Abstract
Background
Carotid body tumors (CBTs) are rare entities for which surgical resection remains the gold standard. Given their hypervascularity, preoperative embolization is often used; however, controversy exists over whether a benefit is associated. Proponents of embolization argue that it minimizes blood loss and complications. Critics argue that cost and stroke outweigh benefits. This study aimed to investigate the impact of embolization on outcomes following CBT resection.
Methods
Patients undergoing CBT resection were identified using the Healthcare Cost and Utilization Project State Inpatient Database for 5 states between 2006 and 2013. Patients were divided into 2 groups: carotid body tumor resection alone (CBTR) and carotid body tumor resection with preoperative arterial embolization (CBETR). Descriptive statistics were calculated using arithmetic means with standard deviations for continuous variables and proportions for categorical variables. Patients were propensity score matched on the basis of sex, age, race, insurance, and comorbidity prior to analysis. Risk-adjusted odds of mortality, stroke, nerve injury, blood loss, and length of stay (LOS) were calculated using mixed-effects regression models with fixed effects for age, race, sex, and comorbidities.
Results
A total of 547 patients were identified. Of these, 472 patients underwent CBTR and 75 underwent CBETR. Mean age was 54.7 ± 16 years. Mean number of days between embolization and resection was 0.65 ± 0.72 days (range 0–3). When compared with CBTR, there were no significant differences in mortality for CBETR (1.35% vs. 0%, P = 0.316), cranial nerve injury (2.7% vs. 0%, P = 0.48), and blood loss (2.7% vs. 6.8%, P = 0.245). Following risk adjustment, CBETR increased the odds of prolonged LOS (odds ratio 5.3, 95% confidence interval 2.1–13.3).
Conclusions
CBT resection is a relatively rare procedure. The utility of preoperative tumor embolization has been questioned. This study demonstrates no benefit of preoperative tumor embolization.
INTRODUCTION
Carotid body tumors (CBTs) are rare tumors of the neck with an incidence of 1 in 30,000, but can make multiple appearances per year in a busy vascular surgery practice. First described by von Haller in 1743, these tumors of neural crest origin can be a frequent cause of neck masses in vascular and head and neck surgery. Also known as paragangliomas, these tumors are most commonly found in the carotid body. Patients generally present with a painless, slowly enlarging lateral neck mass that can lead to dysphagia or odynophagia secondary to compression. There are both heritable and nonheritable CBTs.1 Since the 1930s the carotid body has been described as a chemoreceptor that monitors the body’s oxygen tension. It is stimulated by hypoxia, hypercapnia, and acidosis with subsequent regulation of blood pressure, heart rate, and respiration.1 Given these responsibilities, these tumors are often associated with conditions producing chronically decreased oxygen tension including chronic obstructive pulmonary disease and living at high altitude.2,3 They also tend to be more common in women given lower baseline hemoglobin levels.
Surgery remains the mainstay of treatment for CBTs in patients with appropriate operative risk. Resection is ideal as some tumors (approximately 10%) are malignant at presentation, there is no reliable screening mechanism to follow progression of the tumor, and there is a paucity of data to suggest that correction of underlying hypoxemia will cause the tumor to regress.4 Multiple challenges are encountered in the surgical treatment of these tumors largely attributed to their complicated anatomic location and robust vascularity. Anatomically, CBTs are described by the Shamblin classification, based on the involvement of the extracranial carotid vessels.5 Shamblin group I tumors are localized between the external carotid artery (ECA) and internal carotid artery and do not involve the vessels; group II tumors are adherent or partially surround the vessels; and group III tumors are large and encase the vessels. The latter groups pose a particularly difficult surgical resection.5
The vascular supply to the CBT arises from the ECA. Devascularization of the tumor prior to surgical excision was popularized in the 1980s and remains a standard step in the treatment algorithm; however, a consensus has yet to be reached on the benefit of this procedure. Previous literature has argued both sides.1,6 Controversy remains as to whether preoperative embolization of CBTs improves outcomes in this rare patient population. The majority of the literature published on this topic includes small single-center retrospective reviews with no definitive conclusion to support preoperative embolization. The objective of this study is to determine the impact of preoperative CBT embolization on inpatient mortality, blood loss, and postoperative neurologic events through the use of a large administrative dataset.
MATERIALS AND METHODS
Data Sources
We obtained patient-level data from the Healthcare Cost and Utilization Project (HCUP) State Inpatient Database (SID) for 5 states including California, Florida, New York, Washington, and Iowa across the years 2006–2013 to perform a retrospective cross-sectional review. The SID is an administrative, all-payer dataset aggregated by the Agency for Healthcare Research and Quality to inform health-related decisions. It contains discharge information for 98% of admissions in 48 states.7 As purchasing all 48 state databases could be cost prohibitive, the selected states were chosen to provide a heterogeneous geographic representation of the United States. The Institutional Review Board at our institution deemed the study exempt from review as the data are deidentified, protected, and publically available.
Patient Characteristics
Patients included for study were adults in any of the aforementioned 5 states that carried a diagnosis of a CBT and underwent subsequent resection. This group was then split into 2 groups, those that underwent CBT resection alone and those that underwent CBT resection with preoperative arterial embolization. The diagnosis and procedures were identified by International Classification of Diseases, ninth Revision, Clinical Modification (ICD-9) codes for CBT (194.5, 227.5, 227.6, 237.3), CBT excision (39.8,398.9), and embolization (39.72). These codes were cross-referenced with previous literature8 as well as with professional coding software. We extracted demographic data from HCUP including sex, race (Caucasian, African-American, Hispanic, other), insurance status (Medicaid, private, self-pay), comorbid conditions (obesity, hypertension, and diabetes), and Charlson comorbidity index (CCI; Deyo modification). This score ranges from 0 to 12 and is a prediction of the 1-year mortality of patients with certain comorbid conditions such as heart disease, HIV, and diabetes.9
Statistical Methods
The primary outcomes of interest were postoperative inpatient mortality defined as patients who died prior to discharge from the hospital, stroke, cranial nerve injury, blood loss (created from combination of ICD-9 codes for intraoperative hemorrhage and acute blood loss anemia), and hospital length of stay (LOS). The diagnoses of cranial nerve injury (950.0–950.9, 352, 352.5–352.6, 352.9), stroke (433.11, 434.01, 434.91, 434.11, 434, 997.00–997.02, 997.09), and blood loss (998.11–998.12, 285.1) were developed using ICD-9 codes from previous literature, and cross-referenced with professional coding software.8 Unadjusted rates and risk-adjusted odds were calculated for all the above outcomes. Descriptive statistics of the study population were calculated using arithmetic means with standard deviations for normally distributed continuous variables, median with interquartile ranges (IQRs) for non-normal continuous variables, and proportions for categorical variables. There is no true control population in this study given the fact that CBTs are rarely managed nonoperatively. To better account for this and limit confounding from measured covariates, the study population was propensity score matched 1:1 prior to analysis on the basis of sex, race, insurance status, age, and comorbid disease.10 Unadjusted rates of mortality, stroke, cranial nerve injury, and blood loss were calculated using the Fisher’s exact test due to the small number of affected patients. Unadjusted median LOS was calculated using the Wilcoxon rank-sum/Mann–Whitney U-test given its non-normal distribution. The risk-adjusted odds of mortality, stroke, cranial nerve injury, blood loss, and LOS were calculated using a mixed-effects logistic regression model with fixed effects for patient sex, race, insurance status, and comorbid conditions. Model fit was assessed using the Akaike information criterion and C-statistic. All the above statistical analyses were performed using STATA version 13 (StataCorp LP, College Station, TX).
With the advent of big data, predictive modeling techniques have become increasingly useful in unveiling relationships that are not always obvious with traditional linear approaches. A secondary decision tree analysis was performed to determine the factors that predict which patients may benefit from surgical intervention using Python (Python Software Foundation, Python Language Reference, version 2.7.). Decision trees are often used in business applications to predict future sales, or optimal loan borrowers, but can be applied clinically as well. It is a nonparametric methodology that has the ability to efficiently segment populations into meaningful subgroups.11–13 Our dataset was split into testing and training sets, then run using our variable EX_EMB that included patients who underwent preoperative embolization as the Y or dependent variable, with age, sex, race, insurance, and comorbidity variables as X or independent variables. Missing values were replaced with 0 and means for comorbidities and age, respectively.
RESULTS
After querying HCUP for the 5 aforementioned states during the years 2006–2013, we identified 547 patients who carried a diagnosis of CBT and had a subsequent resection. Of these patients, 75 (13.7%) underwent preoperative embolization of their tumors. The mean age of the overall study population was 54.7 years (standard deviation [SD] 15.6). Patients in the excision-only group were significantly older than those who received preoperative embolization (55.4 (SD 15.4) vs. 50.6 years (SD 15.9), P = 0.013). Both groups were predominantly female with a 2:1 female to male ratio, Caucasian, and had private insurance. We examined the rates of obesity, hypertension, and diabetes among the groups and found they did not significantly vary. As a further indication of comorbid disease, each patient was assigned a CCI. The mean indices for each group were 0.71 (SD 1.4) and 0.77 (SD 1.3) for the excision-only and excision and embolization groups, respectively (Table I).
Table I.
Baseline patient characteristics
| Characteristic | Excision alone (n = 453) | Excision + embolization (n = 75) | P value |
|---|---|---|---|
| Age (SD) | 55.4 (15.4) | 50.6 (15.9) | 0.0128 |
| Sex | |||
| Male | 146 (31%) | 24 (32%) | 0.104 |
| Female | 299 (63.4%) | 51 (68%) | 0.104 |
| Comorbid conditions | |||
| Obesity | 50 (11%) | 8 (11.4%) | 0.918 |
| Hypertension | 211 (46.5%) | 28 (40%) | 0.311 |
| Diabetes | 62 (13.7%) | 13 (18.6%) | 0.274 |
| Charlson comorbidity index | 0.71 (1.4) | 0.77 (1.3) | 0.7119 |
| Race | |||
| White | 269 (61.3%) | 46 (62.2%) | 0.894 |
| African-American | 45 (10.3%) | 6 (8.1%) | 0.894 |
| Hispanic | 78 (17.8%) | 15 (20.3%) | 0.894 |
| Other | 47 (10.7%) | 7 (9.5%) | 0.894 |
| Insurance status | |||
| Medicare | 134 (28.4%) | 21 (28%) | 0.350 |
| Medicaid | 40 (8.5%) | 11 (14.7%) | 0.350 |
| Private | 271 (57.4%) | 38 (50.7%) | 0.350 |
| Self-pay or other | 27 (5.7%) | 5 (6.7%) | 0.350 |
Mean age is represented as SD. All others are represented as frequencies with associated proportions. Charlson comorbidity index ranges from 0 to 12. P value is significant at α < 0.05.
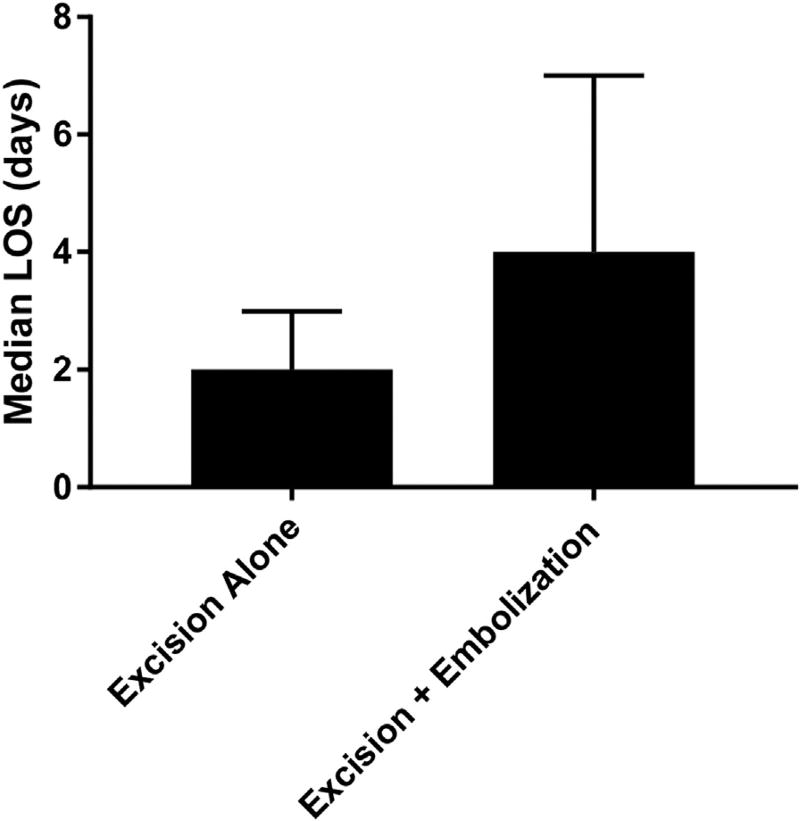
We decided a priori to investigate mortality and common postoperative complications as the primary outcomes of the study. The secondary outcome of interest was LOS in an effort to evaluate hospital resource utilization (Table II). The overall unadjusted inpatient mortality rate was quite low at 0.68% being that there was only a single mortality in the excision-alone group, and no recorded mortalities in the excision with preoperative embolization group (P = 1.000). The postoperative complications evaluated were stroke, cranial nerve injury, and blood loss. The rate of stroke was exactly the same for both groups (4.1%, P = 1.00). Following risk adjustment, we were able to confirm that neither preoperative embolization nor solitary excision leads to increased odds of stroke (odds ratio [OR] 1.07, 95% confidence interval [CI] 0.16–7.2). Cranial nerve injury was exceedingly rare in this study population, with only 2 occurrences in the excision-only group and none noted in the preoperative embolization group. As a result, the risk-adjusted odds could not be accurately calculated. Surprisingly, the unadjusted rate of blood loss was higher in the preoperative embolization group (6.8% vs. 2.7%, P = 0.442), but this did not reach statistical significance even after risk adjustment (OR 4.01, 95% CI 0.63–25.3). LOS was significantly longer in patients who underwent preoperative embolization with a median LOS of 4 days (IQR 3–7) compared with 2 days (IQR 1–3) in the excision-alone group (P = 0.0157; Fig. 1). Following risk adjustment, the same remained true, as patients with preoperative embolization had increased odds of a prolonged LOS (OR 5.27, 95% CI 2.1–13.3).
Table II.
Primary outcomes of the study included mortality, stroke, cranial nerve injury, blood loss, and length of stay
| Outcome | Excision alone | Excision + embolization | P value | OR (95% CI) |
|---|---|---|---|---|
| Mortality (%) | 1 (1.35%) | 0 | 1.000 | a |
| Stroke (%) | 3 (4.05%) | 3 (4.05%) | 1.000 | 1.07 (0.16–7.2) |
| Cranial nerve injury (%) | 2 (2.7%) | 0 | 0.497 | a |
| Blood loss (%) | 2 (2.7%) | 5 (6.8%) | 0.442 | 4.01 (0.64–25.3) |
| Length of stay (IQR) | 2 (1–3) | 4 (3–7) | 0.0157 | 5.3 (2.1–13.3) |
Frequencies of each variable are included with associated rates. Median length of stay includes interquartile ranges. P value is significant at α < 0.05.
Too few occurrences to perform meaningful risk adjustment.
Fig. 1.
Median length of stay was significantly increased in patients with preoperative embolization (2 vs. 4 days, P < 0.001). P value is significant at α < 0.05. Risk-adjusted odds of prolonged length of stay were increased for patients undergoing preoperative embolization.
As a secondary analysis, we utilized a decision tree predictive model to see which patients, if any, would benefit from preoperative embolization (Appendix A). Decision trees start with one “node” or group, containing the entire sample, then the algorithm examines all possible independent or splitting variables and selects the one that results in binary groups that are most different from one another with respect to the dependent variable, embolization or not, in our case.13,14 Once no further splits can be made, a terminal node is created. The independent variables that cause the splits are deemed most important. We found that the 5 most important variables distinguishing patients who should undergo preoperative embolization were age, female sex, Hispanic race, private insurance, and a comorbid diagnosis of hypothyroidism. Variable importance was determined based on Gini scores, which is a measure of inequality of a distribution. Larger values of Gini score indicate greater difference with respect to the prevalence of the dependent variable in the 2 subgroups13,14 (Table III). Further research is needed to delineate the impact that each of these characteristics may have on the preoperative decision-making process.
Table III.
Based on decision tree analysis, the following are the top 5 variables that predicted a need for preoperative embolization: age, private insurance, female sex, Hispanic race, and preoperative hypothyroidism
| Variable | Variable importance score (Gini) |
|---|---|
| Age | 0.432 |
| Private insurance | 0.057 |
| Sex: female | 0.053 |
| Race: Hispanic | 0.051 |
| Comorbidity: hypothyroidism | 0.049 |
DISCUSSION
The results of this study indicate that embolization of CBTs does not improve perioperative morbidity and mortality. In our cohort, the inpatient mortality was low in both groups at 0.68%, and only a single death was noted in the excision-only group. This is consistent with previous literature that cites mortality rates between 0% and 7.4%.6,15 Attempting to reduce surgical blood loss has been one of the principal arguments by proponents of preoperative embolization.16,17 Several reports prior to the 1980s indicated a mean perioperative transfusion requirement of 2.1 units.5,11,18 Other series noted a mean blood replacement of 2 L per patient. We did not find a significant difference between the 2 groups for blood loss and interestingly showed a trend toward a higher blood loss in the embolization group. Opponents to preoperative embolization cite concern for an increase in perioperative neurologic events including stroke in patients undergoing preoperative embolization rather than surgical resection alone.18 The rates of stroke presented here were fairly consistent with other literature that cites rates between 0% and 8%.6,15 Interestingly, the rates were exactly the same between our 2 groups.
The rate of cranial nerve injury/palsy in this population has a wide range in the literature spanning 0–49%.6 The majority of these injuries are temporary palsies involving the hypoglossal and marginal mandibular nerve caused by excessive retraction.18 Cranial nerve injuries were overall very rare in our cohort with only 2 patients in the excision-only group. It is unclear whether this is a result of omission in coding, whereas most patients have a temporary palsy, physicians may not code it as such. As a result, we were likely including patients with true permanent cranial nerve injuries, which tend to be much less frequent.
Another critique of using preoperative CBT embolization is the increased LOS associated with the procedure. Some would weigh this increased cost and LOS against the benefits of decreased blood loss and fewer neurovascular injuries. In our findings, LOS was significantly increased by 2 days in the embolization group without any of the benefits of decreased blood loss or decreased neurological events.
In a time where medical cost and surgical outcomes are so highly scrutinized, further investigation should be undertaken in the form of a randomized prospective trial. This would likely have to involve multiple high volume centers and multiple specialties including vascular surgery, head and neck surgery, and interventional radiology. Outside the simple question of embolization or not, the answer may be in the type of embolic agent or dynamic imaging to better select patients who would benefit from the procedure.
There are many limitations to our study in large part due to the use of an administrative dataset to delineate clinical information. First and foremost, the use of an administrative dataset requires the use of billing ICD-9 codes. Unfortunately, these codes often lack granular clinical details such as disease severity. In the case of CBTs, clinical severity is measured using the Shamblin classification for which there are no associated codes. Given that larger tumors with a higher Shamblin classification are generally those that are embolized preoperatively, our analysis cannot reliably identify the small subset of patients who may benefit from preoperative embolization of their CBTs. This may be manifested in the longer LOS and higher rates of hemorrhage seen in our embolization group. This also impacts the variables selected in our decision tree analysis. Tumor size or involvement with vascularity may have been indicated as important variables in predicting patients appropriate for preoperative embolization, had that data been available. Additionally, coding differences within and between hospitals remain a large source of selection bias, but we hoped to control for some of this with our risk adjustment strategies, propensity score matching, and the use of both linear and nonlinear approaches to the analysis. Our propensity match also helps eliminate bias in measured covariates, but we must be aware that there could be unmeasured covariates that are not accounted for in our analysis. Additionally, tumor size usually plays a large role in whether patients get preoperative embolization. Given the limitations of ICD-9 codes and the data available in our dataset, we do not have access to data to indicate the size of the tumors in these patients. Finally, we pulled patients from 5 representative states, but as a result, the results may not be generalizable to all populations.
CONCLUSION
We did not find a benefit of preoperative CBT embolization in relation to blood loss, stroke, cranial nerve injury, and hospital LOS. This will likely continue to be a controversial topic in the management of CBTs.
Supplementary Material
Acknowledgments
We would like to thank the Loyola University Department of Surgery One:MAP Analytics team as well as DePaul’s Predictive Analytics Program for their contributions, support, and review of this manuscript. We would also like to acknowledge the Loyola University Burn Shock Trauma Institute and the support of NIH T32 GM08750–16.
Footnotes
Presented as an Oral Presentation at the 27th Annual Meeting of the Vascular and Endovascular Surgery Society, Steamboat Springs, CO, February 2–5, 2017.
Disclosure: The authors have no conflicts of interest or financial disclosures.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.avsg.2017.06.149.
References
- 1.Kafie FE, Freischlag JA. Carotid body tumors: the role of preoperative embolization. Ann Vasc Surg. 2001;15:237–42. doi: 10.1007/s100160010058. [DOI] [PubMed] [Google Scholar]
- 2.Saldana MJ, Salem LE, Travezan R. High altitude hypoxia and chemodectomas. Hum Pathol. 1973;4:251–63. doi: 10.1016/s0046-8177(73)80012-7. [DOI] [PubMed] [Google Scholar]
- 3.Chedid A, Jao W. Hereditary tumors of the carotid bodies and chronic obstructive pulmonary disease. Cancer. 1974;33:1635–41. doi: 10.1002/1097-0142(197406)33:6<1635::aid-cncr2820330625>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Knight TT, Gonzalez JA, Rary JM, et al. Current concepts for the surgical management of carotid body tumor. Am J Surg. 2006;191:104–10. doi: 10.1016/j.amjsurg.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Lahey FH, Warren KW. Tumors of the carotid body. Surg Gynecol Obstet. 1947;85:281–8. [PubMed] [Google Scholar]
- 6.Sajid MS, Hamilton G, Baker DM. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007;34:127–30. doi: 10.1016/j.ejvs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 7.HCUP Databases. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. Rockville, MD: [Accessed January 15, 2017]. Available at: www.hcup-us.ahrq.gov/sidoverview.jsp. [Google Scholar]
- 8.Vogel TR, Mousa AY, Dombrovskiy VY, et al. Carotid body tumor surgery: management and outcomes in the nation. Vasc Endovascular Surg. 2009;43:457–61. doi: 10.1177/1538574409335274. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Austin PC, Mamdani MM, Stukel TA, et al. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med. 2005;24:1563–78. doi: 10.1002/sim.2053. [DOI] [PubMed] [Google Scholar]
- 11.Rosen IB, Palmer JA, Goldberg M, et al. Vascular problems associated with carotid body tumors. Am J Surg. 1981;142:459–63. doi: 10.1016/0002-9610(81)90375-5. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn M, Johnson K. Applied Predictive Modeling. Vol. 26. New York: Springer; 2013. [Google Scholar]
- 13.Lemon SC, Roy J, Clark MA, et al. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–81. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 14.Podgorelec V, Kokol P, Stiglic B, et al. Decision trees: an overview and their use in medicine. J Med Syst. 2002;26:445–63. doi: 10.1023/a:1016409317640. [DOI] [PubMed] [Google Scholar]
- 15.Dent TL, Thompson NW, Fry WJ. Carotid body tumors. Surgery. 1976;80:365–72. [PubMed] [Google Scholar]
- 16.Power AH, Bower TC, Kasperbauer J, et al. Impact of preoperative embolization on outcomes of carotid body tumor resections. J Vasc Surg. 2012;56:979–89. doi: 10.1016/j.jvs.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Murphy TP, Brackmann DE. Effects of preoperative embolization on glomus jugulare tumors. Laryngoscope. 1989;99:1244–7. doi: 10.1288/00005537-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Patetsios P, Gable DR, Garrett WV, et al. Management of carotid body paragangliomas and review of a 30-year experience. Ann Vasc Surg. 2002;16:331–8. doi: 10.1007/s10016-001-0106-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.