Abstract
Osteonecrosis is the devitalization of bone and consequent lytic changes. In the jaws, osteonecrosis is a pathological consequence of prior radiation therapy (osteoradionecrosis) or certain anti-resorptive medications. Here we review the pathogenesis and clinical manifestations of these lesions, and describe the spectrum of radiological findings in these conditions, and highlight the similarities and differences between the imaging appearances of these two entities.
Keywords: osteoradionecrosis, medication-related osteonecrosis
Introduction
Bone is a specialized connective tissue that provides structural support and a microenvironment that facilitates several physiologic functions, including production of red and white blood cells, and calcium homeostasis. The cellular elements of bone include osteoblasts (bone-forming), osteoclasts (bone-resorbing), and osteocytes. The dynamic nature of bone is manifested in its constant structural adaptation to mechanical forces, local disease, and systemic hormonal influence. Continual turnover of bone is a net result of a regulated balance between osteoblastic and osteoclastic activity. External insults such as therapeutic ionizing radiation, and pharmacological interventions such as anti-resorptive agents often disrupt this balance. In the jaws, such altered bone homeostasis is further complicated by the oral cavity environment and the presence of teeth— the inflammatory diseases of teeth (dental caries, periodontal disease), and traumatic disruptions of the oral mucosa serve as portals of infection and subsequent bone inflammation, increasing the risk for pathological consequences of osteoradionecrosis (ORN) or medication-related osteonecrosis (MRONJ). Both these conditions have debilitating effects on the integrity of the jaw bone with structural and functional consequences that significantly impair the quality of life.
The term osteonecrosis broadly refers to devitalization of bone and consequent lytic changes. At some sites, such as the femoral head, osteonecrosis is often a consequence of altered vascular supply leading to avascular (aseptic) necrosis. Although altered perfusion may partly contribute to their pathogeneses, it is important to recognize that ORN and MRONJ are considered discrete entities that differ from such areas of avascular necrosis. Moreover, despite the similarities in their therapy-related causation, ORN and MRONJ are separate entities, characterized by distinctive but frequently overlapping clinical and radiographic manifestations, and are managed by different therapeutic approaches.
Osteoradionecrosis
Definition
Osteoradionecrosis (ORN) is a late complication of radiation therapy and occurs when an area of irradiated bone becomes devitalized. ORN is defined as an area of exposed irradiated bone tissue that fails to heal over a period of three months, without residual or recurrent tumor; and when other causes of osteonecrosis have been excluded.1 There is now increasing recognition that such necrotic changes could occur in the bone even before mucosal breakdown is clinically evident, emphasizing the importance of imaging in the diagnostic workup of this condition.
Pathogenesis, epidemiology and risk factors
The pathogenesis of ORN is not completely understood. Initial theories proposed that mucosal trauma allowed for bacterial entry and subsequent osteomyelitis.2 There is now evidence that microbes are contaminants, and do not have a causal role in ORN.1 A widely-accepted model is that radiation-induced microvascular changes create a state of hypoxia, hypovascularity, and hypocellularity, thereby disturbing normal bone homeostasis, and leading to a chronic, non-healing wound.1 Recent data emphasize the potential role of radiation-induced fibrosis to ORN development—where radiation-induced cell damage triggers a cascade of chronic inflammation and deregulated fibroblastic activity around the blood vessel walls, exacerbating a chronic fibrotic response in both the bone and the overlying mucosa.3
ORN can develop anytime following radiation therapy, and typically manifests 6–12 months following radiation treatment. However, the time to onset is variable, ranging from months to years. The incidence of ORN with current therapeutic delivery systems is approximately 5% to 7%, and is similar with conventional radiotherapy, intensity-modulated radiation therapy (IMRT), and brachytherapy.4 ORN occurs more frequent in the mandible than the maxilla, and is thought to reflect the relatively lower vascularity in the mandible. Furthermore, the mandible is more often in the path of radiation delivery. Although rare, maxillary osteonecrosis may develop, and most often occurs secondary to irradiation for nasopharyngeal cancer.
ORN risk is modulated by several factors (Box 6-1). An important primary determinant is the total radiation dose—ORN is unlikely to occur when the delivered radiation dose is less than 60 Gy, and is rare when the dose is less than 50 Gy. At doses above 66 Gy, ORN risk is increased 11-fold. Besides dose, ORN risk is modulated by other aspects of the radiation delivery protocol, including field size, concomitant chemotherapy, and proximity of the neoplastic lesion to bone. Notably, contemporary approaches to deliver radiation, such as IMRT, offer the advantage of limiting “hot-spots” of high radiation dose deposition in the jaw. Other crucial modifiers of ORN modifier of ORN risk are the presence of carious teeth or periodontal disease, or trauma from dental extractions, and from ill-fitting dentures.
Box 6-1. Factors that increase ORN risk.
Total radiation dose, >60 Gray
Proximity of neoplasm to bone
Larger size of the therapeutic field
Concomitant chemotherapy
Poor oral hygiene
Smoking
Alcohol abuse
Dental extractions and other surgical manipulations of the irradiated area
Trauma from dentures and dental appliances
Clinical manifestations and disease staging
ORN manifests as exposed bone in an area of prior therapeutic irradiation that fails to heal over a period of three months, without residual or recurrent tumor; and when other causes of osteonecrosis have been excluded. The presenting symptoms are non-specific and vary from pain, often neuropathic in nature, paresthesia along the course of the inferior alveolar nerve, trismus, bad breath, and formation of fistulas, often with purulent discharge. Notably, ORN-related symptoms can develop even several years following radiation treatment. ORN is often precipitated by an injury, for example tooth extraction, or by infection from caries or periodontal disease, emphasizing the importance of evaluating these changes in the diagnostic workup of ORN.
Small areas of clinically-evident bone exposure may be asymptomatic and remain stable for months, and can be adequately managed by conservative approaches. Often, the region of bone exposure progresses, and is complicated by repeated episodes of superimposed bacterial infection and osteomyelitis. The tissue destruction may result in skin fistulas, and may be severe enough to cause facial disfigurement.
Current staging or scoring systems are based on response to hyperbaric oxygen therapy, the extent of the osseous necrotic changes, and clinical and radiological parameters.1,5–9 In the absence of a universally-accepted staging system, the system proposed by Store and Boysen9 is a convenient and effective tool for the diagnostic radiologist to communicate disease status to the referring provider (Box 6-2).
Box 6-2. Store and Boysen’s Classification of ORN9.
| Stage | Criteria |
|---|---|
| 0 | Mucosal defects only |
| I | Radiological evidence of necrotic bone with intact mucosa |
| II | Positive radiographic findings with denuded bone intraorally |
| III | Clinically exposed radionecrotic bone, verified by imaging, skin fistulas and infection |
Diagnostic imaging objectives and protocols
The diagnosis of ORN is established based on a positive history of irradiation and exclusion of other causes of necrotic bone exposure. Often, the diagnosis of ORN can be made based on history and clinical findings. ORN management seeks to remove the necrotic tissue, and allow for physiologic repair.
The goals of imaging the patient with suspected or established ORN are listed in Box 6-3. Specifically, imaging seeks to identify potential precipitating causes of ORN, such as dental or periodontal disease. Furthermore, imaging aims to evaluate the extent of ORN, and its impact on the integrity of the bone and vital anatomic boundaries.
Box 6-3. Objectives of imaging for suspected or established ORN.
Evaluate teeth and periodontal structures for potential sources of inflammation
Detect radiographic bone lysis prior to clinical bone exposure
Detect presence and define extent of lytic bone changes
Detect cortical destruction in the jaw
Detect periosteal bone formation
Evaluate mandibular bone integrity and assess the risk for pathological fracture
Evaluate integrity of the mandibular canal
Evaluate integrity of the nasal cavity and maxillary sinus
Rule out presence of recurrent malignancy
Considering that the diagnostic assessments are almost all related to hard tissue alterations, conventional radiography and CT are the preferred imaging modalities. Typically, the initial radiographic examinations include conventional dental and panoramic radiographs to provide detailed evaluation of the teeth, periodontal ligament and surrounding alveolar bone. If clinical bone exposure is detected, or when lytic changes are detected on conventional imaging, multi-planar imaging is indicated to better assess the severity of bone loss and its impact on the adjacent structures, and is accomplished by either cone-beam CT (CBCT) or multi-detector CT (MDCT) imaging.
Depending on the extent of the bone changes, limited to medium field of view CBCT is usually adequate. Given the marked variation of the jaw bone trabecular density and architecture, the clinically-unaffected contralateral side must be included in the examination for comparison When assessment of the adjacent soft tissue is indicated, MDCT imaging should be selected. The imaged MDCT volume must encompass the maxillofacial region, starting at the frontal sinuses and extending through the mandible, with axial reconstructions parallel to the dental occlusal plane, using both soft tissue and bone algorithms. Reformations in the coronal and sagittal planes are useful to better depict the effects on the mandibular canal and sinus borders.
Radiographic appearances of osteoradionecrosis
Patients with prior irradiation of the head and neck are typically monitored periodically for ORN development. Often, the presenting symptoms may be non-specific in nature, with no clinically-evident bone exposure, and in these cases imaging is an important component to detect early, and frequently, preclinical ORN. When bone exposure is evident, and the clinical presentation is consistent with ORN, imaging is warranted to assess the extent of the disease and its impact on the surrounding vital structures, and on the integrity of the jaw.
Typically, the first line of diagnostic imaging of the patient with suspect ORN is accomplished with dental periapical and panoramic radiography. These techniques are readily available, relatively inexpensive and convenient, and deliver minimal radiation dose to the patient.10 Initial changes of ORN are manifested as subtle areas of altered trabecular architecture and rarefaction. Given that dental infection is often a precipitating factor in ORN development, the teeth should be examined closely for signs of caries, and periodontal and periapical inflammations (Fig 1A). Notably, widening of the periodontal ligament space along the mandibular tooth roots is a common finding in the irradiated mandible,11 and in the absence of adjacent bone destruction, it requires no management. As the disease progresses, frank lytic bone destruction becomes radiographically evident, and produce a patchy radiolucent appearance with radiodense islands of necrotic bone, or sequestrum, contrasted against the radiolucent lytic changes (Fig 1B). The extent of bone destruction may be severe enough to compromise the integrity of the mandible and lead to a pathological fracture. It is important to recognize that the radiographic manifestation of ORN is not specific, and must be differentiated from other causes of bone necrosis, osteomyelitis, and recurrent malignancy.
Figure 1.
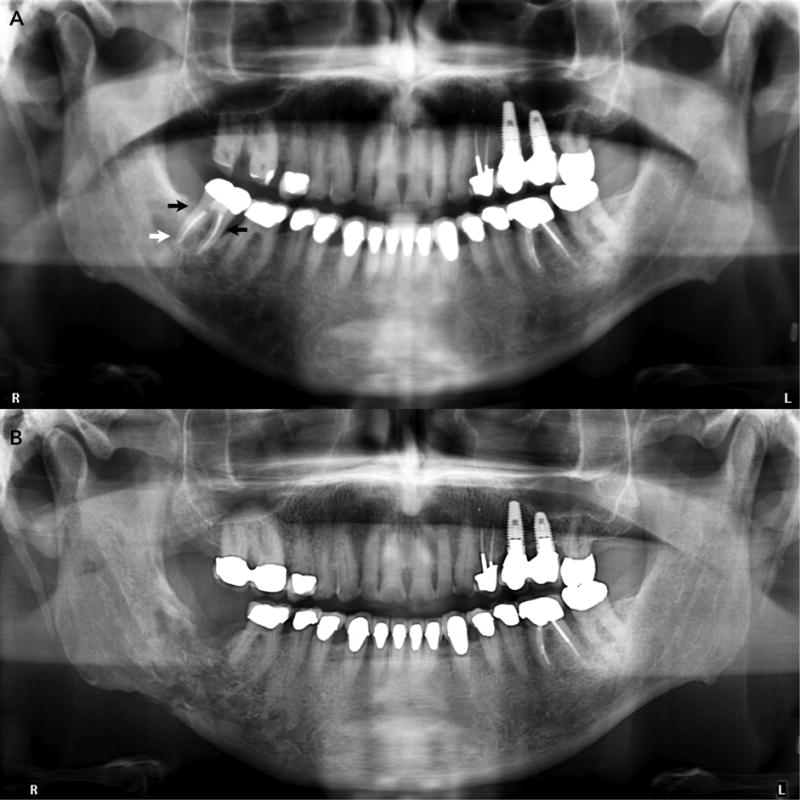
A. Panoramic radiograph taken to evaluate the teeth and jaws in a post-radiation therapy patient shows presence of periodontal bone loss around the mandibular second molar (black arrows). A periapical radiolucency is present at the distal root of the tooth (white arrowhead), suggestive of local microbial infection and inflammation, and potentially early ORN.
B. Panoramic radiograph of same patient taken two years later shows extensive patchy and irregular lytic changes in the right posterior mandible extending to partly destroy the cortical borders of the mandibular canal, and the inferior mandibular cortex.
Although panoramic radiography is appropriate as the initial radiographic evaluation of the ORN patient, it is limited by its two-dimensional nature, and often underestimates the extent of the osseous changes, relative to CT.12 Thus, when the clinical suspicion is high, and panoramic radiography is inconclusive, CT is the imaging modality of choice. Indeed, CT imaging better depicts cortical destruction, central necrosis and sequestration, and in general, provides a better assessment of the extent and impact of the bony changes (Figs 2–4).12 Both CBCT and MDCT are adequate to provide information on osseous changes. CBCT, especially when performed with limited field of view protocols, provides high resolution imaging of the teeth and periodontal structures, and is more sensitive than conventional 2-dimensional imaging for detection of dental disease.13 However, the contrast resolution of CBCT is inadequate to provide detailed assessment of soft-tissue changes. Certain clinical situations dictate the need for soft-tissue imaging—including superimposed osteomyelitis and cellulitis, or a soft tissue mass that may represent recurrent malignancy. In such scenarios, contrast-enhanced MDCT imaging should be the modality of choice.
Figure 2.
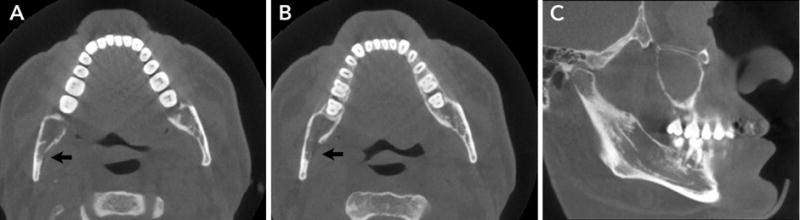
A and B. Axial sections show erosion of the lingual cortex of the mandibular ramus.
B. Sagittal section shows lytic lesions in the mandibular body with loss of mandibular canal cortication.
Figure 4.
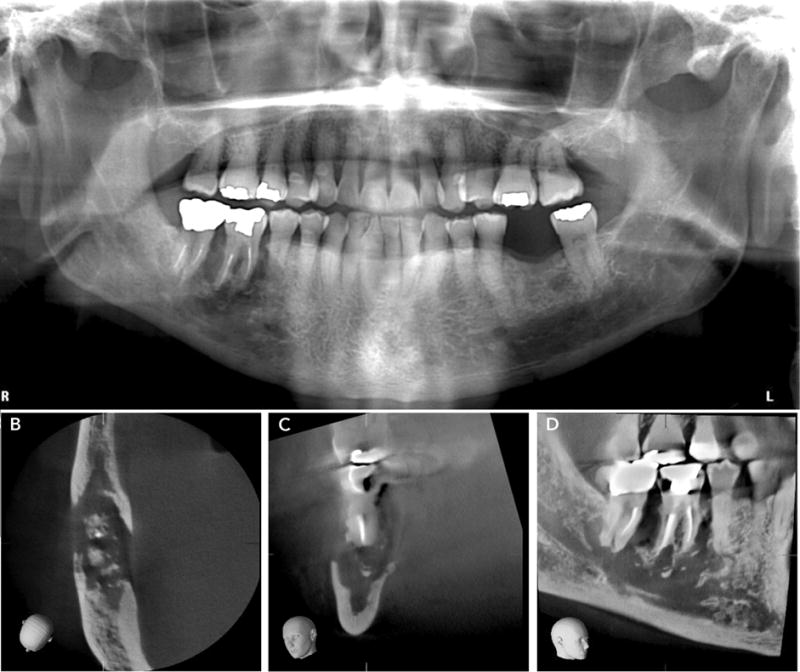
A. Panoramic radiograph of a patient with clinically-evident bone exposure in the mandibular right molar region shows irregular lysis around the mandibular first molar.
B and C. Axial and coronal sections show extensive cortical erosion on the buccal and lingual plates, with small bony sequestrae.
D. Sagittal section shows extent of the lesion inferiorly to the mandibular cortex and ablation of the mandibular canal cortex.
MRI can depict early ORN-induced changes in the bone marrow, prior to its clinical manifestation. This is detected as a reduced intensity of the bone marrow signal on T1-weighted MR imaging, and as an increase in bone marrow signal intensity, on T2-weighted images.14,15 MR imaging also depicts cortical erosions and changes in the adjacent soft-tissues.14,15 However, because most of the diagnostic objectives are met with CBCT or MDCT imaging, application of MR imaging to the routine ORN diagnostic workup has been limited.
Medication-related osteonecrosis of the jaws (MRONJ)
Definition
Medication-related osteonecrosis of the jaws (MRONJ) is osteonecrosis that develops associated with anti-resorptive or anti-angiogenic therapy.16 Anti-resorptive therapies are used to manage skeletal-related events associated with bone metastases (such as from the breast, prostate, and lung), and the lytic lesions of multiple myeloma. In addition, anti-resorptive agents are used to decrease fracture risk in patients with osteoporosis or to manage other bone diseases, such as Paget’s disease and fibrous dysplasia of McCune-Albright Syndrome (MAS). As with ORN, these necrotic lesions typically manifest as areas of non-healing, exposed bone. To clarify its difference from other causes of exposed bone, the American Academy of Oral and Maxillofacial Surgery (AAOMS) has developed specific diagnostic criteria as detailed in Box 6-4.16
Box 6-4. Diagnostic criteria for medication-related osteonecrosis of the jaws (MRONJ)16.
Current or previous treatment with anti-resorptive or anti-angiogenic agents; and
Exposed bone or bone that can be probed through an intraoral or extraoral fistula(e) in the maxillofacial region that has persisted for more than eight weeks; and
No history of radiation therapy to the jaws or obvious metastatic disease to the jaws
Pathogenesis, epidemiology and risk factors
The most recognized risk factor for MRONJ development is prior anti-resorptive therapy, either with bisphosphonates or the RANK-ligand inhibitor, Denosumab. Other anti-angiogenic drugs have been implicated, but MRONJ results less frequently with them. The risk of developing MRONJ is primarily dependent on the indication for its use. In cancer patients, treatment with the bisphosphonate, zolendronate, or with RANK Ligand-inhibitor Denosumab, increases the risk of MRONJ 50– to 100–fold, relative to cancer patients treated with placebo. Contemporary estimates for MRONJ development following zolendronate therapy range from 0.7% to 6.7%.16 Likewise, the risk with Denosumab therapy ranges from 0.7% to 1.9%.16 In contrast to cancer patients, the risk for developing MRONJ among patients with osteoporosis following either Denosumab or zolendronate therapy is considerably lower, and ranges from 0.001% to 0.01%.17 This reflects an MRONJ risk that is more than 100 times lower than that in cancer patients, and emphasizes that MRONJ development correlates with the total antiresorptive dose.
The pathophysiology of ONJ remains to be fully elucidated. Its association with anti-resorptive medications suggests a central role of osteoclastic inhibition that leads to an imbalance in bone turnover. An altered immune response, inhibition of angiogenesis, increased risk for infection, and direct toxicity of anti-resorptive medications on soft and hard tissues of the oral cavity have also been proposed to underlie disease pathogenesis. Like ORN, local inflammation and surgical trauma are thought to precipitate the development of MRONJ.16,17
Clinical manifestations and disease staging
To facilitate disease stratification, the AAOMS has defined criteria for staging of precilinical and clinical MRONJ. This staging system (Box 6-5) is also used to direct appropriate medical, and conservative and radical surgical intervention, and thus provides the radiologist with a clinically-relevant tool to communicate disease severity and complications to the referring clinician.
Box 6-5. Staging of MRONJ*.
| Stage | Exposed or necrotic bone | History and clinical findings |
|---|---|---|
| At risk | No clinical evidence | |
| Stage 0 | No clinical evidence | Non-specific clinical and radiographic findings |
| Stage 1 | Exposed and necrotic bone, or fistulae that probes to bone | Asymptomatic with no evidence of infection |
| Stage 2 | Exposed and necrotic bone, or fistulae that probes to bone | Associated with infection, Pain and erythema in the region of the exposed bone with or without purulent drainage |
| Stage 3 | Exposed and necrotic bone, or fistulae that probes to bone | Pain, infection, and one or more of the following:
|
Adapted from Ruggiero et al.16
Diagnostic objectives of imaging
The diagnosis of MRONJ is essentially based on historical and clinical data. Imaging is an important component of the overall workup to monitor and manage patients at risk for MRONJ, or with established MRONJ. Specifically, imaging seeks to identify precipitating factors such as dental or periodontal disease. Furthermore, imaging aims to evaluate the extent of MRONJ to facilitate its staging and subsequent management.
As with ORN, most the diagnostic tasks evaluate hard tissue alterations, and conventional radiography and CT are adequate in most patients with suspect or established MRONJ. Initial radiographic examinations include conventional dental and panoramic radiographs that serve to detect dental disease and screen at-risk patients. CBCT and MDCT protocols are identical to those described above for ORN.
Imaging of the patients at risk, or with suspect or established MRONJ
Patients being managed for osteoporosis with anti-resorptive therapy are at a much lower risk for MRONJ development.17 When asymptomatic, they do not require any imaging beyond that directed to evaluating dental and periodontal disease. Such imaging is often accomplished at the dentists’ offices with conventional intraoral and panoramic imaging.
High-dose anti-resorptive treatment, used to manage skeletal metastases, carries a much higher risk for MRONJ development. Patients on these treatment regimens benefit from regular dental examinations, including clinical evaluation, intraoral and panoramic radiographs as appropriate, and limited to medium field of view CBCT imaging that encompasses the entire jaw being imaged for comparison purposes, and is often useful in identifying potential dental disease, that increase the risk for MRONJ (Fig 5). Imaging of these asymptomatic patients provides a baseline to monitor future subtle changes that may permit early detection of necrotic bone changes. When clinical changes suggest establishment of MRONJ (Stages 1–3), imaging is indicated to assess the extent of the changes, and the potential for complications such as pathologic fracture or disruption of the nasal cavity and maxillary sinus (Fig 6). While MR imaging has the potential to contribute to early preclinical detection of disease, its use for the care of the MRONJ patient is limited.
Figure 5.
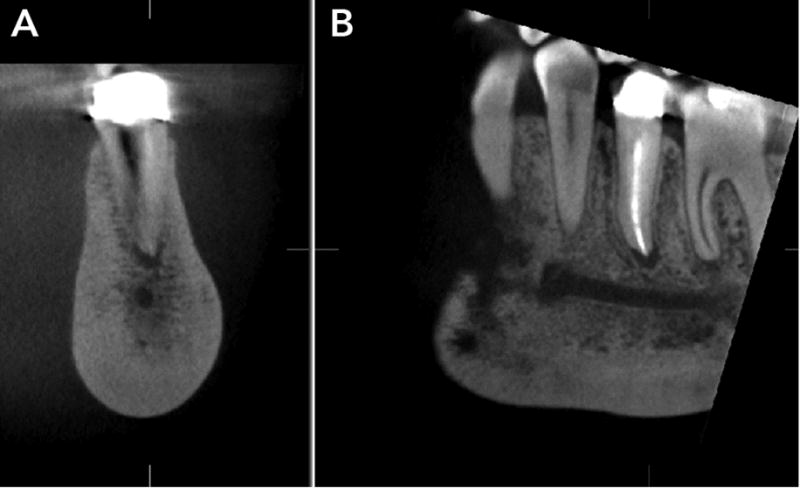
A and B. Coronal and sagittal CBCT sections through the mandibular premolar show intense sclerosis that alters the trabecular pattern of the mandibular body, and widening of the apical periodontal ligament, indicating potential residual odontogenic inflammation.
Figure 6.
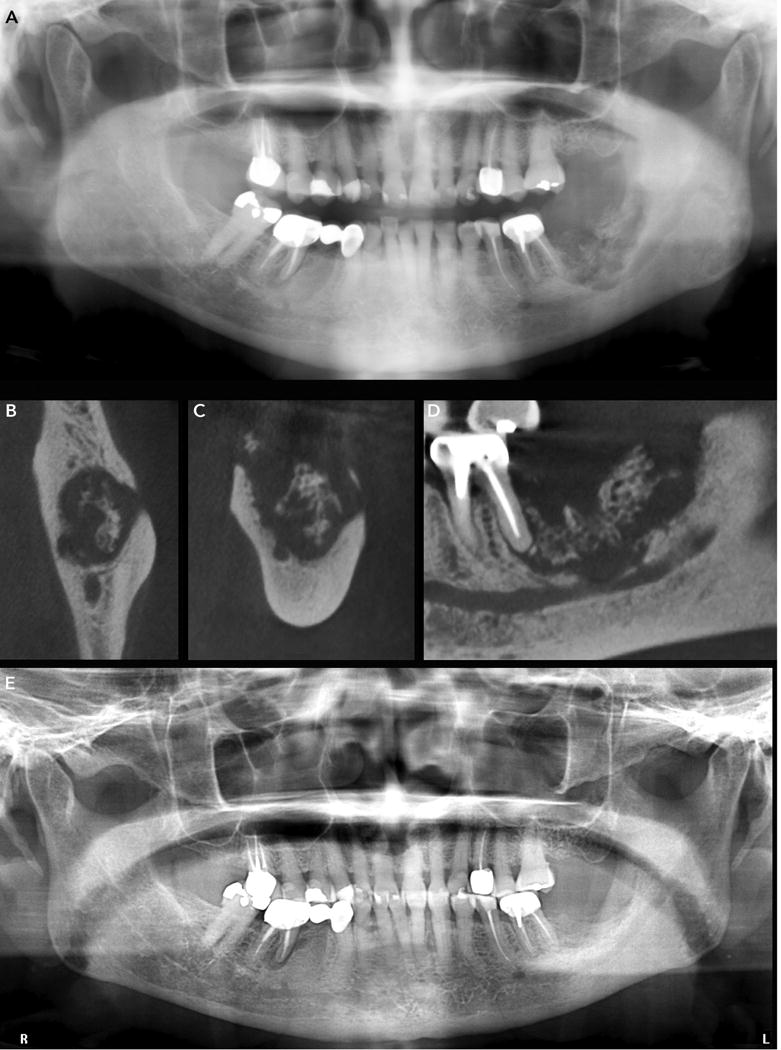
A. Panoramic radiograph of a patient with clinically-evident bone exposure in the mandibular left molar region shows irregular bone destruction that extends into the ascending ramus, and inferiorly to the mandibular canal.
B, C, and D. Axial, coronal and sagittal sections demonstrate marked cortical erosion on the buccal and lingual plates, with multiple small bony sequestrate, and loss of the cortication of the mandibular canal. Note extent of the lesion inferiorly to the mandibular cortex and ablation of the mandibular canal cortex.
E. Panoramic radiograph taken 2 years following conservative management shows osseous healing at the previously necrotic site in the left mandibular molar and retromolar region.
Spectrum of radiological appearances of MRONJ
Initial panoramic and intraoral radiographs are essential to provide an overall assessment of the dental arches. Frank necrotic changes may be visualized, although their extent is likely less than the true lesion (Fig 6). As described above, panoramic radiography is limited by its two-dimensional nature, and may underestimate the presence or extent of necrosis. Ambiguous results on this imaging should trigger more advanced imaging, with CT, either CBCT or MDCT. However, in certain clinical situations the radiologist should anticipate the need for soft-tissue imaging, in which case contrast-enhanced MDCT imaging or MRI will be the imaging modality of choice.
Radiographic changes include lytic and sclerotic changes. Lytic changes are apparent as initially as areas of altered trabecular architecture and density, that progress to frank, patchy radiolucent bone destruction with cortical erosion and marked bone destruction. The lytic changes may extend to disrupt anatomic boundaries of the maxillary sinus and nasal cavity, neurovascular canals, and the cortical boundaries of the mandible with consequent pathological fracture. Non-healing extraction sockets or surgical defects should be identified. Of note, lytic and sclerotic changes are often seen even in the absence of frank bone exposure, emphasizing the need to consider the clinical and radiographic data for appropriate staging of the disease. Notably, recognition of Stage 0 ONJ is particularly important— 50% of patients with Stage 0 disease progress to more advanced MRONJ disease.18,19
Sclerotic changes in ONJ manifest as localized to widespread diffuse osteosclerosis. These changes may be marked and involve the entire height of the mandibular body (Fig. 7). Often, the sclerotic changes are accompanied by periosteal bone formation, and this can be exuberant and cause anatomic expansion of the mandible (Fig 8). As the necrotic changes progress and coalesce, they isolate islands of necrotic bone sequestra.
Figure 7.
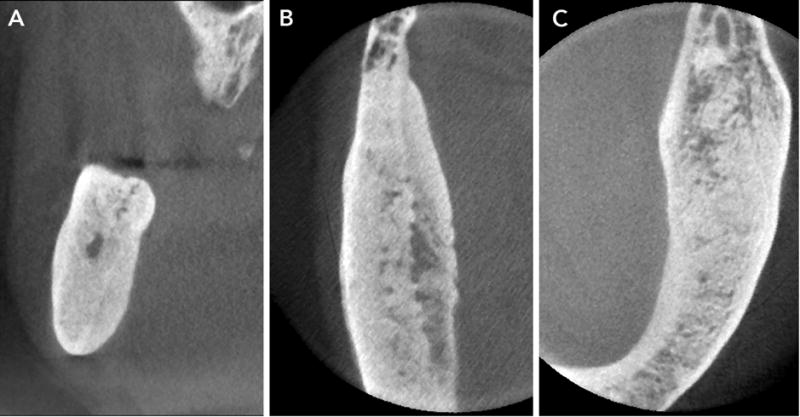
A–C. CBCT sections demonstrate marked alteration of normal trabecular pattern with intense sclerosis and effacement of the trabecular pattern.
Figure 8.
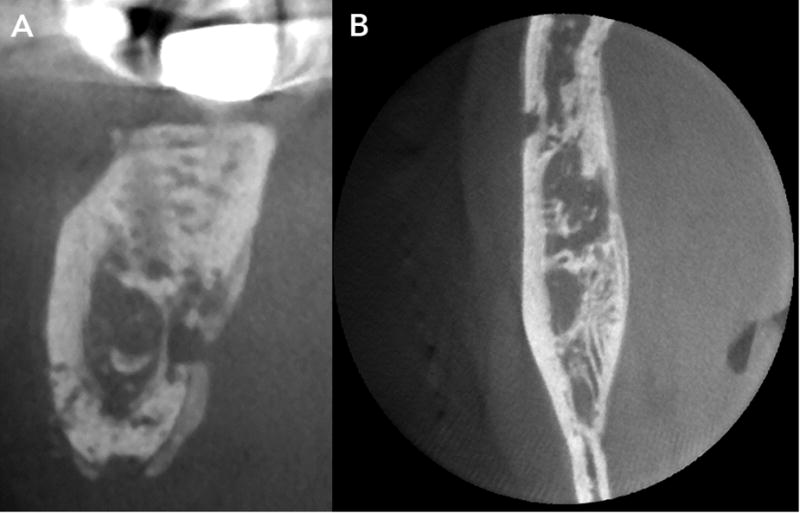
A–B. CBCT sections demonstrate patchy bony destruction, cortical erosion and periosteal bone formation. Note layers of periosteal that are parallel to the cortical plate.
Although the radiographic appearance of ORN and MRONJ can overlap, in general the prominent osteoclastic activation and attenuation of osteoblastic function during ORN result in a rarefying appearance with loss of cortical outlines and trabecular density. On the other hand, osteoclastic inhibition in MRONJ produces an overall increased trabecular and cortical density with frequent pronounced periosteal bone formation.
Lytic lesions of MRONJ must be differentiated from recurrent malignancy, or metastatic lesions in the jaw. Both, MRONJ and malignancy have similar destructive appearances, often with periosteal bone formation. The periosteal new bone formation in MRONJ is regular, typically, paralleling the existing cortex. In contrast, periosteal bone formation in metastatic malignancies is more often disorganized with radiating spicules of new bone that are perpendicular to the existing cortex. Given that a large subset of MRONJ patients are managed for metastatic bone disease, this distinction is particularly important (Fig 9). Finally, these patients may receive periodic FDG-PET/CT examinations (Fig 10) and interval analyses on the bony changes may provide important information of the nature of the MRONJ lesion.
Figure 9.
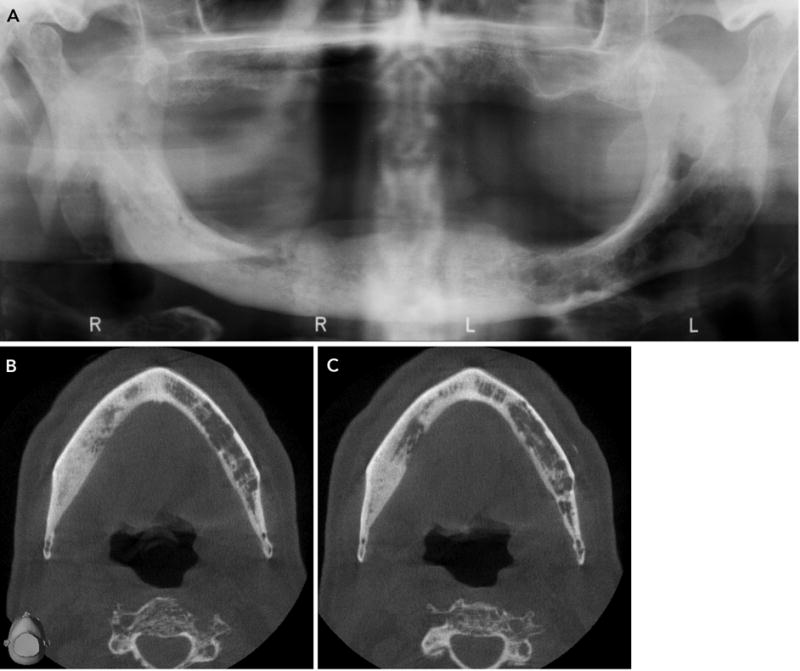
A. Panoramic radiograph of an edentulous patient who received anti-resorptive therapy to manage bone lesions of multiple myeloma shows intense sclerosis that extends through the right mandibular body. Patchy lytic lesions with endosteal cortical erosion are present in the left mandibular body, and likely represent bony lesions of multiple myeloma. B and C. Axial CBCT sections demonstrate the extent of the sclerotic changes that characterize anti-resorptive therapy, and the lytic changes of multiple myeloma.
Figure 10.
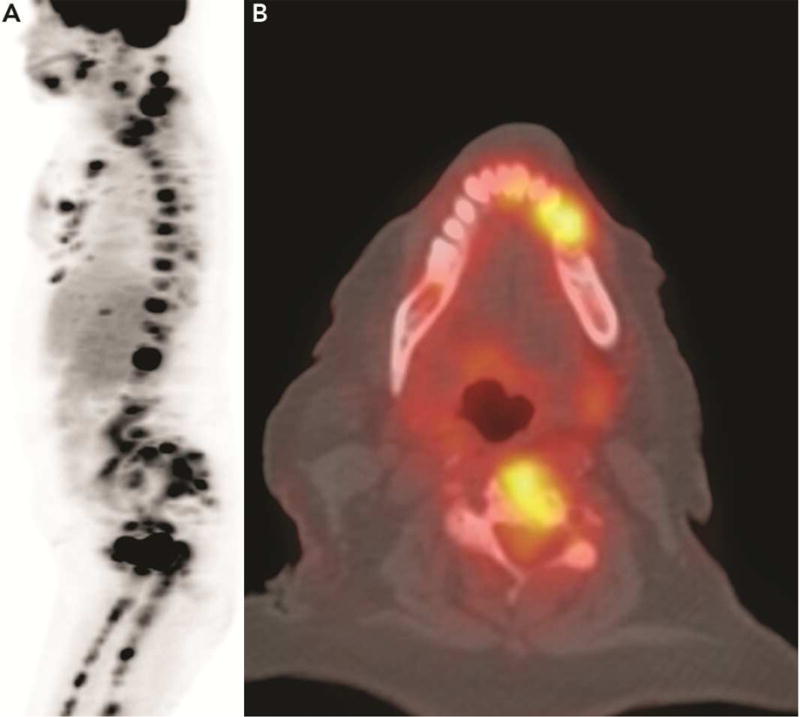
A. Full body FDG-PET imaging to evaluate a patient with multiple metastases to the bone and pleura shows two areas of increased uptake in the jaws. B. Axial fused PET-CT image shows the area of increased uptake in the mandibular body. Note also increased uptake in the cervical vertebra.
Summary
Osteoradionecrosis and Medication-Related Osteonecrosis of the jaw are two distinct entities with different etiologies. Although they share some radiographic features, understanding their pathogenesis and their radiographic subtleties as well as obtaining a good patient history should facilitate diagnosis and differentiation from other similar appearing entities, such as osteomyelitis.
Figure 3.
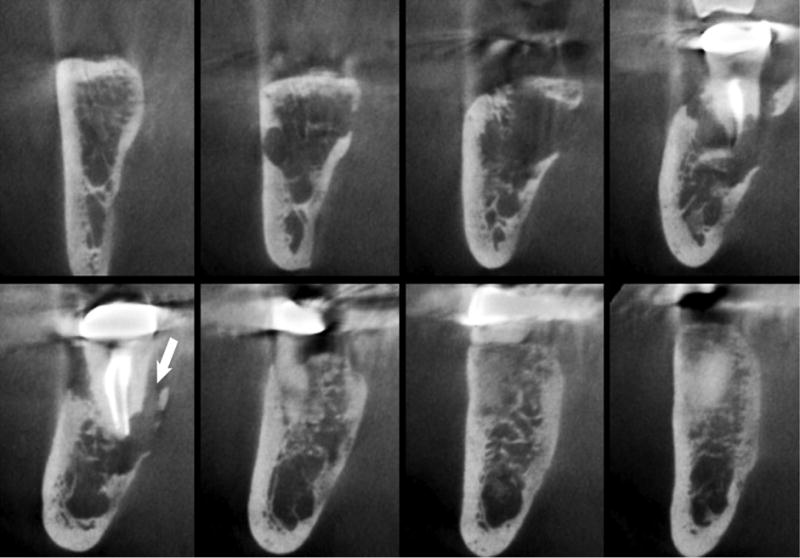
Consecutive coronal sections show irregular lysis of the trabecular bone. Note the erosion on the lingual cortex. A trough-like defect is noted along the root surface and with partial sequestration of bone of the lingual plate.
Key Points.
Osteonecrosis of the jaws is a consequence of prior radiation therapy or certain anti-resorptive medications. The diagnosis of osteonecrosis is made clinically, based on the presence of devitalized exposed bone.
Radiologic imaging, often with CT, is essential in identifying risk modulators, defining disease extent, and the assessing impact of the disease on adjacent structures—which are key elements that guide management of these patients.
Imaging appearances of osteoradionecrosis and medication-related osteonecrosis encompass a broad spectrum, ranging from lytic to mixed to sclerotic osseous changes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Tetradis has served as a consultant and receives funding from Amgen, Inc.
References
- 1.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 2.Meyer I. Infectious diseases of the jaws. Journal of oral surgery (American Dental Association: 1965) 1970;28(1):17–26. [PubMed] [Google Scholar]
- 3.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73(2):119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Peterson DE, Doerr W, Hovan A, et al. Osteoradionecrosis in cancer patients: the evidence base for treatment-dependent frequency, current management strategies, and future studies. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18(8):1089–1098. doi: 10.1007/s00520-010-0898-6. [DOI] [PubMed] [Google Scholar]
- 5.Glanzmann C, Gratz KW. Radionecrosis of the mandibula: a retrospective analysis of the incidence and risk factors. Radiother Oncol. 1995;36(2):94–100. doi: 10.1016/0167-8140(95)01583-3. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JB, Wong FL, Stevenson-Moore P. Osteoradionecrosis: clinical experience and a proposal for classification. J Oral Maxillofac Surg. 1987;45(2):104–110. doi: 10.1016/0278-2391(87)90399-5. [DOI] [PubMed] [Google Scholar]
- 7.Notani K, Yamazaki Y, Kitada H, et al. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck. 2003;25(3):181–186. doi: 10.1002/hed.10171. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz HC, Kagan AR. Osteoradionecrosis of the mandible: scientific basis for clinical staging. Am J Clin Oncol. 2002;25(2):168–171. doi: 10.1097/00000421-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Store G, Boysen M. Mandibular osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin Otolaryngol Allied Sci. 2000;25(5):378–384. doi: 10.1046/j.1365-2273.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 10.White SC, Mallya SM. Update on the biological effects of ionizing radiation, relative dose factors and radiation hygiene. Aust Dent J. 2012;57(Suppl 1):2–8. doi: 10.1111/j.1834-7819.2011.01665.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan KC, Perschbacher SE, Lam EW, et al. Mandibular changes on panoramic imaging after head and neck radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(6):666–672. doi: 10.1016/j.oooo.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Store G, Larheim TA. Mandibular osteoradionecrosis: a comparison of computed tomography with panoramic radiography. Dentomaxillofac Radiol. 1999;28(5):295–300. doi: 10.1038/sj/dmfr/4600461. [DOI] [PubMed] [Google Scholar]
- 13.Fayad MI, Nair M, Levin MD, et al. AAE and AAOMR Joint Position Statement: Use of Cone Beam Computed Tomography in Endodontics 2015 Update. Oral surgery, oral medicine, oral pathology and oral radiology. 2015;120(4):508–512. doi: 10.1016/j.oooo.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Chong J, Hinckley LK, Ginsberg LE. Masticator space abnormalities associated with mandibular osteoradionecrosis: MR and CT findings in five patients. AJNR American journal of neuroradiology. 2000;21(1):175–178. [PMC free article] [PubMed] [Google Scholar]
- 15.Hermans R. Imaging of mandibular osteoradionecrosis. Neuroimaging Clin N Am. 2003;13(3):597–604. doi: 10.1016/s1052-5149(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 16.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Khan AA, Morrison A, Hanley DA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 18.Bedogni A, Fusco V, Agrillo A, Campisi G. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ) Oral Dis. 2012;18(6):621–623. doi: 10.1111/j.1601-0825.2012.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Ryan FS, Khoury S, Liao W, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg. 2009;67(7):1363–1372. doi: 10.1016/j.joms.2009.03.005. [DOI] [PubMed] [Google Scholar]


