Abstract
Background
Y chromosome DNA from male epithelial and sperm cells a detected in vaginal samples after unprotected sex in experimental studies. We assessed the strength of this association in an observational setting to examine the utility of Y chromosome DNA as a biomarker of recent sexual behaviors in epidemiological studies.
Methods
The HITCH cohort study enrolled 502 women attending a university or college in Montréal, Canada, and their male partners from 2005–2010. Participants completed self-administered questionnaires. We used real-time PCR to test women’s baseline vaginal samples for Y chromosome DNA, and assessed which sexual behaviors were independent predictors of Y chromosome DNA positivity and quantity with logistic and negative binomial regression.
Results
Y chromosome DNA positivity decreased from 77% in women in partnerships reporting vaginal sex 0–1 days ago to 13% in women in partnerships reporting last vaginal sex >=15 days ago (adjusted odds ratio=0.09, 95%CI: 0.02–0.36). The mean proportion of exfoliated vaginal sample cells with Y chromosome DNA was much lower for women who reported always using condoms (0.01%) than for women who reported never using condoms (2.07%) (adjusted ratio 26.8, 95%CI: 8.9–80.5). No association was found with reported oral/digital sex frequency or concurrency of partnerships.
Conclusions
Y chromosome DNA quantity is strongly associated with days since last vaginal sex and lack of condom use in observational settings. Y chromosome DNA quantity may prove useful as a correlate of recent vaginal sex in observational studies lacking data on sexual behavior, such as surveillance studies of human papillomavirus infection prevalence.
Keywords: Sexual behavior, Sexual partners, Sexually transmitted infection prevention, Y chromosome
Introduction
Detection of Y chromosome DNA in women’s vaginal tract is associated with recent sexual activity and condom use with a male partner.(1–6) Y chromosome DNA is present in male epithelial cells shed during sexual intercourse and in Y-chromosome carrying sperm cells. It may be detected in 87–100% of women the day after unprotected vaginal intercourse, and may be detected in some cases up to 15 days after.(3,4,6,7) Y chromosome DNA is rarely detected in women who have abstained from intercourse for more than two weeks or who consistently use condoms.(1,3,6)
Y chromosome DNA may be a particularly useful biomarker in studies of human papillomavirus (HPV) infection and sexual transmission.(7) Though most HPV transmission is thought to occur through desquamation of infected epithelial cells,(8) HPV is also known to infect sperm cells.(9) Biomarkers may be an interesting option where collecting self-reported data is impractical. Sexual behavior is difficult to capture and is subject to desirability bias, non-response, and faulty recall in self-reports.(10,11) Many studies lack detailed sexual behavior data, either because they were not included in the questionnaire or due to the secondary analysis of specimens collected for other purposes such as pap tests (ex: (12,13)). Some previous HPV infection studies have used infection with other sexually transmitted infections (STI) as biomarkers to help control for sexual risk (ex. (14,15)), but these have their limitations as most sexual encounters will not lead to STI acquisition. Prostate-specific antigen, another biomarker of semen exposure, has low usefulness for HPV/STI research due to its rapid decay.(2,6)
Y chromosome DNA has been studied as a biomarker of sexual exposures mostly under highly controlled conditions, where small samples of highly motivated women agreed to self-regulate their sexual activity according to an experimental protocol, or where women are experimentally inseminated.(1–4,6,16) However, data to assess its association with sexual behaviors in observational settings are still lacking,(17) and it is still unclear to what extent experimental data is generalizable to observational populations. In this study, our objective was to ascertain whether Y chromosome DNA positivity and quantity are associated with self-reported sexual behaviour, and identify which behaviors are independent predictors of Y chromosome DNA positivity and quantity in an observational setting.
Materials and Methods
STUDY DESIGN
We analyzed secondary data from participants recruited to the HITCH (HPV Infection and Transmission Among Couples Through Heterosexual Activity) cohort study.(18–21) The study was designed to study HPV transmission among newly formed young heterosexual couples. The study enrolled young women attending a university or college in Montréal, Canada, and their male partners throughout 2005–2010. Eligible couples were in a sexual partnership <=6 months old; women were eligible if they were 18–24 years old and men if they were >=18 years old. The study was approved by the ethical Institutional Review Boards of McGill and Concordia universities. Participants provided written informed consent for study participation, and for the storage and use of their specimens in future studies.
We analyzed cross-sectional data from participants’ baseline clinic visit and questionnaires. Women were asked to schedule visits at a time they were not menstruating. Women self-collected a vaginal specimen at baseline with a Dacron™ swab (Invista, Inc, Wichita, KS) at the clinic after receiving instructions by the research nurse. Both men and women independently completed self-administered online questionnaires on their sexual behaviors with their HITCH partner and with any concurrent/previous partners (see Supplemental Digital Content 1 for questionnaire). Participants were asked to recall the last dates of vaginal sex with current and previous partners. Participants had been instructed to abstain from intercourse in the 24h preceding the specimen collection, but could indicate in the questionnaire if this was not the case. Participants were asked to report on a categorical scale the proportion of all vaginal sex acts with their partner where they had used condoms, the proportion of sexual encounters with their HITCH partner where they received oral/digital sex, and whether a condom had ever slipped, broken, been removed early or applied late during their partnership.
Y CHROMOSOME DNA DETECTION & QUANTIFICATION
We used MasterPure (Epicentre, Madison, Wisconsin) to isolate the DNA from vaginal samples. Cells were lysed and DNA was precipitated in isopropanol. After centrifugation at 14000 rpm for 30 min at room temperature, the supernatant was discarded, and the cell pellet was left to dry and resuspended in 150μL of 20 mmol/L Tris buffer (pH 8.3). We used a real-time polymerase chain reaction (PCR) assay to detect samples with inhibitors.(22) Samples with inhibition were diluted 1/4 and retested for the presence of inhibition. Samples and diluted samples without inhibition were then tested using two PCR assays. The first assay used a validated protocol to detect and amplify the SRY gene sequence, which is specific to Y chromosomes.(23) The second assay quantified the total number of cells in the sample using the amplification of a β-globin gene segment, found in both male and female cells. Samples were evaluated in duplicate with the Light Cycler PCR and detection system (Roche Molecular Systems, Laval, Québec) to quantify the number of copies of SRY and β-globin genes. For the SRY assay, 2μl of treated specimen were amplified in a 20μl reaction mix with 3mM of MgCl2, 0.3 pmoles of primers for SRY-109 (5′-TGGCGATTAAGTCAAATTCGC-3′) and SRY 245 (5′-CCCCCTAGTACCCTGACAATGTATT-3′), and 0.3 pmoles of probe for SRY (5′-AGCAGTAGAGCAGTCAGGGAGGCAGA-3′) with FastStart enzyme. We quantified Y chromosome DNA by comparing the cycle where exponential amplification started for each specimen to a standard curve produced by serial dilutions of DNA from quantified cells of prostatic origin available in our laboratory. We determined the level of analytical sensitivity of the assay using a titration curve produced by serial dilutions of DNA and found a detection limit of one copy of SRY per test (2 μl of extracted DNA). We performed the β-globin PCR assay as previously described,(24) and determined the total quantity of cells using serial dilutions of human genomic DNA (Roche Diagnostics, Indianapolis, Indiana) in 10mM Tris-HCl [ph 8.2]. Sample processing and testing was done by female research assistants only to prevent contamination. All negative controls were negative in PCR assays.
STATISTICAL ANALYSIS
Analyses were restricted to partnerships where the woman’s sample had valid β-globin DNA test results, and which had non-missing data for questionnaire items. Y chromosome DNA in vaginal samples was analyzed as a binary outcome (positive/negative) and a quantitative outcome (the proportion of cells with Y chromosome DNA [male cells] in the vaginal sample). The proportion of male cells was calculated by dividing the number of copies of SRY DNA (1 copy/cell) by the total number of exfoliated cells in the vaginal sample with β-globin DNA (2 copies/cell). We used logistic regression and the exact Cochrane-Armitage test to identify the predictors of Y chromosome DNA positivity, and used negative binomial regression to model the proportion of male cells (using the number cells with Y chromosome DNA as the outcome and the log-transformed total number of exfoliated cells from vaginal samples as the offset). We evaluated as univariate model predictors the sexual behavior questionnaire items we hypothesized might be associated with male cell deposition in the vagina (frequency and time since vaginal sex, condom use, oral/digital sex frequency, whether the woman had concurrent partners). We included in multivariable models only those variables which were significantly associated with Y chromosome DNA outcomes in univariate analyses using a cut-off of p<0.05. We considered that the number of days since a woman’s last vaginal sex was the most recent between either 1) the date she reported last having vaginal sex with her HITCH partner or any other partner, or 2) the date her male HITCH partner reported last having vaginal sex with her. We used the woman’s reports for all other sexual behaviors. In sensitivity analyses we evaluated the influence of condom user error by recoding “always” users as “most times” users if either partner reported any condom breakage, slippage, delayed application, or early removal.
We performed subgroup analyses to explore potential modifying effects of condom use and time since last vaginal sex. We used the Cochrane-Armitage trend test to identify the behaviors which might potentially explain Y chromosome DNA positivity in each subgroup.
Results
ALL WOMEN
At the baseline visit, 494 of the 502 enrolled women had samples with valid β-globin DNA results. Women and their male partners were on average 21.1 and 22.7 years old, and 62.3% of partnerships reported vaginal sex in the past 2 days (Table 1).
Table 1.
Proportion of women Y chromosome DNA positive, and mean proportions of exfoliated vaginal cells with Y chromosome DNA by reported sexual behaviors.
| Reported behavior | Frequency
|
Y chromosome DNA positivity
|
Mean proportion of exfoliated vaginal cells with Y chromosome DNA (%)a
|
Trendb (p-value) | |
|---|---|---|---|---|---|
| N | n (%) | All women | Y chromosome DNA positive women only | ||
| Mean (SD) | Mean (SD) | ||||
| Overall | 494 | 227 (46%) | 0.99% (4.56%) | 2.15% (6.55%) | |
| Days since woman’s last vaginal sexc | <0.0001 | ||||
| 0–1 | 111 | 85 (77%) | 2.95% (8.00%) | 3.85% (8.96%) | |
| 2 | 194 | 105 (54%) | 0.58% (2.88%) | 1.07% (3.85%) | |
| 3 | 67 | 18 (27%) | 0.44% (3.51%) | 1.65% (6.76%) | |
| 4 | 24 | 5 (21%) | 0.08% (0.32%) | 0.37% (0.67%) | |
| 5–7 | 49 | 7 (14%) | 0.00% (0.01%) | 0.03% (0.04%) | |
| 8–14 | 22 | 3 (14%) | 0.01% (0.06%) | 0.10% (0.15%) | |
| >=15 | 23 | 3 (13%) | 0.01% (0.02%) | 0.06% (0.04%) | |
| Missing | 4 | 1 (25%) | 3.82% (7.64%) | 15.29% (0%) | |
| Condom use with HITCH partner | <0.0001 | ||||
| Never (0%) | 66 | 38 (58%) | 2.07% (5.83%) | 3.60% (7.36%) | |
| Rarely (1–25%) | 143 | 89 (62%) | 1.21% (4.73%) | 1.94% (5.89%) | |
| Sometimes (26–75%) | 78 | 43 (55%) | 1.26% (6.12%) | 2.28% (8.14%) | |
| Most times (76–99%) | 98 | 34 (35%) | 0.80% (4.28%) | 2.30% (7.09%) | |
| Always (100%) | 103 | 23 (22%) | 0.01% (0.04%) | 0.07% (0.08%) | |
| Missing | 6 | 0 (0%) | 0% (0%) | ||
| Average frequency of vaginal sex acts with HITCH partner (/week) | <0.0001 | ||||
| <=2 | 108 | 28 (26%) | 0.72% (4.01%) | 2.78% (7.60%) | |
| >2–4 | 183 | 76 (42%) | 0.62% (3.40%) | 1.48% (5.18%) | |
| >4 | 196 | 123 (63%) | 1.51% (5.70%) | 2.41% (7.05%) | |
| Missing | 7 | 0 (0%) | 0% (0%) | ||
| Proportion of sex encounters where woman received oral sex from male HITCH partner | 0.16 | ||||
| Never (0%) | 33 | 9 (27%) | 0.32% (1.54%) | 1.19% (2.89%) | |
| Rarely (1–25%) | 121 | 56 (46%) | 0.74% (3.12%) | 1.60% (4.46%) | |
| Sometimes (26–75%) | 196 | 95 (48%) | 1.16% (5.58%) | 2.40% (7.84%) | |
| Most times (76–99%) | 127 | 57 (45%) | 1.10% (4.70%) | 2.45% (6.81%) | |
| Always (100%) | 17 | 10 (59%) | 1.11% (2.80%) | 1.89% (3.50%) | |
| Proportion of sex encounters where woman received digital sex from male HITCH partner | 0.85 | ||||
| Never (0%) | 4 | 1 (25%) | 0.01% (0.03%) | 0.06% (0%) | |
| Rarely (1–25%) | 53 | 25 (47%) | 1.71% (6.21%) | 3.63% (8.73%) | |
| Sometimes (26–75%) | 158 | 66 (42%) | 0.60% (3.15%) | 1.44% (4.76%) | |
| Most times (76–99%) | 218 | 113 (52%) | 1.09% (5.01%) | 2.11% (6.81%) | |
| Always (100%) | 61 | 22 (36%) | 1.03% (4.47%) | 2.86% (7.19%) | |
| Woman had a concurrent partner | 0.95 | ||||
| No | 426 | 196 (46%) | 1.02% (4.70%) | 2.21% (6.75%) | |
| Yes | 68 | 31 (46%) | 0.80% (3.57%) | 1.75% (5.17%) | |
SD=Standard deviation
Proportions are expressed as percentages. We averaged the individual proportions of all women (N) including those who are negative (0% of cells with Y chromosome DNA), and the proportions of women who are Y chromosome DNA positive only (n).
Cochrane-Armitage exact test.
Latest date between any of the following: last date of vaginal sex woman reported with male HITCH partner, last date of vaginal sex their male HITCH partner reported with her, last date woman reported vaginal sex with previous or concurrent partners.
Y chromosome DNA positivity decreased with the reported number of days since last vaginal sex and the frequency of condom use, and increased with the average frequency of vaginal sex acts per week (Table 1, trend p-values<0.0001). Similar associations were observed with the mean proportion of exfoliated cells with Y chromosome DNA. Y chromosome DNA positivity decreased from 76.6% in women in partnerships reporting sex 0–1 days ago to 13.0% in women in partnerships reporting vaginal sex >=15 days ago. While 22.3% of women who reported always used condoms were positive for Y chromosome DNA, their positive samples had a much lower mean proportion of cells with Y chromosome DNA compared to women who reported not always using condoms (0.07% vs. >=1.94%), and a much lower standard deviation (0.08% vs. >=5.89%).
The reported number of days since last reported vaginal sex, condom use, and frequency of vaginal acts per week were associated in regression models with both Y chromosome DNA positivity and the proportion of cells with Y chromosome DNA (Table 2). Compared to women in partnerships reporting vaginal sex 0–1 days ago, women in partnerships reporting last having vaginal sex >=15 days ago had 0.09 (95%CI: 0.02, 0.36) times lower odds of being positive for Y chromosome DNA, and the proportion of their sample cells with Y chromosome DNA was 0.014 (95%CI: 0.002, 0.084) times lower in adjusted models. Compared to women who reported always using condoms, women who reported never using condoms had 5.18 (95%CI: 2.38, 11.29) times higher odds of being positive for Y chromosome DNA, and their samples had a 26.77 (95%CI: 8.90, 80.47) times higher proportion of cells with Y chromosome DNA in adjusted models. While women who reported never receiving oral sex from their male partner had lower odds of Y chromosome DNA positivity, neither the Wald nor the Cochrane-Armitage test suggested the frequency of oral sex was associated with positivity (p=0.16–0.22). Reported frequency of digital sex and concurrency were also not associated with Y chromosome DNA positivity or quantity. The association between the average frequency of vaginal sex acts and Y chromosome DNA was attenuated once adjusted for days since vaginal sex and condom use. Sensitivity analyses where we corrected for reported condom error (ever breakage, slippage, early removal, or late application) did not improve model fit compared to uncorrected condom use (both R2=0.10) and only attenuated the association with Y chromosome DNA positivity (crude odds ratio 3.36 for always vs. never condom users).
Table 2.
Sexual behaviors associated in regression models with odds of Y chromosome DNA positivity and the proportion of exfoliated cells with Y chromosome DNA in vaginal samples.
| Reported sexual behavior | Y chromosome DNA positivity OR
|
Ratio of proportions of exfoliated cells with Y chromosome DNA
|
||||||
|---|---|---|---|---|---|---|---|---|
| OR (crude) | 95% CI | OR (adj)a | 95% CI | PR (crude) | 95% CI | PR (adj)a | 95% CI | |
| Days since women’s last vaginal sexb | ||||||||
| 0–1 (ref) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 2 | 0.37 | (0.22, 0.62) | 0.39 | (0.22, 0.68) | 0.195 | (0.095, 0.401) | 0.089 | (0.039, 0.207) |
| 3 | 0.11 | (0.06, 0.23) | 0.13 | (0.06, 0.27) | 0.150 | (0.059, 0.383) | 0.060 | (0.019, 0.192) |
| 4 | 0.08 | (0.03, 0.24) | 0.08 | (0.03, 0.25) | 0.026 | (0.007, 0.103) | 0.042 | (0.010, 0.181) |
| 5–7 | 0.05 | (0.02, 0.13) | 0.06 | (0.02, 0.16) | 0.002 | (0.001, 0.005) | 0.003 | (0.001, 0.010) |
| 8–14 | 0.05 | (0.01, 0.18) | 0.06 | (0.01, 0.22) | 0.005 | (0.001, 0.021) | 0.003 | (0.001, 0.014) |
| >=15 | 0.05 | (0.01, 0.19) | 0.09 | (0.02, 0.36) | 0.003 | (0.001, 0.012) | 0.014 | (0.002, 0.084) |
| Condom use with HITCH partner | ||||||||
| Never (0%) | 4.6 | (2.3, 9.0) | 5.2 | (2.4, 11.3) | 138.0 | (49.9, 381.8) | 26.8 | (8.9, 80.4) |
| Rarely (1–25%) | 5.7 | (3.2, 10.2) | 5.5 | (2.9, 10.6) | 80.2 | (34.6, 186.2) | 18.9 | (7.4, 48.2) |
| Sometimes (26–75%) | 4.3 | (2.2, 8.1) | 4.3 | (2.1, 9.0) | 84.1 | (31.9, 222.1) | 10.8 | (3.4, 33.9) |
| Most times (76–99%) | 1.9 | (1.0, 3.4) | 1.6 | (0.8, 3.2) | 53.3 | (21.3, 133.1) | 4.6 | (1.5, 14.2) |
| Always (100%) (ref) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Average frequency of vaginal sex acts with HITCH partner (/week) | ||||||||
| <=2 (ref) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| >2–4 | 2.0 | (1.2, 3.4) | 1.3 | (0.7, 2.4) | 0.9 | (0.4, 2.0) | 2.1 | (0.8, 5.5) |
| >4 | 4.8 | (2.8, 8.0) | 1.9 | (1.0, 3.5) | 2.1 | (0.9, 4.7) | 1.1 | (0.5, 2.5) |
| Proportion of sex encounters where woman received oral sex from male HITCH partner | ||||||||
| Never (0%) | 0.4 | (0.2, 0.9) | 0.3 | (0.1, 1.0) | ||||
| Rarely (1–25%) | 0.9 | (0.6, 1.4) | 0.6 | (0.3, 1.4) | ||||
| Sometimes (26–75%) | 1.0 | 1.0 | ||||||
| Most times (76–99%) | 0.9 | (0.5, 1.3) | 0.9 | (0.4, 2.1) | ||||
| Always (100%) | 1.5 | (0.5, 4.0) | 1.0 | (0.2, 5.4) | ||||
| Proportion of sex encounters where woman received digital sex from male HITCH partner | ||||||||
| Never/Rarely (0–25%) | 1.1 | (0.6, 2.1) | 2.7 | (0.9, 7.7) | ||||
| Sometimes (26–75%) (ref) | 1.0 | 1.0 | ||||||
| Most times (76–99%) | 1.5 | (1.0, 2.2) | 1.8 | (0.9, 3.8) | ||||
| Always (100%) | 0.7 | (0.4, 1.4) | 1.7 | (0.6, 4.9) | ||||
| Woman had a concurrent partner | ||||||||
| No (ref) | 1.0 | 1.0 | ||||||
| Yes | 1.0 | (0.6, 1.7) | 0.8 | (0.3, 2.0) | ||||
adj=adjusted; CI=Confidence Interval; OR=odds ratio; PR=proportion ratio; ref=reference level
Adjusted for reported days since woman’s last reported vaginal sex act, condom use, and average frequency of vaginal sex acts per week.
Latest date between any of the following: last date of vaginal sex woman reported with male HITCH partner, last date of vaginal sex their male HITCH partner reported with her, last date woman reported vaginal sex with previous or concurrent partners.
SUBGROUP ANALYSES
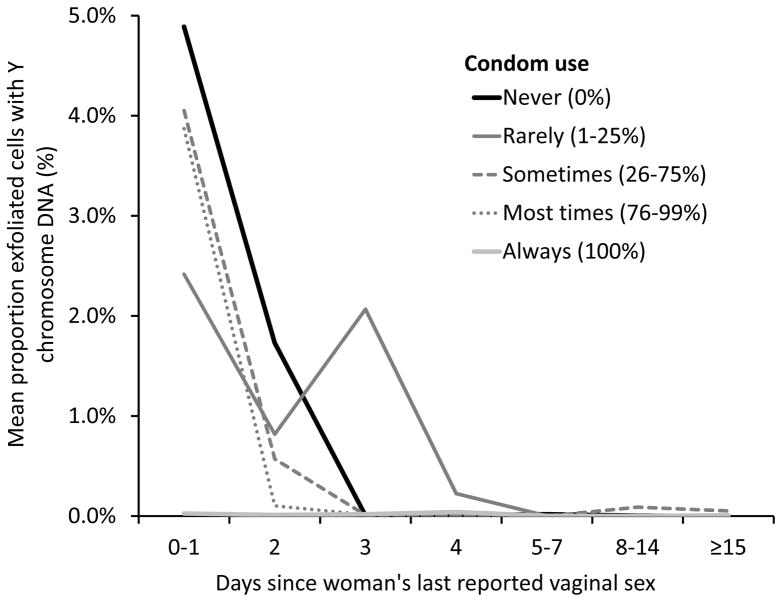
Condom use modified the association between days since last sex and Y chromosome DNA positivity and quantity. Women in partnerships who reported having sex in the last 0–1 day and who reported never using condoms had a 94% probability of being positive for Y chromosome DNA, compared to 28% for those who reported always using condoms (Table 3). Women who reported always using condoms had a very low mean proportion of male cells regardless of days since last vaginal sex (0.00–0.01%), compared to women who reported not always using condoms who had very high mean proportions of male cells if they last had vaginal sex in the last 0–1 (2.41–4.89%) and 2 days (0.10–1.73%) (Figure 1). For women who reported always using condoms, positivity tended to increase with the reported average frequency of vaginal sex acts (p=0.0042) but not with the frequency of digital and oral sex they received from their partner (p=0.26–0.47) (not shown).
Table 3.
Y Chromosome DNA positivity stratified by condom use.
| Reported sexual behavior | Never (0%) condom users
|
Rarely/Sometimes/Most times (1–99%) condom users
|
Always (100%) condom users
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total N | Y chromosome DNA positive n (%) | Trend p-value | Total N | Y chromosome DNA positive n (%) | Trend p-value | Total N | Y chromosome DNA positive n (%) | Trend p-value | |
| Days since woman’s last reported vaginal sexa | <0.0001 | <0.0001 | 0.25 | ||||||
| 0–1 days | 17 | 16 (94%) | 76 | 64 (84%) | 18 | 5 (28%) | |||
| 2 days | 22 | 15 (68%) | 138 | 81 (59%) | 33 | 9 (27%) | |||
| 3 days | 10 | 2 (20%) | 39 | 12 (31%) | 18 | 4 (22%) | |||
| 4–7 days | 8 | 2 (25%) | 44 | 7 (16%) | 21 | 3 (14%) | |||
| >7 days | 8 | 2 (25%) | 22 | 2 (9%) | 13 | 2 (15%) | |||
| Average frequency of vaginal sex acts with HITCH partner (/week) | 0.05 | <0.0001 | 0.0042 | ||||||
| <=2 | 12 | 6 (50%) | 65 | 19 (29%) | 31 | 3 (10%) | |||
| >2–4 | 25 | 10 (40%) | 119 | 59 (50%) | 39 | 7 (18%) | |||
| >4 | 29 | 22 (76%) | 134 | 88 (66%) | 33 | 13 (39%) | |||
SD=Standard deviation
Latest date between any of the following: last date of vaginal sex woman reported with male HITCH partner, last date of vaginal sex their male HITCH partner reported with her, last date woman reported vaginal sex with previous or concurrent partners.
Figure 1.
Mean proportion exfoliated vaginal cells with Y chromosome DNA by days since women’s last reported vaginal sex and frequency of condom use. The sudden increase in mean proportion of cells with Y chromosome DNA in women who reported vaginal sex 3 days ago and rarely using condoms was due to one extreme outlier with 28.7% male cells (all other women in this category had <0.09% male cells).
In the 45 women in partnerships whose last reported vaginal sex was >7 days ago, Y chromosome DNA positivity was highest in those who reported more frequently receiving oral and digital sex from their partner (p=0.02–0.03) (see Table, Supplemental Digital Content 2, women in partnerships whose last reported vaginal sex was >7 days ago). Condom use was not associated with Y chromosome DNA positivity in these women (p=0.76). There were 2 women who reported always using condoms and in partnerships reporting vaginal sex >7 days ago who were Y chromosome DNA positive. Both only had a low proportion of cells with Y chromosome DNA (0.02% and 0.06%). Neither of these women nor their partners reported any condom user error.
Discussion
In this cross-sectional study of young adult heterosexual couples, we found that the self-reported number of days since last vaginal sex and condom use were strongly and independently associated with both Y chromosome DNA positivity and quantity in women’s vaginal samples. Particularly, the quantity of Y chromosome DNA in vaginal samples dramatically decreased with the reported days since last vaginal sex and with increased condom use. While Y chromosome DNA was detected in 22% of women who self-reported always using condoms, the quantity of male cells with Y chromosome DNA in their samples was approximately 50-fold lower compared to women who self-reported never using condoms.
Our results are similar to controlled experimental studies in monogamous women recruited in clinics or universities,(1–4,6,7) which supports generalizing results from experimental studies to an observational setting. Y chromosome DNA positivity and quantity generally decline sharply the first three days after vaginal sex or artificial insemination.(2,4,6,7,25) Our results were comparable to Brotman et al. who found that 87% of women were Y chromosome DNA positive the day after unprotected intercourse, 36–49% positive after 3 days, and 13–18% positive after a week of abstinence.(4) Up to 18% of women have also been found to be still Y chromosome DNA positive 15 days after intercourse in another study.(6) The overall consistency of results between observational and experimental studies suggests results are widely generalizable to other populations of women.
We found a stronger association between sexual behavior and the quantity of Y chromosome DNA than when Y chromosome DNA was considered as a binary outcome (positive/negative). While studies interested in exposure to any unprotected sex may find a binary measure more useful, our data supports using a quantitative measure of Y chromosome DNA as a stronger correlate of time since last sex and condom use. Previous studies which detected Y chromosome DNA in women who reported consistently using condoms concluded that self-reported condom use was inaccurate or that high condom user error prevails.(26,27) However, their population was younger than ours and was interviewed using a different method and so may not be entirely comparable. In our sample the positive women who self-reported always using condoms had approximately 50-fold lower quantities of Y chromosome DNA compared to the positive women who self-report never using condoms. Experimental studies have similarly found low levels of Y chromosome DNA detection even with consistent condom use.(7,25) We did not find that correcting for self-reported ever condom breakage, slippage, or partial use accounted for Y chromosome DNA positivity. While these women could have misreported their condom use or incorrectly used condoms, it is also possible that condoms may not completely prevent deposition of male epithelial cells which are another source of Y chromosome DNA. Our PCR assay was designed to capture Y chromosome DNA from both epithelial or sperm cells, as both can be biomarkers of sexual exposure. Though in our study all quality controls were negative and there was no evidence of false positives due to contamination, we also cannot exclude this possibility.
We generally did not find that the frequency of oral and digital sex women received was associated with Y chromosome DNA positivity and quantity, except in the subgroup of women who last had vaginal sex >7 days ago. Due to the low number of observations these results should be cautiously interpreted. Ghanem et al. found that receptive oral and digital sex within 48 hours of sampling could lead to Y chromosome DNA detection, albeit at much lower quantities than with vaginal sex.(1) It is possible that any deposition from oral and digital sex in our study was masked by the much stronger associations with vaginal sex and condom use.
Like most behavioral studies, we relied on individuals’ self-reported sexual behaviors. We employed various methods to improve measurement validity, ensuring patients knew their information was confidential and using secure web-based self-administered questionnaires. The very strong association between self-reported vaginal sex, condom use, and Y chromosome DNA quantity suggests self-reported sexual behavior was a good measure of actual sexual behavior in our study. The accuracy of self-reporting will depend on the quality of data collection methods and may be lower in other studies and settings (e.g. STI clinics (28)). There is likely to be some misclassification of days since last vaginal sex, as only 51% of partners reported the same date for their last vaginal sex with each other. We used the most recent of both partners’ reported dates because we found a stronger association with Y chromosome DNA than when we used only women’s reported dates (not shown). This suggests that individuals tended to overestimate the date of last sex due to poor recall over time. It is a particular strength of our study that we could use this information from both partners, compared to most studies of sexual behavior which would only have data from one individual. Misreporting is likely to be non-differential according to the outcome as participants were unaware of their Y chromosome DNA results, which would lead to an underestimation of its association with sexual behavior.
Biomarkers of semen exposure have been hailed as objective markers of sexual behavior considered by many superior to self-reporting.(11,17) Most interest in biomarkers to date has been for identifying individuals suspected of misreporting or violating protocol. However, neither biomarkers nor questionnaires have perfect measurement sensitivity or specificity for underlying complex behaviors.(1,2,29) The potential for small depositions from oral or digital sex and the potential for specimen contamination further complicate the interpretation of a positive Y chromosome DNA result.
Nevertheless, the very strong associations we observed suggest that at the aggregate level, Y chromosome DNA quantity in vaginal samples is very strongly correlated to recent sexual activity, and could be useful in epidemiologic analyses for confounder control or as a proxy of some behaviors that could have been measured using questionnaires. We expect biomarkers such as Y chromosome DNA could be especially useful for surveillance of HPV infection in vaccinated and screened population. In a study of HPV infection in Australian women attending cervical cancer screening Tabrizi et al. were unable to control for any differences in sexual behavior pre- and post-HPV vaccination as sexual behavior data only started being collected post-vaccination;(30) the effectiveness of number of HPV vaccine doses is being monitored in Scotland through record linkage and samples from the organised screening program with only birth cohort year and deprivation score available for confounder adjustment.(31) Both of these are examples of studies where biomarkers could provide further information where there is a lack of comparable sexual risk data between periods and cohorts. As more and more jurisdictions start to screen for cervical cancer with HPV testing, there is likely to be increasing data on HPV infection without appended sexual behavior data.
In conclusion, we found that Y chromosome DNA had a similar performance in a large-scale observational study as previously reported in smaller controlled experiments, supporting its utility as a proxy of recent vaginal sex (≤3 days) and lack of condom use in observational settings. Quantitative measures of Y chromosome DNA will be a stronger correlate of these behaviors than binary measures of Y chromosome DNA positivity. Y chromosome DNA was not strongly associated with oral/digital sex frequency and partner concurrency in an observational setting. Biomarkers such as Y chromosome DNA do not replace the need for quality measurements of self-reported sexual behavior. Many complex behaviors will be impossible to study using biomarkers, and self-reported sexual behaviors are generally still found to be very strong predictors of STI infection despite their inherent biases.(20,21) Y chromosome DNA could nonetheless be useful in epidemiological studies as proxies to reduce residual confounding where sexual behavior was not measured or is suspected to be misreported.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported by the Canadian Institutes for Health Research (CIHR) (grants MOP-68893 and CRN-83320 to ELF, and a Postdoctoral Fellowship Award to TM); the US National Institutes of Health (grant AI073889 to ELF); the Reseau Fonds de la Recherche en Santé du Québec (FRSQ) AIDS and Infectious Disease Network (SIDA-MI) (support for optimization of molecular techniques to FC); and supplementary and unconditional funding by Merck-Frosst Canada Ltd and Merck & Co Ltd to ELF. The funders played no role in the writing of the manuscript or the decision to submit it for publication.
Footnotes
Conflicts of Interest: ELF has served as occasional consultant to Merck, GSK, Roche, and BD. His institution has received grants from Merck and Roche. PPT has received payment for lectures by Merck-Frosst Canada and Bayers. FC received grants through his institution for research projects from Roche and Merck, payments for lectures by Merck, Roche, and has participated in an expert group by Merck. All other authors report no potential conflicts of interest.
References
- 1.Ghanem KG, Melendez JH, McNeil-Solis C, et al. Condom use and vaginal Y-chromosome detection: the specificity of a potential biomarker. Sex Transm Dis. 2007;34(8):620–3. doi: 10.1097/01.olq.0000258318.99606.d9. [DOI] [PubMed] [Google Scholar]
- 2.Jamshidi R, Penman-Aguilar A, Wiener J, et al. Detection of two biological markers of intercourse: prostate-specific antigen and Y-chromosomal DNA. Contraception. 2013;88(6):749–57. doi: 10.1016/j.contraception.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis. 2005;32(2):90–4. doi: 10.1097/01.olq.0000149668.08740.91. [DOI] [PubMed] [Google Scholar]
- 4.Brotman RM, Melendez JH, Smith TD, Galai N, Zenilman JM. Effect of menses on clearance of Y-chromosome in vaginal fluid: implications for a biomarker of recent sexual activity. Sex Transm Dis. 2010;37(1):1–4. doi: 10.1097/OLQ.0b013e3181b5f15d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penrose KJ, Richardson BA, Besson G, et al. Y chromosome and HIV DNA detection in vaginal swabs as biomarkers of semen and HIV exposure in women. Sex Transm Dis. 2014;41(11):674–9. doi: 10.1097/OLQ.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurman A, Jacot T, Melendez J, et al. Assessment of the vaginal residence time of biomarkers of semen exposure. Contraception. 2016;94(5):512–20. doi: 10.1016/j.contraception.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baay MF, Francois K, Lardon F, et al. The presence of Y chromosomal deoxyribonucleic acid in the female vaginal swab: possible implications for human papillomavirus testing. Cancer Epidemiol. 2011;35(1):101–3. doi: 10.1016/j.canep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Doorbar J. The papillomavirus life cycle. Journal of Clinical Virology. 2005;32(Supplement):7–15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Foresta C, Patassini C, Bertoldo A, et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One. 2011;6(3):e15036. doi: 10.1371/journal.pone.0015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catania JA, Gibson DR, Chitwood DD, Coates TJ. Methodological problems in AIDS behavioral research: influences on measurement error and participation bias in studies of sexual behavior. Psychol Bull. 1990;108(3):339–62. doi: 10.1037/0033-2909.108.3.339. [DOI] [PubMed] [Google Scholar]
- 11.Gallo MF, Steiner MJ, Hobbs MM, Warner L, Jamieson DJ, Macaluso M. Biological markers of sexual activity: tools for improving measurement in HIV/sexually transmitted infection prevention research. Sex Transm Dis. 2013;40(6):447–52. doi: 10.1097/OLQ.0b013e31828b2f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grün N, Ährlund-Richter A, Franzén J, et al. Oral human papillomavirus (HPV) prevalence in youth and cervical HPV prevalence in women attending a youth clinic in Sweden, a follow up-study 2013–2014 after gradual introduction of public HPV vaccination. Infectious Diseases. 2015;47(1):57–61. doi: 10.3109/00365548.2014.964764. [DOI] [PubMed] [Google Scholar]
- 13.Kavanagh K, Sinka K, Cuschieri K, et al. Estimation of HPV prevalence in young women in Scotland; monitoring of future vaccine impact. BMC Infect Dis. 2013;13:519. doi: 10.1186/1471-2334-13-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaasila M, Koskela P, Kirnbauer R, Pukkala E, Surcel HM, Lehtinen M. Population dynamics of serologically identified coinfections with human papillomavirus types 11, 16, 18 and 31 in fertile-aged Finnish women. Int J Cancer. 2009;125(9):2166–72. doi: 10.1002/ijc.24539. [DOI] [PubMed] [Google Scholar]
- 15.Mesher D, Soldan K, Howell-Jones R, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32(1):26–32. doi: 10.1016/j.vaccine.2013.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacot TA, Zalenskaya I, Mauck C, Archer DF, Doncel GF. TSPY4 is a novel sperm-specific biomarker of semen exposure in human cervicovaginal fluids; potential use in HIV prevention and contraception studies. Contraception. 2013;88(3):387–95. doi: 10.1016/j.contraception.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Snead MC, Black CM, Kourtis AP. The use of biomarkers of semen exposure in sexual and reproductive health studies. J Womens Health (Larchmt) 2014;23(10):787–91. doi: 10.1089/jwh.2014.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology. 2010;21(1):31–7. doi: 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchell AN, Coutlée F, Tellier P-P, Hanley J, Franco EL. Genital Transmission of Human Papillomavirus in Recently Formed Heterosexual Couples. Journal of Infectious Diseases. 2011;204(11):1723–9. doi: 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burchell AN, Rodrigues A, Moravan V, et al. Determinants of prevalent human papillomavirus in recently formed heterosexual partnerships: a dyadic-level analysis. J Infect Dis. 2014;210(6):846–52. doi: 10.1093/infdis/jiu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis. 2010;37(1):34–40. doi: 10.1097/OLQ.0b013e3181b35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez J, de Pokomandy A, Rouleau D, et al. Episomal and integrated human papillomavirus type 16 loads and anal intraepithelial neoplasia in HIV-seropositive men. Aids. 2010;24(15):2355–63. doi: 10.1097/QAD.0b013e32833db9ea. [DOI] [PubMed] [Google Scholar]
- 23.Picchiassi E, Coata G, Fanetti A, Centra M, Pennacchi L, Di Renzo GC. The best approach for early prediction of fetal gender by using free fetal DNA from maternal plasma. Prenat Diagn. 2008;28(6):525–30. doi: 10.1002/pd.2018. [DOI] [PubMed] [Google Scholar]
- 24.Azizi N, Brazete J, Hankins C, et al. Influence of human papillomavirus type 16 (HPV-16) E2 polymorphism on quantification of HPV-16 episomal and integrated DNA in cervicovaginal lavages from women with cervical intraepithelial neoplasia. J Gen Virol. 2008;89(Pt 7):1716–28. doi: 10.1099/vir.0.83579-0. [DOI] [PubMed] [Google Scholar]
- 25.Jadack RA, Yuenger J, Ghanem KG, Zenilman J. Polymerase chain reaction detection of Y-chromosome sequences in vaginal fluid of women accessing a sexually transmitted disease clinic. Sex Transm Dis. 2006;33(1):22–5. doi: 10.1097/01.olq.0000194600.83825.81. [DOI] [PubMed] [Google Scholar]
- 26.Rose E, Diclemente RJ, Wingood GM, et al. The validity of teens’ and young adults’ self-reported condom use. Arch Pediatr Adolesc Med. 2009;163(1):61–4. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum JE, Zenilman J, Melendez J, Rose E, Wingood G, DiClemente R. Telling truth from Ys: An evaluation of whether the accuracy of self-reported semen exposure assessed by a semen Y-chromosome biomarker predicts pregnancy in a longitudinal cohort study of pregnancy. Sexually transmitted infections. 2014;90(6):479–84. doi: 10.1136/sextrans-2013-051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo MF, Warner L, Hobbs MM, Jamieson DJ, Hylton-Kong T, Steiner MJ. Differences in misreporting of sexual behavior over time: implications for HIV trials. Sexually transmitted diseases. 2015;42(3):160–1. doi: 10.1097/OLQ.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59(3):195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 30.Tabrizi SN, Brotherton JML, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. The Lancet Infectious Diseases. 2014;14(10):958–66. doi: 10.1016/S1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 31.Cuschieri K, Kavanagh K, Moore C, Bhatia R, Love J, Pollock KG. Impact of partial bivalent HPV vaccination on vaccine-type infection: a population-based analysis. Br J Cancer. 2016;114(11):1261–4. doi: 10.1038/bjc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.