (Skyline, Version 3.1, UW, Seattle, WA). Collision energies and declustering potential were optimized for each transition. Method building and acquisition were performed using the instrument-supplied software (Analyst, Version 1.5.2, AB Sciex, Framingham, MA). Raw SRM data files were imported to Skyline Version 3.1 software for data processing. Transition quality, peak shape, and peak area boundaries were manually validated. Integrated peak areas were calculated by the software after Savitsky–Golay smoothing, and quantification was based on the ratio of the 12C peptide representing the endogenous sample to the corresponding 13C peptide.
Table 3.
| nHS SD |
nHS PY |
nHS HY |
HS SD |
HS PY |
HS HY |
P Overall |
|
|---|---|---|---|---|---|---|---|
| AIS Head |
0 (0–2.5) | 0 (0–2.0) | 0 (0–2.0) | 0 (0–4.5) | 0 (0–4.0) | 0 (0–3.0) | 0.113 |
| AIS Chest |
0 (0–3.0) | 0 (0–2.0) | 0 (0–2.0) | 3.0 (2.0–3.0) | 3.0 (0–3.0) | 3.0 (0.5–4.0) | <0.001 |
| AIS ABD/Plvs |
0 (0–2.0) | 0 (0–2.0) | 0 (0–2.0) | 0 (0–3.5) | 0 (0–3.0) | 2 (0–4.0) | 0.064 |
| AIS Extrem |
0 (0–2.0) | 0.5 (0–2.0) | 0 (0–2.0) | 2.0 (2–3.5) | 2.0 (0–3.0) | 2.0 (0–3.0) | 0.001 |
| NISS | 19.0 (5–34) | 13.5 (3.5–27.0) | 16.0 (3.0–27) | 41 (17–50) | 34 (27–48) | 41 (33–55.5) | <0.001 |
| Blunt | 54% | 48% | 46% | 64% | 74% | 65% | 0.016 |
| MVC | 20.6% | 23.3% | 23.5% | 24.0% | 38.8% | 29.4% | 0.309 |
| AutoPed | 17.5% | 11.9% | 8.8% | 16% | 18.4% | 23.5% | 0.276 |
| GSW | 30.2% | 30.8% | 32.4% | 20.0% | 14.3% | 23.5% | 0.199 |
| Stabbing | 15.9% | 22.0% | 20.6% | 16.0% | 10.2% | 11.8% | 0.427 |
AIS= abbreviated injury severity score, Extrem = extremity, nHS= non sensitive, HS= t-PA hypersensitive, NISS = new injury severity score, Sen= t-PA sensitive; SD = shutdown; PY = physiologic; HY = hyperfibrinolysis.
Statistical Analysis
SPSS version 23 (IBM, Armonk, NY, USA) was used for statistical analysis. TEG measurements are presented as median and interquartile values. T-PA sensitive versus non sensitive cohorts were contrasted with Mann Whitney U test for continuous variables and chi square for dichotomous variables. Multivariate logistic regression analysis was performed with t-PA sensitivity as the dependent variable, with all significant variables from dichotomous comparisons in a backwards stepwise model to eliminate non-significant variables after adjustment. Overall model significance was determined by area under the curve of a receiver operator characteristic curve (ROCAUC). Fibrinolysis phenotypes characteristics were contrasted with a Kruskall Wallis test for continuous variables or chi square for categorical. Correlations between t-PA and PAI-1 activity levels were correlated with Spearman’s Rho to TEG and clinical variables. Proteomics were normalized to the overall median value of the specific protein analyzed. The values represent the groups median percent change to overall populations median value. Conditional formatting using Excel version 15.23 (Microsoft, Redmond, WA, USA) was used to visually quantify differences between groups medians and proteins. Red represents the 95th percentile of difference in protein levels changes while blue represents the 5th percentile of protein differences (decrease) and white is representative of the 50th percentile (median). Statistical analysis of individual proteins was conducted with a Kruskal Wallis test for overall significance between groups and within each sensitivity cohort with an alpha set to 0.015 for multiple comparisons, and 0.05 considered as a trend.
Results
Patient Population
398 patients were included in the study. Patients were predominantly male (80%) and were a median of 33 years old (IQR 26–48). The median NISS was 18 (IQR 6–34) with 46% of patients sustaining penetrating wounds, with a median admission lactate of 3.9 (IQR 2.7–6.0) and a mortality rate of 13%. The nested cohort of 108 patients with proteomic and ELISA analyses, were predominantly male (71%) with a median age of 33 (28–49), and the median NISS was 41 (IQR 31–50) with 31% of patient sustaining penetrating wounds, and a mortality rate of 24%.
Classification by t-PA Hypersensitivity
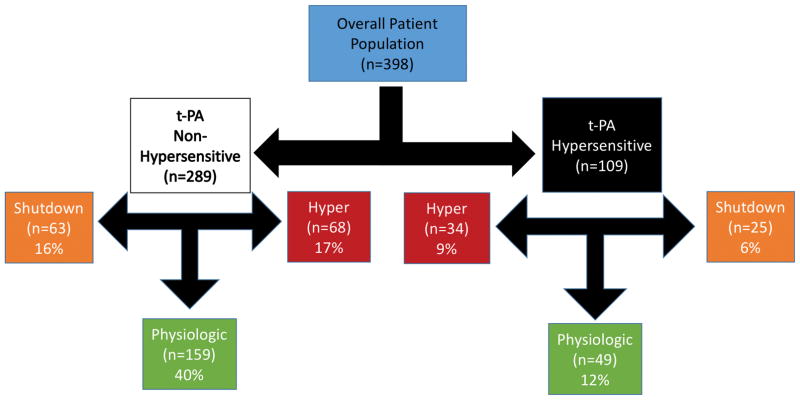
Tissue plasminogen activator sensitivity defined by t-TEG LY30 was present in 27% of patients (n=109). Patient demographic, injury patterns, and initial laboratory values, and coagulation assessment of patients with and without t-PA sensitivity are displayed in Table 1. After conducting a backwards step regression analysis of significant differences between cohorts NISS (P<0.001), INR (P=0.006), platelet count (p=0.033) and ADP platelet function (p=0.001) remained significant predictors of t-PA sensitivity (model ROCAUC 0.852 p<0.001). There was no significant difference in the distribution between fibrinolysis phenotypes within the t-PA HS and nHS cohorts (HY 32% vs 23%; PY 45% vs 55%; SD 23% vs 22% p=0.183 Figure 1). Patients who were t-PA HS had a higher rate of MT (26% vs 3% p<0.001) and increased mortality (33% vs 5% p<0.001).
Table 1.
Variables Associated with t-PA Sensitivity
| nHS | HS | P Value | |
|---|---|---|---|
| Male (n=398) | 79% | 81% | 0.631 |
| Age (n=398) | 33 (26–48) | 34 (26–49) | 0.069 |
| Penetrating (n=398) | 51% | 32% | <0.001 |
| NISS (n=386) | 16 (12–39) | 34 (14–54) | <0.001 |
| ED SBP (n=397) | 110 (90–138) | 100 (79–136) | 0.002 |
| ED GCS (n=394) | 15 (10–15) | 10 (3–10) | <0.001 |
| Lactate (n=244) | 3.6 (2.7–5.1) | 4.9 (2.8–8.5) | 0.001 |
| Ph (n=304) | 7.3 (7.2–7.4) | 7.2 (7.1–7.3) | <0.001 |
| BD (n=304) | 8 (5–12) | 10 (7–16) | <0.001 |
| Hb (n=391) | 13.6 (12.3–14.9) | 12.6 (10.9–14.8) | <0.001 |
| Plt Count (n=391) | 265 (188–329) | 206 (129–260) | <0.001 |
| ADP Plt Function MA (n=305) | 47 (31–57) | 25 (13–36) | <0.001 |
| INR (n=385) | 1.19 (1.10–1.39) | 1.37 (1.15–1.74) | <0.001 |
| Fibrinogen (n=110) | 206 (157–262) | 155 (126–207) | 0.001 |
| D-Dimer (n=100) | 2.7 (0.5 – 6.6) | 8.6 (1.7–20.1) | 0.001 |
| R-TEG ACT | 121 (113–128) | 121 (113–144) | 0.114 |
| R-TEG Angle | 72 (67–75) | 67 (60–72) | <0.001 |
| R-TEG MA | 64 (59–68) | 56 (48–62) | <0.001 |
| R-TEG LY30 | 1.8 (1.0–2.9) | 1.9 (0.9–3.8) | 0.349 |
t-TEG= t-PA challenge TEG, r-TEG= rapid TEG, nHS= non hypersensitive ; HS= t-PA hypersensitive; SD = shutdown; PY = physiologic; HY = hyperfibrinolysis.
Figure 1. Strobe Diagram.
Figure 1 represents a schematic of the breakdown of fibrinolytic phenotypes based off of patient t-PA sensitivity and systemic fibrinolytic activity measured by r-TEG LY30
Stratification by Fibrinolysis Phenotypes
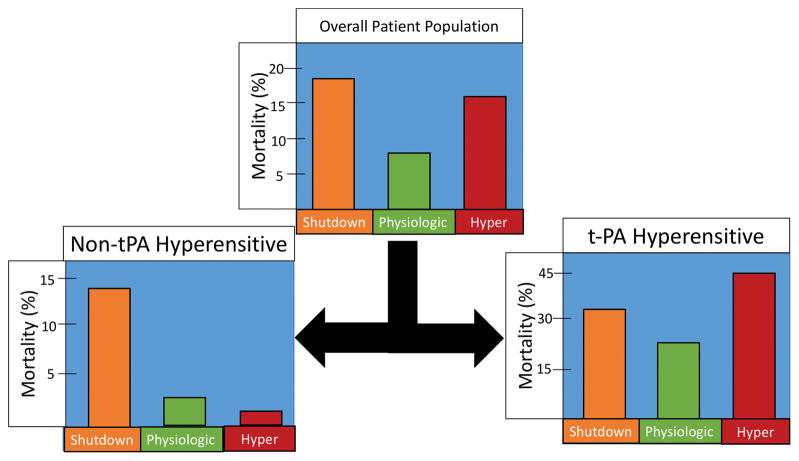
In the overall patient population, the majority of patients had a normal clinical coagulopathy score, but when stratified by fibrinolysis phenotype (not taking into account t-PA HS), hyperfibrinolysis was associated with a higher frequency of clinician appreciated coagulopathy (median 1 IQR 1–3 vs Shutdown 1 IQR 1–2 vs Physiologic 1 IQR 1–2 p=0.008). Hyperfibrinolysis was also associated with the highest rate of massive transfusion (18% vs shutdown 10% vs physiologic 5% p<0.001) and mortality was increased in patients with hyperfibrinolysis and shutdown compared to a physiologic coagulation phenotype (p=0.020 Figure 2).
Figure 2. Mortality Stratified by Fibrinolysis Phenotype.
Figure 2 represents the differences in mortality based on fibrinolytic phenotype that demonstrate a different pattern of survival when patients are stratified by t-PA sensitivity.
Six Phenotypes
Differences between coagulation parameters, coagulopathy score, and massive transfusion rates within the six patient cohorts is listed in Table 2. Within stratum (t-PA HS and nHS) overall differences between phenotypes and clinical coagulopathy scores trended to be higher in both shutdown and hyper (Table 2). The MT rate within stratum remained significantly different between phenotypes with hyperfibrinolysis having the highest rate (47% p=0.002) in the t-PA HS group, while shutdown had the highest rate of massive transfusion (8% p=0.009) in the nHS group. Injury patterns differed by the six phenotypes (Table 3). In general, t-PA HS patients had higher AIS and overall NISS to nHS patients. However, a clear injury pattern or mechanism did not emerge for individual phenotypes with in t-PA vs non t-PA sensitive cohorts. Mortality was also significantly different in the nHS sensitive group (p=0.001 Figure 2). In this cohort, fibrinolysis shutdown had a mortality rate of 14%, which was higher than physiologic (3% p=0.003) and shutdown (1.5% p=0.006). When stratified by t-PA HS, hyperfibrinolysis had a mortality rate of 45%, which was higher than physiologic (25% p=0.049) and shutdown (32% p=0.304) although not significant overall (p=0.139). In the cohort undergoing proteomic analysis, there was no overall difference in NISS (p=0.112) and injury pattern (p=0.336), but differences existed between rates of massive transfusion (p=0.017), and mortality (p=0.015) which had the same pattern as the overall patient population.
Table 2.
Coagulation Variables Between the Six Fibrinolysis Phenotypes
| t-TEG r-TEG |
nHS SD |
nHS PY |
nHS HY |
HS SD |
HS PY |
HS HY |
P Overall |
|---|---|---|---|---|---|---|---|
| ACT | 121 (113–128) | 121 (113–128) | 128 (115–136) | 121 (113–152) | 121 (113–132) | 132 (119–162) | <0.016 |
| Angle | 72 (65–75) | 73 (69–76) | 71 (66–76) | 68 (59–71) | 70 (64–73) | 63 (52–70) | <0.001 |
| MA | 64 (58–66) | 65 (62–68) | 61 (58–66) | 55 (47–62) | 60 (55–64) | 51 (40–60) | <0.001 |
| LY30 | 0.4 (0–0.6) | 1.8 (1.4–2.3) | 3.8 (3.4–5.3) | 0.3 (0.1–0.6) | 1.7 (1.2–2.2) | 9.2 (4.0–51) | <0.001 |
| INR | 1.1 (1.01–1.1) | 1.1 (1.0–1.1) | 1.1 (1.1–1.2) | 1.3 (1.2–1.8) | 1.2 (1.1–1.4) | 1.5 (1.2–2.2) | <0.001 |
| Plt Count | 235 (191–293) | 274 (227–322) | 272 (221–321) | 192 (100–246) | 238 (207–282) | 170 (124–256) | <0.001 |
| ADP Plt MA | 36 (13–54) | 45 (30–57) | 45 (30–57) | 21 (14–34) | 29 (10–36) | 18 (4–41) | =0.005 |
| Coag Score | 1 (1–2) | 1 (1–1) | 1 (1–1) | 2 (1–3) | 1 (1–3) | 3 (1–4) | <0.001 |
| MT | 8% | 1% | 3% | 16% | 16% | 47% | <0.001 |
nHS= non hypersensitive ; HS= t-PA hypersensitive; SD = shutdown; PY = physiologic; HY = hyperfibrinolysis.
t-PA and PAI-1 activity levels
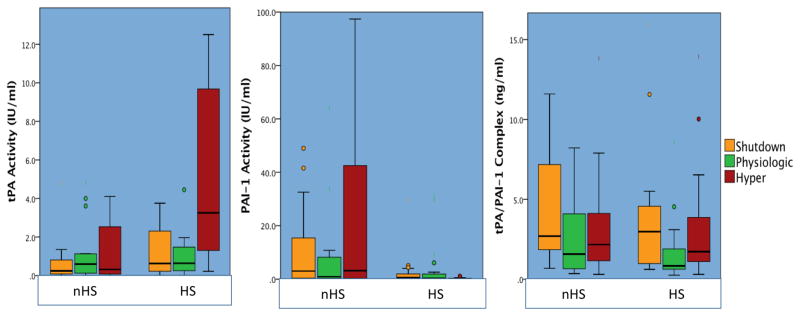
PAI-1 activity and complex levels were available on all 108 patients, 78 patients had t-PA activity levels measured due technical difficulties with assay and limited plasma availability. t-PA activity correlated to R-TEG LY30 (Rho 0.383 p<0.001) T-TEG LY30 (Rho 0.470 p<0.001) and improved when combining variable (T-TEG*R-TEG LY30 Rho =0.537 p<0.001), but did not correlation with platelet function (Rho −0.003 p=0.985). PAI-1 Activity demonstrated a similar trend with R-TEG LY30 (Rho −0.286 p=0.002) and T-TEG (Rho −0.514 p<0.001) but did not improve with combining terms (Rho −0.508 p<0.001) and did not correlate with platelet function (Rho 0.44 p=0.685). Overall t-PA activity (p=0.002) PAI-1 (p<0.001) and t-PA/PAI-1 complex levels (p=0.006) differed between the six phenotypes (Figure 3). In the t-PA HS group all three comparisons were also significantly different (t-PA p=0.003. PAI-1 p=0.009 and complex p=0.044). Only the complex levels differed between phenotypes in the non-sensitive group (p=0.037). The shutdown cohort that were nHS had a median 12-fold reduction in t-PA activity (p<0.001) and 21-times more PAI-1 activity (p=<0.001) compared to t-PA-HS cohort.
Figure 3. t-PA and PAI-1 Activity per Phenotype.
Figure 3 demonstrates differences between the 6 phenotypes of fibrinolysis based on their t-PA and PAI-1 activity and complex levels.
Proteomic analysis
The median percent changes in coagulation proteins compared to the populations overall median levels are displayed in Table 4 and individual relative protein levels are displayed in supplemental Figure 1. Of the 19 proteins analyzed 10 (52%) were significantly different overall between the six cohorts. Within the t-PA HS group 5 proteins (26%) remained significant (Table 4), and in the shutdown group no significant differences existed between groups, but there was a trend towards lower urokinase levels in the shutdown group (p=0.029), with trends towards elevated levels of vitronectin (p=0.027) and coagulation factor XIII (p=0.043 Table 4) for both shutdown and hyperfibrinolysis in the nHS cohort.
Table 4.
Proteomic Analysis
| Non-tPA Sans | tPA Sens | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shutdown | Physiologic | Hyper | Within P | Shutdown | Physiologic | Hyper | Within P | Overall P | |
| Alpha-1-Anti-trypsin | 3.3 | −3.0 | 18.3 | 0.063 | 2.7 | −3.5 | −26.3 | 0.067 | 0.010** |
| Alpha-2-antiplasmin | 23.5 | 5.2 | 32.5 | 0.077 | −18.6 | −1.1 | −42.3 | <0.001** | <0.001** |
| alpha-2-macroglobulin | −2.1 | −14.5 | 13.0 | 0.195 | −8.0 | 5.0 | −12.8 | 0.373 | 0.372 |
| C1 esterase inhibitor | 5.0 | 2.2 | 13.4 | 0.155 | −6.6 | 14.1 | −27.7 | 0.039* | 0.01* |
| Coagulation Factor XIII A chain | 15.1 | 0.8 | 13.9 | 0.245 | −13.8 | 6.4 | −18.4 | 0.088 | 0.016** |
| Coagulation Factor XIII B chain | 18.9 | −1.4 | 16.1 | 0.043* | −8.1 | −0.1 | −27.8 | 0.017* | <0.001** |
| Fibrinogen alpha chain | 6.5 | −6.4 | 17.2 | 0.220 | −13.6 | −5.4 | −30.6 | 0.126 | 0.014** |
| Fibrinogen beta chain | 10.6 | −6.2 | 34.0 | 0.064 | −6.4 | −1.2 | −25.6 | 0.317 | 0.025* |
| Fibrinogen gamma chain | 17.2 | −5.4 | 22.8 | 0.337 | −7.2 | −2.6 | −27.4 | 0.230 | 0.052 |
| Plasminogen light chain A | 17.4 | 1.8 | 14.5 | 0.354 | −2.4 | 13.0 | −36.7 | 0.020* | 0.013** |
| Plasminogen light chain B | 18.2 | 5.8 | 17.1 | 0.244 | −6.2 | 8.1 | −33.2 | 0.026* | 0.010** |
| Protein C | 15.8 | 5.9 | 8.4 | 0.854 | −29.2 | 7.0 | −31.1 | 0.006** | 0.003** |
| Protein S | 9.6 | −3.1 | 2.1 | 0.448 | −18.4 | 9.5 | −30.4 | 0.006** | 0.046* |
| TAFI | 16.9 | 3.4 | −1.2 | 0.400 | −6.5 | 0.0 | −35.1 | 0.079 | 0.002** |
| Thrombomodulin | 0.6 | 4.2 | 20.8 | 0.565 | −15.8 | 4.7 | 45.5 | 0.402 | 0.520 |
| Tissue Factor | −0.6 | 4.0 | −2.2 | 0.674 | −1.6 | −4.4 | 7.3 | 0.011** | 0.057 |
| Urokinase-type plasminogen activator | −14.8 | −23.4 | 40.6 | 0.029* | −10.2 | 29.7 | 21.1 | 0.554 | 0.068 |
| Vitronectin | 19.6 | −3.9 | 19.3 | 0.027* | −13.7 | −1.8 | −33.0 | 0.039* | <0.001** |
| Von Willebrand Factor | −1.1 | 6.7 | 4.0 | 0.570 | −9.8 | −24.0 | 41.0 | 0.013** | 0.017* |
=p<0.05
=p<0.015
Discussion
Fibrinolysis phenotypes based on decreased clot strength at 30 minutes from a standard viscoelastic assay (rapid TEG LY30) demonstrate increased mortality at the pathologic extremes of fibrinolysis. Additional stratification of these patients based on sensitivity to t-PA mediated fibrinolysis, however, demonstrates that unique sub-phenotypes exist within the originally described spectrum of fibrinolysis. t-PA sensitivity is associated with a number of clinical indices of shock, increased injury severity, and platelet dysfunction. Within this cohort of t-PA HS patients those with hyperfibrinolysis had the highest mortality rate, followed by fibrinolysis shutdown. However, in the nHS group, fibrinolysis shutdown was associated with a five-fold mortality compared to physiologic and hyperfibrinolytic patients.
The association of pathologic fibrinolysis phenotype and mortality following trauma has been reported previously in both adult (2, 3) and pediatric(4) patients. While increases in t-PA activity and depletion of PAI-1 have been implicated in hyperfibrinolysis(10, 11), the mechanisms driving acute fibrinolysis shutdown remain unclear. A previous hypothesis was that acute fibrinolysis shutdown is driven by increased inhibitors of fibrinolysis(2), however, the current study has shown that patients can be in a state of fibrinolysis shutdown without upregulation of PAI-1 or α2-AP, and unexpectedly can be hypersensitive to t-PA mediated fibrinolysis. The interaction between systemic fibrinolytic activity (measured by rapid TEG LY30) and t-PA sensitivity (measured by t-PA TEG LY30) is significant. Therefore, it is important consider these findings when resuscitating patients, as the distribution of phenotypes within nHS and t-PA HS patients is similar.
Platelets and Plasma Regulation of Fibrinolysis
Platelet alpha-granule contain potent inhibitors of fibrinolysis (15) and sensitivity to t-PA has previously been associated with platelet dysfunction in trauma patients(9). Early descriptions of platelet dysfunction using a viscoelastic assay to measure platelet function suggests that inhibition of the ADP pathway was associated with increased mortality and administration of blood products(16). Recently it has been demonstrated that dysfunction of the ADP pathway in trauma patients is associated with an impaired ability to release platelet granules(17) rather than platelet exhaust (release of all contents resulting in circulating platelets with no granules), which is seen in other clinical scenarios(18). While platelets may be a component of fibrinolysis regulation, plasma is a buffer of t-PA mediated fibrinolysis(19). This study demonstrates a number of proteins become depleted in patients who are t-PA HS and hyperfibrinolytic. Not only is PAI-1 activity decreased, but t-PA’s back up inhibitor C1 esterase(20) are also relatively depleted, along with additional downstream regulators of fibrinolysis including TAFI(21), factor-XIII(22), and α2-AP (23). These results suggest that platelets and plasma proteins both regulate fibrinolysis to a degree, but the manifestation of overt hyperfibrinolysis is dependent on failure of both systems.
This is further supported by only the t-PA HS hyperfibrinolytic patients having significant decrease in fibrinogen and plasminogen. Decreases in both of these proteins is indicative that plasmin has been generated and fibrin/fibrinogen has been depleted supporting overt systemic hyperfibrinolysis. The t-PA HS shutdown patients also have a high rate of mortality, and demonstrate depletion of fibrinolytic regulators, however have decreased clot degradation as measured by rapid TEG. A similar phenotype has been described previously in patients analyzed with ROTEM, in which high plasmin anti-plasmin levels and low fibrinolytic activity was associated with poor outcomes (24). These data suggest that even after PAI-1 is depleted the additional regulators of fibrinolysis can prevent systemic fibrinolysis. The differences in protein levels between the t-PA HS groups suggest mechanistic differences. The higher levels of Von Willibrand factor in hyperfibrinolytic patients suggests t-PA is released from an endothelial source, as pre-stored t-PA is known to co-localized in endothelial Weibel-Palade bodies(25). Several fibrinolytic inhibitors are decreased in both the shutdown and hyperfibrinolytic group. However, complex t-PA and PAI-1 levels are increased in the shutdown group. These shutdown t-PA HS patients have potentially experienced fibrinolysis, but at the time of blood draw had low systemic fibrinolysis activity, which could be the adaptive response to activating the fibrinolytic system.
Shutdown Due to Inadequate Plasminogen Activators?
In the t-PA-non sensitive cohort, there were no significant increases in fibrinolytic regulators between the shutdown and hyperfibrinolysis. Shutdown patients on the other hand, may be deficient in a pro-fibrinolytic mechanism to promote a physiologic state of fibrinolysis that extends beyond t-PA. Animal work has supported that a deficiency of urokinase and upregulation of PAI-1 after lung injury leads to increased lung damage and pulmonary fibrinosis(26). Urokinase (u-PA) levels were relatively low in both of the shutdown phenotypes (t-PA HS and nHS). Interestingly, the highest PAI-1 activity levels were in the hyperfibrinolytic non-t-PA HS group, who also had the highest u-PA levels but the lowest overall mortality. Future investigation into the temporal changes of u-PA after injury may provide mechanistic insight into a potential protective mechanism from fibrinolysis shutdown with this alternative plasminogen activator.
Emerging data suggests that a second wave of fibrinolysis shutdown following trauma is common, and is associated with a large increase in PAI-1 levels several hours of resuscitation(27). This reperfusion shutdown at four hours from injury is associated with PAI-1 levels that are 20-fold higher than the median level of patients in the shutdown non-t-PA HS cohort from this study. patients that in a persistent fibrinolysis shutdown state have nearly a four-fold increase in mortality compared to patients who normalize their fibrinolytic activity measured by TEG(28). From these data, it is unlikely that PAI-1 is the sole driver of acute fibrinolysis shutdown, and additional modifiers such as uPA may play a role in preventing prolonged fibrinolysis resistance.
Clinical Applicability
The clinical relevance of these data is that the patients that are t-PA HS need to buffer their fibrinolytic system, but the majority do not require direct fibrinolysis inhibition. These patients tend to be more severely injured and have higher rates of massive transfusion with overall laboratory coagulation scores suggestive of coagulopathy, except for fibrinolysis (LY30 Table 1). The benefit from an anti-fibrinolytic is likely only limited to those patients within this cohort with an elevated LY30. This may explain why the previous report of using an LY30 of 3% cut off for administering TXA has not been associated with improved mortality(29). However, using a t-PA TEG is not clinically feasible at this time because it is not FDA approved. INR may serve as a surrogate for t-PA sensitivity for the time being, as it has recently been identified that an elevated INR early after injury is due to fibrinogen depletion, presumed from hyperfibrinolysis(30). Our study also identified low fibrinogen, elevated INR, and protein C depletion in t-PA HS hyperfibrinolytic patients, yet INR can also be elevated in shutdown t-PA HS patients, so should not be used as a sole indicator for TXA administration.
Early fibrinolysis shutdown may represent a biomarker of poor outcomes, or unmeasured modifiers of fibrinolysis may be acting to inhibit fibrinolytic activity. While early shutdown may not be preventable, reperfusion shutdown leading to persistent fibrinolysis shutdown(28) can potentially be attenuated by minimizing platelet transfusions in these patients. Platelet transfusions and TXA were both associated with reperfusion fibrinolysis shutdown that persists beyond 12 hours(27). Using a goal directed approach to selectively administer platelets in these patients is a rational approach. Prospective data has demonstrated that empiric high ratios of platelets are associated with no overall improvement in 30-day survival(31). Point of care TEG based resuscitation has prospectively been shown to decreased early platelet transfusions in trauma patients undergoing a massive transfusion and reduce mortality by 50%(32). While this was an unexpected finding from this randomized control trial, data from this study begin to suggest a mechanism for over utilization of platelets in fibrinolysis shutdown leading to increased morbidity and mortality. The dangers of liberal platelet transfusions was recently demonstrated in a randomized control trial to treat hemorrhagic stroke patients taking antiplatelet medication, in which empiric platelet transfusion were associated with increased mortality(33).
This paper was limited to defining patients that were hypersensitive versus non-hypersensitive to t-PA mediated fibrinolysis. In this study, we do not address t-PA resistant (t-TEG LY30 <5th percentile healthy volunteers) patients. This would create nine phenotypes of fibrinolysis, and a loss of statistical power to differentiate outcomes. However, preliminary analysis of patients that are t-PA resistant demonstrates that shutdown has the highest morality rate in this cohort, and that hyperfibrinolysis defined by rapid TEG also exists in this cohort. Another limitation of this study, is that we measured relative protein concentrations between phenotypes using mass spectrometry, and not the activity level of proteases beyond t-PA and PAI-1. Some of these proteins evaluated in the study which were different between phenotypes, but were not statically significant (like TAFI) were due to high variability between patients. There is still a large gap in knowledge in understanding fibrinolysis changes following injury and it is becoming increasingly apparent that this process is not regulated solely by protein C, as previously hypothesized(34).
In conclusion, acute fibrinolysis shutdown is not caused by a single etiology. The differential phenotypes require an ongoing investigation to identify the optimal resuscitation strategy for these patients. Equally as important, not all hyperfibrinolytic trauma patients have pathologic coagulation changes. Only those patients with t-PA sensitivity have confirmation of a dysregulated fibrinolytic system with a pan coagulopathy. We no longer consider an LY30 > 3% an indication for anti-fibrinolytics and have adopted an LY30 >5% as our current threshold, while taking into consideration the implementation of an INR > 1.5 + LY30 >5% for a more definitive indication.
Acknowledgments
This study was supported in part by National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, in addition to the National Heart Lung and Blood Institute UM1-HL120877. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional research support provided by Haemonetics with shared intellectual property.
Footnotes
Presented at the Western Trauma Association, Snowbird UT, March 2017
References
- 1.Stafford JL. The Fibrinolytic Mechanism in Haemostasis: A Review. Journal of clinical pathology. 1964;17:520–30. doi: 10.1136/jcp.17.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. The journal of trauma and acute care surgery. 2014;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. Journal of the American College of Surgeons. 2016;222(4):347–55. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leeper CM, Neal MD, McKenna C, Sperry J, Gaines BA. Abnormalities in fibrinolysis at the time of admission are associated with DVT, mortality and disability in a pediatric trauma population. The journal of trauma and acute care surgery. 2016 doi: 10.1097/TA.0000000000001308. [DOI] [PubMed] [Google Scholar]
- 5.Hardaway RM, Johnson DG. Influence of fibrinolysin on shock. JAMA : the journal of the American Medical Association. 1963;183:597–9. doi: 10.1001/jama.1963.63700070034020a. [DOI] [PubMed] [Google Scholar]
- 6.Killewich LA, Macko RF, Gardner AW, Cox K, Lilly MP, Flinn WR. Defective fibrinolysis occurs after infrainguinal reconstruction. J Vasc Surg. 1997;25(5):858–64. doi: 10.1016/s0741-5214(97)70215-5. discussion 65. [DOI] [PubMed] [Google Scholar]
- 7.Paramo JA, Rifon J, Llorens R, Casares J, Paloma MJ, Rocha E. Intra- and postoperative fibrinolysis in patients undergoing cardiopulmonary bypass surgery. Haemostasis. 1991;21(1):58–64. doi: 10.1159/000216203. [DOI] [PubMed] [Google Scholar]
- 8.Booth NA, Simpson AJ, Croll A, Bennett B, MacGregor IR. Plasminogen activator inhibitor (PAI-1) in plasma and platelets. British journal of haematology. 1988;70(3):327–33. doi: 10.1111/j.1365-2141.1988.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 9.Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. Journal of thrombosis and haemostasis : JTH. 2015;13(10):1878–87. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. The journal of trauma and acute care surgery. 2016;80(1):16–23. doi: 10.1097/TA.0000000000000885. discussion-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 12.Kalady MF, Lawson JH, Sorrell RD, Platt JL. Decreased fibrinolytic activity in porcine-to-primate cardiac xenotransplantation. Molecular medicine. 1998;4(9):629–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Neal MD, Moore HB, Moore EE, Freeman K, Cohen MJ, Sperry JL, Zuckerbraun BS, Park MS, Investigators T. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: A TACTIC proposal. The journal of trauma and acute care surgery. 2015;79(3):490–2. doi: 10.1097/TA.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, Dzieciatkowska M, Sauaia A, Banerjee A, Hansen KC, Silliman C. Plasma Is the Physiologic Buffer of Tissue Plasminogen Activator-Mediated Fibrinolysis: Rationale for Plasma-First Resuscitation after Life-Threatening Hemorrhage. Journal of the American College of Surgeons. 2015 doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, Sauaia A, West B, Banerjee A, Silliman CC. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015;43(1):39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. Journal of the American College of Surgeons. 2012;214(5):739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartels AN, Johnson C, Lewis J, Clevenger JW, Barnes SL, Hammer RD, Ahmad S. Platelet adenosine diphosphate inhibition in trauma patients by thromboelastography correlates with paradoxical increase in platelet dense granule content by flow cytometry. Surgery. 2016;160(4):954–9. doi: 10.1016/j.surg.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Pareti FI, Capitanio A, Mannucci L, Ponticelli C, Mannucci PM. Acquired dysfunction due to the circulation of “exhausted” platelets. Am J Med. 1980;69(2):235–40. doi: 10.1016/0002-9343(80)90383-6. [DOI] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, Dzieciatkowska M, Sauaia A, Banerjee A, Hansen KC, Silliman C. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. Journal of the American College of Surgeons. 2015;220(5):872–9. doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth NA, Walker E, Maughan R, Bennett B. Plasminogen activator in normal subjects after exercise and venous occlusion: t-PA circulates as complexes with C1-inhibitor and PAI-1. Blood. 1987;69(6):1600–4. [PubMed] [Google Scholar]
- 21.Bouma BN, Marx PF, Mosnier LO, Meijers JC. Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U) Thrombosis research. 2001;101(5):329–54. doi: 10.1016/s0049-3848(00)00411-4. [DOI] [PubMed] [Google Scholar]
- 22.Mutch NJ, Koikkalainen JS, Fraser SR, Duthie KM, Griffin M, Mitchell J, Watson HG, Booth NA. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. Journal of thrombosis and haemostasis : JTH. 2010;8(9):2017–24. doi: 10.1111/j.1538-7836.2010.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth NA. Fibrinolysis and thrombosis. Bailliere’s best practice & research Clinical haematology. 1999;12(3):423–33. doi: 10.1053/beha.1999.0034. [DOI] [PubMed] [Google Scholar]
- 24.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. Journal of thrombosis and haemostasis : JTH. 2013;11(2):307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 25.Huber D, Cramer EM, Kaufmann JE, Meda P, Masse JM, Kruithof EK, Vischer UM. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 2002;99(10):3637–45. doi: 10.1182/blood.v99.10.3637. [DOI] [PubMed] [Google Scholar]
- 26.Bhandary YP, Shetty SK, Marudamuthu AS, Gyetko MR, Idell S, Gharaee-Kermani M, Shetty RS, Starcher BC, Shetty S. Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. American journal of physiology Lung cellular and molecular physiology. 2012;302(5):L463–73. doi: 10.1152/ajplung.00099.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore HBME, Gonzalez E, Huebner BJ, Sheppard F, Banerjee A, Sauaia A, Silliman CC. Reperfusion Shutdown: Delayed Onset of Fibrinolysis Resistance after Resuscitation from Hemorrhagic Shock Is Associated with Increased Circulating Levels of Plasminogen Activator Inhibitor-1 and Postinjury Complications. Blood. 2016;128:206. [Google Scholar]
- 28.Meizoso JP, Karcutskie CAt, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent Fibrinolysis Shutdown Associated with Increased Mortality in Severely Injured Trauma Patients. Journal of the American College of Surgeons. 2016 doi: 10.1016/j.jamcollsurg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, Holcomb JB, Cotton BA. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. The journal of trauma and acute care surgery. 2015;78(5):905–11. doi: 10.1097/TA.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 30.Davenport RA, Guerreiro M, Frith D, Rourke C, Platton S, Cohen M, Pearse R, Thiemermann C, Brohi K. Activated Protein C Drives the Hyperfibrinolysis of Acute Traumatic Coagulopathy. Anesthesiology. 2017;126(1):115–27. doi: 10.1097/ALN.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Annals of surgery. 2015 doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A, Majoie CB, Beenen LF, Marquering HA, Vermeulen M, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605–13. doi: 10.1016/S0140-6736(16)30392-0. [DOI] [PubMed] [Google Scholar]
- 34.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Current opinion in critical care. 2007;13(6):680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]