Abstract
Elderly humans over 65 years old are at great risk to pathogenesis by influenza virus infection. However, although influenza vaccines provide effective protection in healthy young adults, protection of elderly adults is substantially lower even with a good match between the vaccine and the circulating influenza virus. To gain insight of the underlying mechanism for the reduced immunogenicity of influenza vaccines in the aged population, we investigated immunogenicity of influenza virus-like particle vaccines in aged mice, which represent a useful model for studying aging associated impairment in immune responses. Specifically, we investigated the effect of inhibiting regulatory T cells in aged mice on induction of protective immune responses by influenza vaccines. Our results showed that injecting anti-CD25 antibodies could down-regulate CD25 on the surface of regulatory T cells and significantly increase the levels of antibody responses induced by VLP immunization in aged mice. Further, the profiles of antibody responses were also changed towards Th1 type by regulatory T cell blockage in aged mice. Moreover, aged mice that were treated by anti-CD25 antibodies prior to vaccination were more effectively protected against lethal influenza virus challenge.
Keywords: Influenza, vaccine, aging, regulatory T cell
1. Introduction
Influenza virus is a highly contagious airborne pathogen and a major threat to public health. Seasonal epidemics of human influenza virus infection cause extensive mortality and morbidity worldwide each year. More gravely, occasionally influenza viruses of zoonotic origin may cross the species barrier to cause large scale human pandemics. Over the past century, there have been four recorded human influenza pandemics in the years 1918, 1957, 1968, and 2009, and all have originated from zoonotic influenza viruses with three from avian-origin and one from swine-origin influenza virus [1–4]. Currently, 18 HA subtypes and 11 NA subtypes have been identified for influenza A virus and of these, 16 HA subtypes and 9 NA subtypes exist in aquatic and migratory birds [5–7]. Among these, only 3 HA subtypes (H1–H3) and 2 NA subtypes (N1–N2) have been recorded in human type A influenza virus epidemics. However, the potential of other avian influenza virus (AIV) subtypes to break the species-barrier and attain transmissibility in humans also poses a grave threat to public health [8, 9]. In recent years, several AIV subtypes have acquired the ability to break species barriers and directly infect humans or other mammalian species, including the highly pathogenic H5N1 avian influenza viruses (HPAIV), H7N7, H9N2, H6N1, H10N8, and H7N9 AIVs [10–15]. Amongst the different AIV subtypes that have caused human infections, H7N9 AIV is of particular concern. Since its emergence in the spring of 2013 in China, the ongoing H7N9 outbreak has caused over 1500 human infections with over 600 deaths. Further, H7N9 AIV infection has been reported to cause more severe symptoms and relatively higher mortality in elderly humans, a population that is also burdened with higher morbidity and mortality rates by seasonal human influenza virus infection.
Elderly humans over 65 years old constitute up to 90% of all mortality associated with seasonal influenza virus infection [16, 17]. Moreover, results from human seasonal influenza vaccine efficacy studies have shown that although these vaccines provide effective protection in healthy young adults with up to 90% efficacy, protection of elderly adults is substantially lower with efficacy between 20 to 70% [18–22]. The reduced immunogenicity of influenza vaccines in aging hosts can be largely attributed to immunosenescence, which is a hallmark of the aging process and is manifested by reduced immune responses to vaccination and increased susceptibility to virus infection [23–25]. Thus, understanding the underlying mechanism of reduced influenza vaccine immunogenicity in aged hosts is critical for developing more effective vaccines to protect the aging population against infection and pathogenesis of seasonal as well as emerging influenza viruses.
One of the prominent characteristics of immunosenescence is the increase in the percentage of regulatory T cells (Treg) in aged hosts [26, 27]. Treg are a subpopulation of CD4 T cells which play important roles in maintaining immunological self-tolerance and controlling immune responses from inducing tissue damage [28]. These cells constitutively express the CD25 on the surface and exhibit suppressive capabilities to T cell proliferation [29]. Notably, a unique molecular marker, Foxp3 (forkhead winged helix protein 3), has been identified that is expressed solely in Treg cells [30, 31]. Several studies showed that injection anti-CD25 antibodies in mice to deplete Treg enhanced induction of immune responses [32, 33]. On the other hand, it was reported that influenza virus infection in aged mice but not young mice resulted expansion of Treg cells [34]. Altogether, results from these studies suggest that regulatory T cells may play an important role in regulating activation of immune responses in aged animals and elderly humans. We have previously shown that immunization with influenza virus-like particles (VLPs) or inactivated influenza virus vaccines (IIV) induced significantly lower levels of antibody responses in aged mice than in young adult mice [35]. Further, it was observed that a higher vaccine dose is required to confer a complete protection against influenza virus infection in the aged mice. In the present study, we investigated the role of Treg in modulating immunogenicity of influenza VLP vaccines in aged mice. Our results showed that injection of anti-CD25 antibodies into aged mice effectively reduced the portion of Tregs in mouse spleens, and resulted in significant increase in vaccine-induced antibody responses that conferred more effective protection of aged mice against lethal influenza virus challenge.
2. Materials and methods
2.1. Mice and influenza virus
Young adult female Balb/c mice (8 week-old) were purchased from the Charles River Laboratory, and housed in isolated chambers at the animal facility of the Emory University until 18-month old for use in immunization and challenge studies. All animal studies were carried out in accordance with guidelines of the Institutional Animal Care and Use Committee (IACUC) at Emory University.
Influenza virus A/PR/8/34 has been maintained in our group over 10 years. Mouse-adapted influenza virus A/PR/8/34 (H1N1) was prepared as lung homogenates from intranasally infected naïve mice and stored at −80 °C in aliquots until use. The titer of the challenge virus stock was first determined in chicken eggs to calculate the 50% egg-infectious dose (EID50) and then the 50% lethal infectious dose (LD50) was determined in young naïve mice (Balb/c, 8 weeks old). It was calculated that the LD50 of the challenge virus stock was approximately 104 EID50.
2.2. Production and characterization of influenza VLPs
Influenza VLPs were produced in Sf9 insect cells by co-expression of influenza virus M1, HA, and NA proteins using the recombinant baculovirus expression system as described in previous studies [35]. Briefly, Sf9 cells (2 × 106/ml) were co-infected with rBV-HA/NA and rBV-M1 at MOIs (multiplicity of infection) of 5 and 2 respectively, and VLPs released into the medium were collected at 60 hr post infection. After clarification of cell debris, VLPs were concentrated by ultra-centrifugation and further purified through a discontinuous sucrose gradient (10–50%). Purified VLPs were then concentrated by ultra-centrifugation and re-suspended in PBS. Protein concentration of VLPs was determined using a BCA assay kit and the VLP preparations were adjusted by PBS to a final protein concentration of 1 mg/ml. Purified VLPs were characterized by Coomassie blue as well as Western blot analysis for the presence of HA and M1 proteins and the amount of incorporated HA proteins was analyzed by densitometry analysis using Fluoro-Chem FC2 Imaging Illuminator coupled with AlphaEaseFC software (Alpha Innotech, San Leandro, CA). The influenza VLPs were further examined by electron microscopy using a Hitachi-H7500 transmission electron microscope (Hitachi. Ltd., Tokyo, Japan) by negative staining with 1% uranyl acetate, following established protocols [36]. Simian immunodeficiency virus (SIV) Gag VLPs were produced using recombinant baculovirus expressing the SIV Gag and purified following the same procedure, and used as control VLPs in this study as indicated.
2.3. Immunization and challenge of mice
Groups of young (8 weeks old) and aged mice (18 months old) were immunized by intramuscular injection (IM) twice at 4-week intervals with 10 or 1 μg of influenza VLPs or 1 ug of IIV vaccines dissolved in 100 ul PBS as indicated for each group. Aged mice (18-month old) were immunized twice with influenza virus-like particles (VLPs) at 4-week intervals. To investigate the effect of Tregs on induction of immune responses, Tregs were depleted by intraperitoneal injection of a monoclonal anti-CD25 antibody, PC61, 3 days prior to each immunization, only before priming immunization, or only before the boosting immunization. The control group mice received rat-IgG isotype control antibodies prior to each immunization. Blood samples were collected at two weeks after each immunization and antibody responses induced by different immunization regimens were compared by ELISA as well as hemagglutination inhibition assays. At four weeks after the final immunization, mice were challenged by 100 LD50 of a mouse-adapted influenza virus A/PR/8/34 and monitored for weight changes and signs of illness on daily basis. At two weeks after the second immunization, serum samples were collected by retro-orbital bleeding, heat-inactivated ay 55 °C for 30 min, and stored in −80 °C until analysis. At four weeks after the second immunization, mice were lightly anesthetized by isofluorane and challenged by intranasal instillation of 100 LD50 (approximately 106 EID50) of the homologous mouse-adapted influenza virus A/PR/8/34 (diluted in 50 μl PBS). Mice were monitored daily for weight changes and signs of disease for 14 days, and mice that exhibited over 25% body weight loss were sacrificed in accordance with IACUC guidelines.
2.4. ELISA
Influenza HA-specific antibodies were measured in individual mouse sera by ELISA following established protocols [37]. A recombinant vaccinia virus expressing an HA-his fusion protein (The ecto-domain of influenza virus A/PR/8/34 HA was fused with a histag segment HHHHHH at its C-terminus) was generated and used to infect Hela cells. At 48 hr post-infection, supernatant was harvested and HA–his protein released into the medium was purified using Ni-NTA agarose beads (QIAGEN, Valencia, CA). Purified HA-his proteins were characterized by Coomassie Blue staining and Western blot. Purified HA-his proteins were used as coating antigens and a standard curve was constructed by coating each ELISA plate with serial 2-fold dilutions of purified mouse IgG with known concentrations (eBioscience, San Diego, CA). The concentrations of HA-specific antibodies in serum samples were calculated using the obtained standard curves and expressed as the amount of HA-specific antibodies in 1 ml of serum samples (ng/ml).
2.5. Hemagglutination inhibition (HAI) assay
The HAI assay was performed following established protocols [38]. Briefly, mouse sera was heat-inactivated at 56 °C for 1 h and then treated with receptor destroying enzyme (Denka Seiken, Tokyo, Japan) at 37 °C overnight according to the manufacturer’s instruction. After treatment, 25-μl aliquots of 2-fold serially diluted serum samples were incubated with 25-μl virus containing 4 HA units of influenza virus A/PR/8/34 at 37 °C for 1 h, followed by incubation with 50 μl of 0.5% chicken red blood cells (LAMPIRE Biological Laboratories, Pipersville, PA) at 25 °C for 45 min. The HAI titer was defined as the reciprocal of the highest serum dilution that inhibited hemagglutination.
2.6. Fluorescence staining and flow cytometry analysis of Treg
Aged mice (18-month old, groups of 3) were treated by IP injection of 400 ug anti-CD25 antibody PC61 of the rat isotype control antibody, and sacrificed 3 days later to prepare splenocytes following established protocols [39]. The splenocytes were either directly stained or stained after 12 hr culturing in RPMI medium plus 10% fetal calf serum. For fluorescence staining of Treg, i.e. the CD25+/FoxP3+ CD4 T cells, splenocytes from each groups of mice (treated with PC61 or control antibodies) were pooled together, washed twice with PBS containing 3% fetal calf serum, and then stained for surface expression of CD8, CD4, and CD25 with PerCP-conjugated rat anti-mouse CD8, FITC-conjugated rat anti-mouse CD4, and APC-conjugated rat anti-mouse CD25 antibodies respectively. The cells were washed twice again with PBS containing 3% fetal calf serum, followed by fixing and permeabilizing with Fix&Perm buffers, and then stained for intracellular expression of FoxP3 proteins with PE-conjugated rat anti-mouse FoxP3 antibody phycoerythrin antibodies, All antibodies and Fix&Perm buffers were purchased from BD Pharmingen (San Diego, CA). After staining, the splenocytes were analyzed by flow cytometry on a BD FACS-Calibre Station (BD Immunocytometry Systems, San Jose, CA) and data was analyzed with the Flowjo 4.2 software (Tree Star, Ashland, OR). Lymphocytes were gated by the size of the cells based on Forward- and Side-Scattering values, and CD4 T cells were gated as CD4+/CD8− cell population, which were then further analyzed for surface expression of CD25 and intracellular expression of FoxP3.
2.7. Statistical Analysis
The average value and standard deviation for the level of immune responses within each group were calculated for comparison and the significance of the differences between the results from different groups was determined by a student t test using the Excel program (Microsoft, Redmond, WA).
3. Results
3.1. Administration of anti-CD25 antibody to aged mice down regulate FOXP3-expressing CD4 T cells
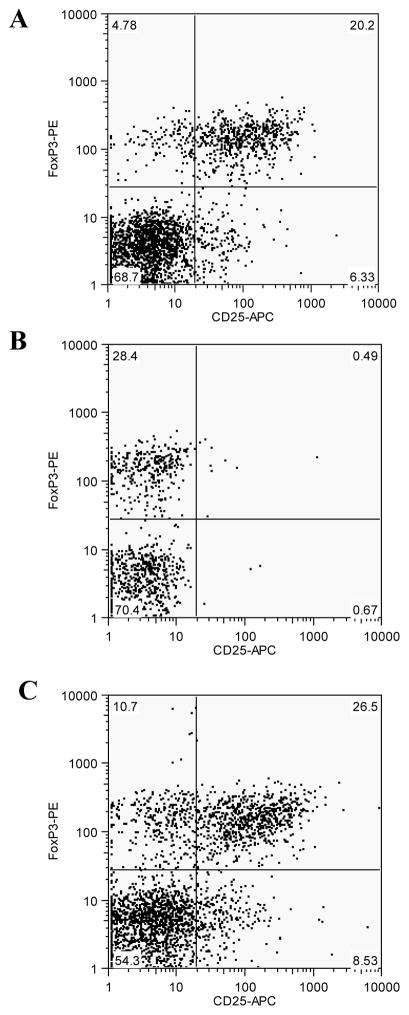
We first investigated the effect of injecting anti-CD25 antibodies on the level of Treg, defined as CD25+/FoxP3+ CD4 T cells, in aged mice. Mice (18 month-old, 3 per group) were treated with 400 ug anti-CD25 monoclonal antibody PC61 or 400 ug rat IgG1 control antibody by IP injection. Three days later, mice were sacrificed and splenocytes were prepared and mixed to determine the level of Tregs for comparison. As shown in Fig. 1A, approximately 20% of the CD4 T cells in control antibody-treated aged mice are CD25+/FOX P3+. In comparison, the percentage of CD25+/FOX P3+ CD4 T cells in PC61-treated aged mice dropped to less than 0.5% (Fig. 1B), representing a 97% reduction in Treg levels. However, it was also noted that with the reduction of CD25+FoxP3+ CD4 T cell population, there is an increase in the CD25-FoxP3+ CD4 T cell population, from about 5% in control mice (Fig. 1A) to about 28% in PC61-treated mice (Fig. 1B). To further investigate this observation, the splenocytes were cultured for 12 hr in complete medium without any stimulant and then stained for Tregs. As shown in Figure 1C, after 12 hr culturing, the percentage of CD25+FoxP3+ CD4 T cells returned to about 26%, that is comparable to the control mice. On the other hand, the levels of CD25-FoxP3+ CD4 T cells dropped to about 10% after 12 hr culturing. These results indicate that the Tregs were not depleted by PC61 antibody treatment. Rather, the antibody treatment led to down-regulation of surface C25, which rapidly recovered after removal of the antibody.
Figure 1. Injection of anti-CD25 antibody PC61 into aged mice results in blockage of surface CD25 expression on Tregs.
Splenocytes from aged mice were stained ofr surface expression of CD4, CD8, CD25, and intracellular expression of FoxP3 as described in Materials and Methods, and data was analyzed with the Flowjo 4.2 software. Lymphocytes were gated by the size of the cells based on Forward- and Side-Scattering values, and CD4 T cells were gated as CD4+/CD8− cell population, which were then further analyzed for surface expression of CD25 and intracellular expression of FoxP3. (A). Direct staining of surface expression of CD25 and intracellular expression of FoxP3 in CD4 T cells from mice that received injection of rat isotype control antibody. (B). Direct staining of surface expression of CD25 and intracellular expression of FoxP3 in CD4 T cells from mice that received injection of anti-CD25 antibody PC61. (C). Staining of surface expression of CD25 and intracellular expression of FoxP3 in CD4 T cells from mice that received injection of anti-CD25 antibody PC61 after 12 hr culturing.
3.2. PC-61 treatment of aged mice augments induction of antibody responses by influenza VLPs
We further investigated whether treatment anti-CD25 antibody PC61 will enhance induction of antibody responses by influenza VLP vaccines in aged mice (18 month-old, Groups of 6). As outlined in Fig. 2A, one group received PC61 prior to both priming and boosting immunizations (Group PB), one group received PC61 only prior to priming immunization (Group P), one group received PC61 only prior to boosting immunization (Group B), one group received IP injection of control antibodies prior to both priming and boosting immunizations (Group NT), and a control group received PC61 prior to both priming and boosting immunizations but received control SIV Gag VLPs in immunizations (Control). As shown in Fig. 2B, aged mice were vaccinated by influenza virus A/PR/8/34 (PR8) VLPs (1 ug per immunization per animal) twice at 4-week intervals with or without PC61 treatment prior to priming or boosting immunizations, and blood samples were collected at 2 weeks after the second immunization for comparing antibody responses.
Figure 2.
(A). Outline of immunization regimen and anti-CD25 antibody treatment schisms for different groups of aged mice. Aged mice (18-month old, 6 per group) were treated by injecting with anti-CD25 antibody PC61 prior to both priming and boosting immunizations (Group PB), only prior to priming immunization (Group P), only prior to boosting immunization (Group B), or injection of isotype control antibodies prior to both priming and boosting immunizations (Group NT). At 3 days after antibody injection, the mice received vaccination by intramuscular injection of 1 ug influenza virus PR8 VLPs as indicated. A control group received anti-CD25 antibody prior to both priming and boosting immunizations but received SIV Gag VLPs in both immunizations (Control). (B). Schematic diagram of immunization, anti-CD25 antibody treatment, blood sample collection, and challenge schedules. Mice were treated with either anti-CD25 antibody PC61 or rat isotype control antibodies at 3 days prior to each immunization. Mice were vaccinated twice at 4-week intervals, Blood samples were collected at 2 weeks after the second immunization for analysis of antibody responses. Mice were challenged at 4 weeks after the second immunization for comparing protective efficacy of different immunization regimens.
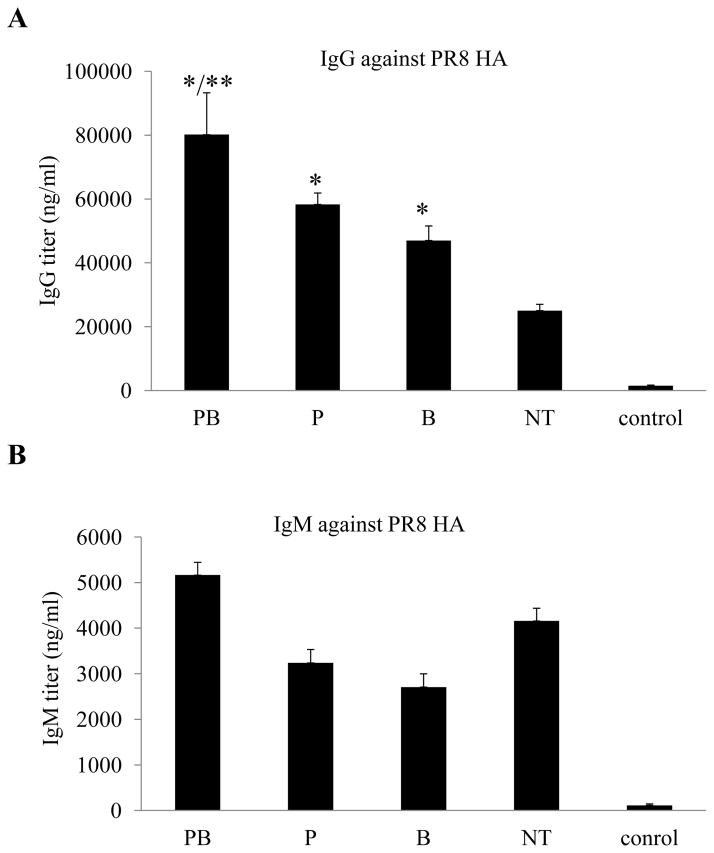
We first compared the levels of total IgG and IgM antibodies against the HA of influenza virus PR8 by ELISA. As shown in Fig. 3A, the IgG levels against PR8 HA in serum samples from Group NT, which received control antibodies prior to both priming and boosting immunizations, reached to about 20000 ng/ml. In comparison, the IgG levels against PR8 HA in serum samples from Group PB, which received PC61 treatment prior to both priming and boosting immunizations, reached to about 80000 ng/ml, a 4-fold increase compared to Group NT (p<0.01). Further, the levels of IgG against PR8 HA in serum samples from Group P and Group B, receiving PC61 treatment prior to only priming or boosting immunization respectively, reached to about 50000 ng/ml, more than 2-fold increase compared to Group NT (p<0.01). In contrast, the levels of IgM antibodies against PR8 HA ranged from 3700 ng/ml to 5200/ng/ml among these groups, varying less than 30%.
Figure 3. Blockage of Treg in aged mice enhances induction of IgG but not IgM antibodies against influenza virus PR8 HA.
Groups aged mice (6 per group, 18-month old) were immunized twice at 4-week intervals by different immunization regimens as shown in Fig. 2. Blood samples were collected at 2 weeks after the second immunization and analyzed for antibody responses against the HA of influenza virus PR8. The levels of antibody responses against HA were determined by ELISA using purified HA proteins as coating antigens, and expressed as the amount of HA-specific antibodies IgG or IgM in 1 ml of serum samples (ng/ml). Error bars indicate standard deviations for each group. (A). IgG antibody levels against the influenza virus PR8 HA. (B). IgM antibody levels against the influenza virus PR8 HA. Data are presented as the mean ± standard deviation. (*) indicate significantly higher compared to Group NT (p<0.01) and (**) indicate significantly higher compared to Group P or Group B (p<0.05).
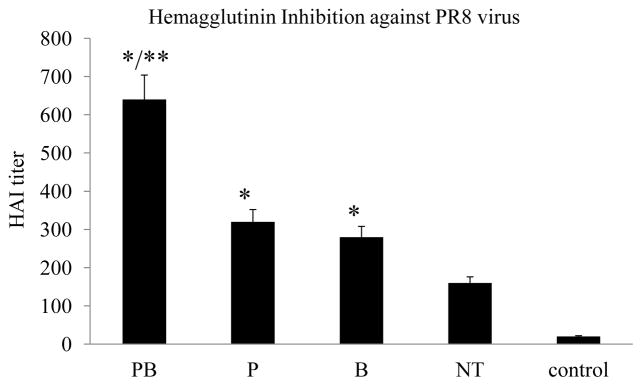
The functional activity of induced antibody responses was analyzed by an HAI assay. As shown in Fig. 4, the HAI titer of sera from Group PB mice that received PC61 treatment prior to both priming and boosting immunizations reached over 1:600 on average, which is approximately 4-fold higher than the HAI titer induced in Group NT mice that received control antibodies (p<0.01). Similarly, the HAI titers of sera from Group P and Group B mice were about 2-fold higher than Group NT (p<0.01). These results showed that treatment with anti-CD25 antibody PC61 also enhanced the functional activity of the induced antibody responses, which correlated with the levels of IgG antibodies against PR8 HA.
Figure 4. Treatment with anti-CD25 antibody increases HAI activity of sera from vaccinated mice.
Groups aged mice (6 per group, 18-month old) were immunized twice at 4-week intervals by different immunization regimens as shown in Fig. 2. Blood samples were collected at 2 weeks after the second immunization and analyzed for their activities to inhibit hemagglutination by influenza virus PR8. The ability of sera to inhibit hemagglutination by influenza virus A/PR/8/34 were determined and expressed as the highest dilution that resulted in complete inhibition of hemagglutination (HAI titer). Error bars indicate standard deviations for each group. Data are presented as the mean ± standard deviation. (*) indicate significantly higher compared to Group NT (p<0.01) and (**) indicate significantly higher compared to Group P or Group B (p<0.05).
3.3. The IgG2a antibody isotype against influenza virus PR8 HA was more drastically enhanced by Treg blockage in aged mice
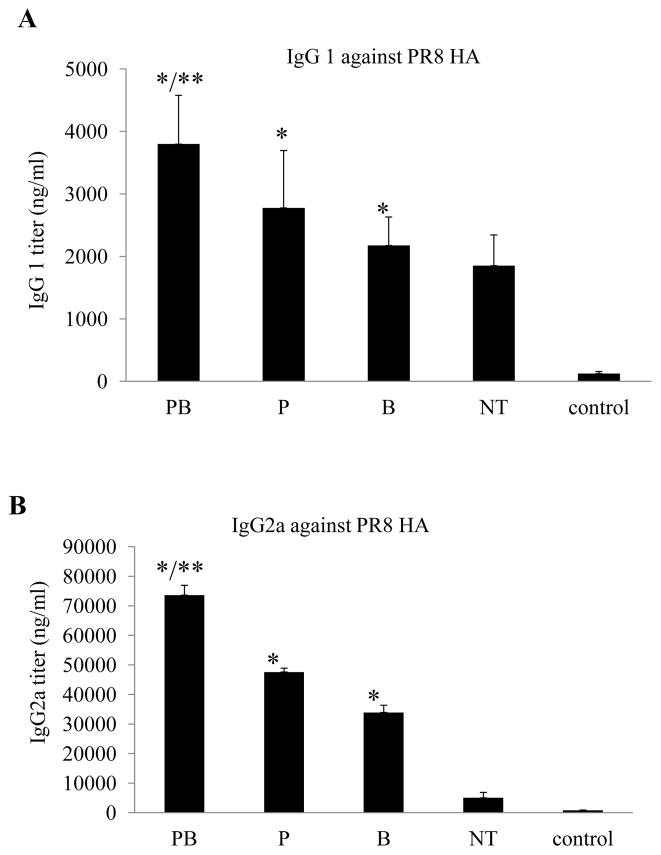
We further compared the levels of IgG1 and IgG2a antibody isotypes induced in different groups of mice. As shown in Fig. 5A, the levels of IgG1 antibodies induced in PC6-treated aged mice were higher than those induced in Group NT that received the control antibodies, increasing by about 3, 2, or 1.5-fold for Group PB, Group P, and Group B respectively (p<0.01). In contrast, as shown in Fig. 5B, the levels of IgG2a antibodies against PR8 HA were drastically enhanced in PC61-treated mice than Group NT mice, increasing by more than 15-fold for Group PB and about 10-fold for Group P and 7-fold for Group B respectively (p<0.01). The relative IgG2a/IgG1 ratio in Group PB mice increased by almost 5-fold compared to Group NT mice. Taken together, these results showed that treatment by anti-CD25 antibody PC61 prior to PR8 VLP immunizations not only enhanced antibody class switch from IgM to IgG but also significantly augmented induction of IgG2a antibodies in aged mice.
Figure 5. The levels of IgG2a antibodies against PR8 HA are more drastically enhanced in aged mice treated with anti-CD25 antibody PC61 prior to immunization with influenza VLPs.
Groups aged mice (6 per group, 18-month old) were immunized twice at 4-week intervals by different immunization regimens as shown in Fig. 2. Blood samples were collected at 2 weeks after the second immunization and analyzed for antibody responses against the HA of influenza virus PR8. The levels of antibody responses against HA were determined by ELISA using purified HA proteins as coating antigens, and expressed as the amount of HA-specific antibodies IgG1 or IgG2a in 1 ml of serum samples (ng/ml). Error bars indicate standard deviations for each group. (A). IgG1 antibody levels against the influenza virus PR8 HA. (B). IgG2a antibody levels against the influenza virus PR8 HA. Data are presented as the mean ± standard deviation. (*) indicate significantly higher compared to Group NT (p<0.01) and (**) indicate significantly higher compared to Group P or Group B (p<0.05).
3.4. Immunization by influenza virus PR8 VLPs after Treg blockage completely protects aged mice against a high dose lethal influenza virus challenge
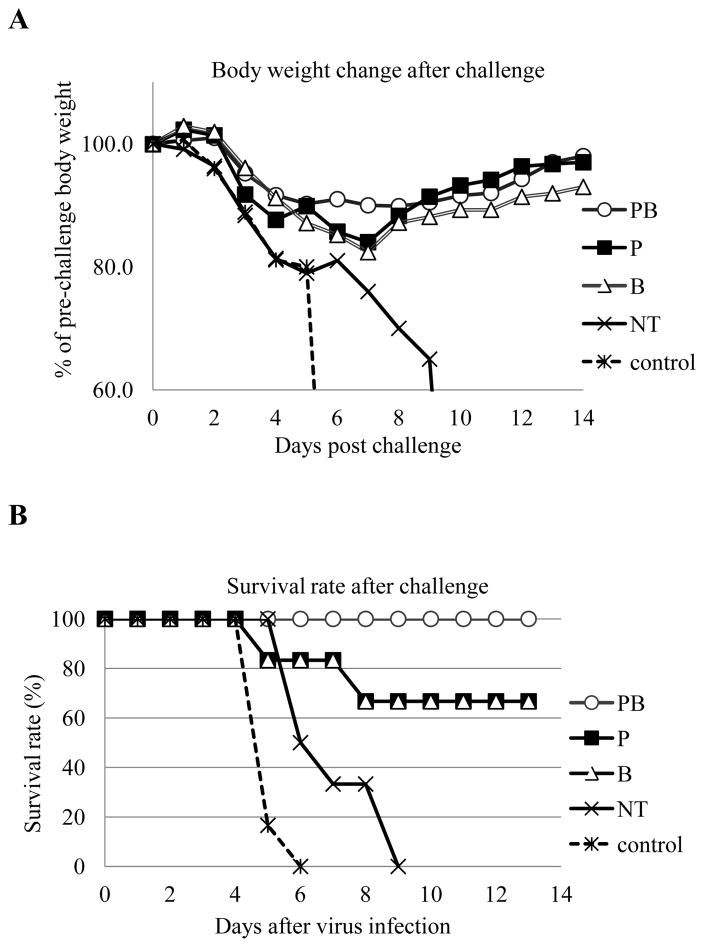
At 4 weeks after the second immunization, aged mice (Groups of 6) were challenged with 100 LD50 of mouse adapted influenza virus A/PR/8/34 to evaluate whether treatment with anti-CD25 antibodies prior to immunizations will improve protective efficacy of influenza VLPs in aged mice. As shown in Fig. 6A, Group PB mice that received PC61 treatment prior to both priming and boosting immunizations were 100% protected, whereas all Group NT mice that received isotype control antibodies as well as Control Group mice succumbed to the challenge. In comparison, mice that received PC61 treatment only prior to priming immunization (Group P) or the boosting immunization (Group B) were partially protected from the challenge at 67%. These results showed that treatment with anti-CD25 antibody PC61 prior to immunization with influenza VLPs significantly enhanced the protective efficacy against a high lethal dose challenge by influenza virus in aged mice. However, it was also observed that all aged mice exhibited significant weight loss (over 10%) after the high dose lethal challenge (Fig. 6B). Nonetheless, mice in Group PB were observed to have the least body weight reduction (less than 15%) after challenge, indicating enhanced protection against pathogenesis by influenza virus infection.
Figure 6. Treatment with anti-CD25 antibody prior to influenza VLP immunizations confers more effective protection of aged mice against a high dose lethal influenza virus challenge.
Groups aged mice (6 per group, 18-month old) were immunized twice at 4-week intervals by different immunization regimens as shown in Fig. 2. At 4 weeks after the second immunization, mice were challenged by intranasal instillation of 100 LD50 of mouse-adapted influenza virus A/PR/8/34. Mice were monitored and weighed daily after challenge, and were sacrificed when found to display severe signs of illness or loss more than 25% body weight in accordance with IACUC guidelines. (A). Mouse survival rates after challenge. (B). Mouse body weight changes after challenge.
4. Discussion
The reduced immunogenicity of influenza vaccines in aging hosts can be largely attributed to immunosenescence, which is a hallmark of the aging process and is manifested by reduced immune responses to vaccination and increased susceptibility to virus infection [23–25]. Results from both clinical studies and animal models suggest that in aged hosts, many aspects of both innate and adaptive immune responses exhibit significant decline [40, 41]. Of note, the levels of Tregs were found to increase significantly in aged hosts. These cells constitutively express the CD25 on the surface and exhibit suppressive capabilities to T cell proliferation [29]. In the present study, we investigated the potential role of Treg in modulating immune responses induced by influenza VLP vaccines in aged mice. We observed that injection of anti-CD25 antibody PC61 into aged mice resulted in drastic loss CD25+/FoxP3+ CD4 T cells (Tregs), but at the same time increased the levels CD25−/FoxP3+ CD4 T cells. Interestingly, by culturing the splenocytes from PC61-treated aged mice for 12 hr, it was found that the levels of CD25+/FoxP3+ CD4 T cells (Tregs) increased back to normal levels, whereas the levels CD25−/FoxP3+ CD4 T cells decreased accordingly at the same time. Conflicting results have been reported as to whether injection of anti-CD25 antibody PC61 will lead to true depletion of Tregs or simply inhibition of their function in mice [42, 43]. Our results here from this study support the idea that injecting anti-CD25 antibody PC61 does not lead to permanent loss of Tregs, but rather result in down-regulating or masking surface CD25 on these cells and thereby blocking their functional activity.
Our results showed that blockage of Treg functions by PC61 treatment prior to immunization significantly increased the levels of antibody responses induced in aged mice by influenza VLP vaccines. These findings are in agreement with previous studies that showed inhibition of Treg functions by injection anti-CD25 antibody PC61 could enhance induction of immune responses in young adult mice [32, 33]. Interestingly, we observed that while the levels IgG against influenza virus PR8 HA increased significantly in mice that received PC61 prior to immunizations, the levels of IgM against PR8 HA were not significantly affected. Further, our results showed that for IgG antibodies against PR8 HA, the levels of the IgG2a isotype were more drastically enhanced by PC61 treatment in aged mice than the levels of the IgG1 antibody isotype. Previous studies have shown that in aged hosts, somatic hypermutation as well as class switching of B cells are impaired and as a result, the ability to produce high affinity antibodies decreases [44, 45]. Further, reduction in the number of naive B cells also limits the repertoire of the antibody response against new antigens [46–48]. Taken together, these results indicate that the high levels of Treg in aged mice may exert an inhibitory activity on antibody class-switch to IgG subclass, and blockage of Treg functions overcomes such inhibition and thereby enhances IgG antibody induction, particularly IgG2a antibody induction. Notably, the levels of IgG antibodies against HA as well as the HAI activity in sera from Group PB are also significantly higher than those in sera from Group P and Group B (p<0.05), indicating blockage of Treg could enhance antibody induction by influenza VLPs in aged mice during both priming and boosting immunizations.
Through assessing the functional activity of antibody responses induced by influenza VLP vaccines, we found that sera from aged mice that received anti-CD25 antibody PC61 treatment prior to both priming and boosting immunizations (Group PB) as well as only priming (Group P) or boosting (Group B) immunizations showed higher levels of HAI activity against influenza virus PR8 than sera from aged mice that received rat-IgG isotype control antibodies prior to priming and boosting immunizations (Group NT). Notably, the levels of HAI activity exhibited a better correlation with the total level of IgG antibodies against PR8 HA but not with a particular IgG isotype. After challenge with a high lethal dose (100 LD50) of mouse-adapted influenza virus PR8, the mice in Group PB were all protected from death, while the mice in Group P and Group B were partially protected. In comparison, all mice in Group NT succumbed to challenge similar as the Control Group mice. Protection aged mice against such a high lethal dose challenge seems to correlate with the levels of total IgG antibodies against HA and the HAI activity of sera from vaccinated mice. Of interest, we have in previous studies showed that aged mice vaccinated with 1 ug of influenza VLPs, similarly as the Group NT in the present study, were completed protected from death against lethal challenge by a lower dose (10 LD50) of the same mouse-adapted influenza virus PR8 [35]. Further, the aged mice in previous studies only exhibited slight body weight loss (less than 10%) after challenge by 10 LD50 mouse-adapted influenza virus PR8. In contrast, mice of all groups in this study exhibited over 10% body weight loss after a high lethal dose challenge (100 LD50). Taken together, these results indicate that full protection of aged hosts against high lethal dose influenza virus infection may require a higher levels of immune response induced by vaccination. This requirement, together with the immunosenescence in aged hosts, poses a difficult challenge to the development of an effective influenza vaccine for the aged population. Further elucidation of deficiencies of influenza vaccines and the mechanism of immune protection against influenza virus infection in aged hosts is of high importance for developing more efficacious influenza vaccine to protect the aged population, especially for developing vaccines against emerging highly pathogenic H5N1 as well as H7N9 avian influenza viruses that are now endemic in poultry species and continuing to cause human infections and outbreaks [49, 50].
Acknowledgments
Portions of this study was supported by funding from the Emory University Global Health Institute, the National Institutes of Health grant AI06652, and the NIH CEIRS contract HHSN272201400004C
Footnotes
Conflict of interest: The authors in this study have no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bean WJ, Schell M, Katz J, Kawaoka Y, Naeve C, Gorman O, et al. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J Virol. 1992;66:1129–38. doi: 10.1128/jvi.66.2.1129-1138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid AH, Fanning TG, Hultin JV, Taubenberger JK. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci U S A. 1999;96:1651–6. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer JR, Kawaoka Y, Bean WJ, Suss J, Senne D, Webster RG. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology. 1993;194:781–8. doi: 10.1006/viro.1993.1319. [DOI] [PubMed] [Google Scholar]
- 4.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012;109:4269–74. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Wu Y, Tefsen B, Shi Y, Gao GF. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014;22:183–91. doi: 10.1016/j.tim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang S, Bai T, Yang L, Wang X, Peng B, Liu H, et al. Sustained live poultry market surveillance contributes to early warnings for human infection with avian influenza viruses. Emerg Microbes Infect. 2016;5:e79. doi: 10.1038/emi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Yu X, Pu X, Yang X, Kou Y, Zhou Y, et al. The diversity of avian influenza virus subtypes in live poultry markets before and during the second wave of A(H7N9) infections in Hangzhou, China. Emerg Microbes Infect. 2015;4:e14. doi: 10.1038/emi.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–21. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 11.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370:520–32. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perdue ML, Swayne DE. Public health risk from avian influenza viruses. Avian Dis. 2005;49:317–27. doi: 10.1637/7390-060305R.1. [DOI] [PubMed] [Google Scholar]
- 14.Yen HL, Webster RG. Pandemic influenza as a current threat. Curr Top Microbiol Immunol. 2009;333:3–24. doi: 10.1007/978-3-540-92165-3_1. [DOI] [PubMed] [Google Scholar]
- 15.Wei SH, Yang JR, Wu HS, Chang MC, Lin JS, Lin CY, et al. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir Med. 2013;1:771–8. doi: 10.1016/S2213-2600(13)70221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–9. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–5. [PubMed] [Google Scholar]
- 19.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Stepanova L, Naykhin A, Kolmskog C, Jonson G, Barantceva I, Bichurina M, et al. The humoral response to live and inactivated influenza vaccines administered alone and in combination to young adults and elderly. J Clin Virol. 2002;24:193–201. doi: 10.1016/s1386-6532(01)00246-3. [DOI] [PubMed] [Google Scholar]
- 21.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 22.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 23.Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- 24.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–46. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–36. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25− Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–94. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 28.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of selftolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J, Lau LL, Shen H. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003;171:4352–8. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- 32.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol. 2005;175:7264–73. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- 33.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–9. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams-Bey Y, Jiang J, Murasko DM. Expansion of regulatory T cells in aged mice following influenza infection. Mech Ageing Dev. 2011;132:163–70. doi: 10.1016/j.mad.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen Z, Ye L, Gao Y, Pan L, Dong K, Bu Z, et al. Immunization by influenza virus-like particles protects aged mice against lethal influenza virus challenge. Antiviral Res. 2009;84:215–24. doi: 10.1016/j.antiviral.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, et al. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–70. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Carrion R, Jr, Ye L, Wen Z, Ro YT, Brasky K, et al. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology. 2009;383:12–21. doi: 10.1016/j.virol.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol. 2008;82:11813–23. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye L, Bu Z, Vzorov A, Taylor D, Compans RW, Yang C. Surface stability and immunogenicity of the human immunodeficiency virus envelope glycoprotein: role of the cytoplasmic domain. J Virol. 2004;78:13409–19. doi: 10.1128/JVI.78.24.13409-13419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu WM, van der Zeijst BA, Boog CJ, Soethout EC. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7(Suppl):94–8. doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- 42.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40:780–6. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 43.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–5. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 44.Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17:378–84. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner SC, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28:8077–84. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allman D, Miller JP. B cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:463–7. doi: 10.1016/j.coi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Liu Y, Xu LT, Jackson KJ, Roskin KM, Pham TD, et al. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J Immunol. 2014;192:603–11. doi: 10.4049/jimmunol.1301384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horm SV, Tarantola A, Rith S, Ly S, Gambaretti J, Duong V, et al. Intense circulation of A/H5N1 and other avian influenza viruses in Cambodian live-bird markets with serological evidence of sub-clinical human infections. Emerg Microbes Infect. 2016;5:e70. doi: 10.1038/emi.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou P, Ma J, Lai A, Gray GC, Su S, Li S. Avian influenza A(H7N9) virus and mixed live poultry-animal markets in Guangdong province: a perfect storm in the making? Emerg Microbes Infect. 2015;4:e63. doi: 10.1038/emi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]