Abstract
Recent research has led to wide acceptance and better understanding of a novel mechanism for cell–cell communication that employs a network of extracellular microvesicles (ExMVs). Derived from the plasma membrane or the endosomal membrane compartment, these small, spherical membrane fragments are secreted from the cell surface or in process of exocytosis from endosomal membrane compartment and 1) with ligands expressed on their surface directly stimulate target cells in a paracrine manner, 2) transfer cell membrane receptors to target cells, or 3) deliver encapsulated mRNA, miRNA, proteins, and bioactive lipids to target cells. This represents an evolutionarily ancient mechanism by which cells signal their presence in the microenvironment, communicate with each other, and affect the biology of neighboring cells. Evidence suggests the pivotal role of ExMVs in almost all biological processes within the body as well as their involvement in certain pathologies. Moreover, liquid biopsies based on deciphering the molecular signature of ExMVs promise to revolutionize laboratory diagnostics. At the same time there are ongoing attempts to employ them as delivery vehicles for drugs as well as therapeutics in regenerative medicine, oncology, and immunotherapy.
Keywords: ExMVs, exosomes, horizontal transfer of RNA and proteins, cell communication, liquid biopsies
Introduction
As with many breakthrough discoveries, the significance of research on microparticles, microvesicles, and exosomes—all recently proposed to be included under the umbrella term “extracellular microvesciles” (ExMVs)—was not immediately apparent [1–5]. The best-known examples initially were platelet-derived ExMVs released from activated platelets as important facilitators in activating the blood coagulation cascade [6] and ExMVs released from maturing reticulocytes and enriched in surplus cell surface receptors shed from maturating red blood cells [3]. By contrast, with regard to the physiological role of ExMVs released from nucleated cells there was initially great skepticism, and ExMVs were believed by many investigators to be merely cell debris or apoptotic bodies released from damaged cells [1,2].
Beginning almost 20 years ago my team became interested in ExMVs based on observations of these small membrane fragments during FACS analysis of apparently healthy normal cells from established cell lines isolated from in vitro cultures. In these analyses, a cluster of events of variable size was consistently visible in the lower left quadrant of the cytogram, which was interpreted by FACS operators as cell debris. To shed more light on this phenomenon we began to isolate these membrane fragments using similar protocols to those employed for isolation of activated platelet-derived ExMVs and discovered that these small, spherical membrane fragments may, if added as stimulators to target cells, enhance their migration and proliferation and induce intracellular signaling [7]. Interestingly, they were also able to transfer cell surface receptors between cells [8]. Most importantly we found that ExMVs transfer mRNA species and proteins between cells that, after delivery, modulate the biological processes of the target cells [9]. Shortly after this seminal observation, several groups followed our lead and produced compelling evidence for a new cell–cell mechanism involving horizontal transfer of bioactive mediators between cells, and this process has now been established as a new and important biological phenomenon [10–13]. All of this has led to exponentially growing interest in the role of ExMVs in physiology and pathology.
In this short review we will summarize the most important findings in the field of ExMVs. We apologize that, because of space constraints, we cannot cite all the excellent work by investigators actively working in this field.
The biological role of ExMVs
Both single-cell and multicellular organisms communicate between cells by several mechanisms. In addition to fine-tuned, ligand-specific, receptor-based interactions that emerged over time during evolution, one of the most developmentally early cell-to-cell communication mechanisms is based on spherical membrane fragments. While larger ExMVs (~100 nm to 1 μm in diameter) are shed from the cell surface by blebbing and budding of the cell plasma membrane, smaller ones (~40–150 nm) are derived from the endosomal cell membrane compartment in the process of exocytosis [1–5]. Smaller ExMVs are known as exosomes in the literature, and in this review, for reasons of simplicity, we will refer to both large and small spherical membrane fragments by the common name ExMVs.
These small, spherical membrane fragments are present in blood plasma, interstitial fluid, cerebrospinal fluid, saliva, urine, bile, synovial fluid, sperm, and breast milk as well as in malignant effusions [3]. ExMVs contain an outer phospholipid layer that surrounds inner content composed of several coding and non-coding RNA species, biologically active proteins (including enzymes), signaling components, transcription factors, bioactive lipids, nucleotides, and certain metabolites [1–13]. In some large ExMVs mitochondria have also been identified [14]. Certain ligands hijacked from microvesicle-producing cells may also be expressed on the surface of ExMVs and may directly stimulate target cells in a ligand receptor-dependent manner [1–7]. Below, we will summarize the most important applications of ExMVs in medical biology, clinical medicine, and diagnostics.
Perspectives on the application of ExMVs in regenerative medicine
One of the known effects of ExMVs is their positive pro-regenerative and anti-apoptotic effect during stem cell therapies in regenerative medicine. For example, in seminal work by Dr. Camussi’s group it was convincingly demonstrated that ExMVs derived from mesenchymal stem/stromal cells exert similar biological effects in in vivo models of tissue and organ regeneration as in intact cells [15]. This observation opened up new therapeutic possibilities for ExMVs in regenerative medicine, as they avoid problems related to the application of cells [15]. Currently, this promising strategy is being tested in several animal models in cardiology, ophthalmology, neurology, hematology, and hepatology.
Moreover, there have been attempts to generate ExMVs more efficiently from a therapeutic point of view by modifying cell lines that serve as their producers. Large-scale isolation of ExMVs for clinical applications is one of the most important challenges in the field. Recent evidence has accumulated that smaller ExMVs are more potent in repairing tissue and organ damage than larger ones [3]. Overall, it is proposed that ExMVs could be isolated from a large-scale ex vivo expansion of cultured cells, for example, mesenchymal stem cells or even induced pluripotent stem cells, which could be employed as producing cell lines [16]. In Figure 1 different approaches to generating more efficient ExMVs are depicted. First, ExMV-producing cells that lack genes encoding histocompatibility antigens would minimize the possibility of cross-immunization with ExMVs by donor HLA antigens (Figure 1A). Next, ExMV-generating cells could be genetically modified to overexpress on their surface i) peptides that protect target cells in damaged organs from apoptosis and stimulate proliferation of the remaining cell population or ii) factors that effectively induce angiogenesis (Figure 1B). This would allow production of more potent ExMVs. Similarly, ExMV-producing cell lines could also be engineered to be enriched for mRNA and regulatory miRNA species that, after horizontal transfer to the damaged tissues, promote regeneration by inhibiting apoptosis and promoting angiogenesis (Figure 1C). Finally, they could also be enriched for cell surface molecules that facilitate their tropism to adhesion-partner molecules present in damaged organs (Figure 1D). We can expect in the near future the first results of clinical trials in which ExMVs are employed in the clinic to ameliorate, for example, cardiovascular complications, non-healing ulcers of the lower extremities, or even to regenerate damaged retina in patients suffering from acute macular degeneration [3].
Figure 1. Different possible approaches to generating more efficient pro-regenerative ExMVs.
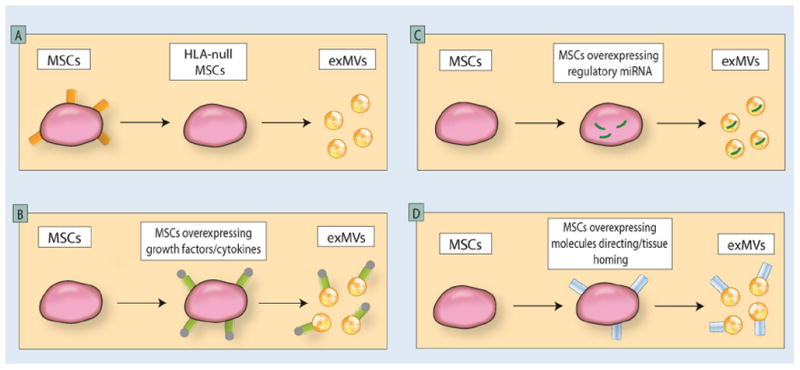
ExMVs for therapeutic purposes could be harvested from large-scale in vitro cultures of producing cell lines—for example, mesenchymal stem cells or induced pluripotent stem cells. Such cell lines may be modified to obtain ExMVs that do not express HLA antigens (Panel A); are enriched in growth factors, cytokines, chemokines, and bioactive lipids that promote regeneration of damaged organs (Panel B); are enriched in mRNA and regulatory miRNA facilitating regeneration of damaged tissues and/or promoting angiogenesis (Panel C); or display on their surface molecules that direct them to, and cause them to be retained in, damaged tissues (Panel D) [adapted from Ratajczak MZ et al; ref. 16].
ExMVs as vehicles for drug delivery
Considering their molecular structure, one can consider ExMVs as a type of physiological liposome. Thus, like liposomes, they could be loaded with bioactive compounds and drugs and employed in the clinic as vehicles for drug delivery [3, 17, 18]. In fact, there are already several reports on using this strategy to deliver therapeutic compounds to cells. As an example, prostate cancer cell-derived ExMvs were used as an effective delivery system of paclitaxel to parental cancer cells, delivering the drug into the malignant cells by an endocytic pathway. Importantly, this approach increased paclitaxel cytotoxicity [17]. In another report, ExMVs were effectively employed to deliver siRNA molecules targeting HER2, an oncogenic receptor tyrosine kinase that critically mediates breast cancer development and progression, to breast cancer cells [18]. The results of first clinical trials using this technology in several clinical settings are expected soon [1–6, 19–21].
ExMVs as diagnostic and prognostic tools
ExMVs exhibit unique molecular signatures that depend on the markers and molecules that they acquire during formation from the cell of origin and thus can be considered a kind of business card with which to identify these cells. These properties have opened the door to exploring their application as diagnostic tools to identify pathologic changes in the body. Based on this approach, ExMVs have emerged as important diagnostic targets in so-called liquid biopsies [3, 22]. Significant effort based on omics technologies is currently being invested to study the profile of ExMV components in several clinical settings, including oncology, cardiology, nephrology, neurology, gastroenterology, and pulmonology [3], in which they could be employed as markers of diseases and have prognostic value. One of the challenges to be overcome is to develop technologies to select ExMVs of interest from biological fluids using unique diagnostic markers.
Unwanted effects of ExMVs
In parallel, evidence has accumulated that ExMVs may play a role in pathology. For example, there are reports that an aberrant molecular composition of ExMVs may be responsible for pulmonary hypertension [4] or eclampsia [23]. Moreover, outdated peripheral blood platelets may be highly enriched for ExMVs that transfer molecules involved in platelet adhesion to endothelium (CD41, CD61, CD62) to tumor cells and thus make cancer cells “sticky” to endothelium and predispose them to metastasis [24]. This may limit the use of outdated platelets in cancer patients. A similar transfer of cell membrane receptors has been observed in many cases; for example, ExMVs derived from glioma cells in glioblastomas transfer the oncogenic receptor EGFRvIII to neighboring cells and thus increase their transforming potential [25]. It has also been reported that ExMVs from leukemic cells negatively affect normal hematopoietic cells in bone marrow [5]. Finally, transfer of HIV-entry receptors by ExMVs from monocytes or blood platelets may render other cells sensitive to entry by M- and T-tropic HIV viruses, respectively [26]. Interestingly, another possible ExMV-mediated mechanism is that prions, HIV, and some other viruses may be carried as cargo inside ExMVs and transmit infection after ExMV fusion with uninfected target cells. This latter possibility of pathogen transfer is known in the literature as the “Trojan horse” effect.
Conclusions
Evidence has accumulated pointing to a pivotal role of ExMVs in almost all biological processes in the body as well as involvement in certain pathologies. A novel, and until now unappreciated, crosstalk between cells has been identified that is mediated by ExMVs. This phenomenon is involved in several physiological processes, from maintaining tissue homeostasis to tissue and organ regeneration, angiogenesis, and cancerogenesis. It will be important to decipher the molecular signature of mRNA species, proteins, and bioactive lipids in ExMVs isolated from normal human individuals, including their dependence on sex and age, and in patients presenting with various health problems. One of the major obstacles to moving this field forward has been the lack of established methods to isolate, measure the concentration of, and purify ExMVs from biological fluids. Currently, several approaches have been proposed to standarize isolation and enumeration protocols, and a broad platform of omics strategies has been employed to decipher this exciting language of cell–cell communication. There is no doubt that we will continue to witness new developments in clinical and diagnostic applications of ExMVs.
Acknowledgments
This work was supported by NIH grants 2R01 DK074720-10 and R01HL112788 and the Stella and Henry Endowment to MZR.
The authors apologize that, because of space limitations, it was impossible to cite and discuss many excellent papers in the field of ExMVs.
Footnotes
Conflicts of interest
None to report.
References
- 1.Hopkin K. Extracellular vesicles garner interest from academia and biotech. Proc Natl Acad Sci USA. 2016;113:9126–28. doi: 10.1073/pnas.1611700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 1006;20:1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 3.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quesenberry PJ, Aliotta J, Deregibus MC, Camussi G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res Ther. 2015;6:153. doi: 10.1186/s13287-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts CT, Jr, Kurre P. Vesicle trafficking and RNA transfer add complexity and connectivity to cell-cell communication. Cancer Res. 2013;73:3200–5. doi: 10.1158/0008-5472.CAN-13-0265. [DOI] [PubMed] [Google Scholar]
- 6.Howard MA, Coghlan M, David R, Pfueller SL. Coagulation activities of plasma microparticles. Thromb Res. 1988;50:145–56. doi: 10.1016/0049-3848(88)90182-x. [DOI] [PubMed] [Google Scholar]
- 7.Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30:450–59. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 8.Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–49. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 10.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, Puente N, Quesenberry P. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25:2245–56. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–8. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 12.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–88. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 26:1166–73. doi: 10.1038/leu.2011.389. 201. [DOI] [PubMed] [Google Scholar]
- 17.Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–37. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell Mol Bioeng. 2016;9:315–24. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angulski AB, Capriglione LG, Batista M, Marcon BH, Senegaglia AC, Stimamiglio MA, Correa A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133+ and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why both Sources are Advantageous for Regenerative Medicine. Stem Cell Rev. 2017;13:244–257. doi: 10.1007/s12015-016-9715-z. [DOI] [PubMed] [Google Scholar]
- 20.Prathipati P, Nandi SS, Mishra PK. Stem Cell-Derived Exosomes, Autophagy, Extracellular Matrix Turnover, and miRNAs in Cardiac Regeneration during Stem Cell Therapy. Stem Cell Rev. 2017;13:79–91. doi: 10.1007/s12015-016-9696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem Cell Rev. 2017;13:226–243. doi: 10.1007/s12015-016-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 23.Gilani SI, Weissgerber TL, Garovic VD, Jayachandran M. Preeclampsia and Extracellular Vesicles. Curr Hypertens Rep. 2016 Sep;18(9):68. doi: 10.1007/s11906-016-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125:1595–603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–9. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nature Med. 2000;6:769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 27.Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak MZ. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]


