Abstract
Dedicator of cytokinesis (DOCK) family are evolutionary conserved guanine nucleotide exchange factors (GEFs) for the Rho GTPases, Rac and Cdc42. DOCK3 functions as a GEF for Rac1, and plays an important role in promoting neurite and axonal growth by stimulating actin dynamics and microtubule assembly pathways in the central nervous system. Here we report a boy with developmental delay, hypotonia and ataxia due to biallelic DOCK3 deletion. Chromosomal single nucleotide polymorphism (SNP) microarray analysis detected a 170 kb homozygous deletion including exons 6–12 of the DOCK3 gene at 3p21.2. Symptoms of our proband resembles a phenotype of Dock3 knockout mice exhibiting sensorimotor impairments. Furthermore, our proband has clinical similarities with two siblings with compound heterozygous loss-of-function mutations of DOCK3 reported in Helbig et al. (2017). Biallelic DOCK3 mutations cause a neurodevelopmental disorder characterized by unsteady gait, hypotonia and developmental delay.
INTRODUCTION
Rho GTPases act as molecular switches that regulate many aspects of cellular functions including cell division, cell migration, cell cycle progression, control of the cytoskeleton, and membrane transport pathways (Schmidt and Hall 2002). Rho GTPases are directly activated by guanine nucleotide exchange factors (GEFs) in response to upstream molecular signaling. Members of dedicator of cytokinesis (DOCK) family are evolutionary conserved GEFs for the Rho GTPases, Rac and Cdc42. There are 11 members of DOCK proteins identified in mammals, characterized by the presence of DOCK homology region (DHR) 1 domain, which binds to phospholipids, and DHR2, which is responsible for GEF activity. DOCK proteins are classified into 4 subgroups based on their sequence homology and domain organization; DOCK-A (DOCK1, DOCK2, and DOCK5), DOCK-B (DOCK3 and DOCK4), DOCK-C (DOCK6, DOCK7 and DOCK8) and DOCK-D (DOCK9, DOCK10 and DOCK11). DOCK-A and DOCK-B proteins specifically activate Rac, and DOCK-D proteins target Cdc42. DOCK-C proteins can activate both Rac and Cdc42 (Gadea and Blangy 2014).
DOCK proteins are involved in a wide range of cellular signaling pathways downstream of membrane receptors. Therefore, DOCK proteins play essential roles in various cellular processes such as cell adhesion, cell migration and regulation of actin cytoskeleton. DOCK proteins are also shown to be key components in pathological processes such as cancer cell migration and invasion (Gadea and Blangy 2014).
To date, DOCK proteins including DOCK2, 6, 7 and 8 have been found as a molecular cause of human diseases related to defects in immune systems or neural development. Biallelic mutations in DOCK2 resulting in loss of function of the protein were found in patients with immunodeficiency (Dobbs et al., 2015). Adams-Oliver Syndrome 2 is caused by autosomal recessive mutations in DOCK6 (Shaheen et al., 2011; 2013; Sukalo et al., 2015). Biallelic loss-of function mutations in DOCK7 were reported as a cause of epileptic encephalopathy (Perrault et al., 2014). Autosomal recessive immunodeficiency due to loss of function mutations in the DOCK8 gene was first reported in Zhang et al. (2009), and since then, DOCK8-related immunodeficiency syndrome has been identified in several hundred patients (reviewed in Zhang et al., 2016).
Recently, Helbig et al. (2017) reported a familial case of biallelic loss-of-function DOCK3 mutations, which involve a paternally inherited 458 kb deletion and a maternally inherited non-sense variant in the gene, and they proposed that biallelic loss-of-function mutations of DOCK3 may cause a neurodevelopmental disorder characterized by developmental disability, hypotonia, and ataxic gait. Here we report a boy with developmental delay, hypotonia and ataxia due to biallelic DOCK3 deletion, further supporting the notion that biallelic DOCK3 mutations cause a neurodevelopmental disorder.
Clinical Report
Proband is a 28-month-old male with developmental delay and hypotonia. His prenatal history was unremarkable, although the information was limited, because he was adopted outside of his biological family. Global developmental delay was noted by 4 months of age. He started sitting at 14 months and walking independently by 22 months. He was not able to pick up small objects at 14–16 months. An emerging pincer grasp was noted at 22 months and remains inconsistent. He has mixed speech/language delays with better receptive than expressive speech. At his current age, he has a few specific words including "ma" and "yea". He can follow some simple commands. While eye contact has been appropriate, he was delayed in pointing and preferred to play alone. At 2 year and 2 months, the Bayley II Mental Scale was administered. He obtained a mental scale raw score of 98, giving him a mental developmental index of less than 50 (normal 100, standard deviation 15) with a level of function of 15 months. At the age of 32 months, his repeat Bayley II mental scale revealed a mental scale raw scare of 108 giving him a developmental level of 17 months.
His growth has been slow, and he has short stature. He has demonstrated cyanotic episodes, which are consistent with breathholding spells. Brain MRI was unremarkable except for slightly bulky and dysmorphic appearance of the corpus callosum. Cardiology evaluation including electrocardiogram and echocardiogram was unremarkable. The family history shows the proband to be one of 9 children to his parents. His family history was non-contributory except that his mother and father are first cousins. There is very limited information about the biological siblings as the patient is adopted. To our knowledge, there are no other siblings with similar features.
On physical exam at 28 months old, his height was 83.9 cm (5th percentile), weight was 11 kg (4th percentile), and head circumference was 47.5 cm (14th percentile). These measurements are consistent with his previous growth curves. His dysmorphic facial features include bilateral epicanthal folds, upturned nasal tip/anteverted nares, and prominent cheeks (Figure 1). He also had small joint laxity. His neurological exam is remarkable for hypotonia and wide spaced, unsteady gait.
Figure 1.
Facial profile of the proband at 2 years and 4 months. Note bilateral epicanthal folds, upturned nasal tip/anteverted nares, and prominent cheeks.
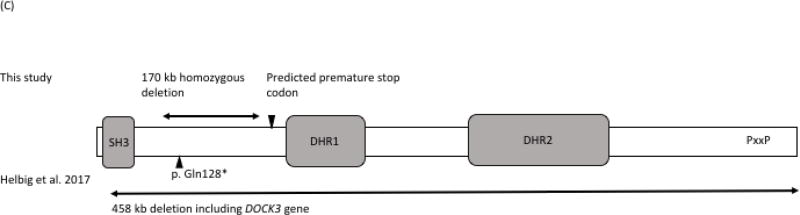
Chromosomal single nucleotide polymorphism (SNP) microarray analysis was carried out using the Illumina CytoSNP850Kv1.1 BeadChip. SNP microarray revealed 11 regions of homozygosity greater than 3 Mb in size, and it detected a 170 kb homozygous deletion including exons 6–12 of the DOCK3 gene at 3p21.2 (arr[hg19] 3p21.2(51,062,402-51,232,768)x0) (Figure 2). The deletion is more likely to result in a frameshift and a premature stop codon within exon 13, and the transcript is predicted to undergo nonsense mediated mRNA decay.
Figure 2.
SNP array demonstrating the homozygous deletion of DOCK3. (A) SNP array analysis revealed a 43.3 Mb region of homozygosity (ROH) on chromosome 3, and captured a 170 kb homozygous deletion within this ROH. Rectangular box demotes the ROH, and arrow indicates the homozygous deletion. (B) Close up view of the SNP array result. Rectangular box indicates the 170 kb homozygous deletion disrupting only DOCK3 gene. (C) Schmatic showing DOCK3 domain structure with mutations reported in this study and Helbig et al., (2017). Domains including SH3 (Src Homology 3), DHR (DOCK homology region) 1, DHR2 and proline-rich region (PxxP) are indicated. Double arrows indicate deletions and arrowheads indicate predicted stop codons. The 458 kb deletion reported in Helbig et al. begins in exon 2, continues through the 3’UTR, and ends in adjacent downstream genomic region.
DISCUSSION
Here we report a boy with developmental delay and hypotonia due to an intragenic homozygous DOCK3 deletion. A recently reported article proposed that biallelic loss-of-function variants in DOCK3 may lead to hypotonia, ataxia, and developmental delay (Helbig et al., 2017). This article reported two siblings with compound heterozygous loss-of-function mutations of DOCK3. Our proband has symptoms similar to the cases described in this report (Table 1). Because of the consistent clinical findings in unrelated individuals with biallelic DOCK3 mutations, DOCK3 deficiency should be regarded as a neurodevelopmental disorder characterized by unsteady gait, hypotonia and developmental delay. We propose “DOCK3-related neurodevelopmental syndrome” to denote this condition.
Table 1.
Clinical features of patients with DOCK3 mutations
| Case 1 | Case 2* | ||
|---|---|---|---|
| Patient 1 | Patient 1 | Patient 2 | |
| Sex | Male | Female | Male |
| Age | 2 years 4 months | 12 years | 11 years |
| Mutation | Homozygous | Compound heterozygous | Compound heterozygous |
| Base change | 170 kb deletion | 458 kb deletion, p. Gln128* | 458 kb deletion, p. Gln128* |
| Clinical findings | Developmental delay, hypotonia, gait ataxia, and facial dysmorphism including bilateral epicanthal folds, upturned nasal tip/anteverted nares, and prominent cheeks | Developmental delay, hypotonia, gait ataxia, and mild dysmorphism including a prominent chin, high arched palate, malocclusion, and long fingers | Developmental delay, hypotonia, gait ataxia, and mild dysmorphism including downslanting palpebral fissures, long face, and a pointed chin |
| Reference | This study | Helbig et al. (2017) |
Two patients in case 2 are siblings.
The degree of developmental delay could be variable among cases with DOCK3 mutations. In Helbig et al. (2017), the first sibling, a 12 year old girl, first walked at 5 years old and had an unsteady gait. She had global developmental delay, hypotonia, and decreased reflexes. At 12 years old, she was not yet speaking. Her brother, an 11 year old boy, walked at 2.5 years old and said his first words at 4 years old. At 11 years old, he had persistent speech delays, hypotonia, and unsteady gait. Both siblings had mild dysmorphic features with a prominent chin, long fingers, long face, and downslanting palpebral fissures. Although the clinical picture of these cases were not provided in the published article, facial dysmorphism described in Helbig et al. (2017) differs from the proband reported herein. Ascertainment of more cases with DOCK3 mutations is required to further delineate the phenotypic spectrum of DOCK3-related neurodevelopmental disorder.
DOCK3, also known as modifier of cell adhesion protein (MOCA) or presenilin-binding protein (PBP) functions as a guanine nucleotide exchange factor for Rac1, and is predominantly expressed in neurons and testis (Kashiwa et al., 2000; de Silva et al., 2003; Namekata et al., 2004). DOCK3 plays an important role in promoting neurite and axonal growth by stimulating actin dynamics and microtubule assembly pathways in the central nervous system (Namekata et al., 2010; 2012). Several studies suggest an involvement of DOCK3 in pathogenesis of neurological diseases such as Alzherimer’s disease (AD), attention deficit hyperactivity disorder (ADHD), and epilepsy. DOCK3 was originally identified as a binding partner of presenilin, of which mutations are the most common cause of familial AD, and found to be deficient in soluble fraction of AD-brains (Kashiwa et al., 2000). DOCK3 was accumulated in neurofibrillary tangles of AD-brains, and stimulate phosphorylation of tau protein (Chen et al., 2001). DOCK3 integrates the neural death signals activated by familial AD-related mutants of amyloid β precursor protein and presenilins (Tachi et al., 2012). In addition, DOCK3 is reported as a candidate gene involved in the pathway leading to ADHD, since a pericentric inversion that disrupts DOCK3 gene co-segregates with an ADHD-like phenotype in extended pedigree (de Silva et al., 2003). A recent report suggests a correlation between increased DOCK3 expression and seizures by analyzing brain tissues of human epilepsy patients and epileptic rat models (Li et al., 2016). Collectively, these findings indicate a significant role of DOCK3 in neurologic function.
Dock3 knockout mice exhibit phenotypic features of gait abnormalities that include limb weakness, ataxia, and impaired ability to swim, indicating sensorimotor impairments. This resembles the symptoms of our proband described above. The mice have neuronal structural changes such as axonal swelling containing abnormal aggregation of neurofilaments and autophagic vacuoles in several brain regions including the spinal cord and cerebellum. These changes are associated with defects in axonal transport via disruption of the axonal cytoskeleton, leading to axonal degeneration (Chen et al., 2009). Given the phenotypic similarities between Dock3 knockout mouse and the patients with DOCK3 mutations, the Dock3 knockout mouse model represents a useful animal model, providing an opportunity to understand the molecular basis of DOCK3-related neurodevelopmental syndrome.
In summary, here we report a second case of biallelic DOCK3 mutation due to homozygous deletion. Given the clinical similarities among the cases with DOCK3 mutations, we provided further evidence that biallelic mutations of DOCK3 lead to a specific DOCK3-related neurodevelopmental syndrome.
Acknowledgments
Grant sponsor: A.I-O received funding from NIH T32 (T32GM008638)
Footnotes
Conflict of interest: None
References
- Chen Q, Peto CA, Shelton GD, Mizisin A, Sawchenko PE, Schubert D. Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration. J Neurosci. 2009;29:118–130. doi: 10.1523/JNEUROSCI.3985-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yoshida H, Schubert D, Maher P, Mallory M, Masliah E. Presenilin binding protein is associated with neurofibrillary alterations in Alzheimer's disease and stimulates tau phosphorylation. The American journal of pathology. 2001;159:1597–1602. doi: 10.1016/S0002-9440(10)63005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva MG, Elliott K, Dahl HH, Fitzpatrick E, Wilcox S, Delatycki M, Williamson R, Efron D, Lynch M, Forrest S. Disruption of a novel member of a sodium/hydrogen exchanger family and DOCK3 is associated with an attention deficit hyperactivity disorder-like phenotype. Journal of medical genetics. 2003;40:733–740. doi: 10.1136/jmg.40.10.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs K, Dominguez Conde C, Zhang SY, Parolini S, Audry M, Chou J, Haapaniemi E, Keles S, Bilic I, Okada S, Massaad MJ, Rounioja S, Alwahadneh AM, Serwas NK, Capuder K, Ciftci E, Felgentreff K, Ohsumi TK, Pedergnana V, Boisson B, Haskologlu S, Ensari A, Schuster M, Moretta A, Itan Y, Patrizi O, Rozenberg F, Lebon P, Saarela J, Knip M, Petrovski S, Goldstein DB, Parrott RE, Savas B, Schambach A, Tabellini G, Bock C, Chatila TA, Comeau AM, Geha RS, Abel L, Buckley RH, Ikinciogullari A, Al-Herz W, Helminen M, Dogu F, Casanova JL, Boztug K, Notarangelo LD. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med. 2015;372:2409–2422. doi: 10.1056/NEJMoa1413462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea G, Blangy A. Dock-family exchange factors in cell migration and disease. Eur J Cell Biol. 2014;93:466–477. doi: 10.1016/j.ejcb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Helbig KL, Mroske C, Moorthy D, Sajan SA, Velinov M. Biallelic loss-of-function variants in DOCK3 cause muscle hypotonia, ataxia, and intellectual disability. Clin Genet. 2017 doi: 10.1111/cge.12995. doi.org/10.1111/cge.12995. [DOI] [PubMed]
- Kashiwa A, Yoshida H, Lee S, Paladino T, Liu Y, Chen Q, Dargusch R, Schubert D, Kimura H. Isolation and characterization of novel presenilin binding protein. Journal of neurochemistry. 2000;75:109–116. doi: 10.1046/j.1471-4159.2000.0750109.x. [DOI] [PubMed] [Google Scholar]
- Li J, Mi X, Chen L, Jiang G, Wang N, Zhang Y, Deng W, Wang Z, Chen G, Wang X. Dock3 participate in epileptogenesis through rac1 pathway in animal models. Mol Neurobiol. 2016;53:2715–2725. doi: 10.1007/s12035-015-9406-9. [DOI] [PubMed] [Google Scholar]
- Namekata K, Enokido Y, Iwasawa K, Kimura H. MOCA induces membrane spreading by activating Rac1. J Biol Chem. 2004;279:14331–14337. doi: 10.1074/jbc.M311275200. [DOI] [PubMed] [Google Scholar]
- Namekata K, Harada C, Guo X, Kimura A, Kittaka D, Watanabe H, Harada T. Dock3 stimulates axonal outgrowth via GSK-3beta-mediated microtubule assembly. J Neurosci. 2012;32:264–274. doi: 10.1523/JNEUROSCI.4884-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekata K, Harada C, Taya C, Guo X, Kimura H, Parada LF, Harada T. Dock3 induces axonal outgrowth by stimulating membrane recruitment of the WAVE complex. PNAS. 2010;107:7586–7591. doi: 10.1073/pnas.0914514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Hamdan FF, Rio M, Capo-Chichi JM, Boddaert N, Decarie JC, Maranda B, Nabbout R, Sylvain M, Lortie A, Roux PP, Rossignol E, Gerard X, Barcia G, Berquin P, Munnich A, Rouleau GA, Kaplan J, Rozet JM, Michaud JL. Mutations in DOCK7 in individuals with epileptic encephalopathy and cortical blindness. Am J Hum Genet. 2014;94:891–897. doi: 10.1016/j.ajhg.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Aglan M, Keppler-Noreuil K, Faqeih E, Ansari S, Horton K, Ashour A, Zaki MS, Al-Zahrani F, Cueto-Gonzalez AM, Abdel-Salam G, Temtamy S, Alkuraya FS. Mutations in EOGT confirm the genetic heterogeneity of autosomal-recessive Adams-Oliver syndrome. Am J Hum Genet. 2013;92:598–604. doi: 10.1016/j.ajhg.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Faqeih E, Sunker A, Morsy H, Al-Sheddi T, Shamseldin HE, Adly N, Hashem M, Alkuraya FS. Recessive mutations in DOCK6, encoding the guanidine nucleotide exchange factor DOCK6, lead to abnormal actin cytoskeleton organization and Adams-Oliver syndrome. Am J Hum Genet. 2011;89:328–333. doi: 10.1016/j.ajhg.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukalo M, Tilsen F, Kayserili H, Muller D, Tuysuz B, Ruddy DM, Wakeling E, Orstavik KH, Snape KM, Trembath R, De Smedt M, van der Aa N, Skalej M, Mundlos S, Wuyts W, Southgate L, Zenker M. DOCK6 mutations are responsible for a distinct autosomal-recessive variant of Adams-Oliver syndrome associated with brain and eye anomalies. Hum Mutat. 2015;36:593–598. doi: 10.1002/humu.22795. [DOI] [PubMed] [Google Scholar]
- Tachi N, Hashimoto Y, Matsuoka M. MOCA is an integrator of the neuronal death signals that are activated by familial Alzheimer's disease-related mutants of amyloid beta precursor protein and presenilins. The Biochemical journal. 2012;442:413–422. doi: 10.1042/BJ20100993. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jing H, Su HC. Recent Advances in DOCK8 Immunodeficiency Syndrome. J Clin Immunol. 2016;36:441–449. doi: 10.1007/s10875-016-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]