Abstract
In this work, we measured the effect of cytochrome c on the NADH-dependent superoxide anion production by synaptic plasma membrane vesicles from rat brain. In these membranes, the cytochrome c stimulated NADH-dependent superoxide anion production was inhibited by antibodies against cytochrome b5 reductase linking the production to this enzyme. Measurement of the superoxide anion radical generated by purified recombinant soluble and membrane cytochrome b5 reductase corroborates the production of the radical by different enzyme isoforms. In the presence of cytochrome c, a burst of superoxide anion as well as the reduction of cytochrome c by cytochrome b5 reductase was measured. Complex formation between both proteins suggests that cytochrome b5 reductase is one of the major partners of cytochrome c upon its release from mitochondria to the cytosol during apoptosis. Superoxide anion production and cytochrome c reduction are the consequences of the stimulated NADH consumption by cytochrome b5 reductase upon complex formation with cytochrome c and suggest a major role of this enzyme as an anti-apoptotic protein during cell death.
Abbreviations: Cb5R, Cytochrome b5 reductase; DTPA, Diethylenetriaminepentaacetic acid; DHE, Dihydroethidium; E+, Ethidium; FAD, Flavin adenine dinucleotide; NADH, Reduced nicotinamide adenine dinucleotide; NBT, Nitroblue tetrazolium nitroblue tetrazolium; SPMV, Synaptic plasma membrane vesicles; TB, Terrific Broth terrific Broth; SOD, Superoxide dismutase; XA, Xanthine xanthine; XO, Xanthine oxidase
Keywords: Cytochrome c, Superoxide anion, NADH oxidase, Cytochrome b5 reductase, Neurons
Highlights
-
•
Cyt c stimulates the NADH-dependent O2·- production by SPMV.
-
•
Antibodies against Cb5R inhibit the Cyt c-stimulated O2·- production by SPMV.
-
•
The O2·- production by purified Cb5R was assessed through biochemical methods.
-
•
Oxidized Cyt c stimulates the O2·- production by Cb5R upon complex formation.
-
•
Reduced Cyt c and O2·- are the products of the NADH-dependent activities of Cb5R.
1. Introduction
The plasma membrane NADH oxidase activity of cerebellar granule neurons represents a disguisable activity producing superoxide anion (O2·-) as a collateral product of NADH consumption [1], [2], [3], [4]. The plasma membrane constituents associated to this activity are not well defined although it is known that cytochrome b5 reductase (Cb5R) is one of its major components present at the plasma membrane of rat cerebellar granule neurons in culture and of synaptic plasma membrane vesicles (SPMV) from rat brain [1]. This protein increases its association to lipids rafts in apoptosis [2]. In addition, 1–3 h after apoptosis induction an increment of O2·- has been detected at the peripheral neuronal plasma membrane [2]. This event correlates with the observed times for cytochrome c (Cyt c) release from mitochondria to the cytosol, as soon as 1 h after apoptosis induction, although the maximum peak for its release was found at 3 h [2].
In this work, we described the function of Cyt c as activator of the O2·- production by Cb5R, as a component of SPMV, and results were experimentally confirmed with two isoforms of human Cb5R. Due to the important role of Cyt c redox state in apoptosis and its reduction by Cb5R, we propose a function of Cb5R, as one the main defensive components during apoptosis after Cyt c release from mitochondria to the cytosol.
2. Materials and methods
2.1. SPMV preparation
Rat brain SPMV were prepared using a standard procedure as described in [1], [3].
2.2. Human Cb5R isoforms cloning
Cloning of Cb5R isoforms was performed as indicated in [5] using commercially available construct for soluble and primers described in Supplementary material.
2.3. Purification of recombinant human Cb5R isoforms
Clones of Cb5R isoforms were overexpressed in DE3 competent cells (Rosetta Gammi 2, Novagen) and the recombinant protein purified as indicated in [5].
2.4. NADH oxidase activity
NADH oxidase was measured at 37 °C as in [1], [3], [4], [6], [7].
2.5. O2 consumption
O2 consumption was measured using an Oxygraph Plus DW1 (Hansatech instruments) electrode in the same buffer described above, in presence of NADH (50 μM) and purified human recombinant Cb5R isoforms at 37 °C.
2.6. O2·- measurement with NBT
O2·- production by Cb5R was calculated measuring the reduction of NBT in the same buffer described above at pH 7.0, with NBT 200 μM and SOD 1 U/mL at 560 nm at 37 °C using a ε of 27.8 mM−1 cm−1 [8], [9].
2.7. Cyclic voltammetry
Qualitative measurement of the O2·- generated by Cb5R was performed by cyclic voltammetry with a pyrolytic graphite electrode using the thin layer technique (membrane cut off 3.5 kDa) [5]. Cb5R (0.6 mM) or albumins (0.6 mM) as a control were loaded onto the electrode. The set up was completed with a silver/silver chloride (Ag/AgCl) reference electrode and a platinum counter electrode to complete the three electrodes cell configuration.
2.8. O2·- measurement with DHE
O2·- formation was measured by fluorescence using dihydroethidium (DHE) [10]. Measurements were performed at 37 °C in buffer (pH 7.0) potassium phosphate 20 mM, DTPA 0.1 mM, and DHE 2 μM and Cyt c at the concentration indicated in each experiment, using a quartz cuvette. Fluorescence of DHE was measured with 470 nm and 605 nm excitation and emission wavelengths, respectively, and slits of 10 nm. Xanthine/Xanthine oxidase (XA/XO) was used to calibrate the signal.
2.9. Cb5R:Cyt c complex formation
Complex formation was measured at 37 °C as indicated in [5].
3. Results
3.1. O2·- production by SPMV NADH oxidase activity is stimulated by Cyt c
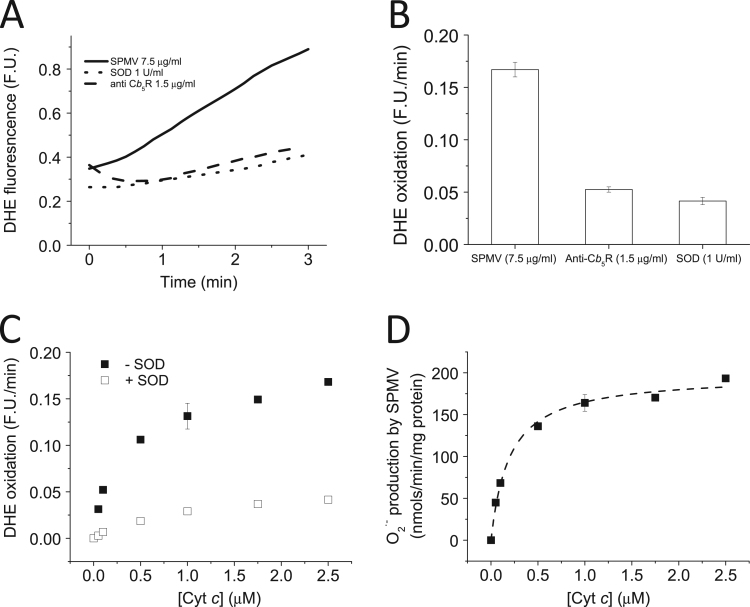
We measured the effect of oxidized Cyt c (Fe3+) on the NADH-dependent O2·- production by SPMV with DHE. Addition of Cyt c (2.5 μM) to the assay produced more than 3-fold increase in the oxidation of DHE, in the presence of SPMV (7.5 μg/mL) and NADH (50 μM) (Fig. 1A, continuous line and B). In addition, SOD added to the assay blocked the Cyt c stimulated DHE oxidation rate by SPMV (Fig. 1A, dotted line and B), pointing out that the increased DHE oxidation rate was due to production of O2·-, as expected for a O2·- responsive dye [11]. The effect of a specific antibody against Cb5R (ProteinTech, Cat #4668234) in this assay was also tested (Fig. 1A, dashed line and B). The O2·- production by SPMV was almost completely inhibited, i.e. ≥ 90 % inhibition, in the presence of the specific antibody against Cb5R. We measured the DHE oxidation rate dependence upon Cyt c (Fe3+) concentration, in the absence (filled squares) and presence of SOD (1 U/mL) (open squares) (Fig. 1C). Addition of increasing concentrations of Cyt c to the assay produced a Cyt c dependent increase of the DHE oxidation rate. Calibration curves for O2·- production vs. DHE oxidation were generated using increasing XO concentrations (Supplementary Fig. S1). Thereafter, we calculated that Cyt c was stimulating the NADH-dependent O2·- production by SPMV almost 20-fold, reaching a maximum value of 192 ± 41 nmoles/min/mg protein, in comparison to the activity measured in absence of Cyt c (10 nmoles/min/mg protein) (Fig. 1D). The NADH dependent O2·- production dependence upon Cyt c concentration yielded a Km for Cyt c stimulation of 0.2 ± 0.03 μM.
Fig. 1.
Cytcstimulated NADH-dependent O2·-production by SPMV. Panel A : Kinetics of the NADH dependent DHE oxidation by SPMV measured by fluorescence in the absence and presence of SOD (1 U/mL) and anti- Cb5R antibody (1.5 μg/mL). DHE oxidation was measured at 37 °C in potassium phosphate 20 mM plus DTPA 0.1 mM (pH 7.0), using a Perkin Elmer spectrofluorimeter with 470 nm and 605 nm excitation and emission wavelengths, respectively, and 10 nm excitation and emission slits. Representative traces of DHE oxidation by SPMV (7.5 μg/mL) in the presence of NADH (50 μM), oxidized Cyt c (Fe3+) (2.5 μM) and DHE (2 μM),in the presence of 1.5 μg/mL anti-Cb5R (dashed line) or 1 U/mL SOD (dotted line) are shown. Panel B: Quantification of the inhibition induced by anti-Cb5R (1.5 μg/mL) and SOD (1 U/mL) on the DHE oxidation rate by SPMV (7.5 μg/mL) in the presence of NADH (50 μM) and oxidized Cyt c (Fe3+) (2.5 μM). Panel C: Dependence of the NADH-dependent DHE oxidation rate by SPMV (7.5 μg/mL) upon Cyt c concentration in the absence (filled squares) or in the presence of SOD (1 U/mL) (open squares). Panel D: NADH dependent O2·- production by SPMV (7.5 μg/mL) dependence upon Cyt c concentration, measured with DHE. All the results shown in this Figure are the average (± standard errors) of experiments done by triplicate.
3.2. Measurement of the O2·- production by recombinant Cb5R isoforms
3.2.1. O2·- production by Cb5R
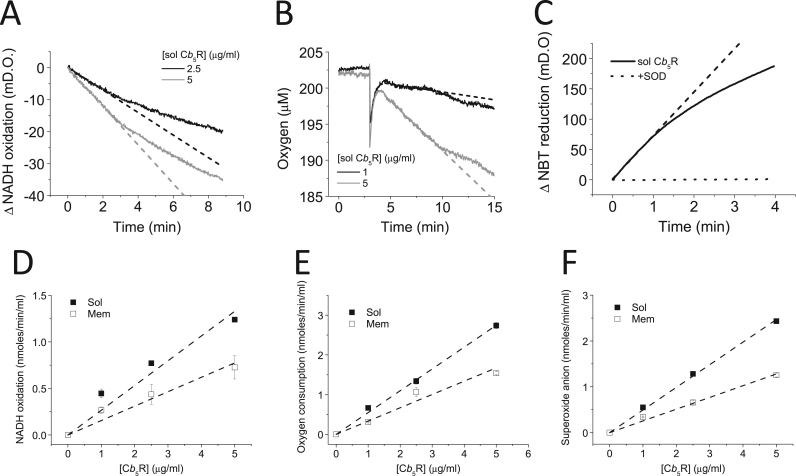
The oxidation of NADH by soluble and membrane purified Cb5R isoforms (Fig. 2 and Supp. Fig. S2A, respectively) was linearly dependent upon protein concentration (Fig. 2D). The calculated NADH oxidase activity of soluble and membrane Cb5R was 0.27 ± 0.02 and 0.15 ± 0.02 μmoles/min/mg of protein, respectively. Under the same experimental conditions the kinetics of O2 consumption, in the presence of NADH, by the soluble and membrane isoform of Cb5R (Fig. 2B and Supp. Fig. S2B, respectively) yielded an O2 consumption rate of 0.54 ± 0.02 and 0.33 ± 0.04 μmoles/min /mg protein for soluble and membrane Cb5R respectively, calculated from the linear regression plot obtained with increasing enzyme concentrations (Fig. 2E). O2·- was measured from the SOD-inhibited NBT reduction (Fig. 2C and Supp. Fig. S2C), yielding a rate of O2·- production by soluble and membrane Cb5R of 0.49 ± 0.02 and 0.26 ± 0.02 μmoles/min /mg of protein, respectively, from the slope of the linear dependence with Cb5R concentration (Fig. 2F). Thus, these results yielded a stoichiometry of ≈ 2 molecules of O2·- generated per molecule of oxidized NADH and a good coherence for the results obtained with these three methods.
Fig. 2.
Correlation between NADH oxidation, O2consumption and superoxide anion production by soluble Cb5R. Panel A : Representative traces of the NADH oxidation by soluble Cb5R (2.5 and 5 μg/mL) are shown. NADH oxidase activity was measured from absorbance decay at 340 nm at 37 °C, in the following assay medium (pH 7.0): potassium phosphate 20 mM, DTPA 0.1 mM, NADH 100 μM in presence of soluble Cb5R 2.5 (black line) and 5 μg/mL (grey line). Dotted lines indicate the slopes used to calculate the activity. Panel B: Oxygen consumption kinetics for soluble Cb5R 1 μg/mL (black line) 5 μg/mL (grey line). The reaction was started by addition of Cb5R at the time marked by a large drop of trace signals. O2 consumption was measured in presence of soluble Cb5R using a Oxygraph Plus DW1 (Hansatech Instruments) electrode, filled with 2 mL of the assay medium indicated in the Panel A. Dotted lines indicate the slopes used to calculate the activity. Panel C: O2·- production by soluble Cb5R was measured with NBT. Representative traces for the kinetics of NBT reduction by soluble Cb5R isoform, and sensitivity to SOD is shown. NBT reduction was measured at 37 °C at 560 nm with soluble Cb5R 2.5 μg/mL in absence (black line) or presence of SOD 1 U/mL (dotted line) in the assay medium indicated in the Panel A, supplemented with NBT 200 μM. The dotted line indicates the slope used to calculate the activity. Panels D, E and F: Dependence upon Cb5R concentration for soluble (filled squares) and membrane (open squares) Cb5R, respectively, of NADH oxidation, oxygen consumption and superoxide production. All the results shown in this Figure are the average (± standard errors) of triplicate experiments.
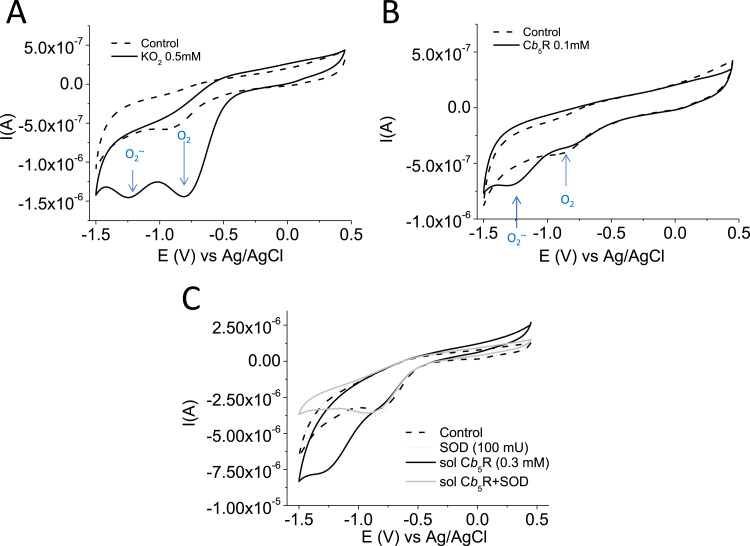
Cyclic voltammetry can be used for experimental assessment of O2·- production [12], [13], [14], [15], [16]. On these grounds, we have used this technique to further confirm O2·- production by Cb5R. The measurement of this radical was first calibrated using KO2 as a model compound. Two peaks appeared dependent on two generated components over the control: one at 1.25 V and another one at 0.8 V that were assigned to O2·- and O2, respectively (Fig. 3A). In presence of Cb5R (panel B), a signal similar to the observed for O2·- (using KO2) appears, at the same potential, and the O2 signal decreased correlating with O2 consumption by the enzyme to generate O2·-. In presence of SOD, the O2·- measured signal generated by Cb5R was equal to control (panel 3C).
Fig. 3.
Qualitative measurement of the O2·-production by soluble Cb5R using cyclic voltammetry. Representative voltammograms of KO2 added to the buffer (continuous line) vs its absence (dashed line) measured using thin layer technique with a membrane (cut off 3.5 kDa) with control/albumin is shown in panel A. Electrolyte: 20 mM Phosphate buffer / 0.1 M KCl pH 7.0 in presence of atmospheric O2. Scan rate: 5 mV/s. Proteins concentration: 0.1 mM. Layer thickness approx. 18 mm. Panel B: Typical voltammograms of Cb5R (continuous line) vs. the control/albumin (dashed line) using thin layer technique with a membrane (Cutoff 3.5 kDa), recorded under the same conditions of Panel A. Panel C: Effect of SOD addition over the O2·- production by soluble Cb5R. Representative voltammograms in the presence of SOD 0.1 U/mL (dotted line) vs. control/albumin (dashed line) are compared to the ones obtained for soluble Cb5R (0.1 mM) (black line) vs. soluble Cb5R (0.1 mM) in presence of SOD (0.1 U/mL) (grey line). Conditions were the same used in previous panels. All the results shown in this Figure are representative of triplicate experiments.
3.2.2. Cyt c stimulated O2·- production by Cb5R
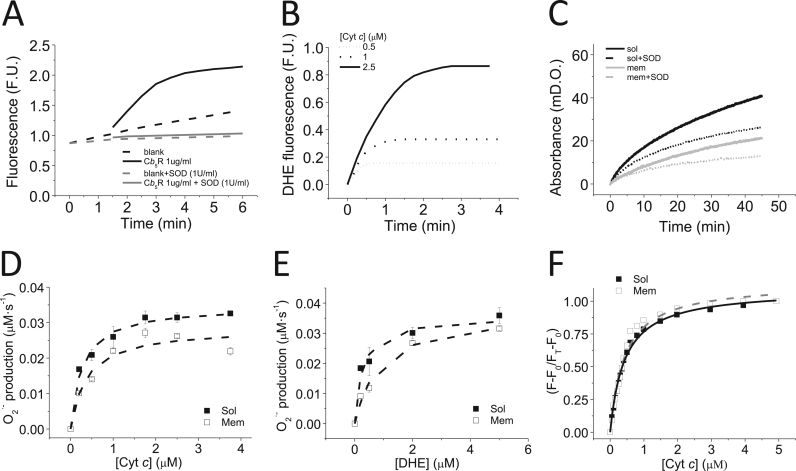
The NADH-dependent DHE oxidation rate by purified Cb5R was almost completely inhibited by the presence of SOD in the assay medium (Fig. 4A). The Cyt c stimulated O2·- production by Cb5R (1 mg/mL) was also reliably monitored with DHE (Fig. 4B) by the dependence upon Cyt c (Fe3+) of the initial DHE oxidation rate. As Cyt c reduction has also been used as an indicator to monitor O2·- production [17], [18], we have experimentally assessed whether the SOD inhibited reduction of Cyt c can reliably monitor the NADH-dependent O2·- production by purified Cb5R. The kinetics of Cyt c reduction by Cb5R in absence (continuous line) and presence of SOD (dashed line) is shown in Fig. 4C. These results showed that SOD (1 U/mL) inhibits by 40-45 % the reduction of Cyt c upon incubation in the assay for 45 min, and about the same reduction of the initial rate of reduction up to 5–10 min. This result is in contrast with the almost complete inhibition by SOD (1 U/ml)of the Cyt c stimulated DHE oxidation by Cb5R, and pointed out that the reduction of Cyt c was the sum of two different kinetic processes: (1) direct reduction by Cb5R which can use Cyt c as a final electron acceptor, and (2) reduction of Cyt c by the O2·- released by Cb5R.
Fig. 4.
DHE oxidation by Cb5R is almost completely inhibited by SOD and Cytcstimulated O2·-production by Cb5R. Panel A: Kinetics of DHE oxidation by soluble Cb5R (continuous line) vs. blank (dashed line) in absence (black line) and presence of 1 U/mL SOD (grey line). DHE oxidation was measured at 37 °C in the following assay medium (pH 7.0): potassium phosphate 20 mM, DTPA 0.1 mM, NADH 50 μM and 2 μM DHE, with a fixed reductase concentration (1 μg/mL or 27.6 nM). Excitation and emission wavelengths 470 nm and 605 nm, respectively, and 10 nm excitation and emission slits. Panel B: Kinetics of DHE oxidation by soluble Cb5R sensitive to SOD (1 U/mL), in the presence of increasing Cyt c concentrations. DHE oxidation was measured as indicated above, in the presence of the Cyt c concentrations listed in the figure. The traces shown are averages of experimental triplicates. Panel C: Representative traces for the kinetics of Cyt c reduction by soluble (black line) and membrane Cb5R (grey line) with Cyt c (3.75 μM), in the absence (continuous lines) and presence (dashed lines) of SOD 1 U/mL. Cyt c reduction was measured at 550 nm at 37 °C in the following assay medium (pH 7.0): potassium phosphate 20 mM, DTPA 0.1 mM, Cyt c 3.75 μM and NADH 100 μM, with soluble or membrane Cb5R (0.1 μg/mL). Panel D: The O2·- production rate by soluble (filled squares) and membrane (open squares) Cb5R dependence upon Cyt c is shown. The DHE oxidation rate was measured as indicated in the Panel A, with a fixed Cb5R (1 μg/mL or 27.6 nM)) and DHE concentration (2 μM) in the assay medium. Panel E: The O2·- production by soluble (filled squares) and membrane (open squares) Cb5R isoforms dependence upon DHE concentration is shown. The DHE oxidation rate was measured as indicated in the Panel A, with a fixed Cb5R concentrations(1 μg/mL or 27.6 nM) and Cyt c (2.5 μM). Panel F: Soluble (filled squares) and membrane Cb5R (open squares) flavin autofluorescence dependence upon Cyt c concentration. Cyt c elicits a large increase of Cb5R-flavin autofluorescence that allows measuring Cb5R: Cyt c complex formation by fluorescence. Y-axis: molar fraction of Cb5R saturated with Cyt c, which has been calculated as follows: ΔF/ΔFmax = (F-F0)/(Fmax-F0), where F is the fluorescence intensity at each Cyt c concentration, and F0 and Fmax are the fluorescence intensity in the absence and saturating concentrations of Cyt c, respectively. Fluorescence measurements were performed at 37 °C in the following buffered solution (pH 7.0): 20 mM potassium phosphate, 1 mM EDTA, and 2 μM Cb5R. Excitation and emission wavelengths 470 nm and 520 nm, respectively, with excitation and emission slits of 10 nm. The averages (± standard errors) of triplicate experiments are shown. Solid and dashed lines are the best non-linear squares fit to the one-binding site equation (F-F0)/(FT -F0) = [Cyt c]/(Kd+[Cyt c]), and yielded values of R2 = 0.99 and 0.98, respectively, for soluble (black line) and membrane (grey line) Cb5R isoforms.
Therefore, for a proper kinetic analysis of O2·- production we measured the dependence of the DHE oxidation rate upon Cyt c and DHE concentration, using a fixed Cb5R concentration and a fixed concentration of one of the twosubstrates for O2·- detection, as indicated in the Supp. material. The data were fit to a two substrate Michaelis-Menten kinetic model (Fig. 4 D and E). To calculate the O2·- production, we calibrated the oxidation of DHE by XA/XO (Supp. Fig. 1B and C). From titration results with different Cyt c concentrations and fixed DHE (2 μM) and Cb5R (1 μg/mL) concentration, we calculated a kcat for O2·- production by soluble and membrane Cb5R of 1.37 ± 0.02 and 1.17 ± 0.02 s−1, with a Km for Cyt c of 0.29 ± 0.01 and 0.42 ± 0.02 μM, respectively (Fig. 4D). From titration with different DHE concentrations and fixed Cyt c (2.5 μM) and Cb5R (1 μg/mL) concentrations, we calculated a kcat for O2·- production by soluble and membrane Cb5R of 1.45 ± 0.11 and 1.49 ± 0.03 s−1 and a Km for DHE of 0.19 ± 0.01 and 0.25 ± 0.04 μM, respectively (Fig. 4E).
3.3. Measurement of Cb5R and Cyt c dissociation constant
The results shown above pointed out that Cyt c behaves as a redox partner of Cb5R, opening the possibility to use flavin autofluorescence of Cb5R to measure the interaction between these two proteins, see e.g. [5].Fig. 4F shows Cb5R flavin autofluorescence intensity dependence upon Cyt c concentration, yielding a large increase of the fluorescence intensity, i.e. between 60 % and 300 % for soluble Cb5R and for membrane Cb5R, at saturating concentration of Cyt c (5 μM). These results revealed that Cyt c interaction with Cb5R can be appropriately monitored by Cb5R flavin autofluorescence. The data can be fit to a hyperbolic curve as indicated in the Material and Methods section, yielding a dissociation constant of the Cyt c/Cb5R complex of 0.40 ± 0.05 and 0.38 ± 0.02 μM for soluble and membrane Cb5R, respectively. Scatchard plot analysis (Supp. Fig. S3) is consistent with the binding of one Cyt c molecule per Cb5R molecule for soluble and membrane isoforms.
4. Discussion
A scheme or the reactions described in this manuscript is shown in Supp. Fig. S4. Our data demonstrate that Cb5R can use O2 as an electron acceptor using NADH as substrate. Although the use of DHE formeasurement of O2·- has been stated to be useful for qualitative purposes in biological systems [19], we achieved to quantify O2·- production by purified Cb5R with DHE, using proper controls (i.e. calibrating the signal with XA/XO in the presence of a large amount of catalase that avoid E+ formation when H2O2 is also produced) as shown in other reports [10], [11], [20]. Stoichiometric ratios between NADH and O2 consumption indicated that Cb5R uses one NADH molecule to reduce two O2 molecules. Moreover, the values obtained for O2·- production correlated with O2 consumption, indicating that the O2 consumption is mainly due to O2·- production (Table 1). With the use of an anti-Cb5R antibody, we confirmed that Cb5R was responsible of the Cyt c (Fe3+) stimulated production by SPMV, since 90 % of the O2·- production was blocked by addition of specific antibodies against Cb5R, added to the assay. As cytochrome P450s also display NAD(P)H-dependent production of O2.- [21] and some cytochrome P450 isoforms are associated the plasma membrane [22], it is likely that cytochrome P450s account for most of the Cb5R-independent O2.- production [22], [23], although we cannot discard other O2.- sources. Our results also show that Cyt c binds to purified Cb5R isoforms with dissociation constants similar to the Km values for the Cyt c stimulated O2·- production by Cb5R isoforms and close to the Km value obtained from the NADH-dependent production of O2·- by SPMV.
Table 1.
O2·- production by human Cb5R.
| Soluble Cb5R | Membrane Cb5R | |
|---|---|---|
| (μmoles/min /mg protein) | (μmoles/min /mg protein) | |
| NADH oxidase | 0.27 ± 0.02 | 0.15 ± 0.02 |
| O2 consumption | 0.54 ± 0.02 | 0.33 ± 0.04 |
| O2·- production (SOD-inhibited NBT reductase) | 0.49 ± 0.02 | 0.26 ± 0.02 |
| Cyt c stimulated O2·- production (DHE) | 4.1 ± 0.2a | 3.5 ± 0.3a |
| Cyt c stimulated O2·- production (DHE) | 4.4 ± 0.1b | 3.8 ± 0.4b |
Values calculated by fitting the data obtained with DHE to one substrate Michaelis-Menten kinetics.
Values calculated by fitting the data obtained with DHE to two substrates Michaelis-Menten kinetics.
In the context of apoptosis, the function of Cyt c reduction by Cb5R can be seen as part of the cellular defense system, because this protein shows a widespread subcellular membrane localization, namely, endoplasmic reticulum, outer mitochondrial membrane and plasma membrane [24]. Cyt c reduction blocks apoptosis since its role in this type of cell death has been mainly attributed to the oxidized form [25], [26], [27]. For this reason, systems with ability to reduce Cyt c have an intrinsic anti-apoptotic function. Noteworthy, the payback for this reduction exerted by Cb5R is the formation of O2·-, a radical also described to be formed in mitochondria upon Cyt c release [28].
Acknowledgments
This work was supported by the Unidade de Ciências Biomoleculares Aplicadas-UCIBIO, which is financed by national funds from FCT/MEC (UID/Multi/04378/2013) and co-financed by the ERDF under the PT2020 Partnership Agreement (POCI-01–0145-FEDER-007728). Experimental work was also partially supported by funding from Grant BFU2014-53641-P of the Spanish Ministerio de Economía y Competitividad and Ayuda a Grupos de la Junta de Extremadura (GR15139 to Group BBB008) co-financed by the European Funds for Structural Development (FEDER). AKSA and SF thank FCT/MCTES for the post-doctoral and pre-doctoral fellowship grants (SFRH/BPD/100069/2014 and SFRH/BD/84543/2012, respectively), which are financed by national funds and co-financed by FSE. We would like to thank Susana Ramos for her initial assistance in the preparation of the membrane isoform of Cb5R.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.11.021.
Contributor Information
Alejandro K. Samhan-Arias, Email: alejandro.samhan@fct.unl.pt.
Carlos Gutierrez-Merino, Email: carlosgm@unex.es.
Appendix A. Supplementary material
Supplementary material Fig. S1. Calibration curves for O2·-production vs DHE oxidation. Panel A: The DHE oxidation rate at 37 °C was calibrated with XA (0.4 mM) with increasing concentrations of XO (18, 36, and 52 mU/mL) in the following assay medium (pH 7.0): potassium phosphate 20 mM, DTPA 0.1 mM, pluscatalase (3 U/mL) and NADH 50 μM. Panel B: XA/XO (same concentrations used above) was used to calibrate the O2·- production using the SOD-sensitive Cyt c reduction assay under the same conditions of Panel A, in the absence and presence of 1 U/mL of SOD. Panel C: Calibration curve for DHE oxidation rates in the presence of XA (0.5 mM) at fixed XO concentrations (16, 36 and 52 mU/mL of XO) vs superoxide anion production measured by the reduction of Cyt c inhibited by SOD at the same XA/XO concentrations. All the results shown in this Figure are the average (± standard errors) of triplicate experiments. Dashed lines indicate the best linear regression fits, R2 0.99 in all cases. Fig. S2. Correlation between NADH, oxygen consumption and superoxide anion production by membrane Cb5R. Panel A: Representative traces for NADH oxidation by membrane Cb5R measured at 340 nm and 37 °C, in the following assay medium (pH 7.0) potassium phosphate 20 mM, DTPA 0.1 mM and NADH 100 μM, in presence of membrane Cb5R 2.5 (black line) and 5 μg/mL (grey line). Dashed lines are the slopes used to calculate the NADH oxidase activity. Panel B: O2 consumption kinetics at 37 °C for membrane Cb5R 1 μg/mL (black line) 5 μg/mL (grey line) measured with an Oxygraph Plus DW1 (Hansatech Instruments) electrode, filled with 2 mL of the assay medium indicated in Panel A. The large drop of the traces monitors the time at which Cb5R was added and dashed lines are the slopes used to calculate the O2 consumption activity. Panel C: O2·- production by membrane Cb5R measured with NBT. Representative traces for the kinetics of NBT reduction (wavelength 560 nm) by 2.5 μg/mL of the membrane Cb5R isoform at 37 °C in the assay medium, indicated in Panel A, supplemented with NBT 200 μM, in absence (black line) or presence (dotted line) of SOD 1 U/mL. The dashed line indicates the slope used to calculate the initial NBT reduction rate. All the results shown in this Figure are representative of triplicate experiments. Fig. S3. Determination of the stoichiometry of the human Cb5R: Cb5complex. The Cb5R-flavin fluorescence was titrated with Cyt c at different soluble (Panel A) and membrane (Panel B) Cb5R concentrations at 37 °C in potassium phosphate 20 mM plus DTPA 0.1 mM buffer (pH 7.0). Cb5R concentrations: 1 μM (square), 2 μM (circle) and 5 μM (up triangle), for soluble (filled symbols) and membrane isoforms (open symbols). Fluorescence intensity was measured with 470 nm and 520 nm excitation and emission wavelengths, respectively, and 10 nm excitation and emission slits. Panel C: The fluorescence intensity increase (F-F0) of soluble and membrane Cb5R autofluorescence dependence upon Cyt c concentration (symbols indicate the same Cb5R concentrations used in Panels A and B). All curves for Cb5R autofluorescence dependence upon Cyt c fit well to an hyperbolic curve (dashed line), with non-statistically significant differences in the half saturation value, pointing out that the binding affinity was not significantly altered in the Cb5R concentration range used in these assays. Panel D: Scatchard-like plot of the data obtained in the Cb5R-flavin fluorescence titration with Cyt c assuming a 1:1 stoichiometric ratio of the complex Cb5R:Cb5. Bound and free [Cyt c] have been calculated as follows: [Cyt c]bound = molar fraction of Cb5R saturated with Cyt c x [Cyt c]total, and [Cyt c]free = [Cyt c]total -[Cyt c]bound. The molar fraction of Cb5R saturated with Cyt c has been calculated as follows ΔF/ΔFmax = (F-F0)/(Fmax-F0), where F is the fluorescence intensity at each Cyt c concentration, and F0 and Fmax are the fluorescence intensity in the absence and saturating concentrations of Cyt c, respectively. The data lead to a value of 1.0 ± 0.07 for the intercept with the X-axis, meaning 1 Cb5 binding site per Cb5R molecule. The average of the plots obtained at different Cb5R concentrations is shown as a continuous line and SD error is shown as dashed lines. Fig. S4. O2·-production by Cb5R (panel A) and its stimulation by Cytc(panel B) upon complex formation with Cytc. Panel A shows the production of O2·- (red) by Cb5R (yellow backbone) (PDB file: 1UMK, is the surface representation of the crystal model for erythrocyte NADH-Cb5R) using NADH and O2 as substrates (in black). Panel B shows the two observed pathways for Cyt c reduction (pink backbone) by Cb5R upon complex formation with oxidized Cyt c (Fe3+) (dark red backbone). Cyt c can be reduced through electron transfer from Cb5R or through the stimulated superoxide anion production generated upon formation of the complex between Cb5R and Cyt c. In both cases NADH is used as a substrate. Arrow thickness indicates the stimulation of activities.
Supplementary material
References
- 1.Samhan-Arias A.K., Garcia-Bereguiain M.A., Martin-Romero F.J., Gutierrez-Merino C. Clustering of plasma membrane-bound cytochrome b5 reductase within 'lipid raft' microdomains of the neuronal plasma membrane. Mol. Cell. Neurosci. 2009;40:14–26. doi: 10.1016/j.mcn.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Samhan-Arias A.K., Marques-da-Silva D., Yanamala N., Gutierrez-Merino C. Stimulation and clustering of cytochrome b5 reductase in caveolin-rich lipid microdomains is an early event in oxidative stress-mediated apoptosis of cerebellar granule neurons. J. Proteom. 2012;75:2934–2949. doi: 10.1016/j.jprot.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Samhan-Arias A.K., Martin-Romero F.J., Gutierrez-Merino C. Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free. Radic. Biol. Med. 2004;37:48–61. doi: 10.1016/j.freeradbiomed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Samhan-Arias A.K., Gutierrez-Merino C. Purified NADH-cytochrome b5 reductase is a novel superoxide anion source inhibited by apocynin: sensitivity to nitric oxide and peroxynitrite. Free. Radic. Biol. Med. 2014;73:174–189. doi: 10.1016/j.freeradbiomed.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Samhan Arias A.K., Almeida R.M., Ramos S., Cordas C.M., Moura I., Gutierrez-Merino C., Moura J.J.G. Topography of human cytochrome b5/cytochrome b5 reductase interacting domain and redox alterations upon complex formation. BBA Bioenerg. 2017;1859:78–87. doi: 10.1016/j.bbabio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Samhan-Arias A.K., Duarte R.O., Martin-Romero F.J., Moura J.J., Gutierrez-Merino C. Reduction of ascorbate free radical by the plasma membrane of synaptic terminals from rat brain. Arch. Biochem. Biophys. 2008;469:243–254. doi: 10.1016/j.abb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Samhan-Arias A.K., Garcia-Bereguiain M.A., Gutierrez-Merino C. Hydrogen sulfide is a reversible inhibitor of the NADH oxidase activity of synaptic plasma membranes. Biochem. Biophys. Res. Commun. 2009;388:718–722. doi: 10.1016/j.bbrc.2009.08.076. [DOI] [PubMed] [Google Scholar]
- 8.Bielski B.H.J., Shiue G.G., Bajuk S. Reduction of nitro blue tetrazolium by CO2- and O2- radicals. J. Phys. Chem. 1980;84:830–833. [Google Scholar]
- 9.Sirota T.V. Use of nitro blue tetrazolium in the reaction of adrenaline autooxidation for the determination of superoxide dismutase activity. Biochem. Suppl. Ser. B: Biomed. Chem. 2012;6:254–260. doi: 10.18097/pbmc20135904399. [DOI] [PubMed] [Google Scholar]
- 10.Zielonka J., Vasquez-Vivar J., Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 11.Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vasquez-Vivar J., Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free. Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 12.Hayyan M., Hashim M.A., AlNashef I.M. Superoxide ion: generation and chemical implications. Chem. Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 13.Costentin C., Robert M., Saveant J.M. Concerted proton-electron transfers: electrochemical and related approaches. Acc. Chem. Res. 2010;43:1019–1029. doi: 10.1021/ar9002812. [DOI] [PubMed] [Google Scholar]
- 14.Sawyer D.T., Sobkowiak A., Roberts J.L. 2nd ed. Wiley; New York: 1995. Electrochemistry for Chemists. [Google Scholar]
- 15.Araki T., Kitaoka H. The mechanism of reaction of ebselen with superoxide in aprotic solvents as examined by cyclic voltammetry and ESR. Chem. Pharm. Bull. 2001;49:541–545. doi: 10.1248/cpb.49.541. [DOI] [PubMed] [Google Scholar]
- 16.Hayyan M., Mjalli F.S., Hashim M.A., AlNashef I.M. Generation of superoxide ion in pyridinium, morpholinium, ammonium, and sulfonium-based ionic liquids and the application in the destruction of toxic chlorinated phenols. Ind. Eng. Chem. Res. 2012;51:10546–10556. [Google Scholar]
- 17.McCord J.M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J. Biol. Chem. 1970;245:1374–1377. [PubMed] [Google Scholar]
- 18.McCord J.M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 19.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free. Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Rogers S.C., Kavdia M. Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann. Biomed. Eng. 2013;41:327–337. doi: 10.1007/s10439-012-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkman J.B., Jansson I. The many roles of cytochromeb5. Pharmacol. Ther. 2003;97:139–152. doi: 10.1016/s0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- 22.Seliskar M., Rozman D. Mammalian cytochromes P450—Importance of tissue specificity. Biochim. Biophys. Acta (BBA) – General. Subj. 2007;1770:458–466. doi: 10.1016/j.bbagen.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Rezende F., Prior K.-K., Löwe O., Wittig I., Strecker V., Moll F., Helfinger V., Schnütgen F., Kurrle N., Wempe F., Walter M., Zukunft S., Luck B., Fleming I., Weissmann N., Brandes R.P., Schröder K. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic. Biol. Med. 2017;102:57–66. doi: 10.1016/j.freeradbiomed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Samhan-Arias A.K., López-Sánchez C., Marques-da-Silva D., Lagoa R., Garcia-Lopez V., García-Martínez V., Gutierrez-Merino C. J. Neurol. Neuromed. 2016;1:61–65. doi: 10.1007/s00429-015-1036-5. [DOI] [PubMed] [Google Scholar]
- 25.Lagoa R., Samhan-Arias A.K., Gutierrez-Merino C. Correlation between the potency of flavonoids for cytochrome c reduction and inhibition of cardiolipin-induced peroxidase activity. Biofactors. 2017;43:451–468. doi: 10.1002/biof.1357. [DOI] [PubMed] [Google Scholar]
- 26.Borutaite V., Brown G.C. Mitochondrial regulation of caspase activation by cytochrome oxidase and tetramethylphenylenediamine via cytosolic cytochrome c redox state. J. Biol. Chem. 2007;282:31124–31130. doi: 10.1074/jbc.M700322200. [DOI] [PubMed] [Google Scholar]
- 27.Hampton M.B., Zhivotovsky B., Slater A.F., Burgess D.H., Orrenius S. Importance of the redox state of cytochrome c during caspase activation in cytosolic extracts. Biochem. J. 1998;329:95–99. doi: 10.1042/bj3290095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai J., Jones D.P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Fig. S1. Calibration curves for O2·-production vs DHE oxidation. Panel A: The DHE oxidation rate at 37 °C was calibrated with XA (0.4 mM) with increasing concentrations of XO (18, 36, and 52 mU/mL) in the following assay medium (pH 7.0): potassium phosphate 20 mM, DTPA 0.1 mM, pluscatalase (3 U/mL) and NADH 50 μM. Panel B: XA/XO (same concentrations used above) was used to calibrate the O2·- production using the SOD-sensitive Cyt c reduction assay under the same conditions of Panel A, in the absence and presence of 1 U/mL of SOD. Panel C: Calibration curve for DHE oxidation rates in the presence of XA (0.5 mM) at fixed XO concentrations (16, 36 and 52 mU/mL of XO) vs superoxide anion production measured by the reduction of Cyt c inhibited by SOD at the same XA/XO concentrations. All the results shown in this Figure are the average (± standard errors) of triplicate experiments. Dashed lines indicate the best linear regression fits, R2 0.99 in all cases. Fig. S2. Correlation between NADH, oxygen consumption and superoxide anion production by membrane Cb5R. Panel A: Representative traces for NADH oxidation by membrane Cb5R measured at 340 nm and 37 °C, in the following assay medium (pH 7.0) potassium phosphate 20 mM, DTPA 0.1 mM and NADH 100 μM, in presence of membrane Cb5R 2.5 (black line) and 5 μg/mL (grey line). Dashed lines are the slopes used to calculate the NADH oxidase activity. Panel B: O2 consumption kinetics at 37 °C for membrane Cb5R 1 μg/mL (black line) 5 μg/mL (grey line) measured with an Oxygraph Plus DW1 (Hansatech Instruments) electrode, filled with 2 mL of the assay medium indicated in Panel A. The large drop of the traces monitors the time at which Cb5R was added and dashed lines are the slopes used to calculate the O2 consumption activity. Panel C: O2·- production by membrane Cb5R measured with NBT. Representative traces for the kinetics of NBT reduction (wavelength 560 nm) by 2.5 μg/mL of the membrane Cb5R isoform at 37 °C in the assay medium, indicated in Panel A, supplemented with NBT 200 μM, in absence (black line) or presence (dotted line) of SOD 1 U/mL. The dashed line indicates the slope used to calculate the initial NBT reduction rate. All the results shown in this Figure are representative of triplicate experiments. Fig. S3. Determination of the stoichiometry of the human Cb5R: Cb5complex. The Cb5R-flavin fluorescence was titrated with Cyt c at different soluble (Panel A) and membrane (Panel B) Cb5R concentrations at 37 °C in potassium phosphate 20 mM plus DTPA 0.1 mM buffer (pH 7.0). Cb5R concentrations: 1 μM (square), 2 μM (circle) and 5 μM (up triangle), for soluble (filled symbols) and membrane isoforms (open symbols). Fluorescence intensity was measured with 470 nm and 520 nm excitation and emission wavelengths, respectively, and 10 nm excitation and emission slits. Panel C: The fluorescence intensity increase (F-F0) of soluble and membrane Cb5R autofluorescence dependence upon Cyt c concentration (symbols indicate the same Cb5R concentrations used in Panels A and B). All curves for Cb5R autofluorescence dependence upon Cyt c fit well to an hyperbolic curve (dashed line), with non-statistically significant differences in the half saturation value, pointing out that the binding affinity was not significantly altered in the Cb5R concentration range used in these assays. Panel D: Scatchard-like plot of the data obtained in the Cb5R-flavin fluorescence titration with Cyt c assuming a 1:1 stoichiometric ratio of the complex Cb5R:Cb5. Bound and free [Cyt c] have been calculated as follows: [Cyt c]bound = molar fraction of Cb5R saturated with Cyt c x [Cyt c]total, and [Cyt c]free = [Cyt c]total -[Cyt c]bound. The molar fraction of Cb5R saturated with Cyt c has been calculated as follows ΔF/ΔFmax = (F-F0)/(Fmax-F0), where F is the fluorescence intensity at each Cyt c concentration, and F0 and Fmax are the fluorescence intensity in the absence and saturating concentrations of Cyt c, respectively. The data lead to a value of 1.0 ± 0.07 for the intercept with the X-axis, meaning 1 Cb5 binding site per Cb5R molecule. The average of the plots obtained at different Cb5R concentrations is shown as a continuous line and SD error is shown as dashed lines. Fig. S4. O2·-production by Cb5R (panel A) and its stimulation by Cytc(panel B) upon complex formation with Cytc. Panel A shows the production of O2·- (red) by Cb5R (yellow backbone) (PDB file: 1UMK, is the surface representation of the crystal model for erythrocyte NADH-Cb5R) using NADH and O2 as substrates (in black). Panel B shows the two observed pathways for Cyt c reduction (pink backbone) by Cb5R upon complex formation with oxidized Cyt c (Fe3+) (dark red backbone). Cyt c can be reduced through electron transfer from Cb5R or through the stimulated superoxide anion production generated upon formation of the complex between Cb5R and Cyt c. In both cases NADH is used as a substrate. Arrow thickness indicates the stimulation of activities.
Supplementary material