Abstract
Background
It is suggested that the integration of maximal myocardial blood flow (MBF) and coronary flow reserve (CFR), termed coronary flow capacity, allows comprehensive evaluation of patients with known or suspected stable coronary artery disease. As management decisions are predicated on clinical risk, we sought to determine the independent and integrated value of maximal MBF and CFR for predicting cardiovascular death.
Methods
MBF and CFR were quantified in 4,029 consecutive patients (median age 66 years, 50.5% women) referred for rest/stress myocardial perfusion positron emission tomography scans from January 2006 to December 2013. The primary outcome was cardiovascular mortality. Maximal MBF<1.8 ml·g-1·min-1 and CFR<2 were considered impaired. Four patient groups were identified based on the concordant or discordant impairment of maximal MBF or CFR. Association of maximal MBF and CFR with cardiovascular death was assessed using Cox and Poisson regression analyses.
Results
A total of 392 (9.7%) cardiovascular deaths occurred over a median follow-up of 5.6 years. CFR was a stronger predictor of cardiovascular mortality than maximal MBF beyond traditional cardiovascular risk factors, left ventricular ejection fraction, myocardial scar and ischemia, rate-pressure-product, type of radiotracer or stress agent used, and revascularization post-scan (adjusted Hazard Ratio, HR [95% Confidence-Interval, CI]: 1.79 [1.38-2.31], p<0.001 per unit decrease in CFR after adjustment for maximal MBF and clinical covariates, and 1.03 [0.84-1.27], p=0.8 per unit decrease in maximal MBF after adjustment for CFR and clinical covariates). In univariable analyses, patients with concordant impairment of CFR and maximal MBF had high cardiovascular mortality of 3.3% (95%CI: 2.9-3.7%) per year. Patients with impaired CFR but preserved maximal MBF had an intermediate cardiovascular mortality of 1.7% (95% CI: 1.3-2.1%) per year; these patients were predominantly women (70%). Patients with preserved CFR but impaired maximal MBF had low cardiovascular mortality of 0.9% (95% CI: 0.6-1.6%) per year. Patients with concordantly preserved CFR and maximal MBF had the lowest cardiovascular mortality of 0.4% (95% CI: 0.3-0.6%) per year. In multivariable analysis, the cardiovascular mortality risk gradient across the four concordant or discordant categories was independently driven by impaired CFR irrespective of impairment in maximal MBF.
Conclusions
CFR is a stronger predictor of cardiovascular mortality than maximal MBF. Concordant and discordant categories based on integrating CFR and maximal MBF identify unique prognostic phenotypes of patients with known or suspected coronary artery disease.
Journal Subject Terms: Cardiovascular Disease, Coronary Artery Disease, Coronary Circulation, Imaging, Ischemia, Mortality/Survival, Revascularization
Introduction
Cardiovascular (CV) disease is the leading cause of mortality worldwide with ischemic heart disease accounting for more than half of the CV deaths.1, 2 In recent years it has been demonstrated that in addition to coronary artery disease (CAD) of the epicardial vessels, dysfunction of the coronary microcirculation contributes to the increased CV morbidity and mortality.3-5 Coronary flow reserve (CFR), the ratio of maximal myocardial blood flow (MBF) during pharmacologically-induced coronary vasodilation to resting MBF, is an integrated measure of flow through both the large epicardial coronary arteries and the microcirculation.6 CFR has been proposed as an indirect parameter to evaluate the function of the coronary circulation, and its impairment is a strong predictor of CV mortality.7-11
However, CFR can be impaired due to a decrease in maximal MBF or an increase in resting MBF. Therefore, it has been proposed that the integration of CFR with maximal MBF, termed coronary flow capacity, could allow for comprehensive evaluation of patients with known or suspected stable CAD compared with CFR alone.12-14 As management decisions including decision to revascularize are predicated on clinical risk, we sought to determine the independent and integrated value of maximal MBF and CFR in assessing the future risk of CV mortality.
Methods
Study Population
All consecutive patients referred for a rest/stress cardiac positron emission tomographic (PET) scan for stable symptoms at Brigham & Women's Hospital (Boston, MA) between January 1, 2006 and December 31, 2013 were included in this study, excluding patients with prior heart transplantation, healthy research participants, and those whose images were missing or uninterpretable owing to poor image quality. A total of 486 unique patient studies from 2006 to 2013 were not interpretable due to poor image quality or technical issues and were excluded. In cases of repeat PET evaluations during the study period, only the earliest evaluable study was included. The study was approved by the Partners Healthcare Institutional Review Board with waiver of informed consent and conducted in accordance with the institutional guidelines. Demographic factors and key elements of the patient's history, including risk factors and medication use, were ascertained at the time of the study by patient interview and review of medical records.
PET Imaging
A standard PET–computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI) was used to image all patients. Patients abstained from caffeine and methylxanthine-containing substances and drugs for 24 hours before their scans. Maximal hyperemic and resting MBF were measured with rubidium-82 (1480–2200 MBq) or N-13 ammonia (700–900 MBq) as the flow tracer, as described previously.15, 16 A standard intravenous infusion of dipyridamole, adenosine, regadenoson, or dobutamine was used as the stress agent based on the prevailing preferred stress agent in our laboratory and patient characteristics such as dobutamine in patients with chronic obstructive lung disease or asthma with wheezing, and adenosine in patients with end-stage renal disease. Heart rate, blood pressure, and 12-lead electrocardiogram were recorded at baseline and every minute during and after pharmacological stress. Maximal hyperemic and resting MBF (in ml · g-1· min-1) were computed from the dynamic stress and rest imaging series, respectively, using compartmental tracer kinetic model lingand commercially available software (Corridor4DM; Ann Arbor, MI), as described previously.15-17 CFR for each patient was calculated as the ratio of maximal MBF at peak hyperemia to resting MBF for the entire left ventricle. The results of CFR and MBFs were not reported clinically and hence, did not influence downstream clinical decision making.
Using a standard five-point scoring system, semi-quantitative 17-segment assessment of the gated myocardial perfusion images was performed by experienced observers.18 Summed rest and stress scores were calculated as the sum of individual segmental scores on the respective images, and their difference was recorded as summed difference score with higher scores reflecting larger areas of myocardial ischemia and/or scar. Summed rest, stress, and difference scores were converted into percentages of total myocardium by division with the maximum possible score of 68 and multiplication by 100. Rest LV ejection fraction (LVEF) was calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, MI).
Outcome Assessment
The vital status of all patients was ascertained by integrating data from the Social Security Administration's Death Master File, the National Death Index, and the Partners Healthcare Research Patient Data Registry from January 1, 2006 to December 31, 2015. For each study patient who had died, two independent reviewers blindly adjudicated hospital records and death certificates to determine the cause of death. In case of disagreement on the cause of death, consensus adjudication was performed. The primary outcome of interest was CV death due to any cause. Non-CV death was censored.
Statistical Analysis
All statistical analyses were performed with SAS 9.4 (SAS Institute Inc, Cary, NC). A two-sided p-value < 0.05 was considered statistically significant. In fully adjusted multivariable models, at least one covariate data value was missing in 44 (out of 4029, 1%) unique patients and they were excluded from multivariable analyses.
Assessment of independent prognostic value of maximal MBF and CFR
Univariable Cox proportional hazards models were used to assess the effect of flow variables on CV mortality. Ties in failure times were handled using Efron's approximation. The Wald Chi-square statistic was used for inference testing. The proportional hazards assumption was examined by inclusion of a time-varying covariate term and was found to be valid. Extended multivariable Cox models were used to evaluate the independent effect of the flow variables on CV mortality after adjustment for age, sex, hypertension, diabetes, dyslipidemia, dialysis, body mass index, known CAD (including prior revascularization and/or prior MI), LVEF (as continuous variable), summed stress score as an indicator for amount of myocardial scar and/or ischemia, post-PET revascularization, rate-pressure-product (resting systolic blood pressure*resting heart rate) and type of radiotracer or stress agent used for PET imaging. The variables for adjustment were selected based on the clinical knowledge. Revascularization post-PET scan was ascertained from the Partners Healthcare Research Patient Data Registry, hospital records and billing claims, and was used as a time-varying covariate term for the adjustment in the analyses to account for the time to revascularization. To assess the independent effect of maximal MBF or CFR on CV mortality, these flow variables were used as continuous variables in two separate models, one model with CFR (without maximal MBF in the model) and the other model with maximal MBF (without CFR in the model).
Assessment of integrated prognostic value of maximal MBF and CFR
Integration of CFR and maximal MBF was achieved by creating four groups based on whether there was concordant or discordant impairment of these coronary flow indices. CFR < 2 and maximal MBF < 1.8 ml · g-1· min-1 were consideredimpaired.13, 19 Annualized and cumulative CV mortality event rates in these four concordant or discordant groups were assessed using Poisson and Cox regression respectively - both in univariable analyses as well as after adjustment for age, sex, hypertension, diabetes, dyslipidemia, dialysis, body mass index, known CAD, LVEF, summed stress score as an indicator for amount of myocardial scar and/or ischemia, post-PET revascularization within 90 days of PET scan, rate-pressure-product, and type of radiotracer or stress agent used for PET imaging.
In addition, the incremental value of one flow index over the other was also evaluated with flow indices as continuous variables. To assess the incremental effect of maximal MBF and CFR on CV mortality, these flow markers were added together in the same model as continuous variables. The correlation between maximal MBF and CFR (r = 0.55) did not preclude such an assessment (variance inflation factor for maximal MBF and CFR was 1.43; <5 indicated collinearity was not an issue between the variables in the model).
Exploratory sub-group analyses
Pre-defined sub-group analyses were carried out for independent and incremental prognostic value of maximal MBF and CFR for the following groups: age ≥/<65, sex, race (white versus non-white), hypertension, diabetes, obesity (body mass index ≥ 30), known CAD, LVEF ≥/< 50%, and presence of myocardial scar and/or myocardial ischemia.
Risk Reclassification
The potential impact of maximal MBF and CFR on risk stratification was assessed by net reclassification improvement (NRI)20 at two years follow-up. Threshold annual CV mortality rates of < 1%, 1-3% and >3% were used for creating low, intermediate, and high risk groups based on the American College of Cardiology/American Heart Association guidelines for the management of patients with stable ischemic heart disease.21 Details of models used for risk reclassification are described in online supplementary material.
Sensitivity Analyses
Sensitivity analyses for the independent and incremental prognostic value of maximal MBF and CFR were carried out for all-cause mortality as the outcome. The primary analyses of interest were also investigated using Fine and Gray competing risk model22 to account for non-CV death competing with CV death. The incremental prognostic value of maximal MBF was also tested within the sub-categories of severely impaired (< 1.5) and mild to moderately impaired (1.5-2) CFR.
Results
Patient and Imaging Characteristics
Baseline patient and imaging characteristics for the overall study population (n = 4,029) as well as when stratified by four groups with concordant or discordant impairment of CFR or maximal MBF are presented in Table 1. Median age of the overall study population was 66 years, half were women (n = 2,033, 50.5%), and there was high prevalence of CV risk factors. A total of 41% of patients had known CAD and approximately 10% of the patients underwent revascularization within 90 days after the PET scan. The main indications for PET scan were evaluation of chest pain and dyspnea. Seventy-one percent of patients had preserved LVEF (≥50%), and myocardial scar or ischemia burden was moderate with 29% of patients who had combined scar and ischemia burden of over 10% of left ventricular myocardium. Median CFR was 1.72 (25th – 75th percentiles: 1.35 – 2.20) and median maximal MBF was 1.75 (25th – 75th percentiles: 1.24 – 2.39) ml · g-1· min-1. The distribution of CFR and maximal MBF with superimposed distribution of CV deaths is illustrated as a scatterplot in Figure 1.
Table 1. Baseline Patient and Imaging Characteristics.
| Impaired CFR | Preserved CFR | ||||
|---|---|---|---|---|---|
| Variable | Impaired Maximal MBF (n = 1,779) | Preserved Maximal MBF (n = 873) | Impaired Maximal MBF (n = 349) | Preserved Maximal MBF (n = 1,028) | All Patients (n=4,029) |
| Demographics | |||||
| Age, y | 69 (60-78) | 66 (57-76) | 63 (55-71) | 62 (53-71) | 66 (57-75) |
| Women | 673 (37.8%) | 608 (69.6%) | 116 (33.2%) | 636 (61.9%) | 2033 (50.5%) |
| Race | |||||
| White | 1227 (69.0%) | 532 (60.9%) | 235 (67.3%) | 618 (60.1%) | 2612 (64.8%) |
| Black | 254 (14.3%) | 158 (18.1%) | 53 (15.2%) | 191 (18.6%) | 656 (16.3%) |
| Other/Unknown | 298 (16.8%) | 183 (21.0%) | 61 (17.5%) | 219 (21.3%) | 761 (18.9%) |
| Cardiovascular Risk Factors | |||||
| Hypertension | 1566 (88.0%) | 701 (80.3%) | 311 (89.1%) | 753 (73.2%) | 3331 (82.7%) |
| Diabetes | 805 (45.3%) | 287 (32.9%) | 118 (33.8%) | 239 (23.2%) | 1449 (36.0%) |
| Dyslipidemia | 1328 (74.6%) | 544 (62.3%) | 252 (72.2%) | 626 (60.9%) | 2750 (68.3%) |
| Body Mass Index, kg/m2 | 29 (25-35) | 27 (23-32) | 31 (27-37) | 29 (25-34) | 29 (25-34) |
| Body Mass Index ≥ 30 | 824 (46.3%) | 320 (36.7%) | 189 (54.2%) | 434 (42.3%) | 1767 (43.9%) |
| Family history of CAD | 404 (22.7%) | 211 (24.2%) | 93 (26.6%) | 320 (31.1%) | 1028 (25.5%) |
| Tobacco use | 149 (8.4%) | 80 (9.2%) | 46 (13.2%) | 115 (11.2%) | 390 (9.7%) |
| Dialysis | 102 (5.7%) | 56 (6.4%) | 3 (0.9%) | 27 (2.6%) | 188 (4.7%) |
| Cardiovascular History | |||||
| Known CAD | 992 (55.8%) | 273 (31.3%) | 161 (46.1%) | 227 (22.1%) | 1653 (41.0%) |
| Prior MI | 709 (39.9%) | 169 (19.4%) | 106 (30.4%) | 148 (14.4%) | 1132 (28.1%) |
| Prior PCI | 517 (29.1%) | 136 (15.6%) | 94 (26.9%) | 142 (13.8%) | 889 (22.1%) |
| Prior CABG | 376 (21.1%) | 78 (8.9%) | 47 (13.5%) | 60 (5.8%) | 561 (13.9%) |
| Early Revascularization (≤ 90 d post PET) | 310 (17.4%) | 34 (3.9%) | 28 (8.0%) | 35 (3.4%) | 407 (10.1%) |
| Late Revascularization (> 90 d post PET) | 131 (7.4%) | 52 (6.0%) | 20 (5.7%) | 47 (4.6%) | 250 (6.2%) |
| Congestive Heart Failure | 174 (9.8%) | 33 (3.8%) | 26 (7.4%) | 22 (2.1%) | 255 (6.3%) |
| Peripheral Vascular Disease | 159 (8.9%) | 52 (6.0%) | 24 (6.9%) | 35 (3.4%) | 270 (6.7%) |
| Cerebrovascular Disease | 150 (8.4%) | 56 (6.4%) | 20 (5.7%) | 44 (4.3%) | 270 (6.7%) |
| Medications | |||||
| Aspirin | 1223 (68.7%) | 486 (55.7%) | 238 (68.2%) | 577 (56.1%) | 2524 (62.6%) |
| Beta-blockers | 1331 (74.8%) | 502 (57.5%) | 255 (73.1%) | 519 (50.5%) | 2607 (64.7%) |
| Lipid Lowering | 1280 (72.0%) | 509 (58.3%) | 232 (66.5%) | 563 (54.8%) | 2584 (64.1%) |
| ACE Inhibitors | 743 (41.8%) | 331 (37.9%) | 176 (50.4%) | 328 (31.9%) | 1578 (39.2%) |
| Insulin | 396 (22.3%) | 146 (16.7%) | 57 (16.3%) | 89 (8.7%) | 688 (17.1%) |
| Indications | |||||
| Chest pain | 636 (35.8%) | 399 (45.7%) | 153 (43.8%) | 625 (60.8%) | 1813 (45.0%) |
| Dyspnea | 556 (31.3%) | 246 (28.2%) | 95 (27.2%) | 282 (27.4%) | 1179 (29.3%) |
| Pre-operative | 282 (15.9%) | 140 (16.0%) | 40 (11.5%) | 110 (10.7%) | 572 (14.2%) |
| Stress Agent | |||||
| Adenosine | 100 (5.6%) | 42 (4.8%) | 17 (4.9%) | 78 (7.6%) | 237 (5.9%) |
| Dipyridamole | 586 (33.1%) | 427 (49.0%) | 73 (21.0%) | 394 (38.4%) | 1480 (36.9%) |
| Dobutamine | 38 (2.1%) | 21 (2.4%) | 11 (3.2%) | 90 (8.8%) | 160 (4.0%) |
| Regadenoson | 1047 (59.1%) | 382 (43.8%) | 247 (71.0%) | 463 (45.2%) | 2139 (53.3%) |
| Radiotracer | |||||
| N-13 Ammonia | 268 (15.1%) | 68 (7.8%) | 87 (24.9%) | 128 (12.5%) | 551 (13.7%) |
| Rubidium-82 | 1511 (84.9%) | 805 (92.2%) | 262 (75.1%) | 900 (87.5%) | 3478 (86.3%) |
| Hemodynamic Parameters | |||||
| HR, bpm | |||||
| Rest | 69 (61-78) | 73 (65-84) | 64 (57-73) | 67 (60-76) | 69 (61-78) |
| Stress | 81 (71-91) | 88 (78-100) | 80 (72-91) | 88 (78-101) | 85 (74-96) |
| Systolic BP, mm Hg | |||||
| Rest | 142 (125-163) | 148 (131-168) | 140 (122-157) | 145 (129-162) | 144 (127-164) |
| Stress | 131 (114-151) | 137 (119-155) | 131 (115-151) | 137 (120-157) | 134 (117-154) |
| Diastolic BP, mm Hg | |||||
| Rest | 72 (63-80) | 73 (65-81) | 73 (64-82) | 73 (65-82) | 72 (64-81) |
| Stress | 64 (57-73) | 65 (58-73) | 65 (59-75) | 67 (60-76) | 65 (58-74) |
| Rate-Pressure-Product, mm Hg*bpm | 9923 (8350-11733) | 11008 (9164-12728) | 9075 (7535-10672) | 9681 (8364-11413) | 9965 (8436-11859) |
| Imaging Parameters | |||||
| Rest LVEF, % | 51 (38-60) | 63 (56-69) | 53 (42-60) | 62 (55-68) | 57 (47-65) |
| LVEF ≤ 35 % | 369 (20.8%) | 37 (4.2%) | 59 (16.9%) | 28 (2.7%) | 493 (12.3%) |
| LVEF 36-49 % | 432 (24.4%) | 84 (9.6%) | 75 (21.5%) | 94 (9.2%) | 685 (17.1%) |
| LVEF ≥ 50 % | 971 (54.8%) | 751 (86.1%) | 215 (61.6%) | 903 (88.1%) | 2840 (70.7%) |
| Scar, % | 0 (0-10) | 0 (0-0) | 0 (0-4) | 0 (0-0) | 0 (0-2.9) |
| Scar ≥ 10% | 455 (26%) | 43 (5%) | 52 (15%) | 40 (4%) | 590 (15%) |
| Ischemia, % | 3 (0-10) | 0 (0-3) | 0 (0-4) | 0 (0-0) | 0 (0-5.9) |
| Ischemia ≥ 10% | 483 (27%) | 75 (9%) | 35 (10%) | 47 (5%) | 640 (16%) |
| Scar + Ischemia, % | 9 (0-24) | 0 (0-6) | 3 (0-10) | 0 (0-1) | 0 (0-11.8) |
| Scar + Ischemia ≥ 10% | 834 (47%) | 131 (15%) | 103 (30%) | 95 (9%) | 1163 (29%) |
| CFR | 1.37 (1.12-1.60) | 1.65 (1.44-1.83) | 2.26 (2.12-2.54) | 2.49 (2.23-2.90) | 1.72 (1.35-2.20) |
| Maximal MBF, ml · g-1· min-1 | 1.21 (0.96-1.47) | 2.30 (1.99-2.75) | 1.55 (1.33-1.69) | 2.55 (2.15-3.14) | 1.75 (1.24-2.39) |
| Resting MBF, ml · g-1· min-1 | 0.89 (0.73-1.07) | 1.47 (1.26-1.75) | 0.63 (0.54-0.73) | 1.01 (0.86-1.22) | 0.98 (0.78-1.28) |
| Rate-Pressure-Product Corrected Resting MBF, ml · g-1· min-1 | 0.72 (0.58-0.92) | 1.12 (0.91-1.38) | 0.57 (0.45-0.70) | 0.87 (0.69-1.07) | 0.82 (0.62-1.07) |
Continuous variables are presented as median [25th–75th percentile]; Categorical variables are presented as n (%).
Impaired CFR was defined as below 2
Impaired maximal MBF was defined as below 1.8 ml · g-1· min-1
BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CFR, coronary flow reserve; HR, heart rate; LVEF, left ventricular ejection fraction; MBF, myocardial blood flow; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood
Figure 1. Scatter Plot of Coronary Flow Reserve and Maximal Myocardial Blood Flow by Cardiovascular Death.
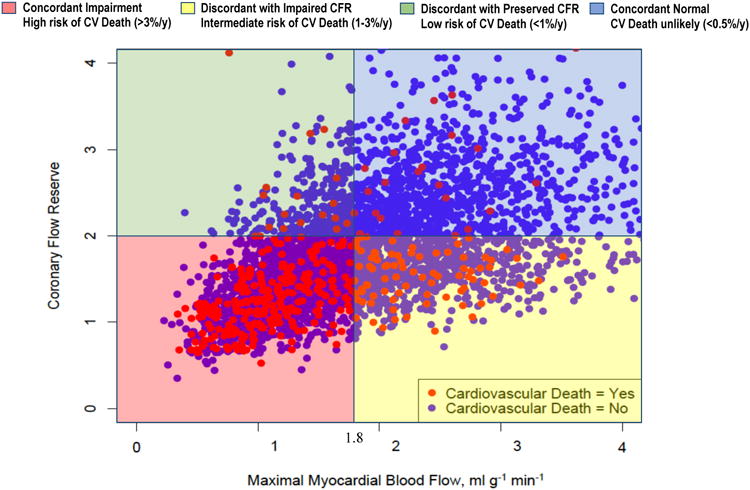
Concordant and discordant impairment of coronary flow reserve and maximal myocardial blood flow identifies unique prognostic phenotypes of patients. Coronary Flow Reserve < 2 and maximal Myocardial Blood Flow < 1.8 ml · g-1· min-1 were defined as impaired. CFR, coronary flow reserve; CV, cardiovascular
Outcomes
After a median follow-up of 5.6 years, there were 1005 total deaths (24.9% of study population), of which 392 were CV deaths (9.7%), Table 2. Patients who suffered CV death were on average older, more likely to be male, and had higher prevalence of hypertension, diabetes, obesity, known CAD, higher burden of myocardial scar and ischemia, lower LVEF, lower CFR, and lower maximal MBF compared with those who did not suffer CV death (Supplementary Table 1). Most CV deaths (n = 349/392, 89%) occurred in patients with impaired CFR (Table 2, Figure 1).
Table 2. Causes of Death.
| Impaired CFR | Preserved CFR | ||||
|---|---|---|---|---|---|
| Cause of Death | Impaired Maximal MBF (n = 1,779) | Preserved Maximal MBF (n = 873) | Impaired Maximal MBF (n = 349) | Preserved Maximal MBF (n = 1,028) | All Patients (n = 4,029) |
| All-cause Death | 594 (33.4%) | 236 (27.0%) | 56 (16.0%) | 119 (11.6%) | 1005 (24.9%) |
| Cardiovascular Death | 269 (15.1%) | 80 (9.2%) | 17 (4.9%) | 26 (2.5%) | 392 (9.7%) |
| Cardiac | 246 (13.8%) | 68 (7.8%) | 16 (4.6%) | 24 (2.3%) | 354 (8.8%) |
| Vascular | 11 (0.6%) | 1 (0.1%) | 1 (0.3%) | 1 (0.1%) | 14 (0.3%) |
| Non-hemorrhagic Stroke | 12 (0.7%) | 11 (1.3%) | 0 (0.0%) | 1 (0.1%) | 24 (0.6%) |
Data presented as n (% of total patients in the group)
Impaired CFR was defined as below 2
Impaired maximal MBF was defined as below 1.8 ml · g-1· min-1
Vascular deaths included deaths related to aortic, mesenteric, renal vascular or peripheral vascular disease excluding coronary or cerebrovascular disease.
CFR, coronary flow reserve; MBF, myocardial blood flow
Independent Prognostic Value of CFR and Maximal MBF
Univariable analysis showed CFR to be a stronger predictor of CV mortality than maximal MBF [hazard ratio, HR (95% confidence interval, CI): 3.37 (2.76-4.11), p<0.001 per unit decrease in CFR, and 2.25 (1.94-2.62), p<0.001 per unit decrease in maximal MBF]. In multivariable analysis, CFR remained a stronger independent predictor of CV mortality than maximal MBF [adjusted HR (95% CI): 1.83 [1.47-2.27], p<0.001 per unit decrease in CFR, and 1.35 (1.13-1.61), p=0.001 per unit decrease in maximal MBF], Figure 2. Risk-reclassification analysis, described below, also supported the stronger independent prognostic value of CFR compared with maximal MBF.
Figure 2. Independent and Incremental Prognostic Value of Coronary Flow Reserve and Maximal Myocardial Blood Flow.
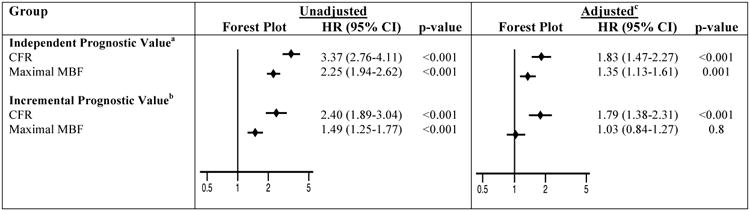
Hazard ratios are expressed per unit decrease in CFR or maximal MBF. aFor assessment of independent prognostic value, CFR or maximal MBF were modeled as continuous variables in separate models. bFor assessment of incremental prognostic value, both CFR and maximal MBF were modeled as continuous variables together in the same model. cAdjusted Cox model includes the following covariates: age, sex, hypertension, diabetes, dyslipidemia, dialysis, body mass index, known coronary artery disease, left ventricular ejection fraction, amount of myocardial scar/ischemia, revascularization post-positron emission tomography scan, rate-pressure-product, type of radiotracer or stress agent. CFR, coronary flow reserve; CI, confidence interval; HR, hazard ratio; MBF, myocardial blood flow
Integrated Prognostic Value of CFR and Maximal MBF
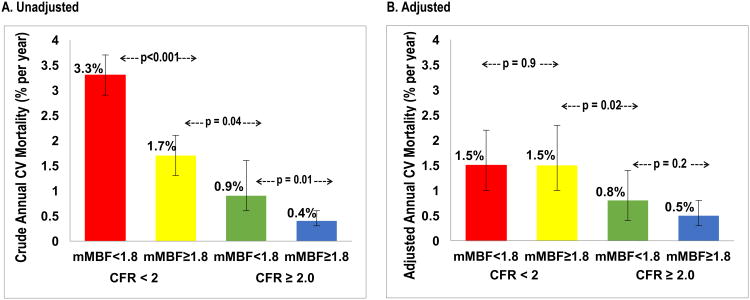
Crude annualized CV mortality rates showed a significant risk gradient for CV mortality in the 4 groups based on the concordant or discordant impairment of CFR and maximal MBF (Figure 3A). Annual CV mortality risk was 3.3%(95% CI: 2.9-3.7%), 1.7%(1.3-2.1%), 0.9%(0.6-1.6%), and 0.4% (0.3-0.6%), respectively, in patients with impairment of both CFR and maximal MBF, impaired CFR with preserved maximal MBF, preserved CFR with impaired maximal MBF, and when both CFR and maximal MBF were preserved (Figure 3A). In adjusted analysis, elevated CV mortality was independently driven by the impairment of CFR irrespective of whether the maximal MBF was impaired or preserved (Figure 3B). Adjusted annual CV mortality was 1.5% (95% CI: 1.0-2.3%), 1.5% (1.0-2.3%), 0.8% (0.4-1.4%) and 0.5% (0.3-0.8%), respectively, in patients with impairment of both CFR and maximal MBF, impaired CFR with preserved maximal MBF, preserved CFR with impaired maximal MBF, and when both CFR and maximal MBF were preserved (Figure 3B).
Figure 3. Annualized Cardiovascular Mortality Rates.
Figure shows annualized CV mortality for the four groups based on concordant or discordant impairment of CFR and maximal MBF. Figure 3A shows crude annualized CV mortality risk. Figure 3B shows adjusted annualized CV mortality risk after adjustment for age, sex, baseline CV risk factors, left ventricular ejection fraction, amount of myocardial scar and ischemia, revascularization post-positron emission tomography scan, rate-pressure-product, and type of radiotracer or stress agent. CFR, coronary flow reserve; CV, cardiovascular; mMBF, maximal myocardial blood flow
CV mortality event curves for the four groups with concordant or discordant impairment of CFR and maximal MBF are shown in Figure 4. As seen with annualized CV mortality, a risk gradient for cumulative CV mortality hazard was observed across the four groups in unadjusted analysis (Figure 4A). At the end of 8.4 years of total follow-up time in the study, cumulative CV mortality rates were 19.6%, 11.3%, 6.4%, and 3.0%, respectively, in patients with impairment of both CFR and maximal MBF, impaired CFR with preserved maximal MBF, preserved CFR with impaired maximal MBF, and when both CFR and maximal MBF were preserved. Adjusted analysis revealed that the CV mortality risk was independently driven by the impairment of CFR but not by whether maximal MBF was preserved or impaired within a category of CFR (Figure 4B).
Figure 4. Event Curves for Cardiovascular Mortality.
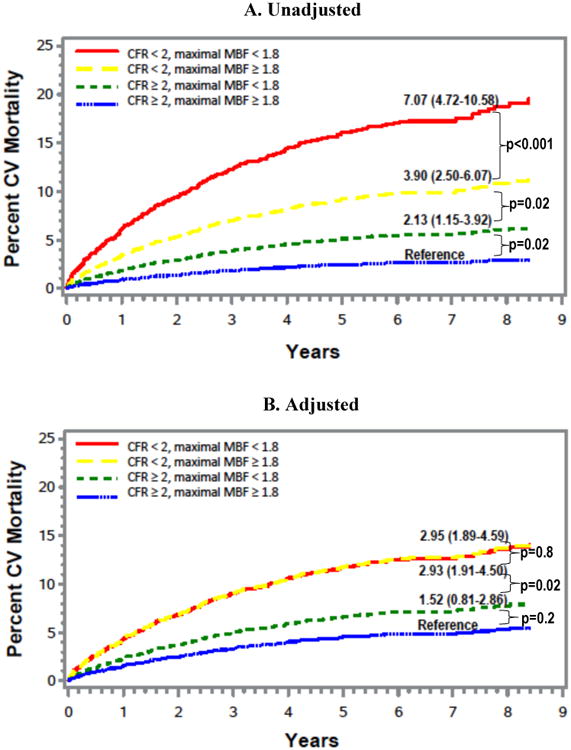
Figure shows event curves for probability of CV mortality over time for the four groups based on concordant or discordant impairment of CFR and maximal MBF. Figure 4A depicts unadjusted event curves, and Figure 4B depicts adjusted event curves after adjustment for age, gender, baseline CV risk factors, left ventricular ejection fraction, amount of myocardial scar and ischemia, revascularization post-positron emission tomography scan, rate-pressure-product, type of radiotracer or stress agent. CFR, coronary flow reserve; CV, cardiovascular; MBF, myocardial blood flow; PET,
Modeling CFR and maximal MBF as continuous variables together in the same model to assess incremental prognostic value of one coronary blood flow variable over the other further supported the stronger prognostic value of CFR over maximal MBF. In the analysis without adjustment for other clinical covariates, both CFR and maximal MBF were associated with CV mortality (HR [95% CI]: 2.40 [1.89-3.04], p<0.001 per unit decrease in CFR, and 1.49 [1.25-1.77], p<0.001 per unit decrease in maximal MBF), Figure 2. However, after adjustment for clinical covariates described above, CFR but not maximal MBF were associated with CV mortality (adjusted HR [95% CI]: 1.79 [1.38-2.31], p<0.001 per unit decrease in CFR after adjustment for maximal MBF and clinical covariates, and 1.03 [0.84-1.27], p=0.8 per unit decrease in maximal MBF after adjustment for CFR and clinical covariates), Figure 2.
Risk-Reclassification
The addition of CFR to the pre-CFR model resulted in the reclassification of 9%, 29%, and 10% of patients at low, intermediate, and high cardiac risk, respectively (Supplementary Figure 1A). The addition of maximal MBF to the pre-MBF model without CFR resulted in the reclassification of 6%, 17%, and 5% of patients at low, intermediate, and high cardiac risk, respectively (Supplementary Figure 1B). The addition of maximal MBF (impaired or preserved) to the pre-risk model with CFR (impaired or preserved) resulted in the reclassification of 5%, 10%, and 1% of patients at low, intermediate, and high cardiac risk, respectively (Supplementary Figure 1C). The categorical and continuous net reclassification indices are detailed in the online supplementary appendix.
Exploratory Sub-Group Analysis
The exploratory sub-group analysis showed a consistently stronger independent prognostic value of CFR for predicting CV mortality compared with maximal MBF across the sub-groups studied (Figure 5). In addition, CFR showed a consistent incremental prognostic value over maximal MBF in most of the sub-groups studied (Figure 6). However, maximal MBF did not show incremental prognostic value over CFR in any of the sub-groups studied (Figure 6).
Figure 5. Exploratory Sub-Group Analysis for Independent Prognostic Value of Coronary Flow Reserve and Maximal Myocardial Blood Flow.
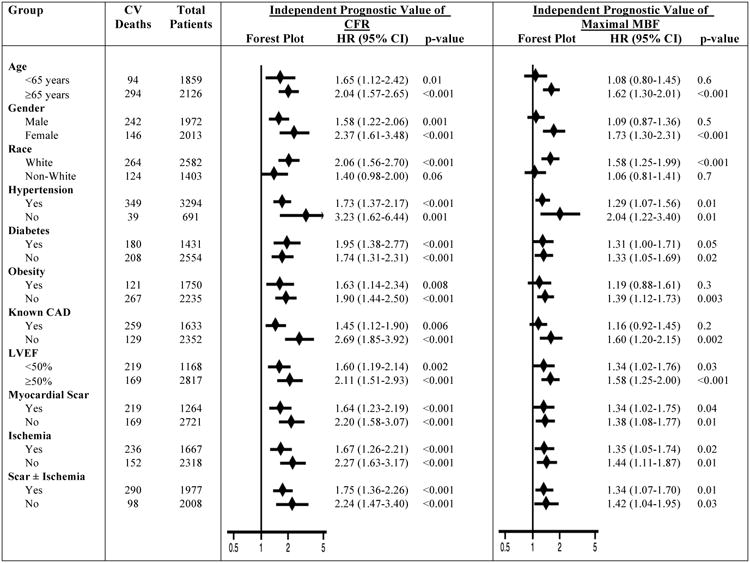
For the assessment of independent prognostic value, CFR or maximal MBF were modeled as continuous variables in separate models. Hazard ratios are expressed per unit decrease in CFR or maximal MBF. Hazard ratios are adjusted for age, sex, hypertension, diabetes, dyslipidemia, dialysis, body mass index, known coronary artery disease, left ventricular ejection fraction, amount of myocardial scar/ischemia, revascularization post-positron emission tomography scan, rate-pressure-product, type of radiotracer or stress agent. A particular variable is excluded from adjustment when it is sub-group of interest. CAD, coronary artery disease; LVEF, left ventricular ejection fraction
Figure 6. Exploratory Sub-Group Analysis for Incremental Prognostic Value of Coronary Flow Reserve and Maximal Myocardial Blood Flow.
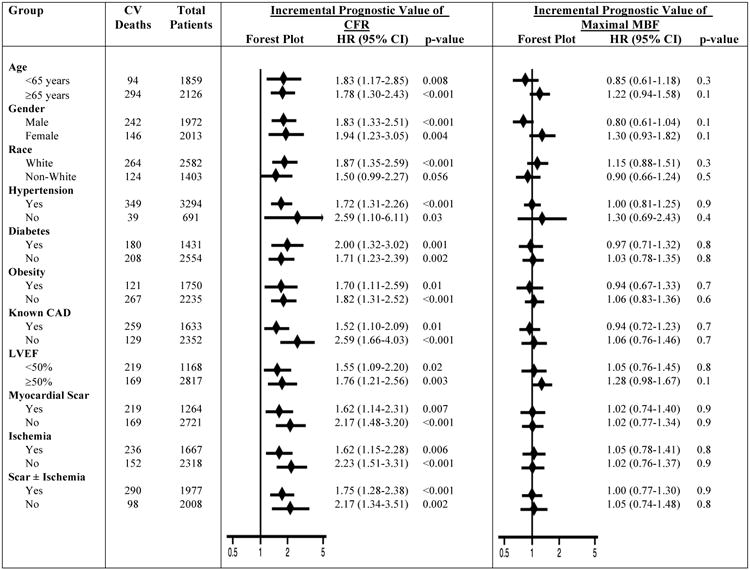
For the assessment of incremental prognostic value, both CFR and maximal MBF were modeled together as continuous variables in the same model. Hazard ratios are expressed per unit decrease in CFR or maximal MBF. Hazard ratios are adjusted for age, sex, hypertension, diabetes, dyslipidemia, dialysis, body mass index, known coronary artery disease, left ventricular ejection fraction, amount of myocardial scar/ischemia, revascularization post- positron emission tomography scan, rate-pressure-product, type of radiotracer or stress agent. A particular variable is excluded from adjustment when it is sub-group of interest. CAD, coronary artery disease; LVEF, left ventricular ejection fraction
Sensitivity Analyses
The conclusions regarding the independent and incremental prognostic value of CFR and maximal MBF were unchanged when non-CV death was included in the model as a competing risk for CV death (Supplementary Figure 2) or when all-cause mortality was assessed as the outcome of interest (Supplementary Figure 3). In our primary analyses, a CFR < 2 was used to define impaired CFR. The results were unchanged when incremental prognostic value of maximal MBF was separately evaluated in groups of mild to moderately (1.5-2) or severely impaired (< 1.5) CFR (Supplementary Figure 4).
Discussion
In this large cohort of 4,029 patients with known or suspected CAD and a median follow-up of 5.6 years, we found that CFR was a stronger independent predictor of CV mortality than maximal MBF. CFR, as a ratio of maximal to resting MBF, may better isolate vasodilator capacity and reduce systematic errors in the measurement of MBF.9 This may partly explain the stronger independent prognostic value of CFR compared with maximal MBF.
Further, we showed that the integrated physiologic assessment of coronary blood flow with groups based on the concordant or discordant impairment of CFR and maximal MBF identified unique prognostic phenotypes of patients. The group with concordantly impaired CFR and maximal MBF had the highest CV mortality (3.3% per year). This group of patients had the highest burden of myocardial scar and ischemia (47% patients in this group with scar + ischemia ≥10% of left ventricular myocardium), suggesting significant underlying obstructive CAD. Our study design does not allow to directly validate the diagnostic utility of CFR and maximal MBF for differentiating epicardial obstruction from diffuse non-obstructive atherosclerosis and microvascular dysfunction due to lack of cardiac catheterization data in the majority of patients. However, our study may provide risk-based guidance for decision of referral to cardiac catheterization. For example, in the group with concordantly impaired CFR and maximal MBF, the risk of CV death is high as is the likelihood for multivessel disease.23, 24 Therefore, angiographic (invasive or computed tomographic) evaluation may generally be necessary to define the specific phenotype of CAD (i.e. predominantly obstructive CAD, diffuse non-obstructive atherosclerosis, and microvascular dysfunction). In contrast, the group with concordantly normal CFR and maximal MBF had the lowest CV mortality risk (0.4% per year) and are unlikely to have flow limiting CAD and, consequently, coronary angiography would be rarely necessary. The discussion on discordant groups follows below.
Discordant Group with Impaired CFR and Preserved Maximal MBF
Patients with impaired CFR and preserved maximal MBF had an elevated CV mortality risk of 1.7% per year. The mechanism of increased risk of CV mortality in patients with impaired CFR and preserved maximal MBF (indicating high resting MBF) was not investigated in our study. However, the elevated CV mortality risk in these patients persisted even after correcting for the rate-pressure-product, suggesting that the risk is dependent on factors beyond simply a hemodynamic effect from increased myocardial workload. The majority (608/873, 70%) of this discordant group were women. The scar and ischemia burden was low with large area of scar and ischemia (≥10% left ventricular myocardium) present in only 15% of patients in this group, suggesting predominantly underlying non-obstructive CAD, a common phenotype in women with impaired CFR.11 Further, the studies utilizing PET to measure MBF have shown that women have higher resting MBF than men.19, 25 Whether the elevated CV mortality risk in this group is a reflection of high CV risk inherent to women or is directly related to underlying flow alterations is not known. However, in our analysis, even after adjustment for various CV risk factors, the CV mortality in this group remained high with adjusted CV mortality risk similar to that of the group with concordantly impaired CFR and maximal MBF. Therefore, there may be an unmet need for initiation or intensification of lifestyle and/or pharmacological preventive therapies for cardiovascular risk reduction in this group of patients that needs evaluation in randomized trials. As women disproportionately represent this group, targeting it may help reduce the gender gap in cardiovascular outcomes.
Discordant Group with Preserved CFR and Impaired Maximal MBF
The patients with preserved CFR but impaired maximal MBF had low risk of CV mortality (0.9% per year). This occurred despite significant scar and ischemia burden (scar + ischemia ≥10% left ventricular myocardium in 30% of patients in this group), suggesting a higher prevalence of underlying obstructive CAD in this group. As this is an observational cohort study, patients' risk was potentially modified by revascularization. However, even after adjustment for post-scan revascularization, the CV mortality risk in this group of patients was low and similar to those with concordantly preserved CFR and maximal MBF (0.8% versus 0.5% per year respectively, p = 0.2). The observed low CV risk in patients with preserved CFR and impaired maximal MBF assessed globally for the entire left ventricular myocardium parallels the coronary vascular territory-specific findings in the invasive literature; patients with decreased fractional flow reserve (a ratio of two pressures under maximal hyperemia; thus, reflecting a ratio of two maximal MBFs) but preserved coronary flow velocity reserve have predominantly focal epicardial stenosis but still have low risk of adverse CV outcomes.5, 26 Whether this group potentially represents patients that may be best served by optimal medical therapy for CAD management, with revascularization reserved for refractory symptoms, needs further investigation in clinical trials.
Limitations
Our study is a single-center observational study and as such has some inherent limitations. We adjusted our analyses for a large number of CV risk factors but there is likely residual and unmeasured confounding. We had broad inclusion criteria, but, given the large sample size, we were able to conduct exploratory sub-group analyses in various patient populations of clinical interest and found consistent results. Moreover, the broad inclusion criteria allow for increased generalizability of our findings. Lastly, as we studied patient-level outcomes in the form of CV mortality risk, the analysis was on a per-patient level (and not per-vessel level) based on the global CFR and maximal MBF for the entire left ventricular myocardium.
Conclusion
In conclusion, CFR is a stronger predictor of CV mortality than maximal MBF. Integrated physiologic assessment of coronary circulatory function based on the concordant or discordant impairment of CFR and maximal MBF identifies unique prognostic phenotypes of patients with known or suspected CAD.
Supplementary Material
Clinical Perspective.
What Is New?
In patients with known or suspected coronary heart disease, coronary flow reserve is a stronger independent predictor of cardiovascular mortality than absolute maximal myocardial blood flow beyond traditional cardiovascular risk factors, hemodynamic load (rate-pressure-product), myocardial scar/ischemia, left ventricular ejection fraction, and post-scan revascularization.
Integrated non-invasive physiologic assessment of coronary circulatory function with concordant or discordant impairment of coronary flow reserve and maximal myocardial blood flow identifies unique prognostic phenotypes in stable coronary artery disease.
What Are The Clinical Implications?
Impaired coronary flow reserve with preserved maximal myocardial blood flow identifies patients at an increased risk of cardiovascular mortality despite lack of myocardial ischemia. These patients may thus be an appropriate target for initiation or intensification of lifestyle and/or pharmacological preventive therapies for cardiovascular risk reduction. As women disproportionately represent this group, targeting it may help reduce the gender gap in cardiovascular outcomes.
Preserved coronary flow reserve even in the presence of impaired maximal myocardial blood flow identifies low risk patients with <1% annual cardiovascular mortality risk. Future trials are needed to identify if there is a role for coronary revascularization in this low risk cohort.
Acknowledgments
Sources of Funding: This study was supported in part by grants 5T32HL094301-07, 5T32076136-12, K23HL135438, and 5R01HL132021-02 from the National Institutes of Health.
Footnotes
Twitter Handles: Ankur Gupta: @AnkurGuptaMD, Marcelo F. Di Carli: @mdicarli
Conflict of Interest Disclosures: Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.
Dr. Venkatesh L. Murthy discloses the following relationships - stock in General Electric, research support from INVIA Medical Imaging Solutions, speaking/consulting fees from Bracco Diagnostics and Ionetix.
Dr. Paolo G. Camici discloses the following relationships – consultant for Servier, speaking fees from Menarini.
All other authors have no relevant disclosures.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–11. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 6.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1076–87. doi: 10.2967/jnumed.108.054478. [DOI] [PubMed] [Google Scholar]
- 7.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–35. doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017;135:566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Hoef TP, Siebes M, Spaan JA, Piek JJ. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J. 2015;36:3312–9a. doi: 10.1093/eurheartj/ehv235. [DOI] [PubMed] [Google Scholar]
- 13.Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA, Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–53. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 14.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovascular imaging. 2012;5:430–40. doi: 10.1016/j.jcmg.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 15.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1062–71. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:783–93. doi: 10.2967/jnumed.106.032789. [DOI] [PubMed] [Google Scholar]
- 17.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46:1264–71. [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 19.Schindler TH. Myocardial blood flow: Putting it into clinical perspective. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2016;23:1056–71. doi: 10.1007/s12350-015-0372-4. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 21.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV, Anderson JL American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126:e354–471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 23.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, Di Carli MF. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:248–55. doi: 10.2967/jnumed.113.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, Klein R, Ruddy TD, Aung M, Garrard L, Beanlands RS. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19:670–80. doi: 10.1007/s12350-011-9506-5. [DOI] [PubMed] [Google Scholar]
- 25.Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–61. doi: 10.1016/s0008-6363(01)00202-4. [DOI] [PubMed] [Google Scholar]
- 26.van de Hoef TP, Echavarria-Pinto M, Escaned J, Piek JJ. Coronary flow capacity: concept, promises, and challenges. The international journal of cardiovascular imaging. 2017;33:1033–1039. doi: 10.1007/s10554-017-1125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.