Abstract
Prenatal exposure to polybrominated diphenyl ethers (PBDEs) have been reported to impair executive function in children, but little is known whether childhood PBDE exposures play a role. Using the Health Outcomes and Measures of the Environment (HOME) Study, a prospective birth cohort in the greater Cincinnati area, we investigated the association between repeated measures of PBDEs during childhood and executive function at 8 years in 208 children and whether effect modification by child sex was present. We used child serum collected at 1, 2, 3, 5, and 8 years to measure PBDEs. The Behavior Rating Inventory of Executive Function was completed by parents to assess executive function at 8 years. We used multiple informant models to examine childhood PBDEs during several exposure windows. Null associations were observed between early childhood PBDEs and executive function. However, we observed significant adverse associations between a 10-fold increase in concurrent concentrations of BDE-28 (β=4.6, 95% CI 0.5, 8.7) and BDE-153 (β=4.8, 95% CI 0.8, 8.8) with behavioral regulation. In addition, PBDEs at 8 years were significantly associated with poorer emotional and impulse control. No associations were noted between childhood PBDEs and metacognition or global executive function. However, child sex significantly modified the associations, with significantly poorer executive function among males with higher concurrent BDE-153, and null associations in females. Our study findings suggest that concurrent PBDE exposures during childhood may be associated with poorer executive function, specifically behavior regulation. Males may also be more sensitive to adverse associations of concurrent PBDEs on executive function.
Keywords: Polybrominated diphenyl ether (PBDE), neurodevelopment, executive function, postnatal, childhood
1. Introduction
Polybrominated diphenyl ethers (PBDEs) are persistent chemicals that were introduced in the late 1970s to retard fire in commercial polymer-based products, including furniture and electronics. In the 2000s, restrictions on the use of PBDEs were made in Europe, the US, and other countries in light of the potential adverse health effects of PBDEs and their persistence in the environment, biota, and in humans (Siddiqi et al., 2003). Several epidemiologic studies have reported that higher PBDE concentrations during fetal development were associated with decreased full scale intelligence quotient (FSIQ) scores, diminished language and reading abilities, increased problems with hyperactivity and attention, and poorer executive function in children (Braun et al., 2017b; Chen et al., 2014; Cowell et al., 2015; Ding et al., 2015; Eskenazi et al., 2013; Gascon et al., 2011; Herbstman et al., 2010; Roze et al., 2009; Sagiv et al., 2015; Shy et al., 2011; Vuong et al., 2016; Zhang et al., 2017).
One study has examined childhood PBDEs and executive function (Sagiv et al., 2015). While no associations were reported between Σ4PBDEs (BDE-47, -99, -100, and -153) at 9 years and executive function at 9 and 12 years, Sagiv et al. (2015) observed poorer parent-reported executive function in females with higher Σ4PBDE concentrations, but not in males. Executive function reflects prefrontal cortex activities and encompasses distinct and inter-related components, including attentional control, cognitive flexibility, goal setting, and information processing, that are necessary for complex activities, academic achievement, as well as daily behavioral and social interactions. PBDE exposures that occur during infancy and childhood may impair brain maturation, particularly with respect to executive function, as it has been shown to have a continued developmental course through adolescence (Anderson et al., 2001a). While various executive function domains have heterogeneous developmental trajectories, rapid incremental advances in executive function parallel the major periods of growth of the frontal lobes, which occur from birth to 2 years, 7–9 years, and 16–19 years (Anderson et al., 2001a; Anderson et al., 2001b). Further, PBDE concentrations are higher among infants, toddlers, and children compared to the adult population (Costa and Giordano, 2007; Schecter et al., 2005; Toms et al., 2008; Toms et al., 2009). The objective of the present study was to investigate the relationship between PBDE concentrations during childhood (1–8 years) and executive function at 8 years and to examine effect modification by child sex.
2. Materials and Methods
2.1 Study participants
This study included children from the Health Outcomes and Measures of the Environment (HOME) Study, a well-characterized, ongoing prospective pregnancy and birth cohort in Cincinnati, OH, USA. Details regarding recruitment, eligibility criteria, biospecimen collection, environmental samples, neurobehavioral assessments, as well as follow-up visits are described in detail by Braun et al. (2017a). Briefly, 468 pregnant women at 16±3 weeks of gestation were enrolled during 2003–2006 from nine prenatal clinics and 390 remained to deliver live singleton infants. To be included in the present study, children had to have had at least one PBDE measure during childhood and an assessment of executive function at 8 years. The institutional review boards at the Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention (CDC) approved this study.
2.2 Assessment of childhood PBDEs
Postnatal PBDEs were measured from blood samples collected at 1, 2, 3, 5, and 8 years using gas chromatography/isotope dilution high-resolution mass spectrometry. Information regarding postnatal PBDE measurement procedures (e.g., quality assurance, lipid adjustment) have been described previously (Vuong et al., 2017a). PBDE measurements less than the limit of detection (LOD) were replaced with the following: LOD/√2. Detection frequencies of select PBDE congeners (-28, -47, -99, -100, and -153) examined in this study are listed in Supplemental Table S1. A total of 208 children with an assessment of executive function at 8 years had PBDE concentrations measured at least once during childhood. However, only 49 (24%), 77 (37%), 18 (9%), 20 (10%), and 44 (21%) children had 1, 2, 3, 4, and 5 PBDE measures during childhood, respectively. Due to limited serum availability from ages 1–3, only a subset of the children who came for follow-up had sufficient serum for PBDE measurements. Thus, we were missing 122 (59%), 139 (67%), 139 (67%), 67 (32%), and 16 (8%) of the 208 children with PBDE measurements at ages 1, 2, 3, 5, and 8 years, respectively. Multiple imputation using the Markov Chain Monte Carlo (MCMC) method was utilized to estimate missing PBDE concentrations for children who had at least one PBDE measurement during childhood. Detailed procedures to produce 100 imputed datasets using multiple imputation models can be found elsewhere (Vuong et al., 2017b).
2.3 Behavior Rating Inventory of Executive Function (BRIEF)
To assess executive function at 8 years, the BRIEF, a valid and reliable questionnaire (Gioia et al., 2000a, b; Skogerbo et al., 2012), was completed by a parent who had extensive contact with the child within the past 6 months. The BRIEF comprises of 86 items and is designed to assess executive function abilities during everyday activities at home, school, and community settings. Behaviors are rated as either: never, sometimes, or often a problem. Raw scores were converted to standardized T-scores based on sex-specific norms for the age as described in the BRIEF manual. Questionnaire responses were used to derive a summary measure from eight clinical scales, referred to as the global executive composite. These clinical scales also yield two broad indexes: 1) behavioral regulation index (scales: inhibit + shift + emotional control); and 2) metacognition index (scales: initiation + working memory + plan/organize + organization of materials + monitor). BRIEF T-scores have a mean of 50±10, with higher scores indicating poorer performance. While scores 1.5 SDs (standard deviation) above the mean are clinically significant (Gioia et al., 2000a), we defined BRIEF scores 1 SD above the mean (≥60) as “at risk” of a clinically relevant executive function problem due to our modest sample size (Supplemental Table S2).
2.4 Statistical analyses
To examine PBDE neurotoxicity at different exposure windows during childhood, we used multiple informant models to estimate βs and 95% confidence intervals (CIs) between repeated measures of log10-transformed lipid-adjusted PBDE concentrations (BDE-28, -47, -99, -100, -153, and their sum [ΣPBDEs]) with BRIEF scores at 8 years using the imputed datasets (Horton et al., 1999; Litman et al., 2007). Multiple informant models uses a non-standard version of generalized estimating equation that allows for repeated measures of PBDEs during childhood. Details regarding multiple informant models have been described by Sanchez et al. (2011). We modeled each PBDE congener individually, including all five windows of exposure (1, 2, 3, 5, and 8 years). Statistically significant interaction terms between child age and PBDEs indicates a potential window of vulnerability to PBDE neurotoxicity. We report β estimates for each exposure time, because several interaction terms (PBDEs×age) had a p<0.10. We also investigated whether childhood PBDEs are associated with having an “at risk” BRIEF score (≥60) using multiple informant models to generate odds ratios (ORs) and 95% CIs. To determine whether effect measure modification by child sex was present between childhood PBDE concentrations and executive function, we included interaction terms between PBDEs (continuous), child sex (binary), and child age (categorical), as well as all 2-way interactions. We also examined non-linear exposure response using separate generalized additive models (GAMs) for each window of exposure for childhood PBDEs and executive function. All models included the following covariates based on bivariate analysis with executive function (p<0.10) (as categorized in Table 1): maternal age, race/ethnicity, household income, child sex, maternal serum blood lead level, maternal depression (Beck et al., 1996), prenatal vitamin use, maternal IQ (Wechsler, 1999), marital status, and Home Observation for Measurement of the Environment (HOME) score.
Table 1.
Child serum concentrations of ΣPBDEs (ng/g lipid) and BRIEF summary measures at age 8 years by maternal and child characteristics, HOME Studya
| Maternal and Child Characteristics | n | ΣPBDEs 1 year | ΣPBDEs 8 years | Behavior Regulation Index | Metacogniton Index | Global Executive Composite |
|---|---|---|---|---|---|---|
| GM (GSD) | GM (GSD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Maternal age at enrollment, yearsc | ||||||
| <25 | 59 | 100.7 (2.1) | 39.3 (2.2) | 49.5 (10.1) | 50.9 (11.3) | 50.6 (10.6) |
| 25–34 | 117 | 130.0 (2.4) | 49.0 (2.1) | 47.3 (9.5) | 47.7 (10.8) | 47.4 (10.4) |
| ≥35 | 31 | 112.9 (2.2) | 34.2 (2.3) | 48.3 (10.4) | 48.9 (11.6) | 48.8 (11.4) |
| Race/ethnicityd, e, f | ||||||
| Non-Hispanic White | 122 | 119.0 (2.2) | 44.0 (2.2) | 46.5 (9.4) | 47.0 (10.6) | 46.7 (10.1) |
| Non-Hispanic Black and Others | 85 | 117.4 (2.4) | 43.0 (2.2) | 50.4 (10.1) | 51.3 (11.4) | 51.2 (10.9) |
| Household Incomeb,d, e, f | ||||||
| <$40,000 | 89 | 130.6 (2.2) | 45.8 (2.2) | 50.1 (9.6) | 51.4 (10.9) | 51.2 (10.3) |
| $40,000–$79,999 | 65 | 144.5 (2.1) | 44.3 (2.2) | 46.4 (10.3) | 47.5 (11.8) | 47.0 (11.4) |
| ≥$80,000 | 53 | 78.4 (2.4) | 39.4 (2.2) | 46.8 (9.0) | 46.0 (9.8) | 46.0 (9.6) |
| Maternal Depressiond, e, f | ||||||
| Minimal/mild | 185 | 116.1 (2.2) | 44.6 (2.1) | 47.5 (9.9) | 48.2 (11.1) | 47.9 (10.8) |
| Moderate/severe | 20 | 138.2 (2.8) | 36.0 (2.7) | 53.5 (7.9) | 55.1 (9.6) | 55.1 (7.8) |
| Home Observation for Measurement of the Environment scored, e, f | ||||||
| ≥40 | 118 | 106.8 (2.4) | 43.4 (2.2) | 46.5 (10.1) | 46.7 (11.1) | 46.4 (10.8) |
| 35–39 | 40 | 128.3 (2.3) | 48.4 (2.5) | 51.0 (10.0) | 52.8 (10.9) | 52.3 (10.3) |
| <35 | 34 | 147.0 (2.1) | 42.9 (1.8) | 49.6 (7.6) | 50.4 (9.2) | 50.4 (8.6) |
| Marital statusb,d, e, f | ||||||
| Married/living with partner | 151 | 107.0 (2.3) | 43.2 (2.1) | 46.6 (9.3) | 47.5 (10.7) | 47.0 (10.2) |
| Not married, living alone | 56 | 155.2 (2.1) | 44.6 (2.3) | 52.1 (10.1) | 52.3 (11.4) | 52.6 (11.0) |
| Maternal Vitamin Used, e, f | ||||||
| Daily | 159 | 113.7 (2.3) | 43.0 (2.2) | 47.3 (9.7) | 47.8 (11.2) | 47.6 (10.7) |
| <Daily | 34 | 124.0 (2.5) | 48.8 (2.2) | 49 (10.4) | 50.5 (11.1) | 50.1 (10.8) |
| Never | 14 | 165.0 (2.0) | 38.8 (2.0) | 54.5 (7.2) | 55.6 (7.3) | 55.4 (6.9) |
| Child Sex | ||||||
| Male | 93 | 124.2 (2.2) | 44.0 (2.3) | 47.6 (10.4) | 48.9 (11.9) | 48.4 (11.3) |
| Female | 115 | 113.4 (2.3) | 43.3 (2.1) | 48.3 (9.4) | 48.7 (10.5) | 48.6 (10.1) |
Abbreviations: BRI, Behavioral Regulation Index; GM, geometric mean; GSD, geometric standard deviation; GEC, Global Executive Composite; MI, Metacognition Index; SD, standard deviation.
ΣPBDEs: Sum of BDE-28, -47, -99, -100, and -153
Frequencies may not add to the total number of participants because of missing values.
p < 0.05 for:
ΣPBDEs at 1 year;
ΣPBDEs at 8 years;
BRI;
MI;
GEC (two-sided p values using ANOVA or t-test
We performed a sensitivity analysis to re-examine research questions using the original, non-imputed data to alleviate the concerns of imputed exposures mostly in early childhood (1–3 years). In other sensitivity analyses, we made additional adjustment for prenatal PBDE concentrations, blood lead concentrations at 8 years, and whether the child was ever breastfed. SAS version 9.4 and R version 3.2.3 were used for statistical analyses, and graphs were produced using GraphPad Prism version 7 and R version 3.2.3.
3. Results
3.1 Participant characteristics
ΣPBDE concentrations at 1 year were significantly lower among children who were from homes with higher household incomes and who had mothers that were married or living with a partner as compared to those from lower income households and children of mothers who were not married or living alone (Table 1). At 8 years, compared to mothers <25 or ≥35 years, concentrations of ΣPBDE were significantly higher in children who had mothers who were 25–34 years. Better (lower) scores on behavioral regulation index, metacognition index, and global executive composite were observed among children who were non-Hispanic White and who were from households with higher HOME Inventory scores and incomes as compared to non-Hispanic Black and other race/ethnicities and children of households with lower HOME inventory scores and incomes. Children who also performed better on the BRIEF had mothers who were minimally/mildly depressed, were married or living with a partner, and who had taken a daily vitamin supplement during pregnancy as compared to children of mothers who were moderately/severely depressed, were not married or living alone, and who did not take daily supplementation. Overall, concentrations of ΣPBDEs in the HOME Study children were highest at 1 year (99.4 ng/g lipid) and gradually declined as children reached 8 years (45.2 ng/g lipid) (Supplemental Table S1).
3.2 Childhood PBDE concentrations and executive function
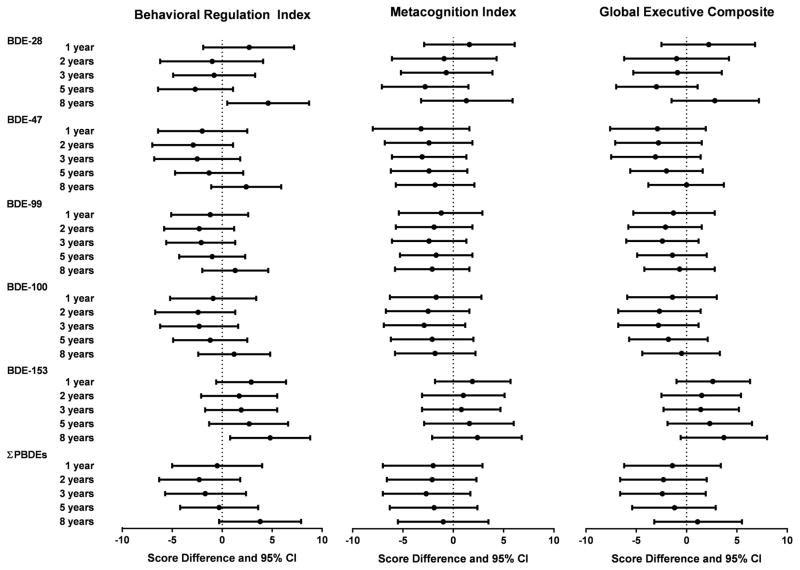
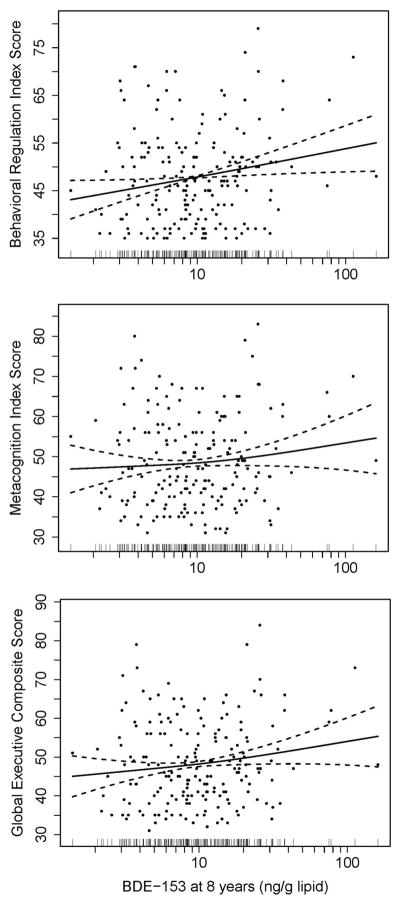
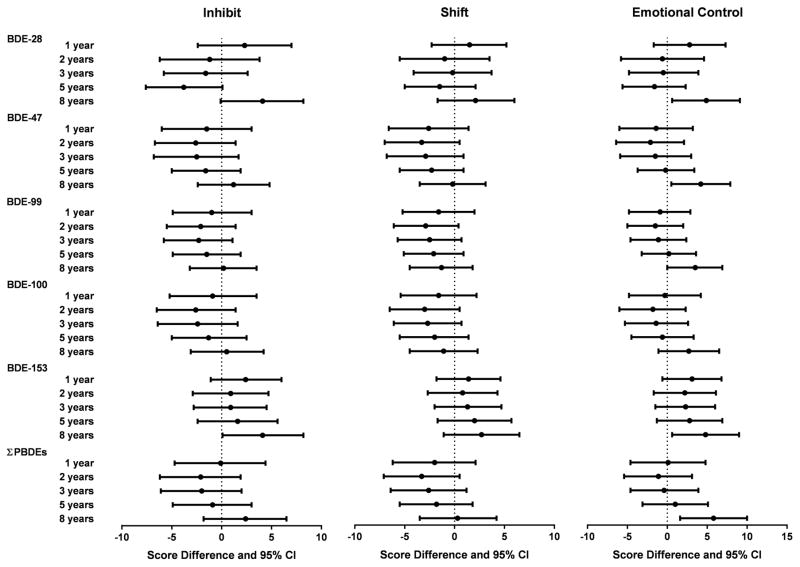
Null associations were observed between childhood PBDEs and metacognition index and global executive composite (Figure 1). However, poorer performance on behavioral regulation index, as indicated by higher scores, was noted with increased concentrations of BDE-153 at 8 years. Higher BDE-153 concentrations at 8 years were associated with worse performance on all BRIEF summary measures linearly in the GAM models (Figure 2). Statistically significant impairment in behavioral regulation index was observed with a 10-fold increase in BDE-153 (β=4.8, 95% CI 0.8, 8.8) and BDE-28 at 8 years (β=4.6, 95% CI 0.5, 8.7). The association between ΣPBDEs at 8 years and behavioral regulation index was also borderline significant, with an increase of 3.8 points (95% CI −0.3, 7.9) with a 10-fold increase in ΣPBDE concentrations. Most of the significant associations observed between PBDEs at 8 years and behavioral regulation index were driven by associations in the emotional control subscale (Figure 3). Statistically significant impairment in emotional control was noted with 10-fold increases in 8 year concentrations of BDE-28 (β=4.9, 95% CI 0.6, 9.1), BDE-47 (β=4.2, 95% CI 0.5, 7.9), BDE-99 (β=3.5, 95% CI 0.01, 6.9), BDE-153 (β=4.8, 95% CI 0.6, 9.0), and ΣPBDEs (β=5.8, 95% CI 1.6, 10.0). We also observed a significant adverse association between BDE-153 at 8 years and scores on the inhibit (impulse control) subscale and a borderline significant association with BDE-28 at 8 years (Figure 3).
Figure 1.
Estimated score differences and 95% confidence intervals in BRIEF summary measures of executive function scores at 8 years by a 10-fold increase in child serum concentrations of PBDEs (ng/g lipid), HOME Study. Adjusted by maternal age, race/ethnicity, household income, child sex, maternal blood lead level, maternal depression, vitamin use, maternal IQ, marital status, and Home Observation for Measurement of the Environment Score
Figure 2.
Scatter plots of child serum concentrations of BDE-153 (ng/g lipid) at 8 years and BRIEF summary measure scores at age 8 years with generalized additive model curve fitting. Data points represent data from each child. Solid lines represent the natural cubic spline of the adjusted association, and dotted lines represent 95% CIs. Distribution of BDE-153 is illustrated by vertical bars on the log10-transformed x-axis. Adjusted by maternal age, race/ethnicity, household income, child sex, maternal blood lead level, maternal depression, vitamin use, maternal IQ, marital status, and Home Observation for Measurement of the Environment Score
Figure 3.
Estimated score differences and 95% confidence intervals in the BRIEF subscales of behavior regulation index scores at 8 years by a 10-fold increase in child serum concentrations of PBDEs (ng/g lipid), HOME Study. Adjusted by maternal age, race/ethnicity, household income, child sex, maternal blood lead level, maternal depression, vitamin use, maternal IQ, marital status, and Home Observation for Measurement of the Environment Score
Although not statistically significant, a 10-fold increase in BDE-153 at 8 years was associated with increased metacognition index and global executive composite scores by 2.4 (95% CI −2.1, 6.8) and 3.7 (95% CI −0.6, 8.0) points, respectively (Figure 1). Further, a 10-fold increase in BDE-153 at 3 years was significantly associated with higher odds of having an “at risk” metacognition index score (OR=2.0, 95% CI 1.1, 8.1) (Supplemental Table S3). In contrast, several PBDEs were associated with better (lower) scores on metacognition index, behavior regulation, and global executive composite, although none were statistically significant (Figure 1).
3.3 Child sex differences
While null associations were found between childhood PBDEs and metacognition index and global executive composite, significant adverse associations were present when we examined by child sex. Specifically, effect measure modification by child sex was noted between BDE-153 at 8 years and behavior regulation index, metacognition index, and global executive composite (pinteraction<0.005) (Supplemental Table S4). Higher concentrations of concurrent BDE-153 were associated with significantly poorer scores in males, but not in females for behavioral regulation index (Males: β=7.9, 95% CI 3.5, 12.4; Females: β=1.6, 95% CI −3.1, 6.4), metacognition index (Males: β=7.6, 95% CI 2.7, 12.5; Females: β= −2.9, 95% CI −8.1, 2.4) and global executive composite (Males: β=8.2, 95% CI 3.5, 13.0; Females: β= −0.8, −5.8, 4.2). Similar results were also observed between ΣPBDE concentrations at 8 years and behavioral regulation index and global executive composite scores (pinteraction<0.02), with males performing significantly worse.
3.4 Sensitivity analyses
Our results were similar when we examined the original, non-imputed data and when we performed separate additional adjustments for prenatal PBDEs, blood lead levels at 8 years, and breastfeeding (yes/no).
4. Discussion
We examined concentrations of PBDEs during childhood (1–8 years) in relation to executive function at 8 years in a prospective cohort in the Cincinnati, OH. After adjusting for multiple individual-level potential confounders, including sociodemographics, maternal IQ and depression, and the child rearing environment, we found that concurrent concentrations of BDE-28 and BDE-153 were significantly associated with impairments in behavioral regulation in children. While earlier childhood concentrations of PBDEs were not associated with impairments in executive function we found that 10-fold increases in concurrent concentrations of BDE-28 and BDE-153 were associated with approximately a 5-point increase on the behavioral regulation index. All PBDE congeners (except BDE-100) and ΣPBDEs at 8 years were significantly associated with poorer emotional control, with a ~4–6-point increase. Only concurrent BDE-28 and BDE-153 concentrations were associated with impaired impulse control. Null associations were observed between childhood PBDEs and metacognition and global executive functioning. However, child sex significantly modified the relationship between concurrent BDE-153 concentrations and all BRIEF composite measures, with significant adverse associations observed in males with regard to behavior regulation, metacognition, and global executive function while there were null associations in females. Further, we observed a linear relationship between concurrent concentrations of BDE-153 and all BRIEF summary measures.
The exact mechanisms of PBDE neurotoxicity are unclear, but two general models of action affecting brain development have been postulated. Postnatal PBDEs have been shown to alter thyroid hormone homeostasis in animal models (Driscoll et al., 2009; Rice et al., 2007; Zhou et al., 2001). Several epidemiological studies have reported thyroid hormone disruption in children with higher concentrations of postnatal PBDEs (Han et al., 2011; Jacobson et al., 2016; Kicinski et al., 2012; Leijs et al., 2012; Xu et al., 2014). Thyroid hormones are vital for proper brain development in utero, and during childhood. Thyroid hormones regulate brain gene expression during the postnatal period, influencing myelination, cerebellum development, glial cell proliferation, neuronal differentiation, and synapse formation (Bernal, 2000). PBDEs may interfere with thyroid hormone transport via competitive binding to thyroid hormone transport protein transthyretin (TTR) or directly interacting with thyroid hormone receptors (Ibhazehiebo et al., 2011; Meerts et al., 2000; Richardson et al., 2008). In addition, PBDE metabolites (OH-PBDEs) are more structurally similar to thyroxine and triiodothyronine and have a higher affinity to TTR and thyroxine-binding globulin (Marchesini et al., 2008; Meerts et al., 2000). The second mode of action for PBDE neurotoxicity is by directly affecting brain cells, particularly neuronal and glial cells. PBDEs have been found to induce apoptotic neuronal death through oxidative stress, disrupt signal transduction, interfere with calcium signaling and homeostasis, and decrease neuron and oligodendrocyte differentiation (Costa et al., 2014).
Only one other study has investigated the impact of PBDEs on executive function. In the CHAMACOS (Center for the Health Assessment of Mothers and Children of Salinas) cohort in California, USA, PBDE concentrations were measured in serum from 546 children at 9 years of age and executive function was assessed using the BRIEF at both 9 and 12 years (Sagiv et al., 2015). Null associations were reported between concurrent concentrations of Σ4PBDEs (−47, −99, −100, −153) and behavioral regulation index, metacognition index, and global executive composite at 9 years. The relation between Σ4PBDEs at 9 years and BRIEF composite measures at 12 years yielded null findings as well. Sagiv et al. (2015) additionally examined Σ4PBDEs at 9 years and repeated measures of BRIEF composite scores at 9 and 12 years using generalized estimating equations (GEE) and reported no associations with behavioral regulation (β=1.4, 95% CI −0.5, 3.4), metacognition (β=1.3, 95% CI −0.8, 3.3), and global executive composite (β=0.8, 95% CI −1.3, 3.0). These findings from the CHAMACOS Study do not align with the results from our study where a significant adverse association was observed between concurrent concentrations of BDE-28 and BDE-153 and behavior regulation index and its subscale inhibit, and between concurrent PBDE congeners and ΣPBDEs and emotional control.
Previously, both the HOME Study and the CHAMACOS Study reported impairment in executive function in children with increased concentrations of prenatal PBDEs. In the HOME Study, 10-fold increases in prenatal BDE-153 were associated with a 3-point increase in behavioral regulation (95% CI 0.60, 5.86), and higher odds of having a score ≥60 was reported with both behavioral regulation (OR=3.92, 95% CI 1.76, 8.73) and global executive composite (OR=2.34, 95% CI 1.05, 5.23) in children 5 and 8 years of age (Vuong et al., 2016). In the CHAMACOS Study, adverse associations were observed between prenatal Σ4PBDEs and metacognition (β=3.3, 95% CI 0.4, 6.3) and global executive composite (β=3.1, 95% CI 0.2, 6.04) in children at 9 years (Sagiv et al., 2015). It is unclear why there is a discrepancy between the HOME Study and CHAMACOS Study for postnatal PBDEs and executive function. Several factors may have contributed to the divergent conclusions. First, PBDE concentrations in the HOME Study (GM of BDE-47 at 8 years: 20.7 ng/g lipid; interquartile range [IQR]: 10.1–39.3 ng/g lipid) are lower than that of the CHAMACOS Study (GM of BDE-47 at 9 years: 35.2 ng/g lipid; IQR: 20.2–64.3 ng/g lipid). Differences in sociodemographics and behavior of study participants in these two cohorts may have influenced childhood PBDE concentrations. First, the CHAMACOS Study is comprised of Mexican Americans, while the HOME Study mainly consists of non-Hispanic white and black women and children. Educational attainment of mothers in the CHAMACOS Study are lower than HOME Study mothers, with 75% having less than a high school education compared to approximately 10% in the HOME Study. Over 70% of families in the CHAMACOS Study had annual incomes that were below the poverty level, whereas 43% of families in the HOME Study had an annual income <$40,000. Third, within the CHAMACOS Study itself, there was a change in the cohort profile with the second wave of child recruitment at 9 years. The second wave of children were less likely to have been breastfed and more likely to live below the poverty level, two factors that may influence PBDE serum concentrations. We also examined repeated measures of PBDE concentrations during childhood with BRIEF assessments completed at 8 years, whereas the CHAMACOS Study examined one measure of PBDEs at 9 years and repeated measures of executive function at 9 and 12 years. Lastly, the CHAMACOS Study reported Σ4PBDEs that did not include BDE-28, which was significantly associated with behavior regulation and emotional control in our study.
Child sex appears to modify the association between concurrent PBDE concentrations and executive function at 8 years, with males performing more poorly on the BRIEF assessment than females. In particular, BDE-153 concentrations at 8 years were associated with significantly higher scores on behavioral regulation, metacognition, and global executive functioning, while females had mixed null associations. The observed effect modification by child sex may be due to differences in PBDE accumulation in the placentas. Leonetti et al. (2014) reported that PBDE concentrations in placentas of women collected in North Carolina, US were higher in male infants compared to females. They also observed differences in the associations between PBDEs and altered thyroid hormone endpoints, including thyroid hormone sulfotransferase (SULT) activities, by child sex. However, the CHAMACOS Study reported that females performed significantly more poorly on a subscale of metacognition (organization of materials) than males (Sagiv et al., 2015). However, previously, poorer scores in behavior regulation were reported among males at 8 years with increased prenatal BDE-153 concentrations in the HOME Study (Vuong et al., 2016), while no sex differences were observed in the CHAMACOS Study for associations between prenatal PBDEs and executive function (Sagiv et al., 2015). Given the lack of consensus between the two studies, it is difficult to draw any conclusions about whether sex modifies PBDE neurotoxicity.
This study had several notable strengths, including its use of a well-established prospective cohort that enabled us to examine repeated measures of PBDEs during childhood. We also used the BRIEF to assess executive function, which has been shown to have a high test-retest reliability across clinical scales (Gioia et al., 2000b). We accounted for a comprehensive list of potential confounders, including sociodemographics, maternal IQ and depression, a nurturing home environment, as well as exposure to other potential neurotoxicants. Adjusting for blood lead concentrations at 8 years did not change our overall conclusions. Further, additional adjustment for prenatal PBDEs resulted in similar findings. Sixth, multiple imputation was utilized to estimate missing concentrations of PBDEs for children in the HOME Study that had at least one measurement during childhood. Imputed concentrations were in line with measured concentrations of PBDEs, with only slightly higher GMs at 2 and 3 years of age. Lastly, we utilized a statistical method that allowed the examination of repeated PBDE concentrations during childhood and executive function.
Our study also had several limitations. Approximately 46% of children were not included in the present study due to missing information on PBDEs or an assessment of executive function at 8 years. However, these excluded children were similar to those included in the study on all aspects (PBDE concentrations and sociodemographics) aside from maternal marital status. Children who were included in the present study were more likely to have mothers who were not married or living alone compared to those who were excluded. Executive function was assessed by a parent who had extensive contact with the child within the past 6 months, but misclassification is a concern because executive function assessment relied on one parent’s perspective. Having an additional parent or a teacher complete the BRIEF might be a more reliable measure than a single assessment of executive function by only one parent. In addition, the BRIEF was not correlated with other measures of executive function in the HOME Study (Barnard et al., 2015). Co-pollutant exposures may also influence the association between childhood PBDEs and executive function. While we utilized a statistical model that was able to examine repeated measures of PBDE exposure, we did not explore more advanced statistical methods that would allow the examination of chemical mixtures and interactions that occur in the real-world setting. In addition, despite having differing toxicological profiles due to varying chemical structures (e.g., number and position of bromines), PBDE congeners are highly correlated with each other and those included in this analysis have long half-lives. This makes it difficult to completely tease out associations that are specific to one PBDE congener itself. However, it is worth noting that BDE-153 has been observed to have higher accumulation in brain tissue and lower metabolism and excretion rates than congeners BDE-47, -99, and -153 (Staskal et al., 2006). Lastly, although we observed significant adverse associations between concurrent PBDE concentrations and behavioral regulation, emotional control, and impulse control at age 8 years, we cannot negate PBDEs’ potential neurotoxicity at earlier stages during childhood. We did not find significant associations between PBDE concentrations at 1–3 years. However, this may be due to selection bias as we do not know whether executive function of those lost to follow-up were significantly different from those who completed the BRIEF assessment at 8 years. A large percentage of PBDE measurements (59–67%) were also imputed at ages 1–3 years, which could have contributed to our null findings. Further, null associations between PBDEs during early childhood may also be influenced by developmental toxicity of PBDEs in neuronal tissues. Synapse pruning, myelination, and neurotransmitter release may be influenced. However, synapse pruning occurs earlier, while myelination takes a much longer time to complete (Tierney and Nelson, 2009). Neurotransmitter release and receptor binding are more impacted by continuous exposure to neurotoxicants, including PBDEs. Observed associations between 8 year PBDE concentrations and impaired executive function may be a result of latent, chronic exposure to PBDEs. This finding may be a result of cumulative PBDE exposure from gestational development, infancy, and early childhood that has influenced the developmental trajectory of executive function. In addition, we do not have PBDE measures at ages 6 and 7 years to examine whether exposures at these time points are adversely associated with executive function at age 8 years. Thus, it is difficult to conclude with certainty that solely cross-sectional PBDE concentrations are associated with poorer executive function and that earlier exposure to PBDEs during childhood do not adversely impact executive function.
5. Conclusions
Toxicological and epidemiologic studies have consistently shown that prenatal exposure to PBDEs adversely impact neurodevelopment (Costa et al., 2014; Herbstman and Mall, 2014). We addressed critical questions related to postnatal PBDE exposures and specific domains of neurodevelopment. Findings from the present study indicate that childhood PBDEs, particularly BDE-28 and BDE-153 at 8 years, may adversely impair execution functions, including behavior regulation and emotional and impulse control. It is unclear whether concurrent PBDE concentrations are adversely associated with executive function or whether these findings reflect cumulative exposure to PBDEs that resulted in the observed impairments.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES020349, R01 ES024381, R01 ES014575, R00 ES020346, T32ES010957, P30ES006096; EPA P01 R829389). Dr. Chen also received partial support from the National Natural Science Foundation of China (NSFC 21628701). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Abbreviations
- BRIEF
Behavior Rating Inventory of Executive Function
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CHAMACOS Study
Center for the Health Assessment of Mothers and Children of Salinas
- FSIQ
full scale intelligence quotient
- GAM
generalized additive model
- GEE
generalized estimating equations
- HOME Study
Health Outcomes and Measures of the Environment Study
- MCMC
Markov Chain Monte Carlo
- PBDE
polybrominated diphenyl ether
- PCB
polychlorinated biphenyls
- OR
odds ratio
- SD
standard deviation
- TTR
transthyretin
Footnotes
Competing financial interest declaration:
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001a;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Northam E, Hendy J, Wrenall J. Developmental neuropsychology: A clinical approach. Psychology Press; New York: 2001b. [Google Scholar]
- Barnard H, Rao R, Xu Y, Froehlich T, Epstein J, Lanphear BP, Yolton K. Association of the Conners’ Kiddie Continuous Performance Test (K-CPT) Performance and Parent-Report Measures of Behavior and Executive Functioning. J Atten Disord. 2015 doi: 10.1177/1087054715578271. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bernal J. In: Thyroid Hormones in Brain Development and Function. De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. Endotext; South Dartmouth (MA): 2000. [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017a;46:24. doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, Lanphear BP, Chen A. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology. 2017b;62:192–199. doi: 10.1016/j.neuro.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjodin A, Dietrich KN, Lanphear BP. Prenatal Polybrominated Diphenyl Ether Exposures and Neurodevelopment in U.S. Children through 5 Years of Age: The HOME Study. Environ Health Perspect. 2014;122:856–862. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett. 2014;230:282–294. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Lederman SA, Sjodin A, Jones R, Wang S, Perera FP, Wang R, Rauh VA, Herbstman JB. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years. Neurotoxicol Teratol. 2015;52:143–150. doi: 10.1016/j.ntt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Yu J, Cui C, Chen L, Gao Y, Wang C, Zhou Y, Tian Y. Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China. Environ Res. 2015;142:104–111. doi: 10.1016/j.envres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: effects on learning, attention and thyroxine levels. Neurotoxicol Teratol. 2009;31:76–84. doi: 10.1016/j.ntt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int. 2011;37:605–611. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources; Odessa, FL: 2000a. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000b;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Han G, Ding G, Lou X, Wang X, Han J, Shen H, Zhou Y, Du L. Correlations of PCBs, DIOXIN, and PBDE with TSH in children’s blood in areas of computer E-waste recycling. Biomed Environ Sci. 2011;24:112–116. doi: 10.3967/0895-3988.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr Environ Health Rep. 2014;1:101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton NJ, Laird NM, Zahner GE. Use of multiple informant data as a predictor in psychiatric epidemiology. Int J Methods Psychiatr Res. 1999;8:6–18. [Google Scholar]
- Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 2011;119:168–175. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MH, Barr DB, Marcus M, Muir AB, Lyles RH, Howards PP, Pardo L, Darrow LA. Serum polybrominated diphenyl ether concentrations and thyroid function in young children. Environ Res. 2016;149:222–230. doi: 10.1016/j.envres.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicinski M, Viaene MK, Den Hond E, Schoeters G, Covaci A, Dirtu AC, Nelen V, Bruckers L, Croes K, Sioen I, Baeyens W, Van Larebeke N, Nawrot TS. Neurobehavioral function and low-level exposure to brominated flame retardants in adolescents: a cross-sectional study. Environ Health. 2012;11:86. doi: 10.1186/1476-069X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, ten Tusscher GW, Olie K, van Teunenbroek T, van Aalderen WM, de Voogt P, Vulsma T, Bartonova A, Krayer von Krauss M, Mosoiu C, Riojas-Rodriguez H, Calamandrei G, Koppe JG. Thyroid hormone metabolism and environmental chemical exposure. Environ Health. 2012;11(Suppl 1):S10. doi: 10.1186/1476-069X-11-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Hoffman K, Miranda ML, Stanton ME. Associations between PBDEs 2,4,6 tribromophenol and thyroid hormone levels in human placental tissues. 14th Annual Workshop on Brominated & Other Flame Retardants (BFR); Indianapolis, IN. 2014. [Google Scholar]
- Litman HJ, Horton NJ, Hernandez B, Laird NM. Incorporating missingness for estimation of marginal regression models with multiple source predictors. Stat Med. 2007;26:1055–1068. doi: 10.1002/sim.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008;232:150–160. doi: 10.1016/j.taap.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008;226:244–250. doi: 10.1016/j.taap.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K, Villasenor D, Bradman A, Holland N, Eskenazi B. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol Teratol. 2015;52:151–161. doi: 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119:409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Shy CG, Huang HL, Chang-Chien GP, Chao HR, Tsou TC. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87:643–648. doi: 10.1007/s00128-011-0422-9. [DOI] [PubMed] [Google Scholar]
- Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res. 2003;1:281–290. doi: 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerbo A, Kesmodel US, Wimberley T, Stovring H, Bertrand J, Landro NI, Mortensen EL. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG. 2012;119:1201–1210. doi: 10.1111/j.1471-0528.2012.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci. 2006;94:28–37. doi: 10.1093/toxsci/kfl091. [DOI] [PubMed] [Google Scholar]
- Tierney AL, Nelson CA., 3rd Brain Development and the Role of Experience in the Early Years. Zero Three. 2009;30:9–13. [PMC free article] [PubMed] [Google Scholar]
- Toms LM, Harden F, Paepke O, Hobson P, Ryan JJ, Mueller JF. Higher accumulation of polybrominated diphenyl ethers in infants than in adults. Environ Sci Technol. 2008;42:7510–7515. doi: 10.1021/es800719v. [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environ Health Perspect. 2009;117:1461–1465. doi: 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Braun JM, Yolton K, Xie C, Webster GM, Sjodin A, Dietrich KN, Lanphear BP, Chen A. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environ Res. 2017;153:83–92. doi: 10.1016/j.envres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjodin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, Chen A. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res. 2016;147:556–564. doi: 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Xu X, Liu J, Zeng X, Lu F, Chen A, Huo X. Elevated serum polybrominated diphenyl ethers and alteration of thyroid hormones in children from Guiyu, China. PLoS One. 2014;9:e113699. doi: 10.1371/journal.pone.0113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjodin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ Health Perspect. 2017;125:746–752. doi: 10.1289/EHP478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.