Abstract
Purpose
Lung cancer is the leading cause of cancer-related mortality in the United States. Radiation, a common component of treatment, can cause acute damage to critical organs including the lungs and the heart, but the serious toxicities from radiotherapy alone is relatively rare. A recent addition to the treatment regimen is immunotherapy, such as anti-PD-1 antibody, which blocks the inhibition of activated T cells. Combining anti-PD-1 treatment and thoracic radiation has potential for improving the outcomes of locally advanced lung cancer over traditional chemoradiation regimens, but the effect of combining these therapies on non-malignant lung tissue has not yet been investigated in preclinical models.
Materials and Methods
6–8 week old C57Bl/6 mice were treated with either anti-PD-1 antibody or control IgG with or without thoracic radiation (20Gy), and survival was monitored as an end point to determine any potential increase of toxicity from the combination therapy. Immune cell infiltration into the irradiated cardiac and lung tissues was analyzed via flow cytometry and histologically.
Results
At 21 days post-radiation, 70% of animals in the IgG + radiation group survived, significantly more than the anti-PD-1 + radiation group (36%; p=0.0169). T cell counts were significantly elevated in both cardiac and pulmonary tissues after combination therapy as compared to anti-PD-1 antibody alone (heart: 6.1 vs 22.4, p<0.001; lung 3.4 vs 20.8, p<0.001) or control IgG plus radiation (heart 11.3 vs 22.4, p<0.05; lung 12.2 vs 20.8, p<0.05) in flow cytometric studies. Histologic analysis confirmed this increase in the comparison of anti-PD-1 antibody alone versus antibody plus irradiation (heart: 464 vs 679 cells per field, p<0.001; lung: 780 vs 1109, p<0.001) and control IgG plus radiation or combination therapy (heart: 526 vs 679 cells per field, p<0.001; lung: 848 vs 1109, p<0.05).
Conclusions
Combining anti-PD-1 antibody and thoracic irradiation results in T cell infiltration into lung and heart tissue and increases mortality in a preclinical model. We conclude that healthy tissue damaged by irradiation is more susceptible to further damage by activated T cells.
Keywords: PD-1, thoracic irradiation, lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States, with the American Cancer Society estimating that ~155,870 Americans will die of the disease in 2017(1). 85% of these are non-small cell lung cancers (NSCLC)(2,3), which has a 49% 5 year survival overall rate; this number drops to 1% for metastatic (stage IV) disease (1). Traditional treatments have centered around chemotherapy and radiation treatment, providing both systemic and local treatment for inoperable locally advanced lung cancer patients.
Although it can be an extremely effective therapy, thoracic radiation can have both acute and long-term detrimental effects to the highly radiation-sensitive lung tissue, in the form of pneumonitis and fibrosis, respectively, which can greatly affect post-treatment quality of life. Up to 15% of patients develop pneumonitis within 2–3 months of chest radiation (4,5). Evidence for an immunological component in radiation pneumonitis is strong: several groups have found that patients meeting radiological (evidence of pulmonary infiltrates on chest x ray) and clinical criteria respond positively to steroid treatment, with disease relapse on discontinuation of treatment (6). T cells constitute an important part of the immune cells infiltrating the lung tissue (7–11), and patients have elevated CD4/CD8 ratios in bronchoalveolar lavage fluid (6, 9–11). Importantly, this increase is also seen in animal models of radiation pneumonitis (12–14). However, it is still controversial whether cells from the innate and adaptive immune system directly contribute to radiation-induced tissue damage or only modulate disease progression. In this regard there is evidence from preclinical and clinical investigations that T cells constitute an important part of the immune cells infiltrating the lung tissue upon irradiation of the thoracic region (7–11).
In addition to pulmonary damage, cardiovascular effects such as pericardial effusion/carditis can be seen acutely after thoracic radiation (15–25). Although cancer survivorship has been improving over the last several decades, this cohort has to contend with a new source of potential health complications, including cardiovascular disease, which is now the second leading cause of death in this group (8). In particular, cardiac fibrosis and remodeling can cause extensive pathology that can severely limit cardiac function (26–27). Multiple kinds of cancer may be treated with thoracic radiation, and in many of these cohorts, increased post-radiation cardiovascular disease has been studied, including breast cancer (28–33), Hodgkins lymphoma (34–35). As serious these complications are, their incidence is low in patients treated with radiation alone (36–37).
The relatively recent addition of immunotherapeutic treatment has proven immensely beneficial for cancer patients, including some with lung cancer. One target of immunotherapy is PD-1, a member of the same family as CTLA-4, which functions to regulate T cell activity by inhibiting activated T cells upon engaging its ligand, PD-L1. PD-L1 expression is upregulated on tumor cells, including breast (38–39), pancreatic (41), colorectal (39–40), ovarian (39, 42), brain (43), and lung cancers (39, 44–46). Isolation of tumor infiltrating lymphocytes has shown that these cells express increased levels of PD-1 (47), indicating that they are active, yet susceptible to the protective PD-L1 upregulation seen almost ubiquitously across tumor types. Clinical trials have shown the efficacy of both anti-PD-1 and anti-PD-L1 blocking antibodies in enhancing the anti-tumor activity of chemo- and radiation therapy, and several drugs have been FDA approved for lung cancer treatment, including atezolizumab (TECENTRIQ, Genentech Oncology), erlotinib (TARCEVA, Astellas Pharm), and nivolumab (Opdivo, Bristol-Myers Squibb). Pembrolizumab (Keytruda, Merck) has been approved as a first-line treatment for stage IV NSCLC with a high level of PDL1. PD1 inhibitors are being combined with thoracic RT to treat stage III NSCLC, in some studies with concurrent initiation of both therapies.
Although the efficacy of anti-PD-1 treatment is being heavily investigated, and the deleterious effects of radiation on thoracic organs has been well-established, the effect of combining these therapies on non-malignant lung tissue has not yet been investigated. Here, we provide evidence that the combination of anti-PD-1 antibody and thoracic irradiation results in T cell infiltration into lung and heart tissue that increases mortality in an animal model ubiquitous in the study of cancer.
Materials and methods
Animals
C57Bl/6 mice were and bred in the pathogen-free animal facility. All protocols were approved by the Institutional Animal Care And Use Committee (IACUC) and complied with the Guide for the Care and Use of Laboratory Animals. Commercially prepared food and water were provided without restriction.
Survival analysis
6–8 week old C57Bl/6 mice were pretreated with 200ug of either control IgG or anti-PD-1 antibody in 100uL PBS 4 days before irradiation, 2 days before irradiation, and just before irradiation. Full thoracic x ray irradiation was applied, with the head, neck, abdomen, and lower body shielded with a custom-designed lead cylinder. Five mice were irradiated in parallel. One irradiation provided 20Gy at 170cGy/min through a 1/4mm copper 1mm aluminum filter. Control animals were exposed to 0Gy. After radiation, mice were returned to a quarantine facility and monitored daily with weighing and behavior observation. They received booster injections of 100ug of antibody 3, 7, 10, 14, and 17 days post-irradiation to maintain circulating levels. Mice were followed until death or weight loss of >20%, at which point they were sacrificed using carbon dioxide and death was confirmed with cervical dislocation, as per IACUC-approved protocols. Mice surviving until 21 days post-irradiation were sacrificed using carbon dioxide and death was confirmed with cervical dislocation.
Lung tissue analysis
Lung tissue was collected from sacrificed animals and ground through a 70um filter. The resulting suspension was rinsed with DMEM with 10% fetal bovine serum and placed on ice to await staining and flow cytometric analysis.
Cardiac tissue analysis
Cardiac tissue was collected from sacrificed animals, cut into 2–3mm slices, and ground through a 70um filter. Red blood cells were lysed. The resulting suspension was rinsed with DMEM with 10% fetal bovine serum and placed on ice to await staining and flow cytometric analysis.
Flow cytometry
All samples were pretreated with CD16/CD32 FcR blocker before staining. Cells were labeled with anti-mouse CD3. Staining was performed as per the manufacturer’s protocols. Data was collected on a BD LSRII Flow Cytometer, and analyzed using FlowJo software.
Histological analysis and quantification
At the time of death, the lungs of each animal were collected for histological analysis, as previously described (48). At the time of death, the thorax and neck were dissected to expose the trachea and thoracic organs. The trachea was cannulated using a 22 gauge needle attached to rubber tubing filled with PBS, and the lungs allowed to fill via gravity. The lungs were then removed and placed in formalin. After removal of the lungs, the cardiac vasculature was flushed with PBS and the heart removed and placed in formalin. Whole organs were embedded in paraffin, and 5μm thick slices were mounted on slides. Slides were stained with anti-CD3 antibody, appropriate for paraffin-preserved samples, as per the manufacturer’s protocols. Slides were photographed at 10× (heart) or 20× (lung) magnification and the number of positively staining cells calculated by blinded analysis.
Statistical analysis
Kaplan-Meier analysis was used to determine significant differences in survival. ANOVA with pairwise comparison was performed with Prism 5.0 software to accommodate multiple groups. Statistical significance was set at the level of p < 0.05.
Results
Combining thoracic radiation and T cell stimulation decreases survival
We first examined whether the combination of thoracic radiation and anti-PD-1 antibody would decrease survival as compared to irradiation and a control IgG antibody or treatment with anti-PD-1 antibody alone. Mice were pretreated with control or anti-PD-1 antibody and irradiated as described above. The first death was seen on day 4, in the IgG plus radiation group; the first deaths in the anti-PD-1 plus radiation group were seen on day 7 (Figure 1). The last deaths were seen on day 12 and 14, respectively. At 21 days post-radiation, 100% of animals in the antibody-only groups were still alive. 70% of animals in the IgG + radiation group survived, significantly more than the anti-PD-1 + radiation group (36%; p=0.0169).
Figure 1.
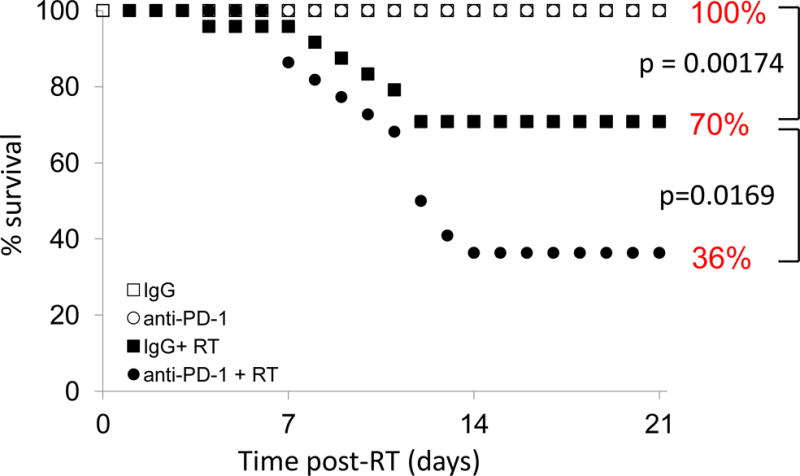
Anti-PD-1 antibody decreases survival after thoracic radiation. Combining anti-PD-1 antibody and 20Gy irradiation significantly decreases survival. IgG + RT first death: day 4. Anti-PD-1 + RT first death: day 7. Day 5 survival: IgG + RT 96%, all other groups 100%. n=20–25 animals per group.
Combination immunotherapy and radiation significantly increases T cell influx into thoracic organs
Flow cytometric analysis of T cells isolated from cardiac tissue or lung tissue showed a significant increase in the number of immune cells present after treating animals with both radiation and anti-PD-1 antibody as compared to anti-PD-1 antibody alone (p=0.0003; p=0.0006) or control IgG and radiation (p=0.02; p=0.03). Anti-PD-1 alone did not significantly alter the T cell count in either organ over treatment with control IgG (Figure 2A). In the heart, the difference in infiltration was not different after control IgG and radiation as compared to control IgG alone, although a significant difference was seen in the lungs (p=0.02).
Figure 2.
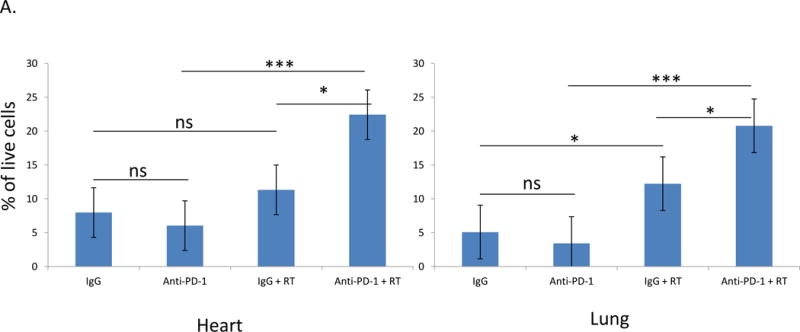
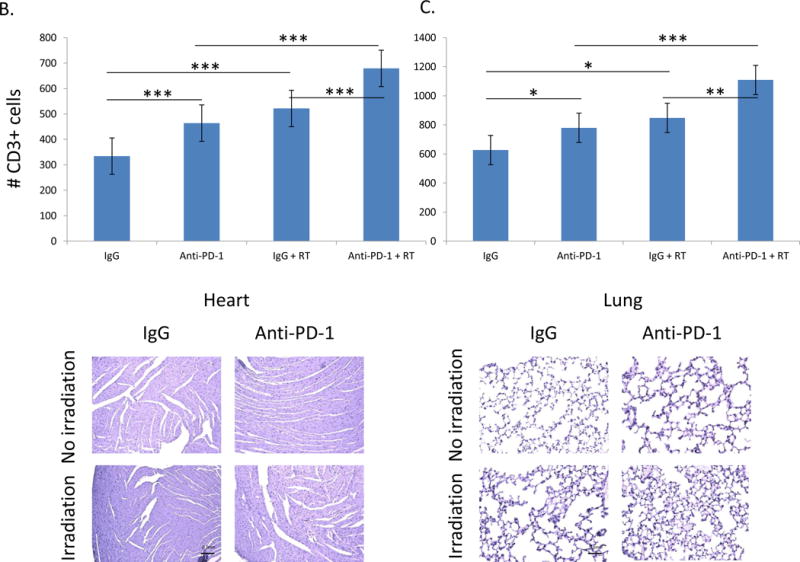
CD3+ cells are significantly increased in the heart and lungs of animals receiving immunotherapy and thoracic irradiation. A. Combining irradiation and anti-PD-1 significantly increase the number of T cells isolated from heart and lung tissue. Irradiation alone significantly increased the number of T cells in the lung. Error bars represent standard error. B and C. Analysis of CD3+ stained samples showed a significant increase in T cells in both the heart and lungs after treatment with radiation, with anti-PD-1 antibody, and with combination treatment. Three views of each sample, 10 samples per group, were analyzed by a blinded observer. Analysis of heart (B) and lung (C) tissue involved calculation of the average number of cells per field and comparison between groups. Representative samples of tissues collected from each treatment group are shown below. *=p<0.05, **=p<0.01, ***=p<0.001
Histological analysis supports these results. The differences in the cardiac tissues (Figure 2B) were significant when comparing control IgG against anti-PD-1 (p=0.000003) or control IgG and radiation (p=8.8×10−11), or comparing anti-PD-1 antibody to anti-PD-1 plus radiation (p=7.1×10−10). Comparing both groups receiving radiation also revealed a significant difference (p=0.0000002). The differences in the lung tissues (Figure 2C) also revealed significant differences when comparing control IgG against anti-PD-1 (p=0.008) or control IgG and radiation (p=0.001), or comparing anti-PD-1 antibody to anti-PD-1 plus radiation (p=0.0009). Comparing both groups receiving radiation also revealed a significant difference (p=0.008).
Discussion
Cancer immunotherapy officially began in the early 1980s when the Rosenberg group used adoptive therapy to treat several different types of cancers, infusing lymphocytes and attempting to induce tumor regression (49). In the 30 years since, therapy has become much more specific and much more aggressive, in some cases resulting in impressive decreases in tumor growth. With the growing popularity of immunotherapy, many models have been developed to study how alteration of immune cell function can affect tumors. Combination immuno- and chemotherapy has been studied in a variety of cancers, and several studies combining immuno- and radiotherapy are underway, with promising preliminary results. This paper reflects the preliminary yet critical findings of increased mortality after immune cell infiltration in animals treated with a combination of radiation and anti-PD-1 blocking antibody, an immunostimulatory molecule.
Combining immunotherapy with traditional chemotherapy has been studied in many cancers, including bladder (50), esophageal (51), urothelial, hepatocellular (52), colorectal (53), and non-small cell lung cancer (54), with promising results reporting synergisitic effects but an accompanying increase in side effects. This may be due to the fact that both treatments are systemic, leading to nonspecific activity that can affect more than the target tissues. Radiation is a commonly used tool in the treatment of many cancers, and it can be applied selectively to limit damage to non-cancerous tissue. Exploring the combination of radiation and immunotherapy is an exciting new area being studied in many tumor types, including melanomas (55), and breast (56–57), colorectal (58), pancreatic (59), prostate (60), and lung (61) cancers.
In addition to analyzing the efficacy of this treatment in tumors being directly irradiated, the combination of anti-PD-1 and radiation has been analyzed for abscopal anti-tumor effects, with a small number of patients showing decreased metastatic disease. Radiation is able to affect change at abscopal sites via its effects on the immune system. At the site of treatment, dead cells release damage-associated molecular patterns (DAMPs), such as ATP, which in turn activate local dendritic cells, increasing antigen presentation (62–63). Radiation can also increase the diversity of presented antigens (64) and localize macrophages to the tumor (65). However, these effects are primarily seen in immunostimulatory tumors such as renal cell carcinoma, melanoma, and hepatocellular cancer (66), and are dose- and method-dependent. For less immunogenic cancers, combining radiation with immunomodulatory molecules may provide the boost needed to see anticancer effects at distant sites. Preclinical data regarding the combination of radiation with IL-2, Flt3-L, TLR ligands (63), and CTLA-4 (67–68) have been published, with promising results. A limited number of studies have looked at treating lung cancer patients with radiation and GM-CSF (69) or ipilimumbab (66), but never before have the combination of anti-PD-1 and irradiation been examined (although Deng et al recently published on the combination of anti-PD-L1 antibody and radiation in the TUBO breast cancer model, 70).
This indicates that the effects of combining these treatments is not restricted to the target area (71–72). Although this has potentially beneficial effects for treating metastatic disease, it also puts non-targeted tissue at risk. Typical tumor regression models involve inoculation with tumor cells on a limb, providing easy access to the site for radiation, but lacking an assessment of how surrounding tissues are affected by irradiation. Ectopic models provide more accurate information regarding the role of the tumor microenvironment, as it is located in the tissue from which the tumor would normally develop, but these models are notoriously difficult to replicate in a uniform manner. Thus, most preliminary studies are conducted in orthotopic models, but further exploration is necessary before parallels can be drawn between the model system and it clinical applications. Our lab’s primary interest is lung cancer; therefore, we explored the consequences of combined thoracic radiation and anti-PD-1 antibody to understand how non-malignant tissue may be inadvertently affected during the treatment of lung cancer.
Analysis of damage to non-target tissue has not been reported in these studies. With the growing popularity of immunotherapy, many models have been developed to study how the alteration of immune cell function can affect tumors. We explored the consequences of combined thoracic radiation and anti-PD-1 antibody to understand how healthy tissue may be inadvertently affected during the treatment of lung cancer.
Both radiation (73) and anti-PD-1 antibody (74) given alone have been shown to cause acute pneumonitis in lung cancer patients. Thankfully, most patients with lung cancer will not experience this complication, but for the 10–15% of patients that do, the consequences can be devastating, negatively impacting quality and length of life. In addition, irradiation is known to cause both long- and short-term cardiac damage (75); the effect of anti-PD-1 on cardiac function has not been studied. Analysis of damage to cardiac and pulmonary non-target tissue has not been reported in the abovementioned combination treatment studies.
In our study, animals with no tumor burden showed increased mortality when given a combination of anti-PD-1 and total thoracic irradiation (a single dose of 20Gy, an easily reproducible dose over that typically described as causing mortality in mice). Dosing and strength were optimized for survival to be used as an end point in order to demonstrate a difference in toxicities. The choice of C57Bl/6 mice, typically known for being fibrosis-prone, for these experiments was a result of much research demonstrating physiological (76), cytologic (77–81), and pathological (76, 78, 82–83) evidence of acute pneumonitis in this model in the setting of thoracic irradiation. These animals are prone to developing Th1-weighted responses, and, as the T cell population is weighted towards this response in acute radiation pneumonitis (84), they are indeed an appropriate model for this study. In addition, modeling this effect in animals that require more intervention to develop this response lends weight to the importance and universality of this finding. At 21 days post-radiation, 70% of animals in the IgG + radiation group survived (Figure 1), significantly more than the anti-PD-1 + radiation group (36%; p=0.0169).
Limited analysis of breath rate and ejection fraction differences between these groups provide preliminary evidence that, with the accumulation of activated immune cells, organ function is compromised (data not shown). In addition, we found that cardiac-targeted irradiation does not decrease survival as dramatically as total thoracic irradiation (data not shown), indicating that the significantly decreased survival is due to damage to multiple organs. The consequences of this finding are critical, as patients undergoing combination therapy are at higher risk from treatment-induced pathology. Understanding the origin of this phenomenon is key to specifically targeting cancer cells while inducing as little unnecessary damage as possible.
T cell counts were significantly elevated in both cardiac and pulmonary tissues after combination therapy as compared to treatment with radiation alone, indicating that, while prolonging the action of immune cells may enhance their anti-tumor activity, non-malignant tissue damaged by irradiation is susceptible to accumulation of and further damage by activated T cells. Flow cytometric and histological analysis of lung and cardiac tissue showed a significant increase in the number of immune cells present after treating animals with both radiation and anti-PD-1 antibody (Figure 2). Simply the presence of these cells undoubtedly affected organ efficiency, as infiltrates of any type are known to interfere with normal functioning. Immune cell infiltrates can be particularly damaging, as activated cells can cause damage beyond disruption of the normal structure and connections, including destruction of tissue. Early studies of PD-1 function, using knockout mice to assess its function, show that animals develop immune infiltrates that cause premature mortality. This is the first report of such a finding in an experimental treatment model, indicating that collateral damage induced when normal tissue is exposed to anti-tumor treatments has the potential to negatively affect patient. Preliminary data (not shown) from these infiltrates indicated a higher level of IFNγ expression in T cells isolated from animals in the combination treatment group. Further analysis of these cells’ functionality will be critical to future studies.
The next step is to understand which cell types are responsible for this damage, including a more in-depth analysis of differences between the T cell population in animals that survived radiation alone or combination therapy. Understanding differences in cytokine production and proportional representation of cell subtypes will allow us to explore the influence of combination therapy in particular on these cells. Once a determination has been made as to what T cell subtype(s) is/are responsible for this damage, and whether they are the same types active in the anti-tumor response, alterations in the therapeutic regime designed to protect non-targeted tissue can be more thoroughly explored.
Our study has a few limitations, namely the use of only one strain of mice. We have limited preliminary findings in Balb/c mice showing the same effect, but further exploration of this nature in a variety of genetic backgrounds will shed more light on this phenomenon. In addition, the radiation dose chosen for these experiments is not directly comparable to that which would be used in a clinical setting. 20Gy allowed us to use mortality as the endpoint, whereas translation of these experiments to the clinic would assess morbidity.
There are currently over 60 studies combining radiation and anti-PD-1 therapy in various stages of development, including 12 currently recruiting patients with lung cancer (no lung cancer studies have begun treatments or data collection). None have yet published data on efficacy or complications. Our experiments are attempting to foresee complications that might arise from applying these treatments at the same time, particularly in light of their known complications individually. We are of course limited by variables that exist in the clinic and may have an effect on the development of cardiopulmonary complications, such as genetic variations, COPD, underlying heart disease, or overall functional status, but seeing these effects in animals that have overall healthy tissues makes it even more likely that they will be seen in patients with significant comorbidities. It is our hope that our findings will provide some insight as to potential complications of treatment and, after further study, may help to guide the development of treatment protocols to minimize complications.
In conclusion, although the combination of radiation and immunotherapeutic treatments has the potential to greatly decrease tumor burden and increase survival in lung cancer patients, healthy tissues may also be affected to the extent that the treatment may be as bad as the disease. A more extensive understanding of the mechanisms underlying these findings may shed light on how to best decrease tumor burden with a minimum amount of collateral damage.
Acknowledgments
The authors would like to thank Tingting Zhan, James McGettigan, Scott Waldman, and Laurence Eisenlohr for their assistance and insights.
This work was supported by the Department of Radiation Oncology at Thomas Jefferson University and the work was funded by NCI 1R21CA178229-01 (Principal Investigator Bo Lu). Carey Myers was supported in part by a Dubbs Scholar Fellowship Award from Thomas Jefferson University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Combining immunotherapy and irradiation has the potential to greatly increase survival in cancer patients, but the effects of this combination on surrounding tissues has not been studied. We treated mice with either antibody or anti-PD-1 antibody, with or without thoracic irradiation, monitored survival, then analyzed immune cell infiltration into cardiac and lung tissues. Animals treated with both anti-PD-1 and thoracic irradiation had significantly decreased survival, and significantly increased infiltration of thoracic organs by CD3+ cells.
Neither author has any conflicts of interest to report.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Navada S, Lai P, Schwartz AG, et al. Temporal trends in small cell lung cancer: analysis of the national Surveillance Epidemiology and End-Results (SEER) database [abstract 7082] J Clin Oncol. 2006;24(18S suppl):384S. [Google Scholar]
- 3.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83(3):355–67. doi: 10.4065/83.3.355. 2008. [DOI] [PubMed] [Google Scholar]
- 4.Boffetta P. Epidemiology of environmental and occupational cancer. Oncogene. 2004;23(38):6392–6403. doi: 10.1038/sj.onc.1207715. [DOI] [PubMed] [Google Scholar]
- 5.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung Cancer Following Chemotherapy and Radiotherapy for Hodgkin’s Disease. JNCI J Natl Cancer Inst. 2002;94(3):182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Becciolini A, Biggeri A, et al. Second malignancies in breast cancer patients following radiotherapy: a study in Florence, Italy. Breast Cancer Research. 2011;13:R38. doi: 10.1186/bcr2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40(5):616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–47. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Hemminki K. Inherited predisposition to early onset lung cancer according to histological type. Int J Cancer. 2004;112(3):451–7. doi: 10.1002/ijc.20436. [DOI] [PubMed] [Google Scholar]
- 10.Boffetta P. Epidemiology of environmental and occupational cancer. Oncogene. 2004;23(38):6392–6403. doi: 10.1038/sj.onc.1207715. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SJ, Cheng LS, Lozano G, et al. Lung cancer risk in germline p53 mutation carriers: association between an inherited cancer predisposition, cigarette smoking, and cancer risk. Hum Genet. 2003;113(3):238–43. doi: 10.1007/s00439-003-0968-7. [DOI] [PubMed] [Google Scholar]
- 12.Bailey-Wilson JE, Amos CI, Pinney SM, et al. A major lung cancer susceptibility locus maps to chromosome 6p23-25. Am J Human Genet. 2004;75(3):460–74. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellers TA, Chen YA. New lung cancer susceptibility locus identified: significance and implications for other genome-wide association studies. Cancer Discov. 2012;2(2):110–1. doi: 10.1158/2159-8290.CD-11-0349. [DOI] [PubMed] [Google Scholar]
- 13.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–42. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–7. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 15.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 16.Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43. doi: 10.1038/nrclinonc.2009.183. [DOI] [PubMed] [Google Scholar]
- 17.Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–9. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12:526–42. doi: 10.1038/nrd4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amendola B, Amendola MA, Perez NC, et al. P1.35: The Role of Salvage SBRT in Recurrent Lung Cancer After Previous Radiotherapy: Track: Advanced NSCLC. J Thorac Oncol. 11(10S):S204. [Google Scholar]
- 20.Boyer MJ, Williams C, Kelley MJ, et al. Survival With Stereotactic Body Radiation Therapy (SBRT) and Conventional Radiation Therapy (CRT) in Stage I Non-Small Cell Lung Cancer Patients in the Veterans Affairs System. Int J Radiat Oncol Biol Phys. 96(2S):S9. [Google Scholar]
- 21.Ahmed KA, Creelan BC, Kim S, et al. Safety and Tolerability of Extracranial Radiation Therapy and Immune Checkpoint Inhibitors Among Patients With Metastatic Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 96(2S):S201. [Google Scholar]
- 22.Timmerman R, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA. 2010;303(11):1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Videtic GM, Gomez Suescun JA, Stephans KL, et al. A Phase 2 Randomized Study of 2 Stereotactic Body Radiation Therapy (SBRT) Regimens for Medically Inoperable Patients With Node-Negative, Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;96(2S):S8–9. [Google Scholar]
- 24.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 9:134–42. doi: 10.1038/nrc2587. 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baskar R, Dai J, Wenlong N, et al. Biological response of cancer cells to radiation treatment. Front Mol Bios. 2014;1:24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaney G, Jacob S, Featherstone C, et al. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–37. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 27.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 28.Jones JA, Lutz ST, Chow E, Johnstone PA. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64(5):296–310. doi: 10.3322/caac.21242. [DOI] [PubMed] [Google Scholar]
- 29.Ringborg U, Bergqvist D, Brorsson B, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001-summary and conclusions. Acta Oncol. 2003;42:357–65. doi: 10.1080/02841860310010826. [DOI] [PubMed] [Google Scholar]
- 30.Bernhardt EB, Jalal SI. Small Cell Lung Cancer. Cancer Treat Res. 2016;170:301–22. doi: 10.1007/978-3-319-40389-2_14. [DOI] [PubMed] [Google Scholar]
- 31.Sanborn RE, Patel JD, Masters GA, et al. A randomized, double-blind, phase 2 trial of platinum therapy plus etoposide with or without concurrent vandetanib (ZD6474) in patients with previously untreated extensive-stage small cell lung cancer: Hoosier Cancer Research Network LUN06-113. Cancer. 2017;123(2):303–11. doi: 10.1002/cncr.30287. [DOI] [PubMed] [Google Scholar]
- 32.Li-Ming X, Lu-Jun Z, Qing-Song P, et al. The Importance of Thoracic Radiation Therapy and Radiation Dose on the Prognosis of Extensive-Stage Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;96(2S):E437. [Google Scholar]
- 33.Wei X, Allen PK, Welsh JW, et al. Immediately Concurrent Chemoradiation Therapy Is Associated With Improved 2-Year Overall Survival in Patients With Limited Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;96(2S):E454–5. [Google Scholar]
- 34.Komaki RU, Allen PK, Wei X, et al. Completing Thoracic Radiation Therapy With Concurrent Chemotherapy Within 6 weeks Is Important for Reducing Distant Disease in Patients With Limited-Stage Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;96(2S):E466–7. [Google Scholar]
- 35.Du L, Waqar SN, Morgensztern D. Role for adjuvant chemotherapy in patients with resected small cell lung cancer. J Thorac Dis. 2016;8(8):1891–2. doi: 10.21037/jtd.2016.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monson JM, Stark P, Riley JJ. Clinical radiation pneumonitis and radiographic changes after thoracic radiation therapy for lung carcinoma. Cancer. 1998;82(5):842–850. [PubMed] [Google Scholar]
- 37.Jaworski C, Mariani JA, Wheeler G, et al. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61(23):2319–2328. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 38.Ghebeh H, Lehe C, Barhoush E, et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12(4):R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 40.Singh PP, Sharma PK, Krishnan G, et al. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol Rep. 2015;3(4):289–97. doi: 10.1093/gastro/gov053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomi T, Sho M, Akahori, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 42.Hamanishi J, Mandai M, Ikeda T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. Jour Clin Onc. 2015;33(34):4015–22. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 43.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- 44.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor infiltrating lymphocytes and their PD-1 expression. Clin Can Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 47.Inozume T, Hanada K-I, Wang QJ, et al. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T-cells. J Immunother. 2010;33(9):956–64. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah D, Romero F, Duong M, et al. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci Rep. 2015;12(5):11362. doi: 10.1038/srep11362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Rosenberg SA. Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Response Mod. 1984;3(5):501–11. [PubMed] [Google Scholar]
- 50.Kuusk T, Albiges L, Escudier B, et al. Antiangiogenic therapy combined with immune checkpoint blockade in renal cancer. Angiogenesis. 2017 doi: 10.1007/s10456-017-9550-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Nakamura J, Noshiro H. Promising immunotherapies for esophageal cancer. Expert Opin Biol Ther. 2017;17(6):723–33. doi: 10.1080/14712598.2017.1315404. [DOI] [PubMed] [Google Scholar]
- 52.Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology. 2017;92(Suppl 1):50–62. doi: 10.1159/000451016. [DOI] [PubMed] [Google Scholar]
- 53.Limagne E, Euvrard R, Thibaudin M, et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016;76(18):5241–52. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 54.Remon J, Besse B. Immune checkpoint inhibitors in first-line therapy of advanced non-small cell lung cancer. Curr Opin Oncol. 2017;29(2):97–104. doi: 10.1097/CCO.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Rahman O. PD-L1 expression and outcome of advanced melanoma patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Immunotherapy. 2016;8(9):1081–9. doi: 10.2217/imt-2016-0025. [DOI] [PubMed] [Google Scholar]
- 56.Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–64. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72(13):3163–74. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 58.He C, Duan X, Guo N, et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499. doi: 10.1038/ncomms12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foley K, Kim V, Jaffee E, et al. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381(1):244–51. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alberti C. Prostate cancer immunotherapy, particularly in combination with androgen deprivation or radiation treatment. Customized pharmacogenomic approaches to overcome immunotherapy cancer resistance. G Chir. 2017;37(5):225–35. doi: 10.11138/gchir/2016.37.5.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacco PC, Maione P, Guida C, et al. The combination of new immunotherapy and radiotherapy: A new potential treatment for locally advanced non-small cell lung cancer. Curr Clin Pharmacol. 2016 doi: 10.2174/1574884711666161201123439. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr Breast Cancer Rep. 2016;9(1):45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med. 2016;4(6):118. doi: 10.21037/atm.2016.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treatment Reviews. 2015;41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2):728–34. [PubMed] [Google Scholar]
- 68.McDermott D, Lebbe C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–64. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Golden EB, Formenti SC. Radiation therapy and immunotherapy: growing pains. Int J Radiat Oncol Biol Phys. 2015;91:252–4. doi: 10.1016/j.ijrobp.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes RJ, Schmerling RA, Haddad CK, et al. Analysis of the Abscopal Effect With Anti-PD1 Therapy in Patients With Metastatic Solid Tumors. J Immunother. 2016;39(9):367–72. doi: 10.1097/CJI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 72.Park SS, Dong H, Liu X, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res. 2015;3(6):610–9. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takigawa N, Segawa Y, Saeki T, et al. Bronchiolitis obliterans organizing pneumonia syndrome in breast-conserving therapy for early breast cancer: radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys. 2000;48(3):751–5. doi: 10.1016/s0360-3016(00)00654-4. [DOI] [PubMed] [Google Scholar]
- 74.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. Jour Clin Onc. 2017;35(7):709–17. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taunk NK, Haffty BG, Kostis JB, et al. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heinzelmann F, Jendrossek V, Lauber K, et al. Irradiation-induced pneumonitis mediated by the CD95/CD95-ligand system. J Natl Cancer Inst. 2006;98(17):1248–51. doi: 10.1093/jnci/djj335. [DOI] [PubMed] [Google Scholar]
- 77.Rube CE, Uthe D, Schmid KW, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47(4):1033–42. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 78.Rube CE, Uthe D, Wilfert F, et al. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. Int J Radiat Oncol Biol Phys. 2005;61(5):1482–92. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 79.Hong JH, Chiang CS, Tsao Cy, et al. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75(11):1421–7. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 80.Chen J, Zhang W, Zhang L, et al. Glycyrrhetinic acid alleviates radiation-induced lung injury in mice. J Radiat Res. 2017;58(1):41–7. doi: 10.1093/jrr/rrw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen YH, Chou CH, Shun CT, et al. The Expression of CXCL16 During Lung Irradiation May Lead to Radiation Pneumonitis and Fibrosis Through Inducing Neutrophil and Macrophage Infiltration in Lung Tissue. Int J Radiat Oncol Biol Phys. 2016;96(2):S65–6. [Google Scholar]
- 82.Chen Y, Williams J, Ding I, et al. Radiation pneumonitis and early circulatory cytokine markers. Sem Rad Onc. 2002;12(1):S26–33. doi: 10.1053/srao.2002.31360. [DOI] [PubMed] [Google Scholar]
- 83.Han G, Liu H, Tan W. Differential Activation of Th1/Th2 Immune Reponses in Murine Radiation Induced Lung Injury. Int J Radiat Oncol Biol Phys. 2015;93(3):E510–1. [Google Scholar]


