Abstract
Despite the lifecourse focus of nursing clinical care, nursing research largely remains cross-sectional or process-oriented within silos determined by patient characteristics such as age, acuity, or disease process. Incorporating interdisciplinary lifecourse theory into pediatric nursing research provides the opportunity to expand nursing theories and research beyond practice, age, and disease silos. One such theory is the Lifecourse Health Development (LCHD) framework. LCHD takes a more expansive view of health development from preconception through old age based on the premise that health is a consequence of transactions between genetic, biological, behavioral, social, and economic contexts that change as a child develops over time (Halfon & Hochstein, 2002). LCHD also explains how intergenerational influences and prevention during early life help predict health development and disease over the lifespan. The preventive and lifecourse focus of LCHD is well-aligned with the lifespan wellness foci of pediatric nurses. The purpose of this paper is to introduce pediatric nurse researchers to LCHD and discuss proposed augmentations and implications related to expanding LCHD into pediatric nursing research.
Keywords: life course, lifecourse, health development, nursing theory
Introduction
Nurses touch people at every point of the lifespan. Despite the lifecourse focus of nursing care, nursing theory and research have largely remained cross-sectional or process-oriented within silos determined by patient characteristics such as age, acuity, or disease process. Interdisciplinary lifecourse theories have the potential to guide nursing research towards investigating mechanisms through which disease and health disparities develop, offering a powerful means of primary prevention during early life that is desperately needed in our compartmentalized and overburdened health system.
The Lifecourse Health Development framework (LCHD; Halfon & Hochstein, 2002; Halfon, Larson, Lu, Tullis, & Russ, 2014) is one example of a promising comprehensive lifecourse theory. The LCHD framework integrates biological, social, and environmental foundations of health to explain how factors during early childhood influence health outcomes later in life (Halfon & Hochstein, 2002; Halfon et al., 2014). Enriching our understanding of the mechanisms underlying health development across the lifespan offers significant potential for developing nursing interventions to ameliorate risk and enhance protective factors for individuals during early childhood. Consequently, introducing LCHD into pediatric nursing research offers an opportunity for expanding existing nursing theories into person-centered models of nursing care and research that move beyond practice and disease silos. This paper presents an introduction to the LCHD framework, proposed LCHD augmentations, and LCHD implications for pediatric nursing research.
Background and Introduction to LCHD
Theoretical foundations for LCHD date back to the late 20th century with work by physician David Barker and sociologist Glen Elder, Jr. Barker’s epidemiologic studies (Barker, 1995; Barker, 1998) demonstrated that low birthweight predicted cardiovascular disease in middle adulthood, which informed the theory on the fetal origins of disease (Barker, 1995) and later the developmental origins of health and disease (DOHaD) theory (Gluckman & Hanson, 2004). DOHaD focuses on biological development from preconception to birth and serves as a theoretical foundation for pediatric nursing research (Cota & Allen, 2010; Thiele & Anderson, 2016).
Elder (1998) built an integrated ecological systems model examining changing life trajectories by leveraging longitudinal studies of children who were developing during the sociocultural and economic turmoil of The Great Depression. According to Elder (1998), contextual effects accumulate over time, influencing the trajectory of human development. These early theoretical foundations would later form the theoretical underpinnings of LCHD.
Overview of LCHD
Halfon et al. (2002; 2014) incorporated these theories and expansive multidisciplinary empirical work into LCHD, yielding a coherent, interdisciplinary perspective on health development across the lifespan with a focus on early life exposures and experiences. LCHD is a lifecourse theoretical framework that explains how health develops across the lifecourse, through the accumulation of risk and protective factors (Figure 1), and enables one to adapt to unexpected challenges. LCHD defines health development as a dynamic trajectory that continuously shifts across the lifecourse and is based on six tenets (Table 1). LCHD purports that early lifecourse risk and protective factors influence overall health and disease trajectories, challenging the notion that adult disease mainly results from acute influences. These risk and protective factors arise from complex, multilevel processes, encompassing a wide range of determinants, from biology to policy (Halfon & Hochstein, 2002; Halfon et al., 2014).
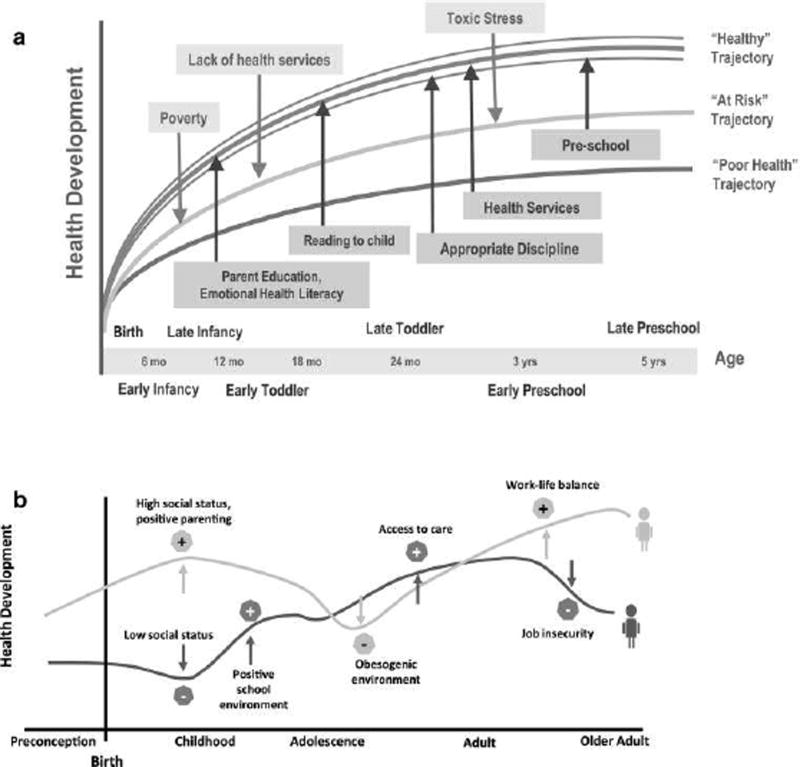
Figure 1.
These two graphics (a and b) illustrate the development of health trajectories based on the impact of various risk, promoting, and protective factors on health development. Graphic a demonstrates how higher or lower health development trajectories are influenced by the relative number and magnitude of risk and protective factors. Graphic b demonstrates how trajectories are not straight, linear, overly determined, or immutable, but can be in a constant state of flux relative to different influences at different points in time. These graphical illustrations of health trajectories challenge the notion that adult disease manifestations have more acute influences; rather, early life course factors begin the health trajectory, influenced by factors along the way. Reprinted with permission from “Lifecourse health development: Past, present, and futures,” by N. Halfon, K. Larson, M. Lu, E. Tullis, & S. Ross, 2014, Journal of Maternal and Child Health, 18(2), p. 352. Copyright 2014 by Springer.
Table 1.
The Six Health Tenets of the Lifecourse Health Development Framework
| Tenet | Description |
|---|---|
| 1 | Health is defined as a set of capacities that develop over time. |
| 2 | Health constantly develops over time, based on by interactions between one’s physiology and their environment. Development occurs during four phases:
|
| 3 | Health develops as a complex, non-linear system over several dimensions, phases, and levels. |
| 4 | Health development is responsive to the social structuring and timing of ecological experiences. |
| 5 | Health development is an evolving process that uses resilience and plasticity to adapt to changing ecological contexts. |
| 6 | Health development is responsive to the timing and synchronization of micro- to macro- level ecological pathways, from molecular to social and cultural functions. |
Note. Table content from “Lifecourse health development: Past, present, and futures,” by N. Halfon, K. Larson, M. Lu, E. Tullis, & S. Ross, 2014, Journal of Maternal and Child Health, 18(2), p. 344-365.
LCHD typifies periods of development as important considerations of how health trajectories develop. Specifically, the period of conception to early childhood is particularly sensitive to the complex, multilevel risk and protective factors that subsequently influence health development. Providing early intervention to ameliorate risk and enhance protective factors during this crucial pediatric developmental period allows children to start with the biological capacity to achieve optimal health and prevents the need for aggressive, often costly, interventions later in life (Shonkoff, 2010).
For example, early exposure to toxic stress resulting from abuse or neglect “primes” the child’s stress response to habitual over-activation, thereby affecting the structure and function of the developing brain (Shonkoff, 2010; Shonkoff et al., 2012). Further, the Adverse Childhood Experiences Study (ACES) demonstrated that childhood adversity predicts numerous adverse health outcomes such as adult depression, heart disease, substance abuse, and cancer, which are all potentially preventable contributors of early disability and mortality (Felitti et al., 1998). Similarly, adolescence is recognized in LCHD as an important developmental and transitional life stage that further lays the foundation for adult health behaviors and outcomes (Halfon & Hochstein, 2002). During adolescence, social contexts and networks play an increasingly important role in health development (Halfon & Hochstein, 2002; Moen & Wethington, 1999). For example, research by Ford and Browning (2011) showed that neighborhood context plays an important role in the perpetuation of risky sexual behaviors and the spread of sexually- transmitted infection among adolescents.
Per LCHD, another important construct in pediatric health development is intergenerational influences (Halfon et al., 2014). For example, children born into poor families have poorer health than children born to families with adequate resources, leading to poor educational attainment and lower future household income, consequently perpetuating intergenerational poverty and illness ( Case, Fertig, & Paxson, 2005; Case & Paxson, 2002; Salsberry, Reagan, & Fang, 2013). Halfon et al. (2014) argue that this intergenerational health and disease transmission should be an increasing focus among pediatric researchers and health care providers. Nurse researchers could play an important role in preventing the intergenerational transmission of adverse health by using LCHD to generate research supporting families and strengthening the capacities of parents.
Halfon et al. (2014) note that major changes in health trajectories are sometimes caused by relatively minute differences in early developmental exposures. Because of these relatively minute differences, LCHD emphasizes that preventive intervention during early life are key to providing the best benefits for a person’s long-term health and may be vastly more cost-effective than interventions delivered later in the disease process.
The expanding focus into preventing and reducing chronic disease burden via early interventions such as prevention, wellness, behavior change, and symptom management across the lifespan is aligned with the Healthy People initiatives and research priorities of the National Institute of Nursing Research 2016 Strategic Plan (National Institute of Nursing Research, 2016). However, much nursing research continues to focus on short-term outcomes and largely cross- sectional models of health behavior change as the U.S. health system labors under the costly and ever-growing burden of complex chronic disease management. Adopting a lifecourse theoretical perspective would enable nurse researchers to shift the focus from short-term outcomes with relatively small measurable impacts to more effectively engage in primary prevention in pediatric populations of adult chronic diseases and examine longitudinal outcomes of this shift.
LCHD consequently offers a perspective that spans the full lifecourse of individuals and characterizes the intergenerational transmission of health and disease. This perspective may have wide-ranging implications for nursing research, and provides a deeper understanding of the current state of health and the health trajectory of individuals, families, and populations.
Critiques of LCHD and Suggested Augmentations for Nursing Research
Despite the many benefits to pediatric nursing research of adopting a lifecourse theoretical model such as LCHD, several critiques of lifecourse models have been identified. These critiques include determinism, the nature of sensitive and critical periods of development, and complexity (Wise, 2003; Wise, 2009).
Determinism
Lifecourse models have been criticized as overly deterministic due to their focus on genetics as the foundation for optimal development (Wise, 2009). However, ongoing work in epigenetics indicates that genes are not destiny but rather are a starting position that may be influenced by multiple social and biological contexts over time (Weinhold, 2006). Halfon et al. (2014) also directly addressed this critique in the updated LCHD model (from the Halfon & Hochstein, 2002 version). LCHD recognizes that genetic makeup is influential to health development but that gene expression is affected by external influences (Halfon & Hochstein, 2002; Halfon et al., 2014). For example, prolonged exposure to adverse experiences during childhood and resulting activation of the body’s regulatory systems can compromise optimal health development by influencing genetic expression towards immediate survival, wearing and tearing body systems, and increasing risk of later disease (Barker, Eriksson, Forsen, & Osmond, 2002). We endorse the Halfon et al. (2014) framework for pediatric nursing research considerations, because in the context of multifactorial, complex disease (e.g., cardiovascular disease), genes are influential for, but not deterministic of, health outcomes (Yu et al., 2016).
Critical and Sensitive Periods
The LCHD model does not differentiate between sensitive and critical developmental periods during childhood and uses the terms interchangeably. This lack of differentiation has garnered robust critique (e.g. Wise, 2009) and limits interpretation on how the particular timing of events during sensitive or critical periods in childhood contributes to varied subsequent outcomes. Differentiating between sensitive versus critical periods is not just a matter of semantics, rather, there is an important, interpretable difference between them described below.
Sensitive periods during child development are defined as periods of increased vulnerability to environmental insult or responsiveness to appropriate stimuli (Knudsen, 2004). They are often characterized by a high degree of neurological plasticity, during which contexts influence the formation of new neuronal circuits and synaptic pruning. This plasticity can be adaptive or maladaptive depending on the context of experiences and the response of the brain (Pickler et al., 2010). For example, animal models have shown that sensory deprivation during childhood results in more profound neurodevelopmental impairments than deprivation during adulthood, suggesting that childhood is a relatively sensitive period for sensory development (Buran et al., 2014; Mowery, Kotak, & Sanes, 2015). Critical periods are times when environmental influences result in permanent developmental sequelae (Knudsen, 2004). For example, during fetal development periods, if an angiotensin converting enzyme inhibitor is consumed by the mother in the second or third trimesters, irreversible adverse effects on nephrogenesis occurs (Ratnapalan & Koren, 2002; Tabacova, Little, Tsong, Vega, & Kimmel, 2003). Thus, while harms occurring during sensitive periods of child development may be amenable to later, often costly and intensive intervention, harms during critical developmental periods are biologically deterministic resulting in long-term consequences for the child. We propose that the LCHD model be expanded to differentiate between critical and sensitive periods of child development, particularly for pediatric nursing research.
Complexity
The purpose of the LCHD framework is to provide a hypothesis-generating, testable model that considers the multidimensional factors affecting child development and health across time, to ultimately shift healthcare towards optimizing population health by preventing chronic disease (Halfon et al., 2014). However, this multifactorial approach has also been criticized. According to Wise (Wise, 2003),
The concern here is that life is too complicated, the human organism is too adaptive, society too dynamic, and clinical and public health interventions too innovative, to plot with any precision life-course health trajectories on the basis of early life experiences (p. 154).
In response, the overall goal of using a LCHD framework in nursing research is to describe, on average for children, the multiple, complex determinants of health across the lifespan, not to perfectly predict individual health trajectories (Figure 1). While life is certainly complex, it is through acknowledgement of that complexity that researchers can more accurately model the effects of early life exposures and contexts on more specific immediate and long-term health outcomes. This does pose additional challenges to pediatric nurse researchers, who must carefully consider sample size and selection, variables to be measured, increased costs, and advanced statistical modeling to better examine this complexity across the lifespan.
Implications for Nursing Research
Despite nursing's historical focus on prevention, much of nursing research remains tethered to short-term outcomes or practice silo approaches. By shifting to a lifecourse perspective through adapting LCHD, pediatric nursing research will have the improved ability to contextualize the lives of children and understand the influence of social, biological, and environmental factors on the development of healthy children into healthy adults. This will lead to evidence-based preventive interventions that maximize health and minimize the risks for later lifecourse disease burden. An example of a research question that may be derived from LCHD and used in nursing research is, “Do children respond differently to stress than adults and does the specific timing of stress particularly affect adult health (Bates, Salsberry, & Ford, 2017)?” Another example is whether interventions to improve asthma controller medication adherence in children can prevent the development of chronic obstructive pulmonary disease in adulthood.
Conclusion
The LCHD framework is a lifecourse theory that was developed in response to a growing body of multidisciplinary, empirical research on the development of health and disease across the lifespan. This theoretical framework is focused on the pediatric population and primary prevention of adult disease by emphasizing optimal health development from preconception through old age and intergenerationally. This preventive focus is well-matched to the changing milieu of health care and is aligned with the wellness foci of pediatric nurses.
Acknowledgments
Manuscript preparation was supported by a Ruth L. Kirschstein National Research Service Award (T32NR014225). The content is based solely on the perspectives of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank Pamela Salsberry, PhD, RN, FAAN for her expert introduction to lifecourse theories and starting the T32 group focused on health development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors report no financial interests or potential conflicts of interest.
Ethical Statement
Randi Bates reports no financial interests or potential conflicts of interest. Lisa Blair reports no financial interests or potential conflicts of interest. Emma Schlegel reports no financial interests or potential conflicts of interest. Colleen McGovern reports no financial interests or potential conflicts of interest. Marliese Nist reports no financial interests or potential conflicts of interest. Stephanie Sealshcott reports no financial interests or potential conflicts of interest. Kimberly Arcoleo reports no financial interests or potential conflicts of interest.
The authors confirm that the content in this manuscript has not been published elsewhere. We confirm this manuscript has not been submitted for publication to any other journal, nor will it be submitted to any other journal. All authors express approval of the manuscript and agree with its submission to the Journal of Pediatric Health Care. If accepted, this manuscript will not be published elsewhere, including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Contributor Information
Randi A. Bates, The Ohio State University, College of Nursing.
Lisa M. Blair, The Ohio State University, College of Nursing.
Emma C. Schlegel, The Ohio State University, College of Nursing.
Colleen M. McGovern, The Ohio State University, College of Nursing.
Marliese Dion Nist, The Ohio State University, College of Nursing.
Stephanie Sealschott, The Ohio State University, College of Nursing.
Kimberly Arcoleo, University of Rochester, School of Nursing.
References
- Barker DJ. The fetal and infant origins of disease. European Journal of Clinical Investigation. 1995;25(7):457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: Strength of effects and biological basis. International Journal of Epidemiology. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and health in later life. 2. Churchill Livingstone; Edinburgh: 1998. [Google Scholar]
- Bates R, Salsberry P, Ford J. Measuring Stress in Young Children Using Hair Cortisol: The State of the Science. Biological Research for Nursing. 2017 doi: 10.1177/1099800417711583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buran BN, Sarro EC, Manno FA, Kang R, Caras ML, Sanes DH. A sensitive period for the impact of hearing loss on auditory perception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(6):2276–2284. doi: 10.1523/JNEUROSCI.0647-13.2014. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. Journal of Health Economics. 2005;24(2):365–389. doi: 10.1016/j.jhealeco.2004.09.008. doi: http://dx.doi.org/10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Case A, Paxson C. Parental behavior and child health. Health Affairs. 2002;21(2):164, 178. doi: 10.1377/hlthaff.21.2.164. [DOI] [PubMed] [Google Scholar]
- Cota BM, Allen PJ. The developmental origins of health and disease hypothesis. Pediatric Nursing. 2010;36(3):157. [PubMed] [Google Scholar]
- Elder GH. The life course as developmental theory. Child Development. 1998;69(1):1–12. [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. doi:S0749379798000178 [pii] [DOI] [PubMed] [Google Scholar]
- Ford JL, Browning CR. Neighborhood Social disorganization and the acquisition of trichomoniasis among young adults in the United States. American Journal of Public Health. 2011;101(9):1696–1703. doi: 10.2105/AJPH.2011.300213. https://doi.org/10.2105/AJPH.2011.300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatric Research. 2004;56(3):311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- Halfon N, Hochstein M. Life course health development: An integrated framework for developing health, policy, and research. The Millbank Quarterly. 2002;80(3):433–479. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: Past, present and future. Maternal and Child Health Journal. 2014;18(2):344–365. doi: 10.1007/s10995-013-1346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EEI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Moen P, Wethington E. Midlife development in a life course context. Life in the Middle: Psychological and Social Development in Middle Age. 1999:3–23. [Google Scholar]
- Mowery TM, Kotak VC, Sanes DH. Transient hearing loss within a critical period causes persistent changes to cellular properties in adult auditory cortex. Cerebral Cortex (New York, N.Y: 1991) 2015;25(8):2083–2094. doi: 10.1093/cercor/bhu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Nursing Research. The NINR strategic plan: Advancing science, improving lives. A vision for nursing science. Washington, DC: National Institute of Nursing Research; 2016. [Google Scholar]
- Pickler R, McGrath J, Reyna B, McCain N, Lewis M, Cane S, Wetzel P, Best A. A model of neurodevelopmental risk and protection for preterm infants. Journal of Perinatal and Neonatal Nursing. 2010;24(4):356–365. doi: 10.1097/JPN.0b013e3181fble70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapalan S, Koren G. Taking ACE inhibitors during pregnancy. Is it safe? Canadian Family Physician. 2002;48:1047–1049. [PMC free article] [PubMed] [Google Scholar]
- Salsberry PJ, Reagan PB, Fang MZ. Disparities in women's health across a generation: a mother-daughter comparison. Journal of Women's Health. 2013;22(7):617–624. doi: 10.1089/jwh.2012.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, & Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–46. doi: 10.1542/peds.2011-2663. [doi] [DOI] [PubMed] [Google Scholar]
- Tabacova S, Little R, Tsong Y, Vega A, Kimmel CA. Adverse pregnancy outcomes associated with maternal enalapril antihypertensive treatment. Pharmacoepidemiology and Drug Safety. 2003;12(8):633–646. doi: 10.1002/pds.796. [DOI] [PubMed] [Google Scholar]
- Thiele DK, Anderson CM. Developmental origins of health and disease: A challenge for nurses. Journal of Pediatric Nursing. 2016;31(1):42–46. doi: 10.1016/j.pedn.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Weinhold B. Epigenetics: The science of change. Environmental Health Perspectives. 2006;114(3):A160–7. doi: 10.1289/ehp.114-a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PH. Framework as metaphor: The promise and peril of MCH life-course perspectives. Maternal and Child Health Journal. 2003;7(3):151–156. doi: 10.1023/a:1025180203483. [DOI] [PubMed] [Google Scholar]
- Wise PH. Confronting social disparities in child health: A critical appraisal of life-course science and research. Pediatrics. 2009;124(Suppl 3):S203–211. doi: 10.1542/peds.2009-1100H. [DOI] [PubMed] [Google Scholar]
- Yu E, Rimm E, Qi L, Rexrode K, Albert CM, Sun Q, Manson JE. Diet, lifestyle, biomarkers, genetic factors, and risk of cardiovascular disease in the nurses’ health studies. American Journal of Public Health. 2016;106(9):1616–1623. doi: 10.2105/AJPH.2016.303316. [DOI] [PMC free article] [PubMed] [Google Scholar]