Abstract
Background
Obstructive sleep apnea (OSA) is associated with atrial remodeling, atrial fibrillation (AF), and increased incidence of arrhythmia recurrence following pulmonary vein isolation (PVI). We aimed to characterize the atrial substrate, including AF triggers in patients with paroxysmal AF (PAF) and OSA.
Methods and Results
In 86 patients with PAF (43 with ≥ moderate OSA [apnea-hypopnea index ≥15] and 43 without OSA [apnea-hypopnea index <5]) right atrial (RA) and left atrial (LA) voltage distribution, conduction velocities, and electrogram characteristics were analyzed during atrial pacing. AF triggers were examined before and after PVI and targeted for ablation. Patients with OSA had lower atrial voltage amplitude (RA, p=0.0005; LA, p=0.0001), slower conduction velocities (RA, p=0.02; LA, p=0.0002), and higher prevalence of electrogram fractionation (p=0.0001). The areas of atrial abnormality were consistent among patients, most commonly involving the LA septum (32/43; 74.4%). At baseline, the PVs were the most frequent triggers for AF in both groups, however following PVI patients with OSA had increased incidence of additional extra-PV triggers (41.8% vs. 11.6%; p=0.003). The 1-year arrhythmia-free survival was similar between patients with and without OSA (83.7% and 81.4%, respectively; p=0.59). In comparison, control patients with PAF and OSA who underwent PVI alone without ablation on extra-PV triggers had increased risk of arrhythmia recurrence (83.7% vs. 64.0%; p=0.003)
Conclusions
OSA is associated with structural and functional atrial remodeling and increased incidence of extra-PV triggers. Elimination of these triggers resulted in improved arrhythmia-free survival.
Keywords: atrial fibrillation, arrhythmia, catheter ablation, obstructive sleep apnea, sleep apnea, atrial substrate, AF trigger
Journal Subject Terms: Atrial Fibrillation, Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Electrophysiology
Introduction
Obstructive sleep apnea (OSA) is exceedingly prevalent in patients with atrial fibrillation (AF).1, 2 Individuals with OSA have 2–4 times increased risk to develop AF as compared to those without OSA. Similarly, individuals with AF have an increased prevalence of OSA, ranging from 10–60%.3 This wide range in the prevalence of OSA in patients with AF stems from differences in study design, patient population, and screening criteria. The coexistence of AF and OSA can be partially attributed to a common risk factor profile, including advanced age, hypertension, obesity, diabetes, and structural heart disease. Nonetheless, there may be a pathophysiologic, mutually-perpetuating, relationship between AF and OSA that includes cardiac electrical and structural remodeling.
OSA has been shown to promote AF via stretch-mediated shortening of atrial refractoriness and slow conduction mediated by collagen deposition and changes in gap junction content and function.4, 5 In particular, mechanical stretch of the thinned-walled atria shortens atrial refractoriness and promotes occurrence of spontaneous atrial premature depolarizations (APD), triggering episodes of AF in animal models of sleep apnea and in humans.3, 6
In this study, we evaluated the occurrence and pattern of atrial remodeling in patients with AF and OSA. We specifically compared the voltage distribution, electrogram characteristics, and conduction properties in patients with paroxysmal atrial fibrillation (PAF) with and without OSA. We also examined the frequency and distribution of AF triggers (pulmonary vein [PV] and extra-PV triggers) in patients with and without OSA. Lastly, we evaluated the role of extra-PV triggers in long-term arrhythmia control of patients with OSA.
Methods
Study Population
The study groups consisted of patients with symptomatic paroxysmal AF without prior diagnosis of sleep apnea referred for sleep study ≤ 90 days before index PVI between August 2013 to March 2016 at 3 institutions: Beth Israel Deaconess Medical Center (Boston, MA), Texas Cardiac Arrhythmia Institute at St. David’s Medical Center (Austin, TX), and University of Miami Miller School of Medicine (Miami, FL). Sleep apnea was evaluated using an in-laboratory overnight polysomnography (PSG) or a home sleep apnea testing device (WatchPAT, Itamar Medical, Israel). Diagnosis of sleep apnea was determined according to the Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine.7 A normal sleep study was defined if the apnea-hypopnea index (AHI) ≤ 5; mild sleep apnea by an AHI range ≥5 and ≤15; and ≥ moderate sleep apnea by AHI≥15. An episode was categorized as obstructive if apneic episodes occurred during respiratory effort. The two study groups included consecutive patients with a normal sleep study and consecutive patients with ≥ moderate OSA. Patients with mild OSA were excluded because of potential overlap in atrial substrate and to decrease misclassification of cases and controls. The two study groups underwent a detailed mapping and ablation protocol as descried in the section below that included PVI plus ablation of extra-PV triggers. The OSA study group is labeled [(+) OSA (+) PVI (+) triggers] and the non-OSA study group is labeled [(−) OSA (+) PVI (+) triggers].
In order to compare the effect of additional extra-PV trigger ablation on arrhythmia control in the study groups, control patients with normal sleep study and with ≥ moderate OSA who underwent PVI alone without mapping and ablation of extra-PV triggers were identified from a prospectively collected cohort: [(−) OSA (+) PVI (−) triggers] and [(+) OSA (+) PVI (−) triggers], respectively.
In order to evaluate the atrial substrate specific for OSA, patients with heart failure (left ventricular ejection fraction ≤50%), significant valvular disease, previous myocardial infarction, untreated hypertension, and/or diabetes were excluded from the study. To examine the association between OSA and atrial substrate and AF triggers independently of the potential effect(s) of continuous positive airway pressure (CPAP) therapy, patients previously treated with CPAP were excluded (patients were allowed to start CPAP therapy after the ablation procedure). Last, we only included patients presenting in sinus rhythm to determine the baseline atrial substrate and avoid measurements related to acute electrical remodeling. The institutional review board of each of the participating centers approved the study protocol.
Electrophysiology study and ablation
The procedures were performed in the postabsorptive state with conscious sedation or general anesthesia according to center and operator preference. Antiarrhythmic drugs (AADs) were discontinued for ≥5 half-lives before the ablation procedure (there were no patients on amiodarone). All patients were chronically anticoagulated for ≥ 4 weeks. The procedures were performed under uninterrupted warfarin (INR range 2–3) or rivaroxaban. In patients taking dabigatran or apixaban, a single dose was often held before the procedure. Unfractionated heparin was administered before the transseptal puncture to maintain an activated clotting time of 300–400 seconds for the duration of the procedure. An intracardiac echocardiography catheter (8Fr AcuNav™, Biosense Webster) was advanced to the RA. Electroanatomical mapping (EAM) of the RA and LA was performed during proximal and distal coronary sinus pacing, respectively (cycle length of 600–800 msec). EAM was performed using Carto®3 (Biosense Webster, Johnson & Johnson) in 182/186 patients, while Rhythmia™ (Boston Scientific, Cambridge, MA) was utilized in 4 patients. Mapping was performed using multielectrode catheters, including circular (Lasso® 10-pole, adjustable circumference, Biosense Webster), pentaray (Pentaray®, interelectrode spacing 2-6-2 mm; Biosense Webster), or basket (Orion™ 64 electrodes, Boston Scientific, Cambridge, MA) catheters. The mapping catheters were advanced into the atria over a long fixed-curved or a steerable sheath. The mapping density was in accordance to the physician’s practice, however with an obligatory minimal cutoff point number of 250 and filling threshold ≤10mm (allowing interpolation between points to be ≤10mm). Ablation was performed using an open irrigation tip radiofrequency catheter (Thermocool® ST™ or Thermocool® SF™, Biosense Webster) with energy of 20–40W. PVI was performed by isolating the left and right pairs of veins en-bloc using either a point-by-point or a continuous ablation approach, according to operator practice. Successful PVI was defined by presence of entrance block (loss of PV ostia potentials) and exit block (failure to capture the LA during pacing from the PV ostia). Persistent isolation for each PV was re-confirmed after ≥15-minute waiting period. In cases of acute PV reconnection, additional radiofrequency applications were performed to re-isolate the PV.
Identification and ablation of AF triggers
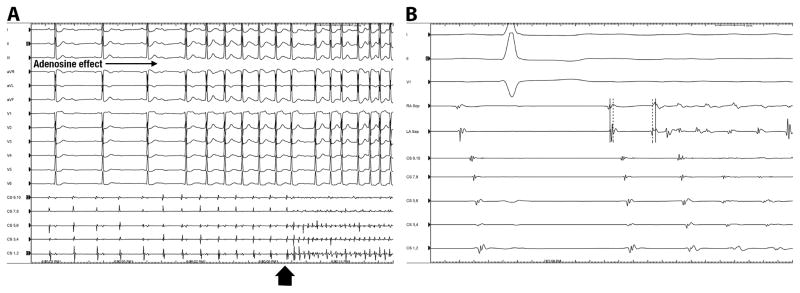
AF trigger was defined as an APD that initiated AF lasting ≥30 seconds. After completion of chamber mapping and before PVI, the ablation catheter was positioned in the right superior PV and the mapping catheter in the left superior PV. Two decapolar catheters were positioned in the coronary sinus (CS) and crista terminalis. An infusion of isoproterenol in increasing doses from 2 to 30 μg/min was given over 10 minutes until development of AF or junctional rhythm. As isoproterenol often results in transient hypotension, phenylephrine (50– 200mcg/min) was simultaneously infused to maintain a systolic pressure >90 mmHg. If AF could not be initiated during the isoproterenol infusion or its wear-off period, isoproterenol infusion was re-started at the maximally achieved dose, and atrial pacing at progressively shorter cycle lengths down to effective refractory period was performed in an attempt to induce AF. Once AF was induced, electrical cardioversion was performed (during isoproterenol infusion) to lower the AF inducibility threshold and trigger initiation of AF as previously described.8 If AF could not be induced with isoproterenol and cardioversion, adenosine was given at a dose sufficient to produce transient AV block; initially at 6mg with incremental doses of up to 48mg. (Figure 1A). Trigger localization was estimated based on the earliest endocardial activation site and pattern of activation from the multiple left and right atrial catheters. Once an AF trigger was identified, the catheters were re-positioned around the zone of earliest activation and electrical cardioversion was performed in attempt to re-induce AF (Figure 1B). Repeat induction of AF was performed to examine the reproducibility of AF induction, the specificity of the initiating APD, and to better localize the its origin.
Figure 1. Identification of AF Triggers.
Panel A, isoproterenol infusion (20μg/min) did not induce atrial fibrillation (AF). However, the concomitant administration of adenosine bolus (48mg) resulted in increased AV block and initiation of AF (arrowhead). Panel B shows initiation of AF from a left atrial premature depolarization (APD). In this case, the initial induction of AF appeared to originate from the inter-atrial septum. Following restoration of sinus rhythm and preparation for a second induction of AF, the catheters were repositioned on the right (RA Sep) and left (LA Sep) inter-atrial septum. Note that during sinus rhythm, activation of the right septum (dotted line) precedes activation of the left septum (dashed line). The sinus beat is then followed by an APD-initiating AF originating from the left septum. Note the reversal of atrial activation with the left septum (dashed line) now preceding the right septum (dotted line).
Following evaluation for AF triggers at baseline, PVI was performed as described above. Evaluation of AF triggers was repeated after PVI in order to identify extra PV triggers. In addition to isoproterenol infusion, adenosine challenge was also performed in an attempt to identify dormant PV conduction, and as an additional provocative test for identification of AF triggers. All extra-PV triggers were targeted for ablation. Ablation of extra-PV triggers was delivered as a cluster of radiofrequency ablation lesions (20–40W; 20–40 seconds) at the site of earliest activation as described above. Following ablation, the provocative measure that resulted in APD-triggered AF was repeated to confirm elimination of the trigger. Additional ablation was performed in attempt to eliminate all AF triggers.
Bipolar voltage distribution and electrogram measurement
Normal atrial bipolar voltage amplitude was defined as bipolar amplitude ≥0.5mV as previously determined for multielectrode mapping catheters with 1mm electrode size and 2mm interelectrode spacing.9 To minimize overestimation of the low voltage area, we defined an area as low voltage only if the bipolar voltage amplitude was ≤0.5mV in ≥3 adjacent points and the electrograms demonstrated fractionation and/or split potentials. Electrograms characteristics, including electrogram duration and presence of fractionations were analyzed offline at uniform gain, and paper speed of 200 mm/s. Normal electrograms were defined as: 1) bipolar voltage amplitude ≥0.5mV; 2) duration ≤50ms; and 3) number fractionations crossing the isoelectric interval ≤5. Electrograms with double potentials at the crista terminalis or at both sides of the septum were considered normal if their individual component duration was ≤50ms.
Atrial conduction time
Total atrial conduction time was determined by the P wave duration before ablation as measured during ostial CS pacing at a constant rate of 600ms. The P wave duration was measured in lead II and averaged over 10 beats. Intra-atrial conduction time was determined separately for the RA and the LA following completion of the EAM. Right atrial conduction time was determined during ostial CS pacing and measured from the site of pacing to the latest RA activation point. Left atrial conduction time was determined during distal CS pacing and measured from the earliest to the latest LA activation point.
Follow-up
Follow-up consisted of clinic visits at 1, 3, 6, and 12 months after the ablation procedure, and at intervals of 6 months afterwards. Holter monitoring of ≥1week duration was performed at least twice during the first year after ablation. Additional visits, electrocardiograms, and Holter monitoring were performed if patients reported arrhythmia symptoms between visits. All AADs were discontinued ≥5 half-lives before the ablation procedure and were not resumed after the procedure. Arrhythmia recurrence was defined as any documented atrial tachyarrhythmia episode lasting for ≥ 30 seconds that occurred after a 4-week blanking period following the ablation procedure. If arrhythmia recurred during the first 4-week period, electrical cardioversion with or without initiation of AADs were considered. If AAD was initiated due to arrhythmia recurrence during the initial period, it was discontinued during the 3-month post ablation clinic visit. Patients with documented recurrence after the blanking period were treated with AADs or repeat ablation. At each clinic appointment, patients were questioned about initiation of CPAP therapy and compliance with therapy.
Statistical Analysis
Continuous variables are reported as mean ± SD (or median and interquartile range, as appropriate) and compared between groups using one-way ANOVA (or Kruskal-Wallis) test. Categorical variables are reported as number and percentage and compared among groups using the Fisher exact test. Event-free survival was estimated by the Kaplan-Meier survival function. Pairwise comparisons of survival rates were made using Mantel-Cox log-rank test. The aim of the comparison was to evaluate the impact of extra-PV triggers ablation on arrhythmia-free survival. Therefore, each of the 2 study groups, with and without OSA, was compared to a respective control group who underwent PVI alone without ablation of extra-PV triggers. The impact of the following variables was assessed in a univariable Cox regression analysis: age, gender, body mass index (BMI), hypertension, diabetes, left ventricular ejection fraction (LVEF), LA area indexed to body surface area (BSA), AF severity index (AF duration since diagnosis and frequency), AF duration, and ablation of extra-PV triggers. Variables demonstrating significant impact on arrhythmia-free survival were then evaluated in a multivariable model. A P value <0.05 was considered statistically significant. Analyses were conducted using SPSS Statistics 22.0 (SPSS, Inc., Chicago, Illinois).
Results
Study Population
Baseline Characteristics
Forty-three patients with ≥ moderate OSA completed the mapping and ablation study protocol, including ≥ 1-year follow-up duration [(+) OSA (+) PVI and (+) trigger]. Forty-five patients with normal sleep study completed the mapping and ablation protocol, however 2 were lost to follow-up, and a total of 43 patients with normal sleep study [(−) OSA (+) PVI and (+) trigger] were analyzed. The control groups that underwent PVI alone without mapping and /or ablation of extra-PV triggers, included 50 patients with OSA [(+) OSA (+) PVI and (−) trigger] and 48 patients without OSA [(−) OSA (+) PVI and (−) trigger]. Patient characteristics are summarized in Table 1. The two OSA groups had larger left atrial dimensions indexed to body surface area (p=0.02). In patients with ≥ moderate OSA, daytime somnolence was reported in 53/91 (58.2%; data was missing for 2 patients)
Table 1.
Baseline clinical characteristics in each study group.
| (+) OSA (+) PVI (+) triggers (n=43) | (−) OSA (+) PVI (+) triggers (n=43) | (−) OSA (+) PVI (−) triggers (n=48) | (+) OSA (+) PVI (−) triggers (n=50) | P value* | |
|---|---|---|---|---|---|
| Male gender | 28 (65.1) | 22 (51.1) | 27 (56.2) | 33 (66.0) | |
| Age, years | 49±12 | 54±14 | 59±12 | 51±15 | 0.42 |
| BMI, kg/m2 | 31±6 | 29±7.5 | 26±9.0 | 32±5.5 | 0.23 |
| Hypertension | 21 (48.8) | 18 (41.8) | 18 (37.5%) | 27(54.0) | 0.62 |
| AF duration (years) | 6.0 (2–8) | 5.5 (1–14) | 4.0 (1–14) | 4.5 (2–7) | 0.30 |
| Frequency(episodes/mon) | 3 (0–4) | 2 (1–5) | 2 (1–4) | 3 (1–6) | |
| Number of failed AADs | 0.8 (0–2) | 0.7 (0–2) | 0.5 (0–3) | 0.4 (0–2) | 0.41 |
| Echocardiographic data | |||||
| LA area indexed BSA (cm2/m2) | 13.2±3.1 | 8.8±1.8 | 9.6±2.4 | 11.2±2.1 | 0.03 |
| LVEF (%) | 60±5 | 62±2 | 63±8 | 60±5 | 0.7 |
| Polysomnography data | |||||
| Mean AHI | 36±26 | 3±2 | 4±2 | 33±22 | <0.0001 |
| Desaturations ≥4%/h (no) | 28±16 | 3±3 | 2±2 | 26±12 | <0.0001 |
P-value for one-way ANOVA (Kruskal-Wallis) among all groups
PVI, pulmonary vein isolation; BMI, body mass index; PAF, paroxysmal AF; AAD, anti-arrhythmic drugs; LA, left atrium; BSA, body surface area; LVEF, left ventricular ejection fraction; AHI, apnea-hypopnea index.
Characterization of atrial substrate
Atrial voltage abnormalities
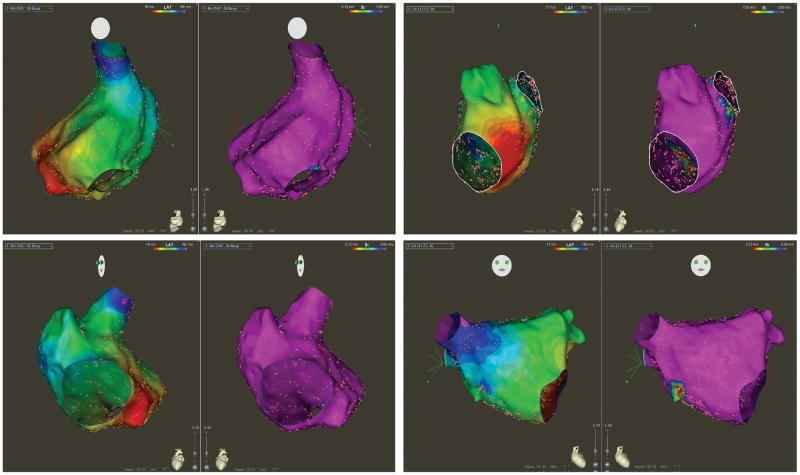
The number of RA data points 272±114 (median 282), and the number of LA data points was 556±320 (median 560). Patients without OSA had normal atrial electrograms and bipolar voltage amplitude with a mean bipolar voltage amplitude of 2.4±1.6mV (median 2; range 0.46–4.4mV). In the RA, bipolar voltage amplitude and electrogram characteristics were normal in all. In the LA, bipolar voltage amplitude and electrogram characteristics were normal in 39/43 (90%). In 4 patients, areas of abnormal bipolar voltage with electrogram fractionation were observed in the anterior roof (2 patients), posterior wall (1 patient), and fossa ovalis (1 patient). In all 4 of these patients, the size of the low voltage area was small (4.4±3.4cm2 [median 4; range 1.4–7.8]). Figure 2 shows a representative example of biatrial voltage map of a patient with a normal sleep study.
Figure 2. Voltage and Conduction Properties in Non-OSA.
The left panel shows a bipolar voltage map (range 0.1–0.5mV) of the right atrium (RA) in postero-anterior (top) and left anterior oblique (bottom) projections during proximal coronary sinus pacing in a patient without OSA. The voltage amplitude is normal. Activation map is presented as isochronal steps of 10ms, demonstrating early activation at the proximal coronary sinus pacing site with smooth and even propagation toward the zone of latest activation at the superior vena cava. The right panel shows bipolar voltage and activation maps of the left atrium at the left lateral (top) and right anterior oblique (bottom) projections during distal coronary sinus pacing. The voltage amplitude is normal (albeit the zone of the fossa ovalis) with normal wavefront propagation from the lateral mitral annulus to the zone of latest activation at the right superior pulmonary vein.
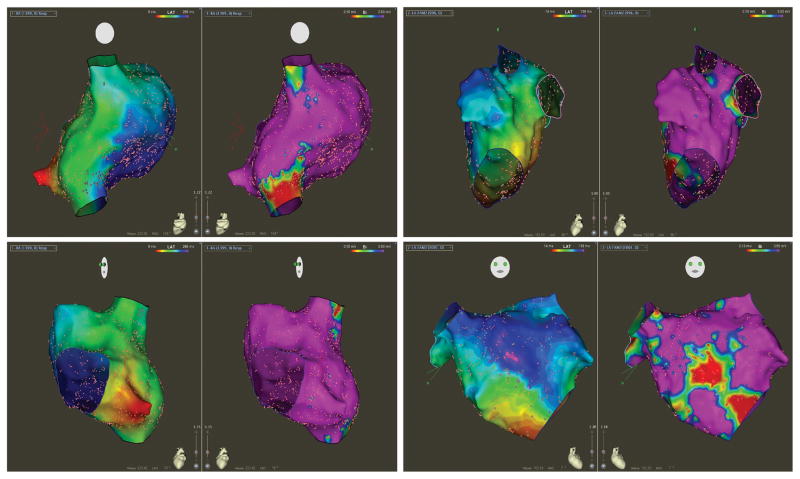
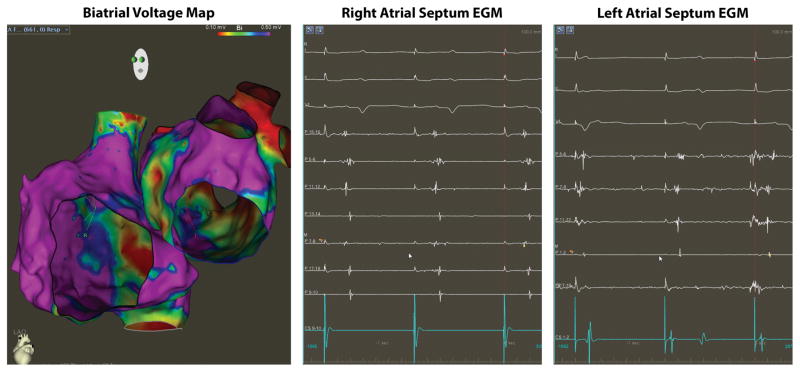
In the OSA group, the prevalence of low bipolar voltage amplitude and abnormal electrograms was significantly greater than the group without OSA (Figure 3). In the RA, areas of low bipolar voltage amplitude with abnormal electrograms were observed in 11/43 (25.5% RA; p=0.0005). The most common areas of abnormality were the septum (72.7%) and the postero-lateral wall (36.4%). The area of RA bipolar voltage amplitude abnormality was small (4.1±3.6cm2 [range 2.5–9.1]). In the LA, low bipolar voltage amplitude with abnormal electrograms was present in 32/43 (74.4%; p=0.0001), with the size of abnormality being relatively large (14.2±5.2cm2 [range 8.6–19.4]). Importantly, the location of tissue abnormality was consistent in 29/32 (90%) patients, involving the anterior septum from the mitral annulus inferiorly, the base of the left atrial appendage (LAA) superiorly, and the fossa ovalis posteriorly. Figure 4 shows an example of a patient with OSA demonstrating voltage abnormality at the right and left atrial septum with electrograms showing delayed and fractionated signals.
Figure 3. Voltage and Conduction Properties in OSA.
The left panel shows bipolar voltage map (range 0.1–0.5mV) of the right atrium (RA) in postero-anterior (top) and left anterior oblique (bottom) projections during proximal coronary sinus pacing in a patient with OSA. This demonstrates an overall normal voltage amplitude, with small areas of lower voltage in the septum. The activation map, similarly presented as isochronal steps of 10ms, demonstrates slow and abnormal conduction in the septum with the mid-posterior septum being activated 258ms after the earliest activation of the proximal coronary sinus. The right panel shows bipolar voltage and activation maps of the left atrium at the left lateral (top) and right anterior oblique (bottom) projections during distal coronary sinus pacing. The voltage amplitude is abnormal with significant area of low voltage at the septum. The activation map during distal coronary sinus is abnormal, with slow conduction over the septum, at the zone of low voltage.
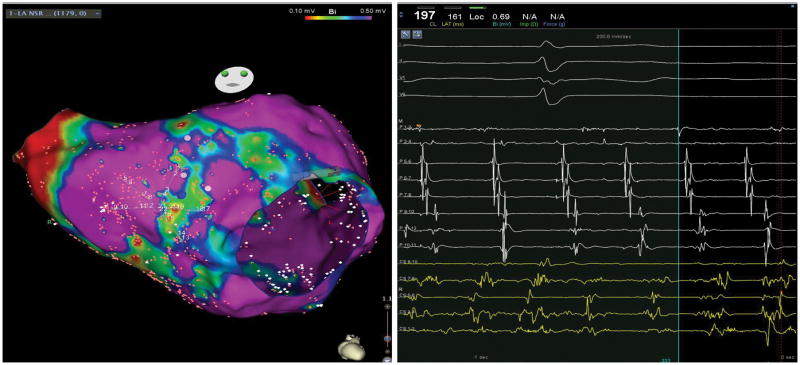
Figure 4. Bi-atrial Voltage and Conduction Abnormalities in OSA.
The left panel shows voltage map (0.1–0.5mV) of the right and left atria from a patient with OSA. Note that the areas of low bipolar voltage primarily involve the inter-atrial septum. The middle panel shows electrogram recorded on the right inter-atrial septum during proximal coronary sinus pacing (CS 9–10) while the right panel shows electrograms recorded on the left inter-atrial septum during distal coronary sinus pacing (CS 1–2). Electrograms were recorded using a pentaray multi-electrode mapping catheter and demonstrates abnormal low and fractionated signals.
Atrial conduction time
Total atrial conduction time was determined as the P wave duration in sinus rhythm before the ablation procedure (heart rate 72 ± 36 beat/minute) and off AADs, beta blockers, or calcium channel blockers. The baseline P wave duration was not statistically different between patients with and without OSA (128±62ms vs. 116±47ms, respectively; p=0.09).
RA conduction time during proximal CS pacing was similar between patients with and without OSA (142±64ms vs. 156±118ms, respectively; p=0.12). However, the pattern of wavefront propagation differed between groups, such that in patients without OSA the area of latest activation was the RAA-SVC junction, while in those with OSA the latest activation was the septal wall (see Figures 2 and 3). Although the total RA activation time was similar between groups, RA conduction velocities were slower in patients with OSA (0.88mm/s vs. 1.22mm/s; p=0.02).
LA conduction time during distal CS pacing was longer in patients with OSA (162±55ms vs. 108±26ms, respectively; p=0.0008). Similarly, LA conduction velocities were significantly slower in patients with OSA (0.78mm/s vs. 1.32mm/s; p=0.0002). The activation pattern was also different, such that the area of latest activation in patients with OSA was at the anterior septum, consistent with the area of low voltage (see Figures 2 and 3).
Triggers of atrial fibrillation
Before PVI, APD-induced AF occurred in 33/43 (76.7%) patients without OSA and in 35/43 (81.3%) of patients with OSA. The most common technique for APD-induced AF initiation was isoproterenol infusion (44.1%), followed by a combination of adenosine bolus during maximal isoproterenol effect (30.9%), and electrical cardioversion during isoproterenol infusion (25%). In 18 patients (20.9%; 10 from the non-OSA group and 8 from the OSA group), AF could not be induced spontaneously or during isoproterenol infusion with and without adenosine, or following cardioversion. The reproducibility of APD-induced AF initiation was examined in 62/68 patients. In these patients, AF was re-initiated in 56/62 (90%) using the same initial technique. The number of initiations per patient was 2.4 (median 2 [1–4)]). In 50/56 (89.2%) patients with ≥1 initiation of AF, the initiating APD had similar activation pattern, suggesting a similar trigger site.
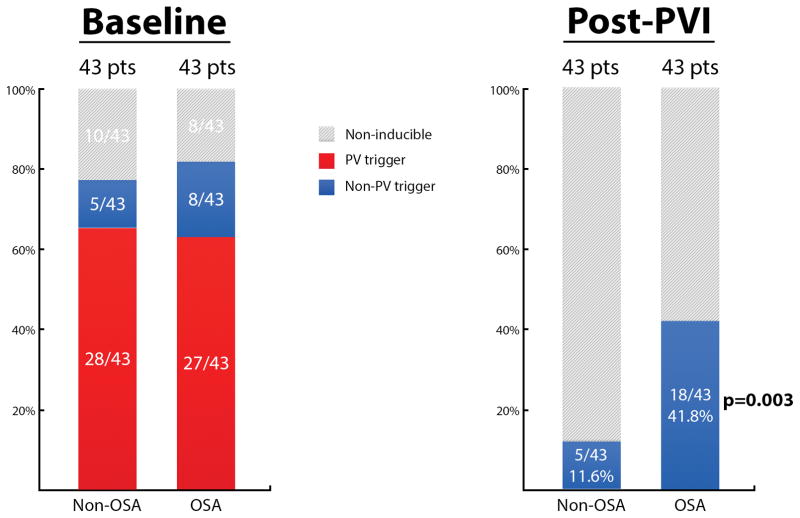
At baseline, the PVs were the most frequent triggers for AF in both the OSA (27/35; 77.1%), and non-OSA group (28/33; 84.8%). The left PVs were the dominant site in both groups (OSA 66.6%, [18/27]; non-OSA 71.4%, [20/28]). PVI was achieved in all patients. The protocol for identifying triggers of AF was then repeated. Patients with OSA had ~3.5-fold increased frequency of residual extra-PV triggers of AF. In patients without OSA, APD-triggered AF was present in 5/43 (11.6%) patients compared to 18/43 (41.8%) patients with OSA (p=0.003). Figure 5 shows the distribution of PV and extra-PV triggers in patients with and without OSA. In the 5 patients without OSA and extra-PV triggers post-PVI, the location was variable and occurred in sites of normal bipolar voltage and electrograms (eustachian ridge, crista terminalis, fossa ovalis, low anterior septum, and coronary sinus). In contrast, in 15/18 (83.3%) patients with OSA and extra-PV triggers, trigger sites were more commonly located within or adjacent to zones of low bipolar voltage and abnormal electrograms, most commonly the left anterior septum (12/15; 80%).
Figure 5.
Distribution of AF Triggers in Patients with and without OSA
During atrial fibrillation, these trigger zones often demonstrated spatiotemporal electrogram dispersion with organized electrical activity. Figure 6 shows a representative example from a patient with OSA and AF that was initiated following PVI from an extra-PV trigger in the left anterior septum. Electrogram recorded in the area of the initiating trigger at the zone demonstrated a relatively organized and stable electrical activity. This pattern of organized spatiotemporal electrogram dispersion during AF was present in 10/15 (66.7%) patients with OSA. Furthermore, ablation at these sites on the left anterior septum during AF resulted in termination of AF in 11/15 patients (73.3%).
Figure 6. Stabilization of Electrical Activity around Zones of Low Voltage.
In this example of a patient with OSA, a pentaray multielectrode mapping catheter was used to map the left atrium during atrial fibrillation (AF). A relatively stable beat-to-beat electrical activity was recoded around the zone of low voltage over the septum. Ablation at this location resulted in termination of AF.
Following ablation of the extra-PV trigger(s), 15 of 23 (65.2%) patients were rendered non-inducible using a similar induction protocol. In the remainder, APD-triggered AF was still present. Similar or additional triggers were identified and ablated in 4 additional patients. Overall, elimination of APD-triggered AF was achieved in 19/23 (82.6%), while in 4/23 (17.4%) elimination of APD-induced AF could not be achieved. The additional ablation time required for elimination of extra-PV triggers was 11min [median 12min, range 3–18min]. Ablation of extra-PV triggers was not associated with additional complications. There were overall 3 complications: 1 groin hematoma in each group, and a single event of intubation-related pharyngeal hematoma in the OSA group. There was no cardiac tamponade, stroke, significant esophageal injury, or heart block. Evaluation of extra-PV triggers following PVI required 12±6 minutes. The potential increase in radiation exposure was evaluated by comparing the X-ray time between the two study groups in whom evaluation and ablation on extra-PV triggers was performed to the two control groups who underwent PVI alone. The X-ray time was similar between the groups. There was no difference in X-ray time (19±11 vs. 17±22min; p=0.21).
Clinical outcome
During a follow-up period of 1 year, 35 of the 43 (81.4%) non-OSA patients and 36 of the 43 (83.7%) OSA patients maintained sinus rhythm following the first PVI. The arrhythmia-free survival rate was similar between the OSA and non-OSA groups (83.7% vs. 79.1%, p=0.53). Two patients from the non-OSA group and three patients from the OSA group were on AADs therapy at 1-year after the index PVI (all on class Ic agents).
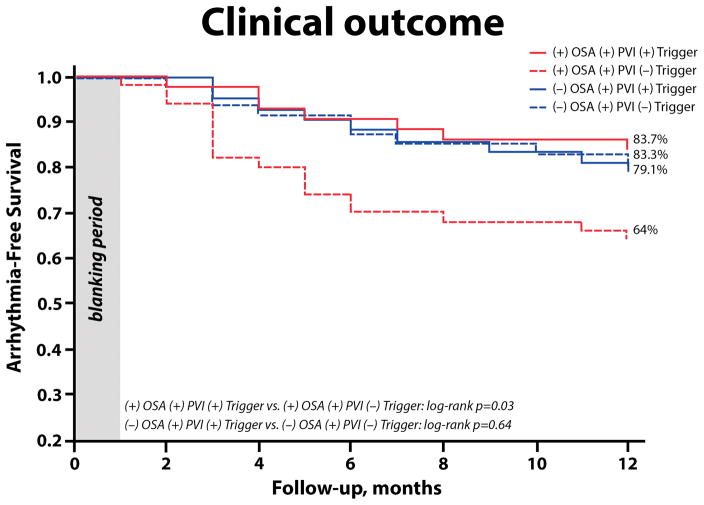
To examine the role of extra-PV trigger ablation on long-term arrhythmia control in patients with and without OSA, we compared arrhythmia-free survival between the OSA study group in whom extra-PV triggers were mapped and ablated [(+) OSA (+) PVI and (+) trigger] and the control group of patients with OSA who underwent PVI alone without mapping and ablation of extra PV triggers [(+) OSA (+) PVI and (−) trigger]. Similarly, patients without OSA who underwent ablation of extra PV triggers [(−) OSA (+) PVI and (+) trigger] were compared to a control group of patients without OSA who underwent PVI alone [(−) OSA (+) PVI and (−) trigger]. In patients with OSA, ablation of extra-PV triggers in addition to PVI resulted in a significantly improved clinical outcome (83.7% vs. 64%; p=0.03, figure 7). In contrast, in patients without OSA, ablation of extra-PV triggers in addition to PVI had no effect on arrhythmia-free survival (79.1% vs. 83.3%; p=0.64).
Figure 7. Kaplan-Meier Survival Curves According to Treatment Groups.
OSA, obstructive sleep apnea; PVI, pulmonary vein isolation
Clinical variables associated with AF recurrence
The effect of baseline characteristics on arrhythmia recurrence was assessed using a stepwise Cox proportional hazards regression model. The [(+) OSA (+) PVI (+) triggers] was compared to the OSA [(+) OSA (+) PVI (−) triggers] group. In univariable analysis (Table 2), LA area indexed to BSA was associated with increased arrhythmia recurrence (HR 1.56; 95% confidence interval 1.06–1.98; p=0.002), while ablation of extra-PV triggers was associated with reduced arrhythmia recurrence (HR 0.42; 95% confidence interval 0.22–0.78; p=0.005). The LA area indexed to BSA and ablation of extra-PV triggers were tested in a multivariable model (Table 2). Ablation of extra-PV triggers remained an independent predictor of reduced arrhythmia recurrence (HR 0.45; 95% confidence interval 0.21–0.86; p=0.02). The LA area indexed to BSA also remained was associated with increased arrhythmia AF recurrence risk (HR 1.32; 95% confidence interval 1.15–3.70; p=0.01). During the follow-up period, 1 patient from the [(+) OSA (+) PVI and (+) trigger] and 2 patients from the [(+) OSA (+) PVI and (−) trigger] initiated CPAP therapy. Statistical analysis excluding these patients on CPAP therapy, demonstrated a similarly significant difference between the groups (p=0.03).
Table 2.
Clinical Variables Associated with AF Recurrence
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| Age | 1.32 | 0.8–1.76 | 0.39 | |||
| Gender | 0.92 | 0.6–1.59 | 0.45 | |||
| BMI | 1.41 | 0.9–1.78 | 0.07 | |||
| Hypertension | 1.21 | 0.7–1.62 | ||||
| Diabetes | 1.4 | 0.8–1.91 | 0.10 | |||
| LVEF | ||||||
| LA area indexed BSA | 1.56 | 1.06–1.98 | 0.002 | 1.32 | 1.15–3.70 | 0.01 |
| AF severity index | ||||||
| Ablation of extra-PV triggers | 0.42 | 0.22–0.78 | 0.005 | 0.45 | 0.21–0.86 | 0.02 |
AF, Atrial fibrillation; BMI, body mass index; LA, left atrium; LVEF, left ventricular ejection fraction; PV, pulmonary veins
Discussion
In this study, we evaluated the anatomical and functional atrial substrate of patients with PAF and moderate-to-severe OSA. The major findings are: 1) PAF in patients with OSA is often associated with patchy areas of low bipolar voltage and slow conduction, predominantly affecting the left anterior septum; 2) these zones are a common source of extra-PV triggers and localized circuits of AF; and 3) ablation of extra-PV triggers of AF is associated with improved clinical outcome in patients with PAF and OSA undergoing catheter ablation.
Repetitive episodes of OSA have been associated with cardiac structural and electrical remodeling. In animal models, repetitive episodes of OSA produce atrial fibrosis as well as important changes in connexin-43 expression and distribution, resulting in atrial conduction slowing and vulnerability for arrhythmias, including AF.4 In addition, repetitive episodes of OSA also result in ventricular remodeling, including LV dilatation, hypertrophy, diastolic dysfunction, along with RV hypertrophy. The natural progression of OSA-mediated atrial remodeling in human is not entirely understood and may well depend on the frequency and severity of OSA, as well as natural compensatory mechanisms, including the genetic makeup; such that some patients with OSA do not have AF, while others have short PAF, with the majority progressing to persistent AF.
Our study found that patients with PAF and OSA have increased incidence of voltage abnormality, primarily involving the left atrial septum. This finding is consistent with a recent report by Dimitri and colleagues, showing reduced biatrial voltage in patients with PAF and OSA.10 While atrial scar is common in patients with persistent AF, it is fairly uncommon in patients with PAF. The increased incidence of atrial scar in these patients may be the result of cardiac remodeling in patients with sleep apnea; however, the cause-and-effect relationship cannot be inferred from this study and requires a separate investigation. Nonetheless, the presence of atrial scar was associated with increased frequency of APDs (likely due to triggered activity) and initiation of AF from areas in and/or around the scar. This presence of atrial fibrosis has been strongly linked to AF, as fibrosis levels measured by cardiac magnetic resonance imaging are higher in AF patients compared to healthy subjects and correlate positively with AF recurrence.11 Moreover, ablation around fibrotic areas identified either by late gadolinium enhancement or EAM has shown to improve ablation success rates.11, 12 Mechanistically, structural and functional tissue heterogeneity associated with fibrosis have generally been linked to arrhythmogenesis, for example in ventricular infarction border zone.13 Such border heterogeneity can be present as large collagen deposits within the functional myocardium, which can slow down or block the propagation of electrical excitation waves, creating conditions for generation and sustenance of reentrant drivers. A recent study demonstrated that atrial areas adjacent to dense fibrosis have high levels of arrhythmogenic activity. Morgan and colleagues created a 3D model of the human atria with varying degree of fibrosis, and myocyte-fibroblast coupling.14 They demonstrated that in models of diffuse fibrosis (such as in elderly patients or patients with hypertension), waves randomly meandered through the atria, whereas in models of patchy and localized fibrosis, such as in patients with OSA, rotors stabilized in the border zones of these patchy fibrosis, where slow conduction enabled the development of localized circuits (rotors) within these relatively small regions. This may explain the relatively high incidence of AF termination we observed with ablation in and around the localized scar area.
Ablation of AF triggers in abnormal atrial tissue resulted in improved clinical outcome with reduced arrhythmia recurrence. Whether this is due to elimination of the extra pulmonary vein triggers or due to ablation of the arrhythmogenic substrate supporting the circuit is unclear. This data is consistent with a recent report by Kottkamp and colleagues that studied the atrial substrate in patients with PAF and recurrent AF despite durable PVI.12 They found that this subset of patients commonly had confluent low voltage areas (<0.5mV); and empiric ablation resulted in an improved clinical outcome with a reduced AF recurrence rate. The study did not include a systematic evaluation for the presence of sleep apnea, and therefore the relative “specificity” of OSA to atrial scar cannot be validated in this cohort. However, the presence of LA septal anterior scar was also found in two patients from the non-OSA group, and is commonly observed in patients with persistent AF. Therefore, OSA may not necessarily represent a unique pathophysiological substrate for AF, but be strongly associated with atrial fibrosis and perpetuation of the arrhythmogenic milieu.
The value of CPAP for arrhythmia control in patients with AF has been evaluated in several observational studies.15–21 Most showed that the CPAP use is associated with reduction in recurrence of AF in patients with OSA. However, the generalizability of these findings is unclear in view of the data’s observational nature. A recent large randomized study in patients with OSA found that therapy with CPAP plus usual care, as compared with usual care alone, did not prevent cardiovascular events, including new-onset AF or strokes in patients with OSA and established cardiovascular disease.22 It is possible that CPAP, like other disease-modifying interventions, exert their maximal benefit in early disease states, especially when complicated by end-organ effects (including atrial scaring). In this regard, Pathak and colleges prospectively examined the impact of aggressive risk factor reduction, including weight, blood pressure, glycemic, and lipid control on the frequency of AF frequency and the response to ablation.23 They found that aggressive risk factor management improved the long-term success of AF ablation (p<0.001). As sleep apnea often accompanies other risk factors, such as obesity, hypertension, and diabetes, it may beneficial to include routine screening and treatment of OSA in patients with early onset of AF.
Data from this study advocates for screening newly diagnosed AF patients for the presence of OSA. This may help identify patients in whom PVI alone may not be insufficient. It may also facilitate earlier diagnosis and treatment of OSA. This strategy may improve the outcomes of patients with AF where conventional therapies appear to have reached their limits. However, there is an urgent need for high-quality data in the form of a large randomized trial to clarify the benefit of screening and treatment of sleep apnea in patients with AF.
Limitations
The major limitations of this study are its relatively small size and lack of randomization. However, the differences in atrial substrate noted between patients with and without OSA may serve as a strong signal towards a larger randomized trial. We did not perform cardiac magnetic resonance imaging in these patients and therefore cannot correlate the magnitude of voltage and electrogram abnormality with late gadolinium enhancement, presence of OSA, and/or extra-PV substrate for AF. Ablation of extra-PV triggers had positive clinical impact in patients with OSA but not in patients without OSA. The low occurrence of extra-PV triggers in the group with a normal sleep study, and the overall small cohort cautions interpretation of this finding. In addition, follow-up monitoring of arrhythmia recurrence was limited to 2 weeks of continuous monitoring plus additional monitoring due to symptoms.
Conclusions
Patients with PAF and OSA demonstrate significant atrial remodeling characterized by structural and functional abnormalities with reduction of bipolar voltage amplitude and slowing of conduction, particularly in the left atrial anterior septum. These changes are associated with an increased incidence of extra-PV triggers for AF. Ablation of this substrate was associated with improved arrhythmia-free survival when compared with OSA patients undergoing PVI alone.
Supplementary Material
WHAT IS KNOWN
There is a strong association between atrial fibrillation (AF) and obstructive sleep apnea (OSA), and it is estimated that 50% of patients with AF also have OSA.
The association between AF and OSA may be related to common risk factors and a mutually-perpetuating pathophysiological relationship between these conditions.
OSA promotes AF via electrical and structural atrial remodeling.
WHAT THE STUDY ADDS
OSA in patients with paroxysmal AF is associated with bi-atrial structural remodeling presenting as patchy areas of low voltage and fractionated electrograms predominantly in the anterior septum.
The pulmonary veins are the major triggers for AF in patients with OSA, however extra-pulmonary vein (PV) triggers are highly common in this patient population.
Ablation of extra PV triggers in patients with paroxysmal AF and OSA is associated with improved clinical outcome compared to pulmonary vein isolation alone.
The high prevalence of OSA in patients with AF coupled with the increased incidence of extra PV triggers calls for universal sleep study in all patients with AF, and an ablation strategy incorporating targeting of extra PV triggers.
Footnotes
Disclosure: Elad Anter receives research grants and speaking honoraria from Biosense Webster, Boston Scientific, Abbott Medical, and Itamar Medical. Luigi Di Biase and Andrea Natale receive consulting and speaking honoraria form Biosense Webster, Boston Scientific, Stereotaxis, and Abbott Medical. Alfred Buxton receives research grants form Medtronic and Biosense Webster. Cory M. Tschabrunn receives consulting from Boston Scientific. Mark E. Josephson received research grants and speaking honoraria from Medtronic. The remainder has no financial disclosures.
References
- 1.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. Journal of the American College of Cardiology. 2007;49:565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Force ESCT, Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M, Kudaiberdieva G, Lane DA, Larsen TB, Lip GY, Lochen ML, Marin F, Niebauer J, Sanders P, Tokgozoglu L, Vos MA, Van Wagoner DR, Document r, Fauchier L, Savelieva I, Goette A, Agewall S, Chiang CE, Figueiredo M, Stiles M, Dickfeld T, Patton K, Piepoli M, Corra U, Marques-Vidal PM, Faggiano P, Schmid JP, Abreu A. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS) Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 doi: 10.1093/europace/euw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki YK, Kato T, Xiong F, Shi YF, Naud P, Maguy A, Mizuno K, Tardif JC, Comtois P, Nattel S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. Journal of the American College of Cardiology. 2014;64:2013–23. doi: 10.1016/j.jacc.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 5.Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1436–43. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 6.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–65. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 7.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017 doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, Supple GE, Garcia FC, Dixit S, Callans DJ, Marchlinski FE. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2016;13:374–82. doi: 10.1016/j.hrthm.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Anter E, Tschabrunn CM, Josephson ME. High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circulation Arrhythmia and electrophysiology. 2015;8:537–45. doi: 10.1161/CIRCEP.114.002737. [DOI] [PubMed] [Google Scholar]
- 10.Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, Antic N, Thornton A, Saint DA, McEvoy D, Antic R, Kalman JM, Sanders P. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:321–7. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 12.Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box Isolation of Fibrotic Areas (BIFA): A Patient-Tailored Substrate Modification Approach for Ablation of Atrial Fibrillation. Journal of cardiovascular electrophysiology. 2016;27:22–30. doi: 10.1111/jce.12870. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford SL, Trew ML, Sands GB, LeGrice IJ, Smaill BH. High-resolution 3-dimensional reconstruction of the infarct border zone: impact of structural remodeling on electrical activation. Circulation research. 2012;111:301–11. doi: 10.1161/CIRCRESAHA.111.260943. [DOI] [PubMed] [Google Scholar]
- 14.Morgan R, Colman MA, Chubb H, Seemann G, Aslanidi OV. Slow Conduction in the Border Zones of Patchy Fibrosis Stabilizes the Drivers for Atrial Fibrillation: Insights from Multi-Scale Human Atrial Modeling. Front Physiol. 2016;7:474. doi: 10.3389/fphys.2016.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 16.Jongnarangsin K, Chugh A, Good E, Mukerji S, Dey S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Boonyapisit W, Pelosi F, Jr, Bogun F, Morady F, Oral H. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. Journal of cardiovascular electrophysiology. 2008;19:668–72. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel D, Mohanty P, Di Biase L, Shaheen M, Lewis WR, Quan K, Cummings JE, Wang P, Al-Ahmad A, Venkatraman P, Nashawati E, Lakkireddy D, Schweikert R, Horton R, Sanchez J, Gallinghouse J, Hao S, Beheiry S, Cardinal DS, Zagrodzky J, Canby R, Bailey S, Burkhardt JD, Natale A. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circulation Arrhythmia and electrophysiology. 2010;3:445–51. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 18.Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y, Ito Y, Kuroki K, Machino T, Yamasaki H, Igarashi M, Sekiguchi Y, Sato A, Aonuma K. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:331–7. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. Journal of the American College of Cardiology. 2013;62:300–5. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, Holmes DN, Peterson ED, Piccini JP, Gersh BJ Investigators O-A. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) American heart journal. 2015;169:647–654. e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, Michaud GF, van der Geest R, Blankstein R, Steigner M, John RM, Jerosch-Herold M, Malhotra A, Kwong RY. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2:e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS Investigators S and Coordinators. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. The New England journal of medicine. 2016;375:919–31. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 23.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. Journal of the American College of Cardiology. 2014;64:2222–31. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.