Abstract
Background
Severe aorto-iliac occlusive disease (AIOD) is traditionally treated with aorto-bifemoral (ABF) or aorto-unifemoral (AUF) bypass. However, cross-femoral bypass (CFB) and hybrid femoral endarterectomy and patch angioplasty with iliac stenting (EPS) have gained popularity as less invasive options. We sought to compare 1-year survival, primary patency, and major amputation rates between open surgical (ABF and AUF) and two less invasive reconstruction techniques (CFB and EPS) using a large, multicenter cohort.
Study Design
This is a retrospective cohort study of patients who underwent either ABF/AUF CFB/EPS for AIOD between 2006 and 2013 in the Society for Vascular Surgery Vascular Quality Initiative registry. Baseline patient and peri-procedural variables were compared. Propensity score matching (PSM) was performed to predict the likelihood of more invasive repair. Kaplan-Meier analysis and Cox models were performed for 1-year survival, primary patency and major amputation.
Results
1872 patients underwent procedures for AIOD, including 1133 ABF/AUF and 739 CFB/EPS. Indication was critical limb ischemia in 47.3% (N=886). Median follow-up time was 305 days (range 10–406). After PSM, the matched cohort included 1094 ABF/AUF and 711 CFB/EPS patients. Multivariate analysis revealed that patient factors and procedure indication were significant predictors of 1-year mortality and major amputation, but not procedure type. ABF/AUF was associated with improved primary patency over CFB/EPS at one year (94.1±1.1% vs. 92.3%±1.5%, hazard ratio 0.65, 95% confidence interval 0.45–.94;P=.02).
Conclusions
In a propensity-matched cohort from a multi-center vascular surgery registry, a direct approach to AIOD (ABF/AUF) demonstrated better 1-year primary patency than commonly-used less invasive strategies. However, treatment approach was not a predictor of 1-year survival or limb salvage, suggesting that patient factors and procedure indication have a greater impact on outcome.
INTRODUCTION
Reconstruction options for aorto-iliac occlusive disease (AIOD) include direct bypass, extra-anatomic bypass, endovascular approaches, and hybrid procedures. The gold standard treatment for severe AIOD has traditionally been direct reconstruction, such as aorto-bifemoral (ABF) and aorto-unifemoral bypass (AUF).1 However, alternative revascularization approaches for AIOD have gained popularity, including extra-anatomic bypass such as cross-femoral bypass (CFB) and hybrid techniques such as common femoral endarterectomy with patch angioplasty and iliac stenting (EPS).2–8 Direct repair provides excellent survival and long-term patency rates, with 0–4% 30-day mortality and 86–92% 5-year primary patency. 9–12 However, open aortic surgery requires more extensive surgical exposure and has an increased physiologic impact that may make these procedures prohibitively high risk in some patients.2,13 The literature directly comparing outcomes for these 2 treatment strategies for AIOD is limited, but the less invasive techniques have become more popular.8,14–16 Thus, we sought to compare survival, primary patency, and major limb amputation between direct revascularization (ABF and AUF) and less invasive techniques (CFB and EPS) using a large, multi-center registry.
METHODS
Data Source
We performed a retrospective study using the Society for Vascular Surgery Vascular Quality Initiative (SVS VQI) registry. 17 The VQI provides an excellent data source and includes peri-procedural variables unique to vascular surgical procedures which are not found in other registries. The suprainguinal bypass and peripheral vascular intervention (PVI) datasets were queried from January 2006 to February 2013. ABF, AUF, or CFB procedures were identified in the suprainguinal dataset, and EPS procedures were identified in the PVI dataset. The registry is updated monthly, but the query dates were restricted to allow for at least one-year (defined as 9–21 months) follow-up for all patients.
Patients
Adult patients aged 18 to 99 were included in the sample. The unit of analysis was patients. Therefore, in instances where patients underwent multiple procedures in the dataset, only the earliest procedure was included in the sample. ABF, AUF, and CFB were excluded if any concomitant infrainguinal bypass or endovascular intervention was performed. Similarly, EPS cases were excluded if vessels other than iliac and femoral arteries were treated. Lastly, emergent procedures and patients with missing data for graft inflow, graft outflow, concomitant procedures, and TASC18 A or B classification were excluded from the sample. This study was approved by the Northwestern University Institutional Review Board and the SVS VQI Research Advisory Committee. The dataset was de-identified before release to investigators.
Procedure and VQI Variables
Procedures within the suprainguinal dataset were identified by graft inflow and outflow. ABF was defined as abdominal aorta for graft inflow and bilateral femoral artery outflow [common femoral (CFA), profunda femoris (PFA), and/or superficial femoral artery (SFA)]. Similarly, AUF was defined as abdominal aorta for graft inflow and unilateral femoral artery outflow. CFB procedures were defined as femoral artery (CFA, PFA, and/or SFA) inflow to contralateral femoral artery outflow. Comparison groups were categorized as direct (ABF and AUF) versus less invasive approaches (CFB and EPS).
The VQI classifies variables as demographic, history, procedure, post-operative and follow-up. History variables included medical co-morbidities, procedure indication, symptoms, prior vascular interventions, and pre-treatment ankle-brachial indices (ABI). Procedure variables include urgency, graft/stent type, arteries treated, and completion imaging results. Post-operative variables included hospital length of stay, early complications, and discharge medications. Follow-up variables included survival, patency, reintervention, and major amputation, which are collected between 9 months and 21 months following the date of the index procedure. Of note, mortality data are supplemented by periodic matching with the Social Security Death Index (SSDI), making it a very reliable endpoint.
In addition to VQI-collected variables, the Vascular Study Group of New England Cardiac Risk Indicator (VSGNE CRI) was used to stratify patients as high and low-risk for postoperative cardiac event. Risk factors such as age, coronary artery disease, congestive heart failure, insulin-dependent diabetes mellitus, chronic obstructive pulmonary disease, renal insufficiency, long-term beta-blocker use, and smoking history are used to calculate the VSGNE CRI score. Patients were considered high-risk if their VSGNE CRI score was greater than or equal to 8, which is associated with a 14.3% risk of an adverse cardiac event.19
Statistical Analysis
Group comparison (ABF/AUF vs. CFB/EPS) and hypothesis testing were performed using Fisher’s exact and chi-square tests for categorical variables and Wilcoxon rank sum for continuous variables. Survival analyses were applied to the matched cohort for the primary study endpoint (one-year survival) and secondary endpoints (one-year primary patency and major amputation). Kaplan-Meier analyses were stratified by procedure class and log-rank tests were applied to assess for statistically significant differences. Stepwise multivariate Cox proportional hazards models were created for the outcomes of interest. Covariates that were statistically significant on univariate analysis (P<.05) were included in the final models.
Propensity Score Matching and Subgroup Analysis
In order to minimize selection bias across comparison groups, we performed propensity score matching (PSM). A stepwise logistic regression model was created using baseline patient characteristic variables that were statistically significant across comparison group. Patients were then matched using a one-to-many caliper of 0.01. To test whether the PSM cohort was successfully balanced, standard differences (SDiff) were calculated for each covariate and deemed appropriately balanced for SDiff<10%.20–22
Subgroup analysis was performed using the matched cohort, which was stratified by operative indication into intermittent claudication (IC) and critical limb ischemia (CLI) groups. We applied a stepwise multivariate Cox proportional hazards model to determine if the statistically significant findings would differ based on operative indication (IC vs. CLI).
Analyses were performed using STATA software, version 13.0 (College Station, Texas).
RESULTS
Sample Overview
Within the combined suprainguinal and PVI datasets, there were 57,146 procedures, of which 2288 were ABF or AUF and 3423 were CFB or EPS. After exclusion for concomitant procedures, emergent cases, TASC lesions A and B, and procedure date after February 2013, the study sample included 1872 patients. Of the 1872 patients, 1133 (60.5%) underwent direct revascularization (ABF N=1076 and AUF N=57) and 739 (39.5%) underwent less invasive procedures (CFB N= 501 and EPS N=238).
Baseline Patient and Procedure Characteristics
Patient and procedure characteristics, as shown in Table I, demonstrated that comparison groups were similar for gender, body mass index, history of positive cardiac stress test, and preoperative medication use. However, for the majority of baseline characteristics, the groups were dissimilar. The most notable differences were: the ABF/AUF group was younger compared to CFB/EPS (median age 60, ABF/AUF vs. 67, CFB/EPS; P<.001), the indication for procedure in ABF/AUF patients was more likely IC (56% vs. 44%; P<.001); and CFB/EPS patients were more likely to have undergone prior lower extremity revascularization procedures (31% vs. 3%; P<.001). Median follow-up time was 305 days (interquartile range 10–406). However, only 1065 patients (56%) had complete one-year follow up.
Table I.
Baseline characteristics (stratified by procedure) and comparison of matched and unmatched cohorts
| Unmatched cohort | Bias reduction in matched cohort | |||
|---|---|---|---|---|
|
| ||||
| Variable | ABF/AUF (N=1133) N (%) |
CFB/EPS (N=739) N (%) |
P value* | |
|
| ||||
| Median age, years | 60 (53–66) † | 67 (60–75) † | <.001 | 93.9% |
|
| ||||
| Female | 466(41.13) | 291 (39.38) | .47 | |
|
| ||||
| Non-white race | 95 (8.38) | 89 (12.04) | .013 | 54.9% |
|
| ||||
| Diabetes | 263(23.21) | 216 (29.23) | .005 | 84.7% |
|
| ||||
| Overweight/obese | 576 (50.84) | 395 (53.45) | .34 | |
|
| ||||
| Coronary artery disease | 241(21.27) | 250(33.83) | <.001 | 94.9% |
|
| ||||
| Prior CABG or PCI | 226 (19.95) | 223 (31.53) | <.001 | 78.8% |
|
| ||||
| Congestive heart failure | 65 (5.74) | 97 (13.13) | <.001 | 74.0% |
|
| ||||
| COPD | 344 (30.36) | 266 (35.99) | .014 | 96.7% |
|
| ||||
| Smoking | 1102 (97.26) | 689 (93.23) | <.001 | 93.6% |
|
| ||||
| Hypertension | 871 (76.88) | 641 (86.74) | <.001 | 61.2% |
|
| ||||
| Dialysis | 6 (0.53) | 22 (2.98) | <.001 | 100% |
|
| ||||
| Positive stress test | 136 (12.00) | 75 (10.15) | .13 | |
|
| ||||
| Statin | 791 (69.81) | 517 (69.96) | .37 | |
|
| ||||
| Aspirin | 791 (69.81) | 516 (69.82) | .51 | |
|
| ||||
| Ambulatory | 1105 (97.53) | 706 (95.53) | .003 | |
|
| ||||
| Indication | 84.6% | |||
| Claudication | 632(55.78) | 312(44.22) | <.001 | |
| Critical limb ischemia | 477(42.10) | 409(55.35) | <.001 | |
|
| ||||
| Prior bypass | 138(12.18) | 146(19.76) | <.001 | 98.8% |
|
| ||||
| Prior percutaneous peripheral intervention | 255(2.51) | 228(30.85) | <.001 | 90.1% |
|
| ||||
| VSGNE-CRI | <.001 | 75% | ||
|
| ||||
| 0–4 | 749 (66.11) | 299 (40.46) | ||
|
| ||||
| 5 to 7 | 312 (27.54) | 306 (41.41) | ||
|
| ||||
| >=8 | 54 (4.77) | 109 (14.75) | ||
|
| ||||
| Overall sample mean percent bias | Unmatched cohort | 23.1% | ||
|
| ||||
| Matched cohort | 3.1% | |||
Bold indicates P<.05
χ2 or Fisher exact tests, when appropriate
N (interquartile range)
ABF, aorto-bifemoral bypass; AUF, aorto-unifemoral bypass; CFB, cross-femoral bypass; EPS, femoral endarterectomy and patch angioplasty with iliac stenting; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; COPD, chronic obstructive pulmonary disease; VSGNE-CRI, Vascular Study Group of New England Cardiac Risk Indicator.
Propensity Score Matching
Based on a stepwise logistic regression model that included preoperative variables, propensity scores were generated for 1816 of 1872 (97%) patients to assess the likelihood of receiving ABF/AUF. The 56 of 1872 patients were excluded from the model because of one or more missing preoperative variables. After applying a one-to-many caliper-matching algorithm, the matched cohort included 1805 patients, of which 61% (N=1094) underwent ABF/AUF and 39% (N=711) underwent CFB/EPS. Testing for balance of the covariates in the PSM cohort showed an overall mean standard difference of 3.1% compared to the mean standard difference of the unmatched cohort of 23.1% (Table 1). The standard differences for individual covariates were all less than 10%, demonstrating a successfully matched sample.
Survival analysis
Survival analysis (Kaplan-Meier) and multivariate Cox proportional hazard regression were performed on the matched cohort to evaluate differences in one-year survival, primary patency, and major amputation between the ABF/AUF and CFB/EPS groups. Kaplan-Meier comparison stratified by procedure type demonstrated a statistically significant difference in survival (95.6%±.6%, ABF/AUF vs. 93.6±.9%, CFB/EPS; P<.001) (Figure 1). However, multivariate Cox regression analysis demonstrated that ABF/AUF was not a significant predictor of survival (P=.96). Advanced age and VSGNE CRI score greater than 8 were negative predictors of survival, while IC as the indication and ambulation status were positive predictors of survival (Table II).
Figure 1.
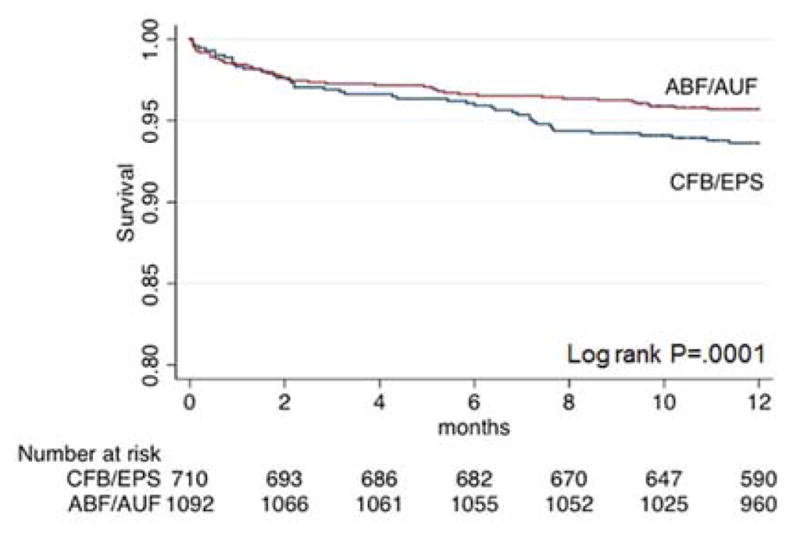
Kaplan-Meier analysis for survival, stratified by procedure
Table II.
Multivariate Cox regression analysis for mortality, primary patency, and amputation in matched cohort
| Survival covariates (N=1802) | Hazard ratio | 95% Confidence interval | P value | |
|---|---|---|---|---|
| ABF/AUF | 1.01 | 0.72 | 1.41 | .96 |
| Age > 65 years old | 1.80 | 1.23 | 2.63 | .002 |
| VSGNE-CRI | 1.23 | 1.15 | 1.32 | <.001 |
| Indication intermittent claudication | 0.48 | 0.34 | 0.68 | <.001 |
| Ambulatory | 0.32 | 0.18 | 0.57 | <.001 |
| Primary patency covariates (N=1182) | ||||
| ABF/AUF | 0.65 | 0.45 | 0.94 | .022 |
| Indication intermittent claudication | 0.44 | 0.30 | 0.65 | <.001 |
| Non-white race | 1.97 | 1.19 | 3.27 | .008 |
| Prior PVI | 1.51 | 1.03 | 2.21 | .034 |
| Amputation covariates (N=1729) | ||||
| ABF/AUF | 1.00 | .49 | 2.03 | .99 |
| Age > 65 years old | 0.40 | 0.19 | .84 | .016 |
| Indication CLI | 15.93 | 3.81 | 66.72 | <.001 |
| Prior major amputation | 10.92 | 4.79 | 24.90 | <.001 |
| CHF | 3.00 | 1.33 | 6.74 | .008 |
| Prior bypass | 2.15 | 1.08 | 4.26 | .029 |
Bold indicates P<.05
HR, hazard ratio; ABF, aorto-bifemoral bypass; AUF, aorto-unifemoral bypass; VSGNE-CRI, Vascular Study Group of New England Cardiac Risk Indicator.
ABF, aorto-bifemoral bypass; AUF, aorto-unifemoral bypass; PVI, peripheral vascular intervention
CLI, critical limb ischemia; CHF, congestive heart failure.
Kaplan-Meier analysis demonstrated improved primary patency for ABF/AUF compared to CFB/EPS (94.1%±1.1% vs. 92.3±1.5% at one year, P=.003). As depicted in Figure 2, at approximately 10 months, both curves had an abrupt decline, which correlates with the loss to follow up period. On multivariate analysis, ABF/AUF was a significant predictor of primary patency after adjusting for IC and race (P=.022) (Table II).
Figure 2.
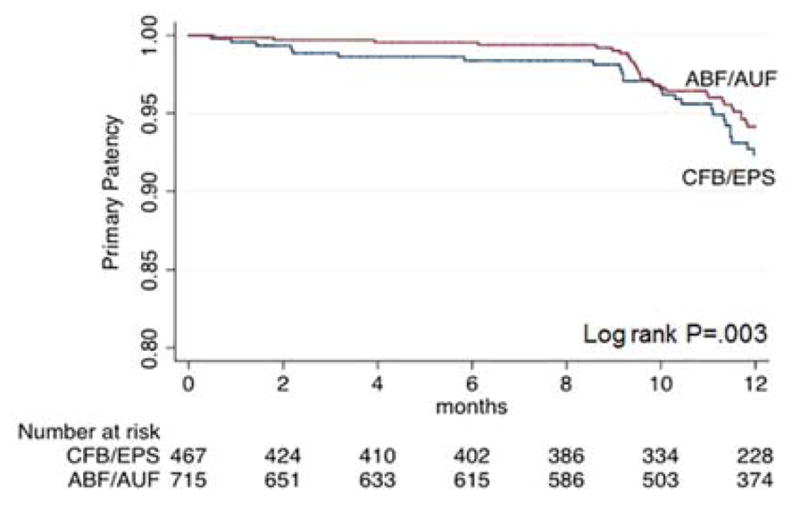
Kaplan-Meier analysis for primary patency, stratified by procedure
Freedom from major limb amputation was not significantly different between the procedure types (97% for both at one year; P=.36) on Kaplan-Meier analysis (Figure 3). Multivariate analysis (Table II) demonstrated that an indication of critical limb ischemia, prior major amputation, CHF, and prior lower extremity bypass were all positive predictors of amputation, whereas age greater than 65 years correlated with limb salvage.
Figure 3.
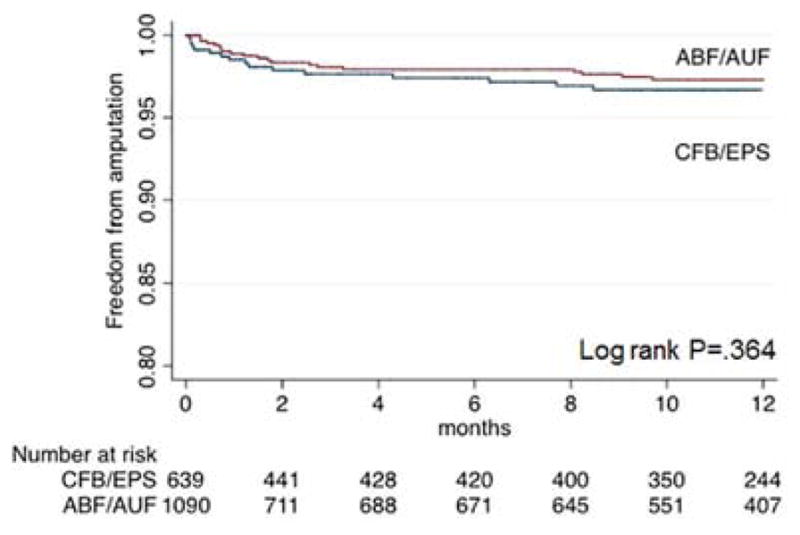
Kaplan-Meier analysis for major amputation, stratified by procedure
Subgroup Analysis
In order to determine if the more severe occlusive disease, i.e., patients with CLI, was the main driver of the difference in primary patency between ABF/AUF and CFB/EPS groups, we next performed a subgroup analysis. The matched cohort consisted of 992 patients (51%) with IC, of whom 655 (66%) were treated with ABF/AUF and 337 (34%) underwent CFB/EPS. Among patients with CLI (N=813), 447 (55%) were treated with ABF/AUF and 366 (45%) underwent CFB/EPS. Multivariate analysis was applied to the CLI and IC subgroups for survival, primary patency, and major amputation.
Within the CLI subgroup of the matched cohort, procedure type was not an independent predictor of mortality (P=.88) (Table III). Age over 65 and increased VSGNE CRI score were positive predictors of mortality; negative predictors were preoperative ambulatory status. Within the IC subgroup, only an increased VSGNE CRI score was a negative predictor of survival (HR 1.33, CI 1.17–1.5) (data not shown).
Table III.
Multivariate Cox regression analysis for mortality, primary patency, amputation in subgroup of patients with CLI
| Survival covariates (N=856) | Hazard ratio | 95% Confidence interval | P value | |
|---|---|---|---|---|
| ABF/AUF | 0.97 | 0.63 | 1.49 | .88 |
| Age > 65 years old | 1.68 | 1.03 | 2.71 | .034 |
| VSGNE-CRI | 1.28 | 1.15 | 1.42 | <.001 |
| Ambulatory | 0.30 | 0.16 | 0.55 | <.001 |
| Primary patency covariates (N=549) | ||||
| ABF/AUF | 0.57 | 0.36 | 0.90 | .017 |
| Non-white Race | 2.13 | 1.14 | 2.99 | .017 |
| Prior PVI | 1.90 | 1.20 | 2.21 | .006 |
| Amputation covariates (N=815) | ||||
| ABF/AUF | 1.01 | .49 | 2.09 | .98 |
| Age > 65 years old | 0.38 | .17 | .83 | .015 |
| Prior major amputation | 9.17 | 3.87 | 21.77 | <.001 |
| Congestive heart failure | 3.19 | 1.41 | 7.21 | .005 |
| Prior bypass | 2.09 | 1.04 | 4.24 | .04 |
Bold indicates P<.05
ABF, aorto-bifemoral bypass; AUF, aorto-unifemoral bypass; VSGNE-CRI, Vascular Study Group of New England Cardiac Risk Index; PVI, peripheral vascular intervention; CLI, critical limb ischemia
In terms of primary patency, multivariate analysis of patients in the CLI subgroup demonstrated that ABF/AUF was a significant predictor of primary patency after adjusting for prior history of PVI and race (P=.017) (Table III). Multivariate analysis of primary patency in the IC subgroup did not identify any predictive variables other than ABF/AUF vs. CFB/EPS (data not shown).
Finally, procedure type was not an independent predictor of amputation in the CLI subgroup, but prior major amputation, CHF and prior lower extremity bypass were all positive predictors of amputation (Table III). As seen in the entire matched cohort analysis, age greater than 65 years was associated with limb salvage (P=.015). Multivariate analysis of primary patency in the IC subgroup did not identify any predictive variables other than age greater than 65 (data not shown).
DISCUSSION
Since the first reports of direct anatomic repair for AIOD in the 1950s,13 there has been a need for less invasive options for reconstruction for patients who are at high risk for an aortic cross-clamp or laparotomy. Although direct repair is associated with good long-term durability, extra-anatomic bypasses and hybrid approaches have emerged as options for patients with contraindications for direct repair and as less invasive options. This paradigm shift toward less invasive approaches for AIOD warrants direct comparison of these contemporary treatment strategies.
We sought to compare outcomes for common reconstruction options for TASC C/D AIOD by performing a retrospective study using a propensity-score matched cohort from a large, multi-center vascular surgery-centric dataset. Axillo-femoral bypasses were not included since reported patency and survival rates are significantly inferior to the other strategies.23–25 We found no difference in survival for more invasive (ABF/AUF) repair compared to less invasive (CFB/EPS) approaches after multivariate analysis. Instead, patient characteristics such as advanced age, VSGNE CRI score greater than 8, indication for surgery (IC vs. CLI), and ambulatory status significantly impacted survival. Age, VSGNE CRI, and ambulatory status were also significant predictors of mortality when we performed a subgroup analysis of patients with CLI, thus highlighting the importance of patient selection as a guiding factor in the treatment of AIOD regardless of revascularization strategy or indication. Although it is difficult to make direct comparisons of our results to those reported in the literature, the lack of statistically significant differences in survival between reconstruction techniques have also been demonstrated in multiple studies9,12,26–28.
The 1-year patency rates for ABF/AUF vs. CFB/EPS were 94% vs 92%, respectively, and although the log-rank tests reached statistical significance, these results must be interpreted with caution. The rate of one-year follow-up for the study sample was 57%, and the long-term follow-up period for the VQI begins at 9 months, which corresponds to the time period when an abrupt decline in patency occurs. Improvement in long-term follow-up is a current quality initiative for the VQI, and will certainly be important to improve the quality of the data.29 The low rate of long term follow up may cause a reporting bias in the observed patency rates, as patients with worse patency in the VQI may be disproportionately over-represented among those who have one-year follow-up. However, there is no reason to think that any reporting bias would be different in our two study groups and in that regard it is still likely that there is a true patency difference between the study groups. Unfortunately, prior reports have different comparison groups for AIOD treatment, and there is little literature that includes ABF, AUF, CFB, and EPB revascularization techniques and has longer-term follow-up intervals (i.e., 3 or 5 years).9,26–28
This study has a number of limitations. First, and perhaps most critically, is missing data and, as mentioned above, the rate of loss to follow-up. There was a substantial amount of missing data for preoperative medication use, preoperative ankle-brachial indices/toe-brachial indices, postoperative variables, and follow-up variables. Schneider et al. (2015)30 noted similar limitations due to loss to follow-up in their study using the VQI registry to compare eversion and conventional carotid endarterectomy, and Siracuse et al. (2016)29 made a similar observation in a comparison of open and endovascular treatment of CLI. In our study, despite excluding patients who underwent procedures after February 2013, there was only modest improvement in one-year follow-up, which subsequently limits the interpretation of primary patency. In addition, given the registry nature of the VQI, we also were not able to correct for variables such as surgeon preference in choosing a procedure type or with surgeon experience.
On the other hand, the size of the VQI dataset is much greater than that used in any precedent article on this topic. Furthermore, this dataset is representative of a large number of participating surgeons and institutions and the data are de-identified with respect to subject and physician. Thus, it is unlikely that that there is a bias against publication of less favorable results, and the results likely represent “real world” outcomes. The VQI dataset also includes granular clinical and surgical variables not available in other surgical datasets such as NSQIP. These strengths of the VQI tend to offset the weakness of incomplete follow-up, although we acknowledge the imperative to improve completeness of follow-up in the VQI. We are hopeful that recent suggestions by the VQI Research Advisory Council to improve long-term follow-up (“Suggestions for Success”)31 will diminish this problem in the future.
Lastly, our study was limited by the observational retrospective design, which has intrinsic bias. Even with the use of propensity score matching, it is difficult to achieve the same quality of results as a randomized control trial. A key limitation of propensity score matching is that only observable differences can be controlled across treatment groups. Although imperfect, the application of statistical methods such as propensity score matching and inverse probability weighting may be the best method to address selection bias in observational studies.21
CONCLUSIONS
In a propensity score matched cohort, a treatment approach for severe AIOD was not a predictor of 1-year survival or limb salvage, suggesting that patient factors and procedure indication have a greater impact on outcome. Although a direct treatment approach demonstrated improved 1-year primary patency compared to less invasive, this interpretation is limited by inadequate follow-up.
Acknowledgments
Funding sources
This work was funded in part by T32HL094293 (to K. Z.) from the National Heart, Lung, and Blood Institute.
Abbreviations
- AIOD
aorto-iliac occlusive disease
- ABF
aorto-bifemoral bypass
- AUF
aorto-unifemoral bypass
- CFA
common femoral artery
- CFB
cross-femoral bypass
- CLI
critical limb ischemia
- EPS
femoral endarterectomy and patch angioplasty with iliac stenting
- IC
intermittent claudication
- PFA
profunda femoris artery
- PSM
propensity score match
- SFA
superficial femoral artery
- VSGNE CRI
Vascular Study Group of New England Cardiac Risk Indicator
Footnotes
Presentation information:
This study was presented at the 2015 Midwestern Vascular Surgical Society Annual Meeting, Chicago, IL, September 10-12, 2015
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pomposelli FB, Clair DG, Geraghty PJ, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. Development. 2015;1:40. doi: 10.1016/j.jvs.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Sachwani GR, Hans SS, Khoury MD, et al. Results of iliac stenting and aortofemoral grafting for iliac artery occlusions. Journal of vascular surgery. 2013;57(4):1030–1037. doi: 10.1016/j.jvs.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Rowe VL, Lee W, Weaver FA, Etzioni D. Patterns of treatment for peripheral arterial disease in the United States: 1996–2005. Journal of vascular surgery. 2009;49(4):910–917. doi: 10.1016/j.jvs.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 4.Self SB, Richardson JD, Klamer TW, et al. Utility of femorofemoral bypass. Comparison of results with indications for operation. The American surgeon. 1991;57(9):602–606. [PubMed] [Google Scholar]
- 5.Mingoli A, Sapienza P, Feldhaus RJ, et al. Comparison of femorofemoral and aortofemoral bypass for aortoiliac occlusive disease. The Journal of cardiovascular surgery. 2001;42(3):381–387. [PubMed] [Google Scholar]
- 6.Deruyter L, Caes F, Van den Brande P, et al. Femorofemoral bypass grafting in high-risk patients. Acta chirurgica Belgica. 1985;86(5):271–276. [PubMed] [Google Scholar]
- 7.Ozkan U, Oguzkurt L, Tercan F. Technique, complication, and long-term outcome for endovascular treatment of iliac artery occlusion. Cardiovascular and interventional radiology. 2010;33(1):18–24. doi: 10.1007/s00270-009-9691-7. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap VS, Pavkov ML, Bena JF, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. Journal of vascular surgery. 2008;48(6):1451–1457. 1457 e1451–1453. doi: 10.1016/j.jvs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Onohara T, Komori K, Kume M, et al. Multivariate analysis of long-term results after an axillobifemoral and aortobifemoral bypass in patients with aortoiliac occlusive disease. The Journal of cardiovascular surgery. 2000;41(6):905–910. [PubMed] [Google Scholar]
- 10.Chiu KWH, Davies RSM, Nightingale PG, et al. Review of Direct Anatomical Open Surgical Management of Atherosclerotic Aorto-Iliac Occlusive Disease. European Journal of Vascular and Endovascular Surgery. 2010;39(4):460–471. doi: 10.1016/j.ejvs.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa R, Marone EM, Tshomba Y, et al. Aortobifemoral bypass grafting using expanded polytetrafluoroethylene stretch grafts in patients with occlusive atherosclerotic disease. Ann Vasc Surg. 2009;23(6):764–769. doi: 10.1016/j.avsg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Burke CR, Henke PK, Hernandez R, et al. A contemporary comparison of aortofemoral bypass and aortoiliac stenting in the treatment of aortoiliac occlusive disease. Annals of vascular surgery. 2010;24(1):4–13. doi: 10.1016/j.avsg.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Cronenwett JL. Rutherford’s Vascular Surgery. 8. Vol. 2. Elsevier; 2014. pp. 1701–1709. [Google Scholar]
- 14.Upchurch GR, Jr, Dimick JB, Wainess RM, et al. Diffusion of new technology in health care: The case of aorto-iliac occlusive disease. Surgery. 2004;136(4):812–818. doi: 10.1016/j.surg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Piazza M, Ricotta JJ, 2nd, Bower TC, et al. Iliac artery stenting combined with open femoral endarterectomy is as effective as open surgical reconstruction for severe iliac and common femoral occlusive disease. Journal of vascular surgery. 2011;54(2):402–411. doi: 10.1016/j.jvs.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Sachwani GR, Hans SS, Khoury MD, et al. Results of iliac stenting and aortofemoral grafting for iliac artery occlusions. Journal of vascular surgery. 2013;57(4):1030–1037. doi: 10.1016/j.jvs.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Peterson BG, Longo GM, Kibbe MR, et al. Duplex ultrasound remains a reliable test even after carotid stenting. Ann Vasc Surg. 2005;19(6):793–797. doi: 10.1007/s10016-005-7976-0. [DOI] [PubMed] [Google Scholar]
- 18.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Journal of Vascular Surgery. 2007;45(1, Supplement):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Bertges DJ, Goodney PP, Zhao Y, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. Journal of Vascular Surgery. 2010;52(3):674–683. e673. doi: 10.1016/j.jvs.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 20.d’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health services research. 2014;49(5):1701–1720. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996:249–264. [PubMed] [Google Scholar]
- 23.Passman MA, Taylor LM, Moneta GL, et al. Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. Journal of vascular surgery. 1996;23(2):263–269. doi: 10.1016/s0741-5214(96)70270-7. discussion 269–271. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JR, McDaniel MD, Walsh DB, et al. Axillofemoral bypass: outcome and hemodynamic results in high-risk patients. Journal of vascular surgery. 1992;15(6):952–962. discussion 962–953. [PubMed] [Google Scholar]
- 25.Naylor AR, Ah-See AK, Engeset J. Axillofemoral bypass as a limb salvage procedure in high risk patients with aortoiliac disease. Br J Surg. 1990;77(6):659–661. doi: 10.1002/bjs.1800770623. [DOI] [PubMed] [Google Scholar]
- 26.Schneider JR, Besso SR, Walsh DB, et al. Femorofemoral versus aortobifemoral bypass: outcome and hemodynamic results. Journal of vascular surgery. 1994;19(1):43–57. doi: 10.1016/s0741-5214(94)70119-9. [DOI] [PubMed] [Google Scholar]
- 27.Huded CP, Goodney PP, Powell RJ, et al. The impact of adjunctive iliac stenting on femoral-femoral bypass in contemporary practice. Journal of vascular surgery. 2012;55(3):739–745. doi: 10.1016/j.jvs.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang RW, Goodney PP, Baek JH, et al. Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease. Journal of vascular surgery. 2008;48(2):362–367. doi: 10.1016/j.jvs.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Siracuse JJ, Menard MT, Eslami MH, et al. Comparison of open and endovascular treatment of patients with critical limb ischemia in the Vascular Quality Initiative. Journal of vascular surgery. 2016;63(4):958–965. e951. doi: 10.1016/j.jvs.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 30.Schneider JR, Helenowski IB, Jackson CR, et al. A comparison of results with eversion versus conventional carotid endarterectomy from the Vascular Quality Initiative and the Mid-America Vascular Study Group. Journal of vascular surgery. 2015;61(5):1216–1222. doi: 10.1016/j.jvs.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Initiative SVQ. VQI Long-Term Follow-up: Suggestions for Success. [Accessed May 2, 2016]. [Google Scholar]


