SUMMARY
Fine control of stem cell maintenance and activation is crucial for tissue homeostasis and regeneration. However, the mechanism of quiescence exit of Tert+ intestinal stem cells (ISCs) remains unknown. Employing a Tert knock-in (TertTCE/+) mouse model, we found that Tert+ cells are long-term label-retaining self-renewing cells, which are partially distinguished from the previously identified +4 intestinal stem cells (ISCs). Tert+ cells become mitotic upon irradiation (IR) injury. Conditional ablation of Tert+ cells impairs IR-induced intestinal regeneration but not intestinal homeostasis. Upon IR injury, Wnt signaling is specifically activated in Tert+ cells via the ROS-HIFs-transactivated Wnt2b signaling axis. Importantly, conditional knock-out of β-catenin/Ctnnb1 in Tert+ cells undermines IR-induced quiescence exit of Tert+ cells, which subsequently impedes intestinal regeneration. Our results strongly suggest that Wnt signaling-induced activation of Tert+ ISCs is indispensable for intestinal regeneration, which unveils the underlying mechanism of how Tert+ stem cells undergo quiescence exit upon tissue injury.
Keywords: intestinal stem cells, Tert, intestinal regeneration, Wnt/β-catenin, ROS-HIFs-Wnt2b
eTOC Blurb
Suh et al. define Tert+ cells as essential stem cells for intestinal regeneration, and unveil how Tert+ intestinal stem cells escape from the quiescent state and repopulate into progenitor cells upon tissue injury.
INTRODUCTION
Stem cells (SCs) are consistently or conditionally activated for tissue homeostasis or regeneration (Barker, 2014). The fine tune of SC dynamics is governed by both cell intrinsic (Blanpain, et al., 2006; Lowell, et al., 2000), and extrinsic factors (Choi, et al., 2013; Lim, et al., 2013; Eisenhoffer, et al., 2012; Marinari, et al., 2012). The intestinal epithelium is composed of heterogeneous cell populations (absorptive enterocytes, secretive goblet cells, enteroendocrine cells, and Paneth cells), which originate from ISCs and are rapidly replenished from the crypts to the villi (Sancho, et al., 2003). Two distinct ISCs co-exist in the crypts (Barker, 2014). While the highly proliferative crypt base columnar (CBC) ISCs (Lgr5+) continuously generate intestinal epithelial cells (IECs) for tissue homeostasis (Snippert, et al., 2010), the relatively quiescent position 4 (+4) ISCs, located above the Paneth cells, are involved in intestinal regeneration (Yan, et al., 2012).
Several markers of quiescence ISC with distinct expression patterns have been identified. For example, +4 ISCs are co-localized with Bmi1-expression cells (Yan, et al., 2012; Sangiorgi and Capecchi, 2008), whereas Bmi1 is broadly expressed in the crypt, including the Lgr5+ cells (Itzkovitz, et al., 2012; Munoz, et al., 2012). In Tert transgenic mouse, Tert+ cells are mainly located in the +5 to +8 position (Montgomery, et al., 2011). In addition, proliferative Hopx+ cells are found at +4 after IR (Takeda, et al., 2011). Furthermore, quiescent labeled-retaining cells (LRCs) are thought to be the precursor of secretory cells expressing Lgr5 (Buczacki, et al., 2013). Nonetheless, quiescent ISCs need to be further validated given the various expression of Lgr5 in the crypts. To overcome the current technical limitation in studying SCs, we recently generated a Tert knock-in mouse model (Jun, et al., 2016). Tert, a catalytic subunit of telomerase, is specifically expressed in self-renewing cells including SCs, germ cells, and cancer cells (Flores, et al., 2006). By utilizing Tert expression as a functional SC marker in our study, here, we explored the biology of Tert+ SCs in tissue regeneration.
High-dose ionizing radiation induces the loss of crypt cells, resulting in radiation-induced gastrointestinal syndrome (RIGS) (Saha, et al., 2011). Owing to damaged stem cell population in the crypts, RIGS prevents the replenishment of intestinal epithelium and leads to several pathophysiological conditions including electrolyte imbalance, diarrhea, and weight loss (Zimmerer, et al., 2008). Recent therapeutic strategies for RIGS are transplantation of stromal cells (Saha, et al., 2011), treatment of R-spondin 1 (Bhanja, et al., 2009), or administration of macrophage-derived WNTs (Saha, et al., 2016), suggesting that Wnt/β-catenin signaling might be essential for tissue regeneration. Herein we dissected the mechanism of how ISCs are activated during intestinal regeneration.
RESULTS
Conditional repopulation of Tert + cells upon radiation injury
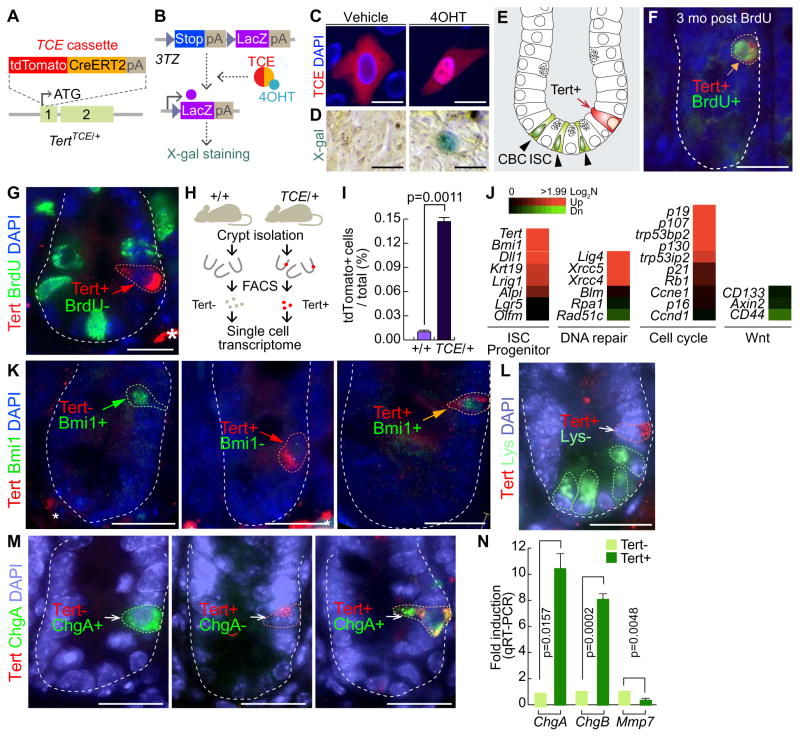
A Tert knock-in mouse (hereafter referred as TertTCE/+) expresses tdTomato-CreERT2 (TCE) driven by the endogenous Tert promoter (Jun, et al., 2016) (Figure 1A). We first tested whether TCE protein conditionally induces Cre-loxP recombination. Tamoxifen (Tam) treatment induced the nuclear translocation of TCE protein and subsequent Cre-loxP recombination, represented by LacZ expression in 3TZ, Cre-loxP recombination reporter cells (Psarras, et al., 2004) (Figures 1B–1D). Employing TertTCE/+ mice, we found that Tert+ cells resided at the +3~+4 position beyond CBC ISCs in the intestine, at a frequency of one Tert+ cell per 120.5 ± 26.50 crypts (Figures 1E, S1A). To determine whether Tert+ cells are quiescent ISCs, we performed a label-retaining cell (LRC) assay by a single dose injection of 5-bromo-2′-deoxyuridine (BrdU) into TertTCE/+ mice (Hsu and Fuchs, 2012; Potten, et al., 2002). Three months after BrdU administration, we detected Tert− :BrdU+ (20.6%), Tert+:BrdU− (43%), and Tert+:BrdU+ (36.4%) in the crypts (Figures 1F and S1C), suggesting that some Tert+ cells are long-term LRCs. Consistently, BrdU incorporation assays (BrdU injection 0.5 hr prior to tissue collection) showed that Tert+ cells were not proliferative in the homeostatic intestine (Figure 1G). Next, we performed single cell gene expression analysis of Tert+ cells isolated from the intestinal crypt using fluorescence-activated cell sorting (FACS) (Figures 1H–1I, S1B). Tert+ cells exhibited the prominent enrichment for Tert and Bmi1 expression (Figure 1J), however, immunofluorescent (IF) staining showed that not all Tert+ cells were Bmi1+ cells (Figures 1K and S1D). The progenitor cell markers (Dll1, Krt19, Lrig1, and Alpi) and non-homologous end joining DNA repair components (Lig4, Xrcc5, and Xrcc4) were also upregulated in Tert+ cells, whereas the expression of homologous recombination DNA repair-related genes (Blm, Rpa1, and Rad51c) did not show any difference between Tert+ and Tert− cells. Interestingly, Tert+ cells exhibited the high expression of cell cycle arrest-related genes (p19, p107, p53, p130, and p21), but no change in Wnt target gene expression (CD133, Axin2, and CD44) compared to Tert− cells (Figure 1J). These results support that Tert+ cells are long-term LRCs. Previously, it was reported that LRCs are secretory precursor cells (Buczacki, et al., 2013). To determine whether Tert+ long-term LRCs share similar markers as secretory precursor cells, we performed IHC for detecting the Paneth cells or enteroendocrine cells. We found that Tert+ cells are not co-localized with lysozyme+ cells (the Paneth cell) (Figures 1L and S1E). Intriguingly, we located a few ChgA+ cells in the crypts as Tert+ cells (Figures 1M and S1F). These results were also confirmed by measuring the expression of ChgA, ChgB, and Mmp7 in Tert+ cells by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Compared to Tert− cells, Tert+ cells displayed an increase of enteroendocrine cell marker genes (ChgA, ChgB), whereas no enrichment of the Paneth cell marker gene (Mmp7) in Tert+ cells was detected (Figure 1N). These results suggest that a small portion of Tert+ cells includes enteroendocrine precursor cells but not Paneth cells, which partially supports the previous study (Buczacki, et al., 2013).
Figure 1. Characterization of Tert+ cells in the intestine.
(A) Generation of the TertTCE/+ knock-in mouse model by gene targeting. tdTomato-CreERT2 (TCE) cassette was inserted in frame into the Tert allele.
(B) Conditional activation of TCE by 4-hydroxytamoxifen (4OHT). 3TZ cells were transfected with a TCE-expressing plasmid. 4OHT-activated TCE induces Cre-loxP recombination, resulting in expression of LacZ, detected by β-galactosidase (X-gal) staining.
(C) Nuclear translocation of TCE by 4-OHT. HeLa cells were transfected with TCE plasmid and treated with 4OHT (100 μM for 24 hr). Scale bars=20μm.
(D) TCE-induced Cre-LoxP recombination by 4-OHT. 3TZ cells were transfected with TCE plasmid, treated with 4OHT (100 μM for 36 hr), and visualized by β-galactosidase (X-gal) staining. Scale bars=20μm.
(E) Illustration of ISCs and Tert+ cells in the small intestine. Tert+ cells are located at position 4 (+4) (arrow), while the crypt base columnar (CBC) ISCs are located at the bottom of crypts (arrowheads).
(F) Label-retaining cell assay. TertTCE/+ mice (2 wk of age) were injected with BrdU and tissues collected at 3 mo of age. Tert+:BrdU+ cell is indicated by an arrow. Scale bars=20μm.
(G) 5-Bromo-2-deoxyuridine (BrdU) incorporation assay. Briefly, TertTCE/+ mice were injected with BrdU (1 mg in PBS) 30 min before tissue collection and the small intestine was subjected to IF staining. Tert+:BrdU− cells are indicated by an arrow. Asterisk: non-specific signal. Scale bars=20μm.
(H–I) Detection and isolation of Tert+ cells using fluorescence-activated cell sorting (FACS).
(J) Single cell gene expression profiling of Tert+ cells compared to Tert− cells.
(K) Tert+ cells partially overlap with Bmi1+ cells in the small intestine.
(L) Tert+ cells do not overlap with lysozyme+ cells (Paneth cell) in the small intestine.
(M) Tert+ cells partially overlap with ChgA+ cells (enteroendocrine cell) in the small intestine. IHC of Tert− :Bmi1+, Tert+:Bmi1−, Tert+:Bmi1+, Tert+:Lysozyme−, Tert− :ChgA+, Tert+:ChgA−, Tert+:ChgA+ cells from the TertTCE/+ mice. Scale bars=20μm.
(N) Expression of ChgA, ChgB, and Mmp7 in Tert+ cells. Tert+ cells were isolated from TertTCE/+ mice and markers for Paneth and enteroendocrine cells were assessed by qRT-PCR. Tert− cells were used as the control sample. Error bars indicate s.e.m.
The representative images are shown; N≥3.
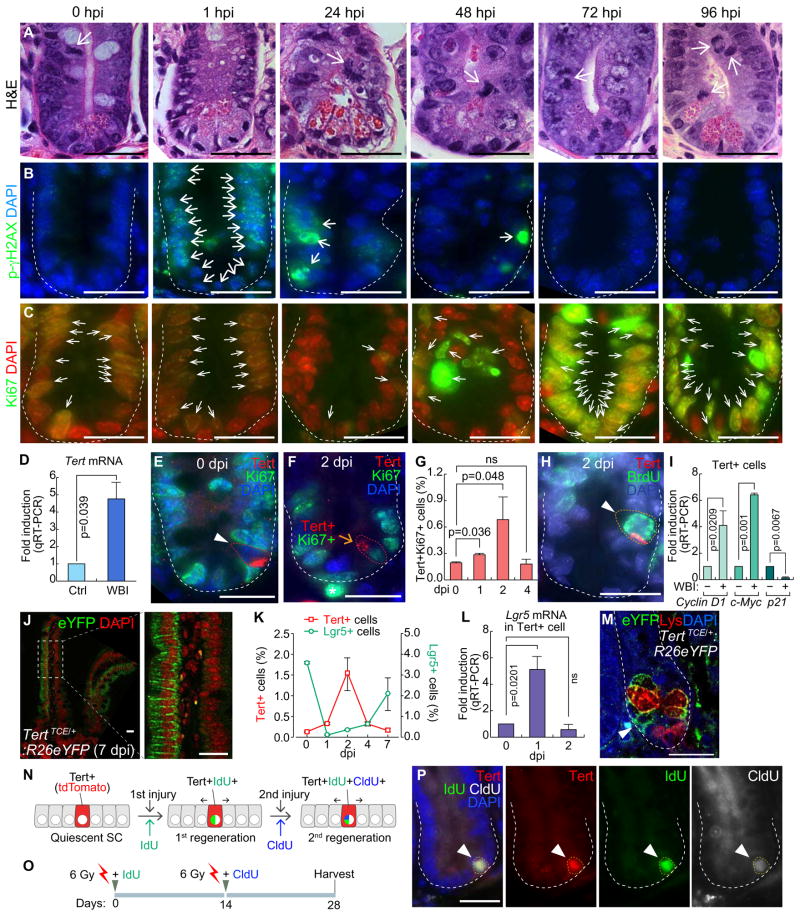
Given the specific expression of Tert in self-renewing cells (Hiyama and Hiyama, 2007), we hypothesized that Tert+ cells are quiescent ISCs but conditionally repopulated upon tissue injury. To test this, mice were exposed to whole body irradiation (WBI; 10 Gy) for injury and monitored for intestinal regeneration. After ionizing radiation (IR), proliferating cells (transit-amplifying and CBC cells) were depleted mainly due to DNA damage as well as apoptosis (Figures 2A–2C, S2A–S2B). Interestingly, despite the massive depletion of IECs by IR, Tert mRNA was markedly upregulated in the crypt, implying the increase of Tert+ cells and/or Tert mRNA during regeneration (Figure 2D). To clarify this, we determined whether IR induces proliferation of Tert+ cells. While Tert+ cells were non-proliferative (Tert+:Ki67−) during intestinal homeostasis, Tert+ cells became proliferative after IR (Figures 2E–2H). The number of proliferative Tert+ cells was increased until 2 dpi (days post injury) (more than four-fold) and restored at 4 dpi (Figure 2G). Single cell gene expression analysis of Tert+ cells at 1 dpi showed an increase of Cyclin D1 and c-Myc expression but decreased expression of p21 (Figure 2I), supporting the notion that IR induces the proliferation of Tert+ cells. Next, we asked whether mitotically activated Tert+ cells generate IECs by employing lineage-tracing assay. TertTCE/+:R26eYFP strain treated with Tam and IR exhibited repopulation of Tert+ cells into IECs during regeneration (Figure 2J). Furthermore, given the co-existence of proliferative (Lgr5+) and quiescent (+ 4) ISCs in the intestinal crypts, we assessed the cell population kinetics between Tert+ cells and Lgr5+ ISCs during intestinal regeneration. Upon IR injury, most Lgr5+ cells were depleted at 1 dpi and then recovered through 7 dpi. Conversely, Tert+ cells were markedly increased until 2 dpi and returned to the basal level at 7 dpi (Figures 2K and S2C). Intriguingly, Lgr5 mRNA expression in Tert+ cells was significantly elevated at 1 dpi (Figure 2L) and the progeny of Tert+ cells (YFP+) were found between the Paneth cells at the bottom of the crypt (Figure 2M). These results imply that Tert+ cells might serve as a reservoir for Lgr5+ cells during intestinal regeneration.
Figure 2. Quiescence exit of Tert+ cells upon radiation injury.
(A–C) Regeneration of intestinal crypt upon IR injury. Mice (5 wk) were treated with whole body irradiation (WBI; 10 Gy; a single dosage). H&E staining (A); DNA damage foci (phospho-gamma histone H2AX) (B); proliferation (Ki67) (C). Hour post injury (hpi); scale bars=20μm.
(D) Tert mRNA upregulation in the crypts after WBI (10 Gy). qRT-PCR of intestinal crypts at 24 hpi.
(E) Quiescence of Tert+ cells during intestinal homeostasis. Tert+:Ki67− cells (arrowhead) of TertTCE/+ mice. Day post injury (dpi); scale bars=20μm.
(F) Activation of Tert+ cells during regeneration (10 Gy, 2 dpi). Tert+:Ki67+ cells (arrow) of TertTCE/+ mice. Scale bar=20μm.
(G) Quiescence exit of Tert+ cells by WBI. TertTCE/+ mice treated with WBI (10 Gy) were collected (0, 1, 2, and 4 dpi) for assessing Tert+:Ki67+ cells using FACS.
(H) Mitotic activation of Tert+ cells by WBI. TertTCE/+ mice were treated with WBI (10 Gy). At 2 dpi, BrdU (1mg/ml, i.p.) was administrated 30 min before tissue collection and the small intestine was subjected to IF staining. Tert+:BrdU+ cells (arrowhead). Scale bars=20μm.
(I) Expression of cell cycle-related genes in Tert+ cells. TertTCE/+ mice were treated with WBI (10 Gy, 24 hpi) and subjected to Tert+ cell isolation from intestinal crypts using FACS and qRT-PCR for Cyclin D1, c-Myc, and p21 expression.
(J) Lineage-tracing of Tert+ cells. TertTCE/+:R26eYFP mice were treated with 4OHT and WBI (10 Gy). At 7 dpi, cryosectioned, small intestine samples were analyzed for YFP expression.
(K) Comparative analysis of Tert+ and Lgr5+ cell populations after WBI (10 Gy). FACS analysis of Tert+ cells (tdTomato) from TertTCE/+ and Lgr5+ cells (GFP) from Lgr5EGFP-IRES-creERT2 mouse models.
(L) Expression of Lgr5 in Tert+ cells. TertTCE/+ mice were treated with WBI (10 Gy; 0, 1, 2 dpi) and subjected to Tert+ cell isolation from intestinal crypts using FACS and qRT-PCR for Lgr5 expression.
(M) Lineage-tracing of Tert+ cells. TertTCE/+:R26eYFP mice were treated with 4OHT and WBI (10 Gy). At 7 dpi, YFP+ cells (arrowhead) were found at the bottom of the crypt in between Paneth cells (lysozyme+ cells).
(N–P) Sequential injury-induced Tert+ cell activation. Illustration of Tert+ cell activation by consecutive tissue injuries (N). Scheme of mouse treatment (O). Of note, instead of a lethal dose (10 Gy), a sub-lethal dose WBI (6 Gy) was used, with IdU and CldU. Tert+:IdU+:CldU+ cells were visualized (28 dpi; arrowhead) (P).
The representative images are shown; N≥3; error bars indicate s.e.m.; ns: non-significant (P≥0.05).
To be defined as SCs, SCs should repopulate into progenitor cells and self-renew. Having observed the repopulation of Tert+ cells into IECs by injury (Figures 2J and 2M), we next asked whether Tert+ cells are activated by successive injuries. We treated TertTCE/+ mice with sequential IR injuries (6 Gy, nonlethal dose but sufficient to induce intestinal injury) to label the dividing cells along with the simultaneous injection of thymidine analogs (IdU for the first round of injury and CldU for the second round of injury) (Figures 2N–2P). We found that Tert+ cells were labeled with both IdU and CldU after two consecutive regenerations (28 days later) (Figure 2P). Of note, due to the rapid replenishment of intestinal epithelium (3–5 days), only LRCs remain with IdU or CldU labeling. These results further suggest that Tert+ cells are quiescent ISCs, which are conditionally repopulated into IECs during intestinal regeneration.
Impaired intestinal regeneration by conditional ablation of Tert+ cells
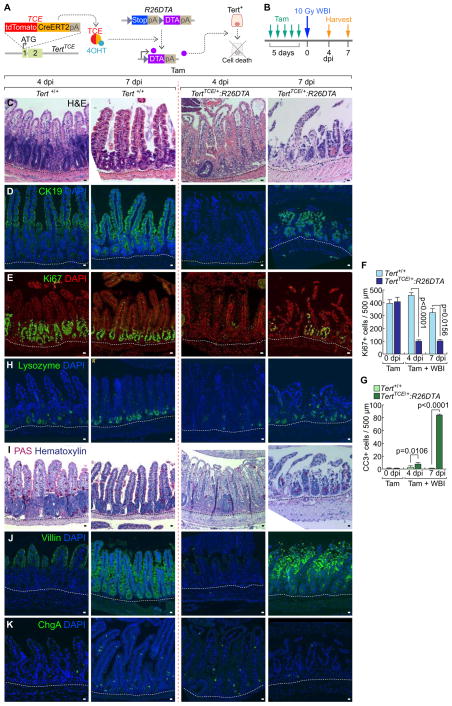
Having determined the quiescence exit of Tert+ cells upon intestinal injury, we next asked whether Tert+ cells are indispensable for intestinal regeneration. To address this, we generated a TertTCE/+:R26DTA compound strain. Upon Tam administration, diphtheria toxin A (DTA) is specifically expressed in Tert+ cells, which leads to their selective removal by cell death (about 70% removal of Tert+ cells) (Figures 3A–3B, S3A–S3B). Before cell ablation experiments, we tested whether de novo generation of Tert+ cells occurs. TertTCE/+:R26DTA mice were injected with Tam to deplete Tert+ cells and assessed for Tert+ cells using FACS. We found that Tert+ cells were not generated up to 30 days after Tam administration, which excludes the potential de novo generation of Tert+ cells in our experimental setting (Figures S4A–S4B). Next, we asked whether removal of Tert+ cells impairs intestinal regeneration. Intriguingly, TertTCE/+:R26DTA strain treated with Tam and IR (experimental group) showed the significant loss of intestinal epithelium integrity, decreased proliferation, and increased apoptosis in IECs of the crypts (Figures 3C–3G, S4C). It is noteworthy that other control groups without IR (Tert+/+ and TertTCE/+:R26DTA treated with Tam) displayed no defects in intestinal homeostasis (Figures S3C–S3J), indicating that Tert+ cells are not essential for normal intestinal homeostasis. Additionally, upon Tert+ cell ablation followed by IR, the intestinal epithelium showed abnormal lineage development of the Paneth cell, goblet cell, enterocyte, and enteroendocrine cell throughout the villi and crypts (Figures 3H–3K) as well as the increased mouse mortality (Figure S3K). These results suggest that Tert+ cells are indispensable for intestinal regeneration.
Figure 3. Impaired intestinal regeneration by conditional ablation of Tert+ cells.
(A) Illustration of conditional ablation of Tert+ cells (TertTCE/+:R26DTA). 4OHT treatment activates TCE, which leads to the expression of diphtheria toxin A (DTA) for Tert+ cell ablation.
(B) Scheme of mouse treatment.
(C–K) Impairment of intestinal regeneration by Tert+ cell ablation. H&E staining (C); cytokeratin 19 (CK19) (D); Ki67 (E); lysozyme (Paneth cell, H); PAS (goblet cell, I); villin (enterocyte, J); chromogranin A (ChgA; enteroendocrine cell, K). Of note, Tam-treated TertTCE/+:R26DTA did not affect intestinal homeostasis (Figures S3B–S3I). Quantification of the number of Ki67+ cells (F) and CC3+ cells (G) per 500 μm region of crypts. Scale bars=20μm; dot lines indicate the basal membranes below crypts.
The representative images are shown; N≥3.
IR-activated Wnt/β-catenin signaling in Tert+ cells via ROS-HIFs-Wnt2b
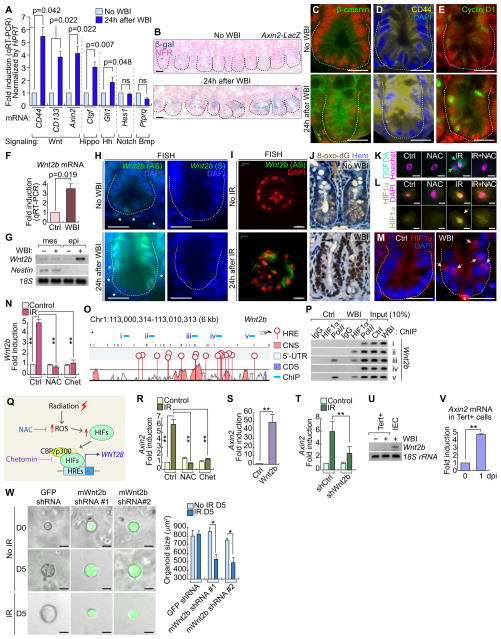
Next, we sought to dissect how tissue injury triggers the quiescence exit of Tert+ cells for intestinal regeneration. To identify specific signaling pathway(s) involved in Tert+ ISC activation, we performed gene expression analysis of several candidate signaling pathway target genes. We found that IR markedly upregulated Wnt target genes (CD44, CD133, and Axin2) in the crypts (Figure 4A), which was further confirmed by the increase of β-catenin reporter activity (X-gal staining of Axin2-LacZ mice), β-catenin protein, and β-catenin target gene expression (CD44 and Cyclin D1) (Figures 4B–4E, S5A). These results suggest that IR activates Wnt/β-catenin signaling in the crypts, which implies the possible involvement of Wnt signaling in Tert+ ISC activation.
Figure 4. IR-activated Wnt/β-catenin signaling in the crypts and Tert+ cells.
(A) IR-induced upregulation of Wnt signaling target genes (CD44, CD133, and Axin2). qRT-PCR of intestinal crypts isolated from mice treated with WBI (10 Gy, 24 hpi).
(B–E) IR-induced Wnt/β-catenin signaling activation in intestinal crypt. WBI (10 Gy, 24 hpi). X-gal staining in Axin2-LacZ intestine (B); IHC for β-catenin (C); CD44 (D) Cyclin D1 (E). Scale bars=20μm.
(F) Wnt2b upregulation in crypts by WBI (10 Gy, 24 hpi). qRT-PCR.
(G) IR-induced Wnt2 upregulation in the crypt epithelial cells. WBI (10 Gy, 24 hpi). Semi-quantitative (sq) RT-PCR.
(H) Wnt2 upregulation in crypts by WBI (10 Gy). Fluorescence in situ hybridization (FISH). AS: antisense; S: sense probes; scale bars=20μm; asterisks: Wnt2b+ mesenchymal cells.
(I) Wnt2 upregulation in crypt organoids by IR (8 Gy). Fluorescence in situ hybridization (FISH). AS: antisense; scale bars=20μm.
(J) IR-induced ROS generation in the crypt. IHC for 8-Oxo-2′-deoxyguanosine (8-oxo-dG). Scale bars=20μm.
(K) IR-induced ROS generation in CCD841CoN IEC cells (10 Gy, 0.5 hr). DCFDA staining.
(L) IR-induced HIF1α activation via ROS in CCD841CoN. Cells were pre-treated with N-acetyl-L-cysteine (NAC, 1 mM) for 4 hr and exposed to IR (10 Gy). After 24 hr, cells were analyzed by IF staining for HIF1α. Scale bars=20μm.
(M) IR-induced HIF1α nuclear translocation in the crypt. WBI (10 Gy, 24 hpi); scale bars=20μm; asterisks: non-specific signal.
(N) IR-induced Wnt2 transactivation via ROS-HIFs in CCD841CoN. Cells were pre-treated with NAC or chetomin (100 nM) for 4 hr. After IR exposure (10 Gy, 24 hr), cells were analyzed for qRT-PCR.
(O) Wnt2b promoter analysis for hypoxia response element (HRE). Conserved non-coding sequence (CNS); coding sequence (CDS); 5 ChIP amplicons (i–v).
(P) IR-induced recruitment of HIF1α to Wnt2b promoter in the small intestine. Small intestine samples from mice (±WBI [10 Gy], 24 hr) were analyzed for ChIP assays. RNA polymerase II (Pol II) served as a positive control for transcriptional activation.
(Q) Illustration of radiation-induced Wnt/β-catenin signaling activation via ROS-HIFs-Wnt2b.
(R) Inhibition of IR-induced Axin2 by NAC or chetomin. CCD841CoN cells were pre-treated with NAC or chetomin, treated with IR (10 Gy, 24 hr), and analyzed by qRT-PCR.
(S) Wnt2b-activated β-catenin signaling activation. CCD841CoN cells were transfected with Wnt2b expression plasmids. 24 hr after transfection, cells were analyzed by qRT-PCR for Axin2.
(T) Wnt2b mediates IR-induced Wnt/β-catenin signaling activation. CCD841CoN cells were stably transduced with lentiviruses encoding shRNAs against Wnt2b, and then treated with IR (10 Gy, 24 hr) followed by qRT-PCR analysis of Axin2.
(U) No expression of Wnt2b in Tert+ cells. Semi-quantitative (sq)RT-PCR. IECs isolated from the crypts served as positive control.
(V) Axin2 upregulation by IR in Tert+ cells. qRT-PCR of Axin2 in Tert+ cells isolated from TertTCE/+ mice treated with WBI (10 Gy; 1 dpi). The representative images are shown; N≥3; error bars indicate s.e.m. double asterisks (**)=P<0.05.
(W) Knock-down of Wnt2b in crypt single cell organoids. Lentiviruses expressing mouse Wnt2b shRNAs (clone #1~#2) inhibited organoid growth after IR (4 Gy). GFP shRNA was used as a negative control. Scale bars=20μm; asterisks (*)=P<0.05.
Given the pivotal roles of Wnt ligands and agonists in the maintenance of intestinal integrity via β-catenin-mediated gene regulation (Farin, et al., 2012; Kim, et al., 2005), we hypothesized that IR-induced upregulation of Wnt ligands triggers target gene activation. To test this, we assessed the expression of nineteen Wnt ligands in the crypt (± IR). We found that Wnt2b, Wnt4, Wnt5a, Wnt6, Wnt7b, and Wnt9a were upregulated by IR (Figures 4F, S5B). To further examine the localization and expression pattern of these Wnt ligands, we performed fluorescence in situ hybridization (FISH). Among the six Wnt ligands selected, Wnt2b expression was the most prominently upregulated in the intestinal epithelial cells of the crypts after IR (Figure 4H). We found that Wnt4 is specifically expressed in the mesenchyme during intestinal homeostasis (non-IR treated) and in the epithelium during regeneration (IR-treated) (Figure S5E). Wnt5a was found expressed at the crypt-villus junction while Wnt6 and Wnt9a were expressed in the crypt in both normal and regenerating intestine (Figures S5F, S5G, S5I). Lastly, Wnt7b-expressing cells were localized in the mesenchyme and were slightly upregulated by IR (Figure S5H). Upregulation of Wnt ligands from our semi-quantitative RT-PCR might be due to either transcriptional upregulation or increase of expressing cells. It is noteworthy that we observed an increased frequency of Wnt5a, Wnt6, or Wnt9a-expressing cells after IR, but not their transcriptional upregulation (Figures S5J–S5K). However, Wnt2b was the most significantly upregulated by IR at the transcriptional level and the increase of Wnt2b-expressing cells (Figure 4H). In the normal intestine, Wnt2b is expressed in the mesenchymal fibroblasts near the crypts (Valenta, et al., 2016) (Figures 4G–4H). To exclude the involvement of Wnt2b secreted from the mesenchymal cells, we isolated and cultured crypt IECs for the crypt organoid development and the subsequent IR treatment. FISH for Wnt2b showed that IR conditionally activated the Wnt2b expression in the mesenchymal cell-free crypt organoids (Figure 4I).
Next, we asked how IR upregulates Wnt2b in IECs. Given that IR increases reactive oxygen species (ROS) (Azzam, et al., 2012) and subsequently activates hypoxia-inducible factors (HIFs) (Moeller, et al., 2004), we tested whether the ROS-HIFs signaling axis mediates IR-induced Wnt2b upregulation. Indeed, IR increased ROS generation in the crypt and the normal IECs, represented by the increase of 8-Oxo-2′-deoxyguanosine (8-oxo-dG; an indirect marker of ROS) and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; a direct marker of intracellular ROS), respectively (Figures 4J–4K). Moreover, IR induced the nuclear translocation of HIF1α in the crypt IECs and CCD841CoN IECs (Figures 4L–4M). Next, we determined whether IR activates HIFs through ROS. We found that the ROS inhibitor, N-acetyl cysteine (NAC), blocked the nuclear translocation of HIF1α in IR-treated CCD841CoN IECs (Figure 4L). Of note, IR also induced the nuclear translocation of HIF2α (Figure S5L). These data suggest that IR-induced HIF activation is mediated by ROS. Furthermore, we found that IR-induced Wnt2b upregulation was inhibited by NAC or chetomin (Chet, an inhibitor for HIFs) (Figure 4N), suggesting the potential involvement of ROS and HIFs in IR-induced Wnt2b upregulation.
Next, we tested whether HIFs directly transactivate Wnt2b. We identified multiple consensus hypoxia response elements (HRE; acgtg) in the conserved noncoding sequences (CNS) of both human and mouse Wnt2b promoter (Figure 4O), whereas other Wnt ligands (Wnt4, Wnt5a, Wnt6, Wnt7b, and Wn9a) harbor fewer HREs (Figure S5J). These results imply that HIFs-transactivated Wnt2b might be evolutionarily conserved in mammals. Chromatin immunoprecipitation (ChIP) promoter scanning assays showed that HIF1α conditionally occupied HREs at the Wnt2b promoter in mouse small intestine upon IR (Figure 4P). These results suggest that IR induces transactivation of Wnt2b via ROS-HIFs (Figure 4Q). Next, we tested whether IR-transactivated Wnt2b induces Wnt/β-catenin signaling activation in IECs. In CCD841CoN IECs, treatment of either NAC or Chet suppressed IR-induced upregulation of Axin2, a β-catenin target gene (Figure 4R). We also found that ectopic Wnt2b expression is sufficient to activate Wnt/β-catenin signaling, as represented by Axin2 upregulation in CCD841CoN IECs (Figure 4S). Conversely, depletion of endogenous Wnt2b decreased IR-induced Axin2 upregulation (Figures 4T and S5C). These data suggest that IR activates Wnt/β-catenin signaling in IECs via the ROS-HIFs-Wnt2b signaling axis. Interestingly, it is noteworthy that IR-transactivated Wnt2b was only detected in Tert− IECs but not in Tert+ cells in the crypts (Figure 4U). Nonetheless, we found that IR upregulated Axin2 in Tert+ cells (Figure 4V). To provide clearer evidence that Wnt2b is a critical downstream mediator of regeneration, we also performed knock-down assays for Wnt2b in the single cell-driven crypt organoids. Using lentivirus encoding shRNA against mWnt2b, we depleted the endogenous Wnt2b and treated the crypt organoids with IR. We found that Wnt2b knock-down inhibited the organoid growth under IR treated condition (Figures 4W and S5D). It is noteworthy that in the absence of IR treatment, Wnt2b shRNA did not affect the crypt organoid growth. This observation is consistent with the results that Wnt2b KO mice are viable without the defects in the intestinal homeostasis (Tsukiyama and Yamaguchi, 2012). These results suggest that IR activates Wnt/β-catenin signaling in Tert+ cells via ROS-HIFs-Wnt2b signaling axis.
Impaired intestinal regeneration by β-catenin conditional knock-out in Tert+ cells
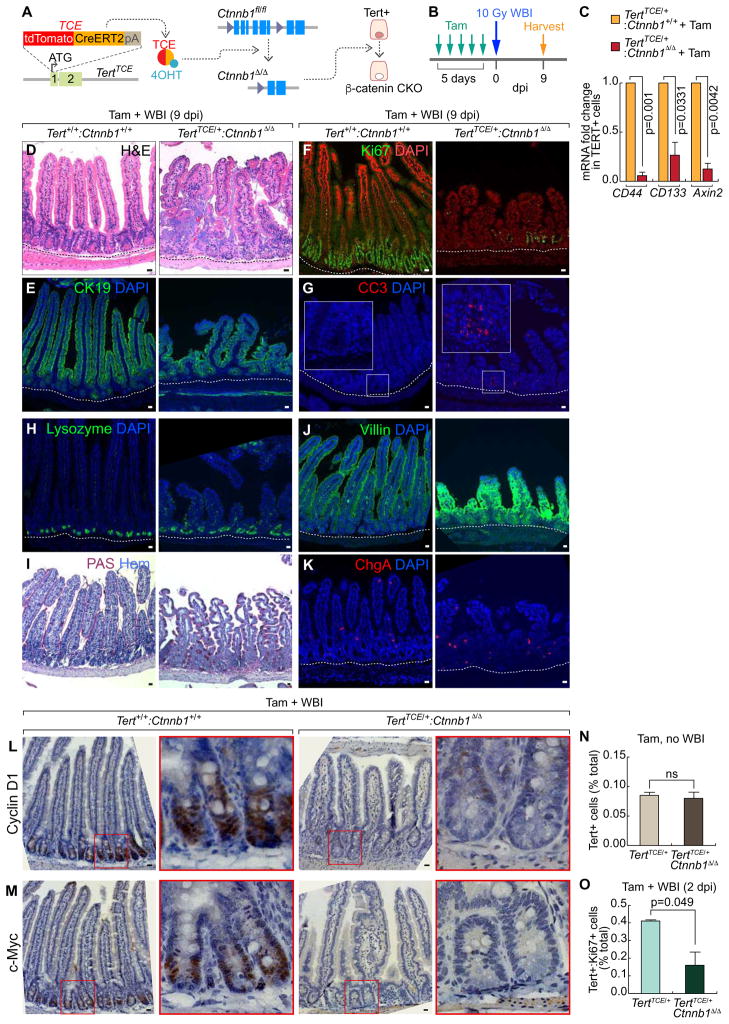
Given the requirement of Tert+ cells for intestinal regeneration (Figure 3) and IR-induced activation of Wnt/β-catenin signaling in Tert+ cells (Figure 4V), we hypothesized that IR-activated Wnt/β-catenin signaling contributes to Tert+ ISC activation and subsequent Tert+ cell-driven intestinal regeneration. To test this, we genetically ablated β-catenin/Ctnnb1 in Tert+ cells by Tam administration into TertTCE/+: Ctnnb1fl/fl mice (Figures 5A–5B). Isolated Tert+ cells from the Tam-treated TertTCE/+: Ctnnb1Δ/Δ strain displayed downregulation of β-catenin target genes (Figure 5C), indicating successful conditional knock-out (CKO) of β-catenin/Ctnnb1. Next, we examined the effects of β-catenin CKO in Tert+ cells on intestinal regeneration. Indeed, Tam and IR-treated TertTCE/+: Ctnnb1Δ/Δ mice showed impaired intestinal regeneration, represented by the loss of epithelium integrity, decreased proliferation, increased apoptosis, and abnormal distribution of IEC lineages (Figures 5D–5K). These results suggest that activation of Wnt/β-catenin in Tert+ cells is crucial for intestinal regeneration.
Figure 5. Impaired intestinal regeneration by β-catenin conditional knockout in Tert+ cells.
(A) Illustration of β-catenin/Ctnnb1 conditional knock-out (CKO) in Tert+ cells (TERTTCE/+;Ctnnb1fl/fl).
(B) Scheme of mouse treatment.
(C) β-catenin CKO-induced downregulation of Wnt/β-catenin target gene expression in Tert+ cells. qRT-PCR of Tert+ cells for CD44, CD133, and Axin2 expression from TertTCE/+ (control) and TertTCE/+:Ctnnb1Δ/Δ (experimental group) treated with tamoxifen.
(D–K) IHC of impaired intestinal regeneration by β-catenin CKO in Tert+ cells. H&E staining (D); CK19 (E); Ki67 (F); CC3 (G); lysozyme (H); PAS (I); villin (J); ChgA (K). Scale bars=20μm.
(L–M) Cell cycle-related gene expression in Tert+/+;Ctnnb1+/+ and TertTCE/+;Ctnnb1Δ/Δ (10 Gy, 7 dpi). Cyclin D1 (L); c-Myc (M). Scale bars=20μm. Cyclin D1 and c-Myc were significantly downregulated by β-catenin CKO in Tert+ cells and WBI.
(N) No loss of Tert+ cells by β-catenin CKO during intestinal homeostasis. Population analysis of Tert+ cells after Tam treatment in TertTCE/+ and TertTCE/+;Ctnnb1Δ/Δ, using FACS. ns: non-significant (p>0.05).
(O) Reduced proliferation of Tert+ cells during intestinal regeneration by β-catenin CKO. Quantification of Tert+:Ki67+ cells of Tam and IR (10 Gy; 2 dpi)-treated TertTCE/+ and TertTCE/+;Ctnnb1Δ/Δ mice, using FACS.
The representative images are shown; N≥3; error bars indicate s.e.m.
Next, we questioned how Wnt/β-catenin engages in Tert+ cell-mediated intestinal regeneration. Given IR-induced upregulation of cell proliferation-related and β-catenin target genes (Ccnd1, c-Myc, Lgr5, and Axin2) in Tert+ cells (Figures 2I, 2L, 4V), we tested whether IR-induced Wnt/β-catenin activation is required for the quiescence exit of Tert+ cells during intestinal regeneration. First, we assessed the effects of β-catenin CKO on the number of the intestinal Tert+ cells. After β-catenin CKO, the number of Tert+ cells was similar between TertTCE/+ and TertTCE/+:Ctnnb1Δ/Δ in the homeostatic intestine (no IR) (Figures 5N, S6A). However, FACS analysis showed that β-catenin CKO in Tert+ cells followed by IR reduced the number of proliferative Tert+ cells (Tert+:Ki67+) (Figures 5O, S6B). Moreover, Ccnd1 and c-Myc transcripts were significantly decreased in the small intestine of TertTCE/+:Ctnnb1Δ/Δ mice upon IR, compared with those in Tert+/+:Ctnnb1+/+ mice (Figures 5L–5M). These results suggest that IR-activated Wnt/β-catenin signaling is required for the transition of Tert+ cells from quiescent to proliferative status upon tissue injury.
DISCUSSION
The quiescent ISCs have been identified as Bmi1 or Hopx-expressing cells as well as BrdU-retaining secretory precursors (Buczacki, et al., 2013; Takeda, et al., 2011; Sangiorgi and Capecchi, 2008). Interestingly, we observed that not all Tert+ cells were colocalized with Bmi1+ cells (see Figure 1K), indicating Tert+ ISCs are somewhat distinguished from Bmi1+ ISCs. Furthermore, unlike Buczacki’s LRCs (Buczacki, et al., 2013), the markers of Tert+ LRCs partially overlapped only with enteroendocrine cells but not Paneth cells (see Figures 1L–1N), which suggest the existence of additional quiescent ISCs. Although we here limited our scope to Tert+ LRCs, it is probable that the different ISCs coordinately serve as multiple sources for repopulation into IECs during intestinal regeneration.
Importantly, given the contrasting kinetics of Lgr5+ and Tert+ cell populations (see Figure 2K), the marked induction of Lgr5 expression in Tert+ cells (see Figure 2L), and Tert+ cell-driven progeny at the bottom of the crypt during regeneration (see Figure 2M), it is highly conceivable that the acute loss of Lgr5+ cells by IR might be compensated by Tert+ cells. Similarly, Hopx+ and Bmi1+ cells give rise to Lgr5+ cells ex vivo (Takeda, et al., 2011; Tian, et al., 2011). This is consistent with the results that neither genetic ablation nor IR-induced removal of Lgr5+ ISCs impairs intestinal integrity (Yan, et al., 2012);(Tian, et al., 2011). Therefore, it is highly likely that despite the removal of Lgr5+ cells, Tert+ cells serve as a reservoir for Lgr5+ cells during regeneration. This is also supported by our results that the ablation of Tert+ cells severely impairs intestinal regeneration (see Figures 3C–3K). Intriguingly, despite the crucial roles of Tert+ cells in intestinal regeneration, the removal of Tert+ cells did not affect intestinal homeostasis (see Figures S3C–S3J). Considering the cell plasticity in various regenerative tissues such as pancreas (Kopp, et al., 2016), intestine (Tetteh, et al., 2016; Asfaha, et al., 2015; van Es, et al., 2012), and lung (Tata, et al., 2013), our working model does not fully exclude the possibility that Tert+ cells are generated from Tert− cells during intestinal regeneration, which should be carefully examined using the compound lineage-tracing experiments. Nonetheless, during intestinal homeostasis, no Tert+ cells were generated (up to 30 days) after Tert+ cell ablation (see Figures S4A–S4B), implying no de novo development of Tert+ cells, at least, during intestinal homeostasis.
Employing our knock-in mouse model, we elucidate that Wnt/β-catenin signaling is required for the transition of Tert+ cells from quiescence to mitotic activation. We observed that IR creates multiple DNA damage foci in most IECs in the crypts (see Figure 2B). IR directly induces DNA damage by random DNA breaks or indirectly through reactive oxygen species (ROS) generated by water hydrolysis (Azzam, et al., 2012). Although excessive ROS induces cell death, a moderate increase of ROS can regulate the activity of several downstream proteins including HIFs (Niecknig, et al., 2012; Gerald, et al., 2004). Indeed, IR increased ROS in the intestine followed by the direct recruitment of HIF-1α to the Wnt2b promoter and subsequent Wnt2b transactivation (see Figures 4J, 4P). Furthermore, given the inhibitory effects of Chet on both HIF-1α and HIF-2α and the protective role of HIF-2α in the intestine (Taniguchi, et al., 2014; Xie, et al., 2014), HIF-2α might also be involved in ROS-mediated Wnt2b transactivation.
We recently found that IR acutely activates Wnt/β-catenin signaling and transactivates LIG4, a core component of non-homologous end joining repair (Jun, et al., 2016). However, the detailed molecular mechanism of IR-activated Wnt/β-catenin signaling remained elusive. Our unbiased screening results of signaling pathways and Wnt ligands led us to select Wnt2b and the Wnt/β-catenin pathway as important mediators of Tert+ cell activation during intestinal regeneration. Wnt2b functionally compensates for the loss of epithelial Wnt3 in crypt organoid culture (Farin, et al., 2012; Goss, et al., 2009), indicating a role of Wnt2b in transducing canonical Wnt signaling. We found that IR markedly upregulates Wnt2b in IECs near +4 but not in Tert+ cells (see Figures 4H, 4U), and Wnt2b knock-down inhibited organoid growth upon IR (see Figure 4W). Of note, Wnt2b was shown to activate canonical Wnt signaling (β-catenin-mediated) (Goss, et al., 2009). We found that IR activates Wnt/β-catenin signaling in Tert+ cells (see Figure 4V), suggesting that IR-induced Wnt2b in IECs activates Wnt signaling in Tert+ cells. Moreover, the results from β-catenin CKO in Tert+ cells clearly demonstrated the requirement of Wnt/β-catenin signaling in Tert+ cell activation and Tert+ cell-driven intestinal regeneration (see Figures 5D–5K). Wnt signaling plays multiple roles in SC regulation including SC maintenance, activation, and differentiation (Lien and Fuchs, 2014). In our experimental condition, we found that β-catenin CKO in Tert+ cells did not affect the number of Tert+ cells but instead, suppressed the quiescence exit of Tert+ cells (see Figures 5N–5O). This is also consistent with the reduced expression of cell proliferation-related genes in β-catenin CKO in Tert+ cells (see Figures 5L–5M). Thus, our results strongly suggest that β-catenin is essential for the quiescence exit of Tert+ cells during intestinal regeneration.
Despite the significance of Tert as a catalytic subunit of telomerase in self-renewing cells, the absence of accurate Tert reporter mouse models made it difficult to study Tert+ cells. Unlike previous transgenic mice generated by pronuclear injection of Tert promoter-CreER DNA (Montgomery, et al., 2011), our model is a knock-in mouse strain established by blastocyst injection of targeted mouse embryonic stem cells. Given the limitation of transgenic mice mainly due to the random integration and uncontrolled copy number of the foreign DNA, gene targeting (knock-in) is more specific and reliable for representing physiological and pathological events of Tert+ cells. Moreover, our Tert knock-in mouse model not only visualizes Tert+ cells with tdTomato fluorescence, but also enables us to perform various cellular and genetic manipulation of Tert+ cells using a CreERT2 cassette. Given that Tert is expressed in the self-renewing cells, future studies employing TertTCE/+ mice in different tissues (pancreas, liver, kidney, and hair follicle) may provide valuable insights into our understanding of tissue SCs. Furthermore, due to the reactivation of telomerase in human cancer, our Tert mouse models can also be used to isolate and characterize self-renewing tumor cells.
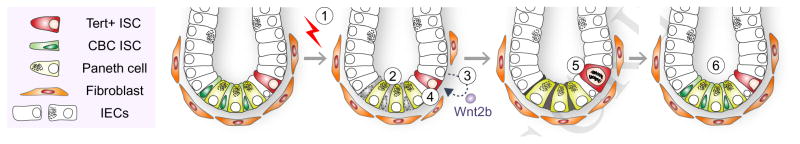
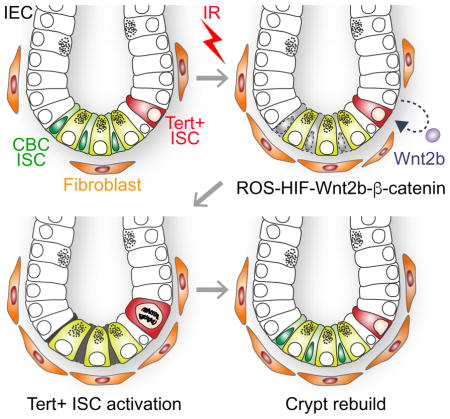
Together, our results define Tert+ cells as essential ISCs for tissue regeneration and unveil how Tert+ ISCs are conditionally activated from the quiescent state upon tissue injury (see Figure 6).
Figure 6. Illustration of working model.
IR induces the death of mitotic cells (TA and CBC ISCs) in the crypts. (1–2). Simultaneously, IR transactivates Wnt2b via ROS-HIFs in IECs (3) and activates Wnt/β-catenin signaling in Tert+ cells (4), which results in the quiescence exit of Tert+ cells (5). Repopulation of Tert+ cells generates the progenitor and differentiated IECs (6). Finally, Tert+ cells re-enter into the quiescent state.
EXPERIMENTAL PROCEDURES
Animals
All mice were maintained in compliance with Institutional Animal Care and Use Committee (IACUC) guidelines of MD Anderson Cancer Center. Male and female mice (older than six weeks) were used for experiments. Gt(ROSA) 26Sortm1(EYFP)Cos/J (Jax strain 006148), Gt(ROSA)26Sortm1(DTA)Lky/J (Jax strain 009669), Ctnnb1tm2Kem/KnwJ (Jax strain 004152), and Axin2LacZ (Jax strain 009120) were purchased from Jackson Laboratory. Genotyping was performed following the Jackson Laboratory’s protocol. Tam was dissolved in corn oil (Fisher) at a final concentration of 10 mg/ml for intraperitoneal (i.p.) administration (50 mg/kg). For intestine injury, mice were exposed to 10 Gy or 6 Gy (non-lethal dose for cell labeling) WBI, and tissues were collected at multiple time points.
Label-retaining cell assay
TertTCE/+ mice were intraperitoneally administrated with 5-bromo-2′-deoxyuridine (BrdU, Sigma) (1 mg) at age 2 weeks. The tissue sample was collected at age 12 weeks and analyzed for Tert+:BrdU+ cells in the crypt.
FACS analysis
Intestinal crypts were isolated from TertTCE/+ mice. For single cell isolation, crypts were digested with Accumax (Stem cell technology 07921) for 20 min and collected through 70 μm (BD 087712) and 40 μm (BD 087711) cell strainers. Next, cells were suspended in phosphate-buffered saline (PBS) with 10% fetal bovine serum (FBS) and stained with SYTOX® Blue (Life Technologies S34857) to exclude dead cells. tdTomato fluorescence was detected by FACS (MoFlo® Astrios™, Beckman Coulter) and sorted into Tert+ and Tert− cells among live cells based on the gate. Sorted Tert+ and Tert− cells were analyzed for single cell gene expression profiling. To quantify the proliferation of Tert+ cells, single cells isolated from crypt were fixed with 4% paraformaldehyde, blocked, incubated with a FITC-conjugated Ki67 antibody, and stained with DAPI. The population of Tert+:Ki67+ was assessed by FACS (Gallios™ 561, Beckman Coulter).
Gene expression analysis
Isolated Tert+ and Tert− cells (≤ 5 cells) were synthesized to complementary DNA (cDNA) using REPLI-g WTA Single Cell Kit (QIAGEN) and analyzed for single cell gene expression. For gene expression analysis, isolated crypts were processed for RNA extraction (QIAGEN RNeasy Mini Kit) and reverse transcription (iScript RT Supermix for RT-qPCR, Biorad). 18S ribosomal RNA (18S rRNA) was used as an endogenous control for normalization. qRT-PCR was performed using intron-spanning primers. Fold induction was quantified using the 2−ΔΔCT method. For Figure 1J, ΔΔCT values were displayed using Heatmap software (bar.utoronto.ca/). Primer sequences are listed in Supplementary Table 1.
Dual-pulse labeling assay
TertTCE/+ mice were treated for the first tissue injury (6 Gy WBI) followed by 5-Iodo-2′-deoxyuridine (IdU, Sigma) (1 mg) i.p. injection. Two weeks later, TertTCE/+ mice were treated for the second tissue injury (6 Gy WBI) followed by 5-Chloro-2′-deoxyuridine (CldU, Sigma) (1 mg) i.p. injection. After two weeks, the tissue was collected and analyzed for Tert+:IdU+:CldU+ cells in the crypts.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed according to the manufacturer’s protocol (Invitrogen, FISH Tag™ RNA Green Kit, with Alexa Fluor® 488 dye). Probes were designed to be complementary to the coding sequences of Wnt2b (441 bp), Wnt4 (421 bp), Wnt5a (437 bp), Wnt6 (476 bp), Wnt7b (570 bp), and Wnt9a (401 bp). PCR products of Wnt ligands were inserted and transformed using TOPO®-TA Cloning (Invitrogen). Linearized plasmids (sense and antisense) were subjected to transcription, purification (anime-modified RNA), and labeling with the fluorescent dye (Alexa Fluor® 488). Intestine tissue slides were deparaffinized, processed for antigen retrieval (proteinase K, 10 μg/ml), and incubated with hybridization buffer (50% formamide, 5× SSC, 100 μg/ml fragmented salmon testes DNA, 50 μg/ml heparin, 0.1% Tween20). Tissue slides were then incubated with probe-fluorescent dye in hybridization buffer (1 μg/ml) at 55°C in a water bath for 20 h. The next day slides were washed with hybridization buffer, 50% hybridization buffer/PBS with 0.1% Tween 20 (PBT), and PBT. Slides were soaked with 70% glycerol/30% PBT and counterstained with 30 nM DAPI (Molecular Probes), and mounted with SlowFade® Gold Antifade Mountant (Thermo). A sense probe was used as a negative control.
In silico promoter analysis
Conserved noncoding sequences (CNSs) were analyzed using the VISTA genome browser (http://www-gsd.ldl.gov/vista/). Briefly, the human and mouse Wnt ligands promoter was analyzed with default options (200-bp window, x-axis; 70% conservancy, y-axis) for potential hypoxia response elements (HREs; balloons). Promoter regions where peak values (y-axis) are above 50% were considered as potent evolutionarily conserved regulated elements.
ChIP assays
Mouse intestines (non-treated and IR) were minced into small pieces, crosslinked with 1% formaldehyde for 15 min RT, and quenched by glycine (0.125 M). After washing with cold PBS, tissues were incubated with lysis buffer [0.5% NP-40, 25 mM HEPES, 150 mM KCl, 1.5 mM MgCl2, 10% glycerol and KOH (pH 7.5)] containing protease inhibitor for 15 min on ice. Cell lysates were centrifuged (5,000 r.p.m. for 5 min), and supernatants were discarded. Cell pellets were then subjected to sonication with ChIP-radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris, pH 8.0; 150 mM NaCl; 0.1% SDS, 0.5% deoxycholate, 1% NP-40 and 1 mM EDTA; 10 times, 30 s on/30 s off), using Bioruptor® Plus sonication device (Diagnode, NJ). After centrifugation (13,200 r.p.m. for 30 min), a supernatant was immunoprecipitated with antibody overnight at 4 °C and was pulled down using Dynabeads® Magnetic Beads (Thermo). Immunoprecipitates were further washed serially with ChIP-RIPA lysis buffer, high salt (50 mM Tris, pH 8.0; 500 mM NaCl; 0.1% SDS, 0.5% deoxycholate, 1% NP-40 and 1 mM EDTA), LiCl wash buffer (50 mM Tris, pH 8.0; 1 mM EDTA, 250 mM LiCl; 1% NP-40 and 0.5% deoxycholate) and Tris-EDTA buffer. Finally, immunoprecipitate crosslinking was reversed by incubation at 65 °C ov ernight and treated with RNase A and proteinase K to extract DNA. ChIP amplicons (i–v) were detected via ChIP-PCR. Primer sequences for ChIP-PCR are available in Supplementary Table 1.
Statistical analyses
The Student’s t-test was used for comparisons of two samples. P values < 0.05 were considered significant. Error bars indicate s.e.m. The number of biological and experimental replicates is ≥ 3, unless otherwise mentioned in Figure Legends.
Supplementary Material
Highlights.
Tert+ cells are quiescent but conditionally repopulated upon tissue injury.
Tert+ cells are indispensable for intestinal regeneration.
ROS-HIFs-transactivated Wnt2b hyperactivates Wnt signaling in Tert+ cells.
β-catenin induces quiescence exit of Tert+ cells.
Acknowledgments
We thank Pierre D. McCrea, Junjie Chen, Seung-Hyo Lee, and Christopher L. Cervantes for helpful comments on the manuscript. This work was supported by the Cancer Prevention and Research Institute of Texas (RP140563), the National Institutes of Health (R01CA193297-01, P50CA098258), the Department of Defense (CA140572), the Duncan Family Institute for Cancer Prevention and Risk Assessment Grant (IRG-08-061-01), a Center for Stem Cell and Developmental Biology Transformative Grant (MD Anderson Cancer Center), an Institutional Research Grant (MD Anderson Cancer Center), a New Faculty Award (CA016672), and a Metastasis Research Center Grant (MD Anderson Cancer Center). The Genetically Engineered Mouse Facility was supported by the MD Anderson Cancer Center Support Grant (CA016672).
Footnotes
AUTHOR CONTRIBUTIONS
H.N.S. and J.-I.P. conceived the experiments. H.N.S., M.J.K., Y.-S.J., E.M.L., S.J., and J.-I.P. performed the experiments. H.N.S. and J.-I.P. analyzed the data. H.N.S. and J.-I.P. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL, Worthley DL, et al. Krt19(+)/Lgr5(−) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015;16:627–38. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009;4:e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–35. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–9. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–33. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–9. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529. e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol. 2006;18:254–60. doi: 10.1016/j.ceb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–94. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–8. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96:1020–4. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol. 2012;13:103–14. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol. 2012;14:106–14. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Jung YS, Suh HN, Wang W, Kim MJ, Oh YS, Lien EM, Shen X, Matsumoto Y, McCrea PD, et al. LIG4 mediates Wnt signalling-induced radioresistance. Nat Commun. 2016;7:10994. doi: 10.1038/ncomms10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–9. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Grompe M, Sander M. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol. 2016;18:238–45. doi: 10.1038/ncb3309. [DOI] [PubMed] [Google Scholar]
- Lien WH, Fuchs E. Wnt some lose some: transcriptional governance of stem cells by Wnt/beta-catenin signaling. Genes Dev. 2014;28:1517–32. doi: 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–30. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–5. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–41. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–91. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niecknig H, Tug S, Reyes BD, Kirsch M, Fandrey J, Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res. 2012;46:705–17. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–8. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- Psarras S, Karagianni N, Kellendonk C, Tronche F, Cosset FL, Stocking C, Schirrmacher V, Boehmer Hv H, Khazaie K. Gene transfer and genetic modification of embryonic stem cells by Cre- and Cre-PR-expressing MESV-based retroviral vectors. J Gene Med. 2004;6:32–42. doi: 10.1002/jgm.442. [DOI] [PubMed] [Google Scholar]
- Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun. 2016;7:13096. doi: 10.1038/ncomms13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–20. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–44. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–4. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, Atwood TF, Xing L, Giaccia AJ. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med. 2014;6:236ra64. doi: 10.1126/scitranslmed.3008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–23. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–13. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T, Yamaguchi TP. Mice lacking Wnt2b are viable and display a postnatal olfactory bulb phenotype. Neurosci Lett. 2012;512:48–52. doi: 10.1016/j.neulet.2012.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantu C, Aguet M, Basler K. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2016;15:911–8. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Xue X, Taylor M, Ramakrishnan SK, Nagaoka K, Hao C, Gonzalez FJ, Shah YM. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol Cell Biol. 2014;34:3013–23. doi: 10.1128/MCB.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–71. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer T, Bocker U, Wenz F, Singer MV. Medical prevention and treatment of acute and chronic radiation induced enteritis--is there any proven therapy? a short review. Z Gastroenterol. 2008;46:441–8. doi: 10.1055/s-2008-1027150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.