Abstract
Objectives
Patients with rheumatoid arthritis (RA) have an excess risk of cardiovascular disease (CVD). We aimed to assess the impact of CVD risk factors, including potential sex differences, and RA-specific variables on CVD outcome in a large, international cohort of patients with RA.
Methods
In 13 rheumatology centers, data on CVD risk factors and RA characteristics were collected at baseline. CVD outcomes (myocardial infarction, angina, revascularization, stroke, peripheral vascular disease and CVD death) were collected using standardized definitions.
Results
5638 patients with RA and no prior CVD were included (mean age: 55.3 [SD:14.0] years, 76% women). During mean follow-up of 5.8 (SD:4.4) years, 148 men and 241 women developed a CVD event (10-year cumulative incidence 20.9% and 11.1%, respectively). Men had a higher burden of CVD risk factors, including increased blood pressure, higher total cholesterol and smoking prevalence than women (all p<0.001). Among the traditional CVD risk factors, smoking and hypertension had the highest population attributable risk (PAR) overall and among both sexes, followed by total cholesterol. The PAR for disease activity score and seropositivity were comparable in magnitude to the PAR for lipids. A total of 70% of CVD events were attributable to all CVD risk factors and RA characteristics combined (separately 49% CVD risk factors and 30% RA characteristics).
Conclusions
In a large international cohort of patients with RA, 30% of CVD events were attributable to RA characteristics. This finding indicates that RA characteristics play an important role in efforts to reduce CVD risk among patients with RA.
Keywords: Cardiovascular disease, rheumatoid arthritis, risk factor, population attributable risk
Introduction
Patients with rheumatoid arthritis (RA) have an increased risk of cardiovascular disease (CVD),[1] which may be attributable to a combination of traditional CVD risk factors and RA-specific characteristics.[2, 3] In a recent meta-analysis, and hypertension, diabetes mellitus, hypercholesterolemia and obesity were shown to increase the risk of CVD in patients with RA.[4]
Successful prevention of CVD requires identification of persons at high risk. However, for patients with RA, established CVD risk calculators have been shown to provide inaccurate estimates.[5, 6] This imprecision may be due to several factors. Despite a high atherosclerotic burden, RA patients have more silent myocardial infarctions (MI) compared to non-RA persons.[7] This asymptomatic atherosclerosis in the carotid and coronary arteries may not be accounted for during CVD risk evaluation.[8–10]
Another factor that may explain the incorrect estimation of CVD is the female preponderance. In the general population, it is well documented that females manifest CVD at older ages than males.[11] Possible differences between the sexes in risk factors associated with CVD have been explored in large general population cohorts.[12–14] It is unknown whether the impact of traditional risk factors on CVD differs between the sexes in patients with RA.
RA is an inflammatory joint disease and the increased risk of CVD is likely also related to disease-specific factors, such as inflammation and disease activity. Inflammation also influences traditional CVD risk factors, such as lipoproteins.[15] Patients with RA are known to have lower lipoprotein levels than non-RA persons [16–18], despite their increased CVD risk, because lipid levels decrease with increasing inflammation. [16, 17, 19, 20] This lipid paradox complicates the CVD risk evaluation of patients with RA. [21] Therefore, it is recommended to assess the lipid profile when the disease is not active. Thus, the importance of each CVD risk factor on CVD outcomes in patients with RA may differ from that in the general population.
The objectives of this study were to assess the impact of CVD risk factors and RA characteristics, including potential sex differences, on CVD outcomes in a large, international cohort of patients with RA.
Patients and methods
Study populations
Thirteen cohorts of patients with RA originating from 10 different countries (UK, Norway, Netherlands, USA, Sweden, Greece, South Africa, Spain, Canada and Mexico) were combined. Patients in each cohort were included based on physician diagnosis of RA and/or fulfillment of 1987 or 2010 American College of Rheumatology criteria for RA. Cohorts followed patients prospectively through study visits at regular intervals or retrospectively through medical record review. Cohorts followed patients prospectively through study visits at regular intervals or retrospectively through medical record review. Data were collected using standardized definitions, but patient management strategies were not standardized. Details regarding these cohorts are located on the consortium website (http://www.atacc-ra.com) and in our previous publication.[22] The study complied with the Declaration of Helsinki, was approved by ethical boards/committees at each center, and informed consent was obtained from the subjects where required. Data were anonymized and aggregated for analyses.
Variable definitions
The primary outcomes were fatal/non-fatal CVD events including acute coronary syndrome (ST-elevation and non-ST elevation myocardial infarction and unstable angina pectoris), chronic ischemic heart disease (stable angina pectoris), coronary revascularization (e.g., percutaneous coronary intervention and coronary artery bypass grafting), CVD death, cerebrovascular events (ischemic cerebrovascular accident and transient ischemic attack) and peripheral vascular events (with and without revascularization procedures, peripheral artery disease). Not included were cases of confirmed cerebral hemorrhage, non-coronary cardiac death, non-ischemic CVD, heart failure and aortic aneurysm.
Traditional CVD risk factors collected at baseline were: age, sex, smoking status (current, former, never), systolic and diastolic blood pressure, lipid levels (total cholesterol [TC], high density lipoprotein cholesterol [HDL-c], low density lipoprotein cholesterol [LDL-c], triglycerides), body mass index (BMI), family history of CVD, diabetes mellitus and hypertension. Data on use of lipid-lowering medications and anti-hypertensives were also collected.
RA-specific factors were collected at baseline including rheumatoid factor (RF) positivity, anti-citrullinated protein antibodies (ACPA) positivity, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and disease activity score including 28 joints (DAS28) using ESR. RF and ACPA were considered positive based on the tests performed at each center. ACPA testing was unavailable in some patients. CRP was unavailable in one cohort, and DAS28 was unavailable in another cohort. Data on use of non-steroidal anti-inflammatory drugs (NSAIDs), Cox-2 inhibitors, glucocorticoids and RA therapies were also collected.
Statistical methods
Descriptive statistics (means, percentages, etc.) were used to summarize characteristics at baseline by sex with comparisons performed using chi-square and rank sum tests. Multiple imputation methods were used to impute missing values for CVD risk factors using 10 repetitions. Log-transformations were used when imputing lipid levels to avoid bias when computing lipid ratios from imputed data. [23]
The cumulative incidence of CVD by sex according to time since baseline and age was estimated using Kaplan-Meier methods. Adjusted curves were obtained using inverse probability weighting. [24] The influence of CVD risk factors and RA characteristics on development of CVD was estimated overall and separately by sex using multivariable Cox models. All models were stratified according to high and low CVD risk centers to account for underlying differences in CVD event rates and to avoid issues with instability of estimates for small centers. Univariable models adjusted for age and multivariable models containing all CVD risk factors and all CVD risk factor plus RA characteristics were examined. As sex differences were not substantially different for adjusted univariable models compared to multivariable models, only the multivariable model results were reported. Estimates for triglycerides were obtained by removing HDL-c and adding triglycerides to the overall multivariable model.
Population attributable risk (PAR) is the proportion of disease in a population that could be prevented by elimination of an exposure or risk factor. PAR is commonly calculated as PAR = (P(D) − P(D|Ē))/P(D) where P(D) is the probability of disease (i.e., CVD), and P(D| Ē) is the conditional probability of disease among individuals without the risk factor.[25] PAR is a function of time, since it depends on the probability of disease and the prevalence of the risk factor, which changes over time. Thus, the cumulative incidence of CVD was used to estimate the probability of CVD over time, and these estimates were obtained from Cox models to allow for adjustment for multiple risk factors. The conditional probability of CVD over time for those without a risk factor was estimated from the same Cox models, but with a target cohort that matched the observed cohort except that it lacked the risk factor of interest. For continuous risk factors, such as lipid levels, target values representing desirable levels were chosen. An overall PAR considering the impact of a collection of risk factors was also obtained by utilizing target cohorts modifying several risk factors simultaneously. The sum of PAR for single risk factors may not equal the PAR for a combination of several risk factors due to overlapping exposures and non-additive effects.[26] Reported values of PAR correspond to 10 years after baseline, as 10 years is a commonly used time horizon for CVD risk assessment. P-values <0.05 were considered to be statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Risk factors
Baseline characteristics of the 5638 included patients with RA without prior CVD are presented in table 1. The mean age (±SD) in the total population was 55.3 (±14.0 years). There was a clear female preponderance (75.9% overall) with even higher female/male ratios among the 2 Hispanic cohorts. Statistically significant differences between the sexes were observed in the majority of the baseline characteristics. Male patients were older and had a higher burden of traditional CVD risk factors, including higher blood pressure/more hypertension and were more often smokers (p<0.001 for all). Higher levels of CRP (p<0.001) were also seen in men compared to women. Women had slightly higher TC, HDL-c and ESR levels and were more frequent users of synthetic and biologic DMARDs (p <0.001 and 0.005, respectively).
Table 1.
Descriptive baseline characteristics of 5638 patients with rheumatoid arthritis without prior cardiovascular disease
| Characteristic | Available n |
Overall (n=5638) | Female (n=4278) | Male (n=1360) | p value |
|---|---|---|---|---|---|
| Length of follow-up, years | 5638 | 5.8 (4.4) | 5.8 (4.4) | 6.0 (4.6) | -- |
| Age, years | 5638 | 55.3 (14.0) | 54.7 (14.4) | 57.2 (12.7) | <0.001 |
| White race | 3945 | 3699 (94%) | 2924 (93.4%) | 775 (95.2%) | 0.056 |
|
| |||||
| RA characteristics | |||||
|
| |||||
| Calendar year of RA diagnosis | 5628 | 1998.4 (9.9) | 1998.1 (10.1) | 1999.2 (9.1) | 0.007 |
| RA disease duration, years | 5628 | 6.4 (9.2) | 6.9 (9.5) | 4.9 (8.0) | <0.001 |
| RF and/or ACPA positive | 5485 | 3949 (72%) | 2982 (71.8%) | 967 (72.7%) | 0.540 |
| ESR, mm/hr | 4737 | 24.8 (21.4) | 25.1 (20.9) | 23.8 (22.8) | <0.001 |
| C-reactive protein, mg/L | 4528 | 15.4 (27.1) | 13.7 (24.8) | 21.1 (33.0) | <0.001 |
| DAS28 | 4448 | 4.0 (1.7) | 4.0 (1.7) | 3.9 (1.7) | 0.466 |
|
| |||||
| Traditional CVD risk factors | |||||
|
| |||||
| Systolic blood pressure, mmHg | 5345 | 136.0 (21.9) | 134.3 (22.0) | 141.3 (20.7) | <0.001 |
| Diastolic blood pressure, mmHg | 5343 | 79.6 (11.1) | 78.5 (11.0) | 83.0 (10.7) | <0.001 |
| Total cholesterol, mmol/L | 4457 | 5.2 (1.1) | 5.2 (1.1) | 5.1 (1.1) | <0.001 |
| Low density lipoprotein, mmol/L | 4364 | 3.1 (1.0) | 3.1 (1.0) | 3.1 (1.0) | 0.077 |
| High density lipoprotein, mmol/L | 4403 | 1.5 (0.4) | 1.5 (0.5) | 1.3 (0.3) | <0.001 |
| Triglycerides, mmol/L | 4240 | 1.4 (0.8) | 1.4 (0.7) | 1.5 (1.0) | 0.001 |
| Current smoker | 5368 | 1148 (21%) | 788 (19.3%) | 360 (28.1%) | 0.001 |
| Ever smoker | 5120 | 2688 (52%) | 1838 (47.1%) | 850 (69.7%) | <0.001 |
| Body mass index, kg/m2 | 5160 | 27.0 (5.4) | 26.9 (5.7) | 27.2 (4.4) | <0.001 |
| Hypertension | 5618 | 2344 (42%) | 1726 (40.5%) | 618 (45.7%) | 0.001 |
| Diabetes mellitus | 5637 | 395 (7%) | 290 (6.8%) | 105 (7.7%) | 0.237 |
| Family history of CVD | 3677 | 876 (24%) | 658 (23.8%) | 218 (23.9%) | 0.931 |
|
| |||||
| Medication | |||||
|
| |||||
| Antihypertensives | 5608 | 1314 (23%) | 1008 (23.7%) | 306 (22.6%) | 0.437 |
| Lipid lowering therapy | 5604 | 510 (9%) | 383 (9.0%) | 127 (9.4%) | 0.395 |
| Synthetic DMARD | 5593 | 2610 (47%) | 2085 (49.1%) | 525 (38.9%) | <0.001 |
| Biologic DMARD | 5592 | 891 (16%) | 762 (18.0%) | 129 (9.6%) | <0.001 |
| Corticosteroid | 5590 | 1527 (27%) | 1177 (27.7%) | 350 (26.0%) | 0.201 |
| Corticosteroid dosage, mg/day | 869 | 5.8 (4.4) | 5.6 (4.2) | 6.4 (5.2) | 0.066 |
Values in table are mean (SD) or n (%). RA: rheumatoid arthritis, CVD: cardiovascular disease, RF: rheumatoid factor, ACPA: anti-citrullinated protein antibody, DAS: disease activity score, ESR: erythrocyte sedimentation rate, HAQ: health assessment questionnaire, DMARD: disease modifying anti-rheumatic drug
Outcomes
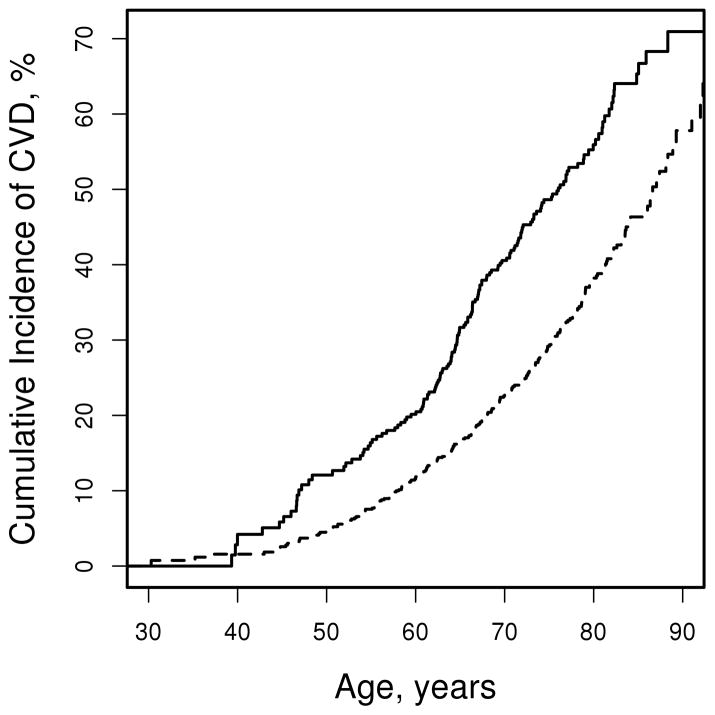
During a mean (±SD) follow up of 5.8 (±4.4) years, 389 patients (148 men and 241 women) developed CVD events (including 166 patients with MI, 88 with stable or unstable angina, 62 with revascularization, 35 with CV deaths, 147 with cerebrovascular events, and 46 with peripheral vascular events). The unadjusted cumulative incidence of CVD at 10 years after baseline was 20.9% (95%CI: 17.3% – 24.4%) among men and 11.1% (95%CI: 9.6% – 12.7%) among women. Adjustment for CVD risk factors had minimal impact on 10 year CVD risk estimates (19.2% among men and 11.4% among women). The cumulative incidence of CVD was higher in men than in women in all ages above 40 years (figure 1).
Figure 1.
Cumulative incidence of cardiovascular disease (CVD) among men (solid line) and women (dashed line) with rheumatoid arthritis according to age
Relative risk
The age-adjusted relative risk of CVD was higher among men than women (hazard ratio [HR]: 1.74; 95%CI: 1.41 – 2.12). Additional adjustment for CVD risk factors reduced the sex effect slightly (HR: 1.49; 95%CI: 1.19 – 1.85; table 2). Age was significantly associated with CVD overall and in both sexes, and the age effect was not impacted by adjusting for other CVD risk factors. TC, LDL-c and triglycerides were all significantly associated with CVD following age adjustment, but no longer reached statistical significance following adjustment for other CVD risk factors. Among men the only lipid measure that was significantly associated with CVD was triglycerides, which remained statistically significant following adjustment for all CVD risk factors. Smoking and hypertension were strong predictors of CVD overall and among both sexes, even after adjustment for all other CVD risk factors. Diabetes mellitus was significantly associated with CVD among women, but did not reach statistical significance among men. BMI was not significantly associated with CVD. Regarding RA characteristics, DAS28 and RF/ACPA positivity were significantly associated with CVD with age adjustment, but were no longer significant after adjustment for all CVD risk factors. There was no evidence of differential effects between sexes regarding the association between CVD risk factors and CVD outcomes (interaction p>0.1 for all) or between RA characteristics and CVD outcomes (interaction p>0.6 for all).
Table 2.
Adjusted hazard ratios for cardiovascular disease for both sexes
| Risk factor | Age-and sex-adjusted hazard ratio (95% CI) | Multivariable-adjusted† hazard ratio(95% CI) | p-value for sex difference | ||
|---|---|---|---|---|---|
|
| |||||
| Cardiovascular disease | Overall | Overall | Women | Men | |
| Male Sex (vs Female) | 1.74 (1.41, 2.13) | 1.49 (1.19, 1.85) | -- | -- | -- |
| Age, years* | 1.70 (1.57, 1.85) | 1.66 (1.52, 1.81) | 1.74 (1.56, 1.94) | 1.54 (1.33, 1.79) | 0.19 |
| Total cholesterol, mmol/L | 1.10 (1.02, 1.20) | 1.15 (0.93, 1.43) | 1.06 (0.80, 1.40) | 1.27 (0.90, 1.77) | 0.94 |
| LDL-c, mmol/L | 1.15 (1.04, 1.27) | 1.00 (0.79, 1.27) | 1.11 (0.81, 1.52) | 0.89 (0.62, 1.29) | 0.29 |
| HDL-c, mmol/L | 0.73 (0.56, 0.95) | 0.67 (0.48, 0.93) | 0.76 (0.51, 1.15) | 0.51 (0.28, 0.94) | 0.82 |
| Triglycerides, mmol/L** | 1.17 (1.06, 1.30) | 1.13 (0.99, 1.29) | 1.08 (0.89, 1.31) | 1.24 (1.01, 1.51) | 0.79 |
| Current smoker | 2.05 (1.58, 2.67) | 1.98 (1.52, 2.58) | 1.79 (1.28, 2.50) | 2.50 (1.56, 4.03) | 0.20 |
| Former smoker | 1.46 (1.14, 1.86) | 1.43 (1.12, 1.83) | 1.36 (1.01, 1.82) | 1.75 (1.10, 2.79) | 0.39 |
| Hypertension | 1.62 (1.31, 2.00) | 1.59 (1.28, 1.96) | 1.40 (1.07, 1.84) | 1.95 (1.38, 2.75) | 0.30 |
| Diabetes mellitus | 1.49 (1.08, 2.06) | 1.39 (0.99, 1.95) | 1.62 (1.06, 2.50) | 1.13 (0.66, 1.96) | 0.42 |
| Body mass index, kg/m2 | 1.01 (0.99, 1.03) | 1.00 (0.98, 1.02) | 1.00 (0.97, 1.02) | 1.00 (0.96, 1.04) | 0.79 |
| Rheumatoid arthritis | |||||
| DAS28 ≥3.2 | 1.40 (1.03, 1.90) | 1.24 (0.91, 1.70) | 1.32 (0.88, 1.98) | 1.12 (0.68, 1.84) | 0.80 |
| RF/ACPA positive | 1.30 (1.02, 1.65) | 1.23 (0.96, 1.56) | 1.26 (0.92, 1.70) | 1.18 (0.79, 1.76) | 0.99 |
| ESR, mm/hr* | 1.02 (0.98, 1.07) | 1.01 (0.97, 1.06) | 1.03 (0.96, 1.09) | 1.01 (0.94, 1.08) | 0.68 |
| CRP, mg/L* | 1.02 (0.99, 1.04) | 1.01 (0.98, 1.04) | 1.01 (0.96, 1.05) | 1.02 (0.97, 1.06) | 0.82 |
LDL-c: Low density lipoprotein cholesterol, HDL-c: high density lipoprotein cholesterol, DAS28: Disease activity score using 28 joints, RF: rheumatoid factor, ACPA: anti-citrullinated protein antibodies, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein; CI: confidence interval
per 10 unit increase
Estimates for triglycerides were obtained by removing HDL-c and adding triglycerides to the overall multivariable model.
All cardiovascular risk factors were included in one multivariable model for each sex, except triglycerides. Each rheumatoid arthritis variables was subsequently added to the overall and sex-specific multivariable models including all the cardiovascular disease risk factors.
Population attributable risk
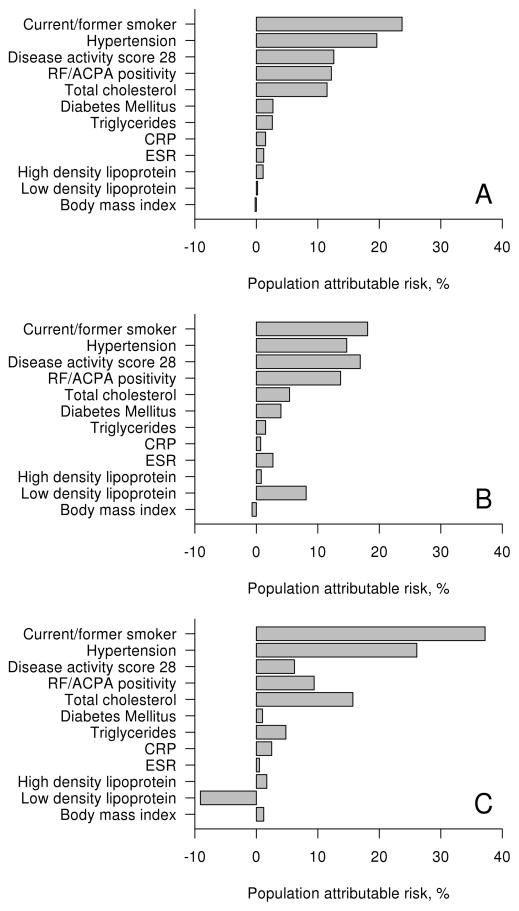
PAR estimates the fraction of CVD events that could be prevented by a potential target intervention. Hence, PAR is a function of risk factor prevalence, effect size and a chosen target. Smoking and hypertension had the highest PAR values overall and among both sexes (figure 2 and table 3). PAR for smoking and hypertension were nearly twice as high among men than women (37.2% vs 18.1% and 26.1% vs 14.7%, respectively), but these differences did not reach statistical significance (p=0.12 and p=0.24, respectively). PAR for TC was 11.5% overall, with an insignificantly higher PAR among men than women (p=0.56). PAR for the remaining CVD risk factors was <10% overall and for both sexes. For the RA characteristics, DAS28 and RF/ACPA positivity had similar PAR to TC (12.6% and 12.2%, respectively). PAR for decreasing DAS28 to 3.2 if >3.2 at baseline was 16.9% among women compared to 6.2% among men (p=0.46). PAR for RF/ACPA was also higher among women than men (13.7% vs 9.4%), but not significantly (p=0.74). PAR for ESR and CRP were <5% overall and for both sexes.
Figure 2.
Population attributable risk for cardiovascular risk factors and RA characteristics overall (panel A), among women (panel B) and among men (panel C).
Table 3.
Population attributable risk for 10 year cardiovascular disease risk by sex
| Risk factor | Target Values | Multivariable-adjusted† Population attributable risk, % |
p-value for sex difference | ||
|---|---|---|---|---|---|
|
| |||||
| Cardiovascular disease | Overall | Women | Men | ||
| Total cholesterol, mmol/L | Decreased to 4.5 mmol/L | 11.5 | 5.4 | 15.7 | 0.56 |
| LDL-c, mmol/L | Decreased to 2.5 mmol/L | 0.2 | 8.1 | −9.1 | 0.41 |
| HDL-c, mmol/L | Increased to 1.2 mmol/L for women, 1.0 mmol/L for men | 1.1 | 0.8 | 1.7 | 0.43 |
| Triglycerides, mmol/L | Decreased to 1.7 mmol/L | 2.6 | 1.5 | 4.8 | 0.29 |
| Current/Former smoker | Never smoked | 23.7 | 18.1 | 37.2 | 0.12 |
| Hypertension | Absence | 19.6 | 14.7 | 26.1 | 0.24 |
| Diabetes mellitus | Absence | 2.7 | 4.0 | 1.0 | 0.39 |
| Body mass index, kg/m2 | Decreased to 25 kg/m2 | −0.2 | −0.7 | 1.2 | 0.81 |
| Rheumatoid arthritis | |||||
| DAS28 ≥3.2 | Decreased to <3.2 | 12.6 | 16.9 | 6.2 | 0.46 |
| RF/ACPA positive | Negative | 12.2 | 13.7 | 9.4 | 0.74 |
| ESR, mm/hr | Decreased to 20 mm/hr | 1.2 | 2.7 | 0.5 | 0.76 |
| CRP, mg/L | Decreased to 5 mg/L | 1.5 | 0.7 | 2.5 | 0.77 |
| All CVD risk factors | 49.0 | 42.8 | 61.1 | 0.29 | |
| All RA characteristics | 30.3 | 39.9 | 18.0 | 0.39 | |
| All CVD and all RA characteristics | 69.6 | 72.7 | 69.3 | 0.87 | |
Abbreviations: LDL-c, Low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol, DAS28, Disease activity score using 28 joints; RF, rheumatoid factor; ACPA, anti-citrullinated protein antibodies; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RA, rheumatoid arthritis
per 10 unit increase
Estimates for triglycerides were obtained by removing HDL-c and adding triglycerides to the overall multivariable model.
All CVD risk factors (including age) were included in one multivariable model for each sex, except triglycerides. Each rheumatoid arthritis variables was subsequently added to each sex-specific multivariable model including all the CVD risk factors.
In aggregate, all the CVD risk factors in table 3 combined explained 49.0% of the CVD risk in patients with RA. The RA characteristics explained 30.3% of the CVD risk, and the CVD risk factors and RA characteristics combined explained 69.6% of the CVD risk. PAR for all the CVD risk factors combined was higher among men (61.1%) than among women (42.8%), but this difference was not statistically significant (p=0.29). In contrast, PAR for all RA characteristics combined was higher among women (39.9%) than among men (18.0%), but not significantly (p=0.39). Furthermore, when both CVD risk factors and RA characteristics were combined, the PAR for men and women were similar (72.7% vs 69.3%, respectively, p=0.82). Additional adjustment for use of anti-hypertensives, lipid-lowering medications, NSAIDs, COX-2 inhibitors, glucocorticoids and family history of CVD had little impact (2.0% increase in PAR).
Discussion
Our study illuminated that both traditional and RA-specific factors had significantly different prevalence among women and men with RA. There was a sex difference in CVD event rates in patients with RA for all ages above 40 years, and this was independent of traditional CVD risk factors and markers of RA disease activity. Despite sex differences in the prevalence of many risk factors, the relative risks for CVD for the risk factors did not differ between the sexes, and this resulted in no significant differences in the PAR between the sexes. Altogether, the CVD risk factors and RA characteristics explained 70% of the PAR for CVD outcomes, leaving 30% unaccounted for.
Our findings among patients with RA of no differential effects of CVD risk factors between the sexes differed from reports in the general population. The INTERHEART study found that smoking had a higher impact on CVD among men than women, and hypertension and diabetes mellitus had a higher impact among women than men.[13] Schnohr et al. also found a higher impact of diabetes mellitus on CVD risk among women compared to men, but no differential sex effect for hypertension.[27] In contrast, they found a higher impact of smoking on CVD among women compared to men.[27] While the relative risk of diabetes mellitus in our study was also higher for women than men, the effect of smoking appeared to be greater in men than women, but neither difference reached statistical significance. The female predominance of RA may have limited statistical power for these comparisons despite the large sample size in this study.
Our findings that smoking and hypertension had the highest PAR were consistent with the findings from the INTERHEART study and Schnohr et al. [27, 28] Our PAR for smoking (18.1% for women and 37.2% for men) were similar to the INTERHEART study (15.8% for women and 44.0% for men), which differed from Schnohr et al. (37% among women and 22% among men). PAR for hypertension also differed between the INTERHEART study (35.8% among women and 19.5% among men) and Schnohr et al. (14% among women and 18% among men), but in this case our PAR (14.7% among women and 26.1% among men) were closer to those of Schnohr et al.. Likewise our PAR for diabetes mellitus (4.0% among women and 1.0% among men) agreed more closely with Schnohr et al. (3% for both sexes) than with INTERHEART (19.1% among women and 10.1% among men). The reasons for these disparate PAR between studies in the general population are unknown. However, there could be differences in smoking exposures or in hypertension control between these study populations.
Concordant with our findings, neither of the general population studies noted differential effects of lipids on CVD between sexes.[13, 27] The relation of LDL-c and CVD events appeared to be U-shaped for both RA and non-RA persons.[29] Low levels of LDL-c have been linked to an increased risk of CVD also in other chronic diseases, such as heart failure and cancer.[30, 31] In our study, the PAR for LDL-c among men was negative, indicating that lowering LDL-c may not reduce CVD risk among men. This is consistent with other reports of a modest impact of lipids on future CVD in RA. In addition, Schnohr et al reported low PAR of 9% among men and 12% among women for hypercholesterolemia, despite high hypercholesterolemia prevalence of 47% among men and 57% among women.[27] With lower prevalence rates of hypercholesterolemia in RA than in the general population, it is not surprising that our PAR for lipids were low.
The RA-specific variables seem to have a greater impact on CVD among women than men. While the prevalence of RF/ACPA positivity and DAS28 levels were similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance. Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR. Furthermore, RA disease duration was longer among women and more women than men were receiving biologic DMARDs at baseline.
Of interest, the PAR for DAS28 and RF/ACPA positivity were comparable in magnitude to the PAR for TC, underscoring the importance of RA characteristics in attributing CVD outcomes among patients with RA. Altogether, RA characteristics accounted for 30% of the CVD risk. These findings indicate that RA characteristics play an important role in efforts to reduce CVD risk among patients with RA.
Strengths of this study include its large sample size comprised of several diverse cohorts of patients with RA, as well as the careful assessment of RA characteristics and CVD risk factors in these cohorts. In addition, most of the cohorts were either population-based or included all consecutive patients, which should reduce selection bias issues. However, several cohorts were developed at referral centers, so the possibility of referral bias cannot be excluded. Limitations include the possibility of differences in measurement of CVD risk factors and CVD events despite agreement on variable definitions across cohorts. Moreover, patient counseling and aggressive treatment of CVD risk at some centers may limit generalizability of our findings. Finally, RA characteristics were measured at the single time point of study inclusion. As RA disease activity fluctuates over time, assessment at a single time point may lead to underestimation of the impact of RA characteristics on development of CVD events.
In this large, international cohort of RA patients, no significant sex differences were observed concerning the risk factors’ effect on future CVD. Furthermore, the importance of various CVD risk factors differed from what is reported from other patient populations. Knowledge regarding the impact of various risk factors on CVD events is essential to individualize CVD risk evaluation and prevention for patients with RA. Optimal management of CVD risk factors continues to be an important goal of CVD risk management in patients with RA as evidenced by the sizeable proportion of CVD risk attributable to CVD risk factors. Furthermore, the substantial proportion of CVD risk attributable to RA characteristics indicates that RA disease activity and severity play an important role in efforts to reduce CVD risk among patients with RA.
Acknowledgments
Funding: This work was supported by a collaborative agreement for independent research from Eli Lilly, a grant from the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health (grant number R01 AR046849), and grants from the Norwegian South East Health Authority (grant numbers 2013064, 2013010). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional ATACC-RA consortium members:
Iris Colunga, Dionicio Galarza, and José Ramón Azpiri-López, Hospital Universitario “Dr. José E. González”, Monterrey, Mexico
Elaine Husni and Robert Overman, Cleveland Clinic, Cleveland, Ohio, US
Jaap Fransen, Department of Rheumatic Diseases, Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands
Solbritt Rantapää-Dahlqvist, University of Umeå, Umeå, Sweden
Daniel Solomon and Katherine Liao, Harvard Medical School Brigham and Women’s Hospital, Boston, MA, United States
Footnotes
Conflict of Interest: Cynthia S. Crowson reports grants from National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health, during the conduct of the study. A. G. Semb has received speaker honoraria and/or consulting fee from Merck/Schering-Plough, Abbott, BMS, UCB, Pfizer/Wyeth, Eli Lilly, and Hoffmann-La Roche. Dr. Kvien reports grants and personal fees from AbbVie, BMS, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Hospira/Pfizer, Merck-Serono, MSD, Mundipharma, Novartis, Oktal, Orion Pharma, Roche, Sandoz, and UCB.
Silvia Rollefstad, Eirik Ikdahl, George D. Kitas, Piet L. C. M. van Riel, Sherine E. Gabriel, Eric L Matteson, Karen Douglas, Aamer Sandoo, Elke Arts, Solveig Wållberg-Jonsson, Lena Innala, George Karpouzas, Patrick H. Dessein, Linda Tsang, Hani El-Gabalawy, Carol Hitchon, Virginia Pascual Ramos, Irazú Contreras Yáñez, Petros P. Sfikakis, Evangelia Zampeli, Miguel A. Gonzalez-Gay, Alfonso Corrales, Mart van de Laar, Harald Vonkeman, and Inger Meek: none declared.
References
- 1.Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis. 2011;70(6):929–934. doi: 10.1136/ard.2010.143396. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67(1):64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–1925. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baghdadi LR, Woodman RJ, Shanahan EM, et al. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117952. doi: 10.1371/journal.pone.0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–424. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74(4):668–674. doi: 10.1136/annrheumdis-2013-204024. [DOI] [PubMed] [Google Scholar]
- 7.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 8.Semb AG, Rollefstad S, Provan SA, et al. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J Rheumatol. 2013;40(4):359–368. doi: 10.3899/jrheum.120621. [DOI] [PubMed] [Google Scholar]
- 9.Semb AG, Ikdahl E, Hisdal J, et al. Exploring cardiovascular disease risk evaluation in patients with inflammatory joint diseases. Int J Cardiol. 2016;223:331–336. doi: 10.1016/j.ijcard.2016.08.129. [DOI] [PubMed] [Google Scholar]
- 10.Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis. 2014;73(4):722–727. doi: 10.1136/annrheumdis-2012-203101. [DOI] [PubMed] [Google Scholar]
- 11.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leifheit-Limson EC, Reid KJ, Kasl SV, et al. The role of social support in health status and depressive symptoms after acute myocardial infarction: evidence for a stronger relationship among women. Circ Cardiovasc Qual Outcomes. 2010;3(2):143–150. doi: 10.1161/CIRCOUTCOMES.109.899815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29(7):932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 14.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 15.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68(4):460–469. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 16.Semb AG, Kvien TK, Aastveit AH, et al. Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the Apolipoprotein-related Mortality RISk (AMORIS) Study. Ann Rheum Dis. 2010;69(11):1996–2001. doi: 10.1136/ard.2009.126128. [DOI] [PubMed] [Google Scholar]
- 17.Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1310–1314. doi: 10.1136/ard.2009.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rantapää-Dahlqvist S, Wållberg-Jonsson S, Dahlén G. Lipoprotein (a), lipids, and lipoproteins in patients with rheumatoid arthritis. Ann Rheum Dis. 1991;50:366–368. doi: 10.1136/ard.50.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters MJ, Voskuyl AE, Sattar N, et al. The interplay between inflammation, lipids and cardiovascular risk in rheumatoid arthritis: why ratios may be better. Int J Clin Pract. 2010;64(10):1440–1443. doi: 10.1111/j.1742-1241.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 20.Svensson KLG, Lithell H, Hållgren R, et al. Serum Lipoprotein in Active Rheumatoid Arthritis and Other Chronic Inflammatory Arthritides: I. Relativity to Inflammatory Activity. Arch Intern Med. 1987;147(11):1912–1916. [PubMed] [Google Scholar]
- 21.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12(3):e0174656. doi: 10.1371/journal.pone.0174656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris TP, White IR, Royston P, et al. Multiple imputation for an incomplete covariate that is a ratio. Stat Med. 2014;33(1):88–104. doi: 10.1002/sim.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Crowson CS, Therneau TM, O’Fallon WM. Technical Report Series No.82, Attributable Risk Estimation in Cohort Studies. Department of Health Sciences Research, Mayo Clinic; Rochester, MN, USA: 2009. [Google Scholar]
- 26.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10(3):195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 27.Schnohr P, Jensen JS, Scharling H, et al. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from The Copenhagen City Heart Study. Eur Heart J. 2002;23(8):620–626. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]
- 28.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 29.Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67(8):2004–2010. doi: 10.1002/art.39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42(11):1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Iribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396–2403. doi: 10.1161/01.cir.92.9.2396. [DOI] [PubMed] [Google Scholar]