Abstract
The use of antiretroviral (ARV) drugs with central nervous system (CNS) penetration effectiveness (CPE) may be useful in the treatment of HIV-associated neurocognitive disorder (HAND) as well as targeting a CNS reservoir in strategies to achieve a functional cure for HIV. However, increased cognitive deficits are linked to at least one of these drugs (efavirenz). As mitochondrial dysfunction has been found with a number of ARVs, and as such can affect neuronal function, the objective of this study was to assess the effects of ARV with high CPE for toxicological profiles on presynaptic nerve terminal energy metabolism. This subcellular region is especially vulnerable in that a constant supply of ATP is required for the proper maintenance of neurotransmitter release and uptake supporting proper neuronal function. We evaluated the effects of acute treatment with ten different high CPE ARVs from five different drug classes on rat cortical and striatal nerve terminal bioenergetic function. While cortical nerve terminal bioenergetics were not altered, striatal nerve terminals exposed to efavirenz, nevirapine, abacavir, emtricitabine, zidovudine, darunavir, lopinavir, raltegravir, or maraviroc (but not indinavir) exhibit reduced mitochondrial spare respiratory capacity (SRC). Further examination of efavirenz and maraviroc revealed a concentration-dependent impairment of striatal nerve terminal maximal mitochondrial respiration and SRC as well as a reduction of intraterminal ATP levels. Depletion of ATP at the synapse may underlie its dysfunction and contribute to neuronal dysfunction in treated HIV infection.
Keywords: antiretrovirals, bioenergetics, neurotoxicity, synaptic mitochondria
Introduction
Potent combination antiretroviral (ARV) therapies have revolutionized the care of those infected with HIV, leading to greatly improved immune function, decreased opportunistic infections, and reduced prevalence of other HIV-related conditions including HIV-associated dementia, the most severe form of neurocognitive manifestations of HIV infection (Eggers et al, 2017; Heaton et al, 2010; Heaton et al, 2011; Letendre, 2011; Saylor et al, 2016). However, HIV-associated neurocognitive disorder (HAND), characterized by a wide spectrum of behavioral, cognitive, and motor dysfunctions, continues to affect approximately 50% of ARV-treated patients (Heaton et al, 2011; Letendre, 2011; Saylor et al, 2016; Valcour et al, 2011).
To treat or prevent HAND, one option is to use ARVs that penetrate the blood-brain barrier to control viral replication in the brain (Letendre et al, 2008; Letendre et al, 2004). However, while treating CNS infection penetration into the brain, these ARVs may compromise central nervous system (CNS) function and contribute to the development of neurocognitive complications (Caniglia et al, 2014; Robertson et al, 2012). Although the molecular mechanisms responsible for ARV-induced CNS side effects remain elusive, growing evidence points to disturbances in brain energy homeostasis and mitochondrial function, particularly studied in the case of the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (Apostolova et al, 2015; Decloedt and Maartens, 2013), the long-term use of which is associated with lower neurocognitive function in HIV infected individuals (Ma et al, 2016). In addition to their presence in the neuronal soma, mitochondria are physically located at the presynaptic nerve terminal, a subcellular location that requires a constant supply of ATP for proper maintenance of active physiological events such as neurotransmitter release and reuptake as well as establishment of electrochemical gradients.
There has been a growing body of evidence that synaptic dysfunction is a critical event in a number of neurodegenerative diseases and neuropsychiatric disorders (Lepeta et al, 2016; Palop et al, 2006; van Spronsen and Hoogenraad, 2010). It has been postulated that one possible mechanism underlying such synaptic dysfunction is altered bioenergetic capacity resulting in a depletion of ATP at the terminus (Flynn et al, 2011). In fact, impaired spare respiratory capacity (SRC), which can result in energy demand outpacing supply, is associated with neuronal death (Yadava and Nicholls, 2007). In this study we evaluate the direct effects of ten different ARV drugs with high CNS-penetration effectiveness separately on rat nerve terminal bioenergetic function, particularly mitochondrial respiration and SRC.
Materials and methods
Reagents
The following reagents were obtained through the AIDS Reagent Program, Division of AIDS, NIAID, NIH: Efavirenz (Cat #4624), Nevirapine (Cat #4666), Abacavir (Cat #4680), Emtricitabine [(−) FTC] (Cat #10071), Zidovudine (Cat #3485), Darunavir (Cat #11447), Indinavir Sulfate (Cat #8145), Lopinavir (Cat #9481), Raltegravir (Cat #11680), and Maraviroc (Cat #11580). Stock solutions of each ARV drug were made in dimethylsulfoxide (DMSO) and stored at −80°C (each ARV drug stock solution concentration was 50 mM, except for abacavir and raltegravir, which were 25 mM). The final ARV dilutions for nerve terminal treatments were prepared in the appropriate assay buffer for each experiment such that the final concentration of DMSO was 0.1%. In vehicle control experiments, nerve terminals were treated with 0.1% DMSO and used as comparisons in the statistical analysis. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO), except the Pierce BCA Protein Assay and ATP Determination Kit, which were purchased from Thermo Fisher Scientific (Rochester, NY).
Animals
Male Long-Evans rats were obtained from Charles River Laboratories International, Inc. (Wilmington, MA). Rats weighing 400-450 grams were housed two per cage in an AALAC-certified animal care facility with a constant 12 h light/dark cycle and fed standard pellet rat chow and water ad libitum. Rats were used for nerve terminal isolations at 5–6 months of age. All rat procedures were carried out under approved University of Nebraska Medical Center Institutional Animal Care and Use Committee protocols.
Nerve terminal isolation
Rats were euthanized by isoflurane inhalation and decapitation, and then cortices and striata were removed and subjected to a nerve terminal isolation using a previously described method with slight modifications (Choi et al, 2009; Dunkley et al, 2008). Briefly, the cortex and striatum were rapidly removed and transferred to separate pre-chilled Dounce homogenizers containing Sucrose Medium: 320 mM sucrose, 1 mM EDTA, 0.25 mM dithiothreitol, pH 7.4. The tissue was homogenized using 10 strokes. The homogenate was centrifuged at 1,000 × g for 10 min. The supernatant was layered on top of a discontinuous Percoll gradient (3, 10, and 23% Percoll in Sucrose Medium) and centrifuged at 31,000 × g for 10 min. The band between 10% and 23% Percoll, which contains the nerve terminals, was collected. The nerve terminal suspension was diluted with Ionic Medium: 20 mM HEPES, 10 mM D-Glucose, 1.2 mM Na2HPO4, 1 mM MgCl2, 5 mM NaHCO3, 5 mM KCl, 140 mM NaCl, pH 7.4 and then centrifuged at 15,000 × g for 15 min. The nerve terminal pellet was resuspended in Incubation Medium: 3.5 mM KCl, 120 mM NaCl, 1.3 mM CaCl2, 0.4 mM KH2PO4, 1.2 mM Na2SO4, 2 mM MgSO4, 15 mM D-glucose, pH 7.4 and the protein concentration was measured using the Pierce BCA Protein Assay.
Mitochondrial respiration
For monitoring respiration, the oxygen consumption rate of mitochondria within nerve terminals was determined in units of picomoles of O2 per minute using a microplate-based respirometer as previously described (Choi et al, 2009; Gerencser et al, 2009). The nerve terminals (15 μg protein/well) were attached by centrifugation to poly-D-lysine coated 96-well cell culture microplates (Seahorse XFe96 Analyzer; Agilent Technologies, Santa Clara, CA). The assay buffer: 3.5 mM KCl, 120 mM NaCl, 1.3 mM CaCl2, 0.4 mM KH2PO4, 1.2 mM Na2SO4, 2 mM MgSO4, 15 mM D-glucose, 10 mM pyruvate, and 4 mg/ml fatty acid-free bovine serum albumin, pH 7.4 containing vehicle (DMSO) or ARV drug was added to each well. The cell culture microplate was incubated at 37°C for 2 hours prior to loading into the XFe96 extracellular flux analyzer. Nonmitochondrial respiration, which corresponds to the average of the three measurement values after the injection of 2 μM rotenone (complex I inhibitor) combined with 2 μM antimycin A (complex III inhibitor), for each well was subtracted from all other values for that well before calculation of the following mitochondrial respiratory parameters: basal respiration (average of the first three measurement values before the injection of 5 μM oligomycin, an inhibitor of complex V); maximal respiration (first measurement value after the injection of 4 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), an uncoupler of oxidative phosphorylation); respiration driving proton leak (average of the three measurement values after the injection of 5 μM oligomycin); respiration driving ATP synthesis (basal respiration minus respiration driving proton leak); and spare respiratory capacity (SRC, maximal respiration minus basal respiration) (Choi et al, 2011).
Experiments testing all ten different ARV drugs: For comparisons across assay plates using independent nerve terminal preparations for each plate, absolute rates of O2 consumption were converted to relative percent response from baseline (third measurement before oligomycin injection) (Salabei et al, 2014). The calculated values for each well were averaged for 4–6 technical replicate wells on one plate to give n = 1 biological replicate. Respiration data are presented as the means ± SEM of three independent experiments with different nerve terminal preparations (n = 3 biological replicates, each comprising 4–6 technical replicates). For ease of visualization the respiration data is shown with the vehicle control compared to each class of ARV drugs individually, versus all on the same graph (all ten ARV drugs were compared to the same vehicle control as each replicate experiment was performed on the same assay plate).
Experiments testing only efavirenz and maraviroc: For comparisons between nerve terminal preparations from different rats run on the same plate, rates of O2 consumption for each well were normalized to μg protein for that well determined using the Pierce BCA Protein Assay (Thermo Fisher Scientific). The calculated values for each well were averaged for 2 technical replicate wells for each rat to give n = 1 biological replicate. Respiration data are presented as the means ± SEM of one experiment with nerve terminal preparations from three different rats (n = 3 biological replicates, each comprising 2 technical replicates).
ATP Assay
Nerve terminal ATP levels were measured using the ATP Determination Kit (Thermo Fisher Scientific). Efavirenz and maraviroc concentrations from 0 to 25 μM were prepared in assay buffer. The nerve terminals were incubated with equivalent dilutions of DMSO, efavirenz, or maraviroc for 2 hours at 37°C, followed by extraction of ATP (Yang et al, 2002), and measurement of luminescence on a SYNERGY HTX multi-mode reader (Bio-Tek Instruments). Oligomycin (10 μM) was used to inhibit mitochondrial ATP synthase. Data are presented as the means ± SEM of one experiment with nerve terminal preparations from three different rats (n = 3 biological replicates).
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). Significance level was determined by performing ANOVA with Bonferonni post-hoc testing (GraphPad Prism 6 software, GraphPad Software, La Jolla, CA).
Results
Effects of ARV drugs on nerve terminal respiration
In order to fully evaluate the effects of acute (2 hour) treatment with different ARV drugs on cortical and striatal nerve terminal respiration, we used a Seahorse XFe96 extracellular flux analyzer. The mean respiration results from three independent experiments are shown for cortical (Fig. 1 and 2, Supplementary Table S1) and striatal (Fig. 3 and 4, Supplementary Table S2) nerve terminals following treatment with 25 μM of one of ten different ARVs (efavirenz and nevirapine; abacavir, emtricitabine, and zidovudine; darunavir, indinavir, and lopinavir; raltegravir; and maraviroc) that are members of five different drug classes: non-nucleoside reverse transcriptase inhibitors (NNRTIs); nucleoside reverse transcriptase inhibitors (NRTIs); protease inhibitors; integrase inhibitors; and entry inhibitors (CCR5 receptor antagonist), respectively, versus vehicle control. These drugs were chosen as all have high CPE ratings (3 or 4 on a 4 point scale) (Letendre, 2011). Acute (2 hour) treatment was used for optimal functional integrity since it is preferable to use nerve terminals within several hours after isolation (Dunkley et al, 2008; Whittaker, 1993; Xu et al, 2013). Our previous work revealed that treatment of primary rat striatal neurons with 25 μM efavirenz for 2 hours reduced ATP levels by 66%, without inducing non-mitochondrial cytotoxicity (Purnell and Fox, 2014), thus, for our 2 hour treatment of nerve terminals we chose to compare the ten different ARV drugs using this dose.
Fig. 1.
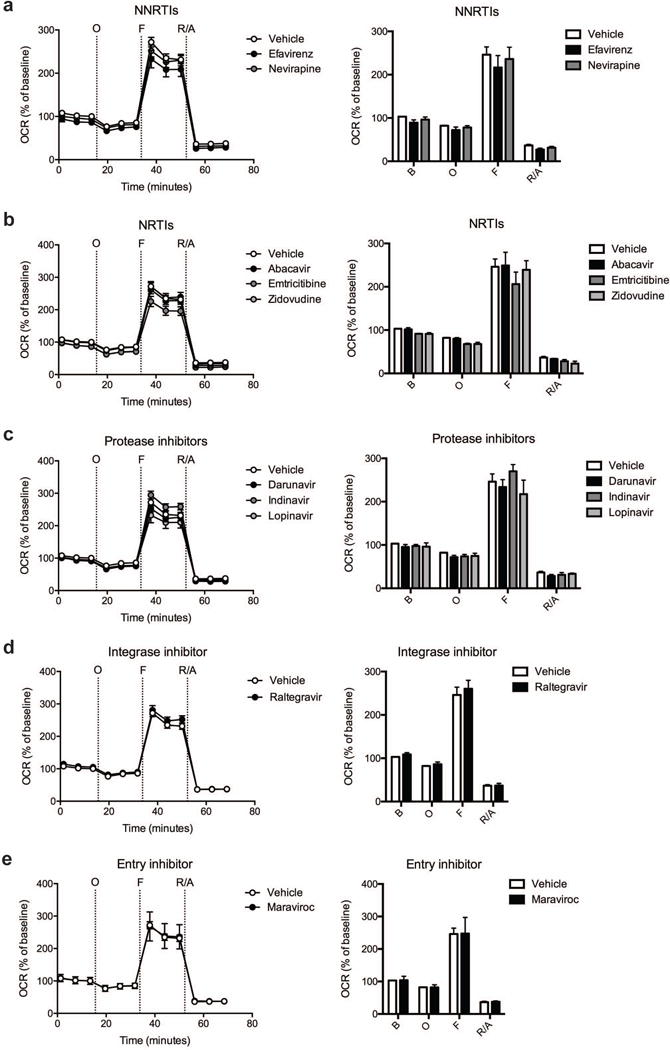
Effect of ARV drug treatment on cortical nerve terminal respiration. The OCR was measured in cortical nerve terminals exposed to vehicle or 25 μM ARV drug for 2 hours. Left: Graphical representation of the relative OCR responses over time expressed as a percent response from baseline (third measurement, before oligomycin injection); sequential additions are indicated as O (5 μM oligomycin), F (4 μM FCCP), and R/A (2 μM rotenone and 2 μM antimycin A). Right: Quantification of the mean OCR in cortical nerve terminals exposed to (a) NNRTIs (efavirenz or nevirapine), (b) NRTIs (abacavir, emtricitabine, or zidovudine), (c) protease inhibitors (darunavir, indinavir, or lopinavir), (d) integrase inhibitor (raltegravir), or (e) entry inhibitor (maraviroc) is shown for respiration under baseline conditions (B) and after the sequential additions (O, F, and R/A). Data (mean ± SEM; n = 3) were compared with those for vehicle control and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test. For ease of visualization the data is presented with the vehicle control compared to each class of ARV drugs individually.
Fig. 2.
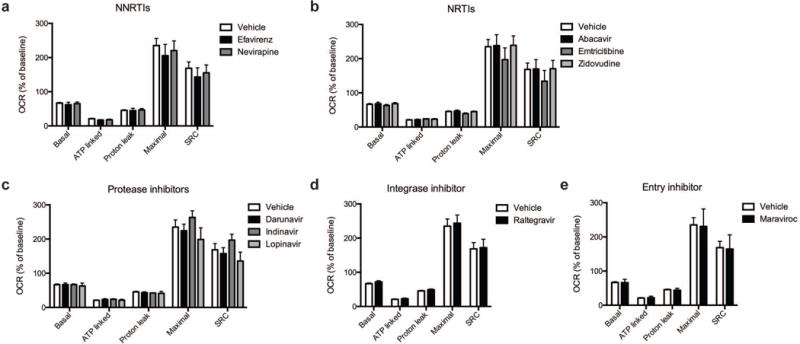
Effect of ARV drug treatment on mitochondrial respiration. Mitochondrial respiratory parameters calculated from the OCR (Figure 1) in cortical nerve terminals exposed for 2 hours to vehicle or 25 μM ARV drug (a) NNRTIs (efavirenz or nevirapine), (b) NRTIs (abacavir, emtricitabine, or zidovudine), (c) protease inhibitors (darunavir, indinavir, or lopinavir), (d) integrase inhibitor (raltegravir), or (e) entry inhibitor (maraviroc) is shown for basal mitochondrial respiration (B minus R/A), ATP linked respiration (B minus O), proton leak (O minus R/A), maximal mitochondrial respiration (F minus R/A), and SRC (F minus B). Data (mean ± SEM; n = 3) were compared with those for vehicle control and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test. For ease of visualization the data is presented with the vehicle control compared to each class of ARV drugs individually.
Fig. 3.
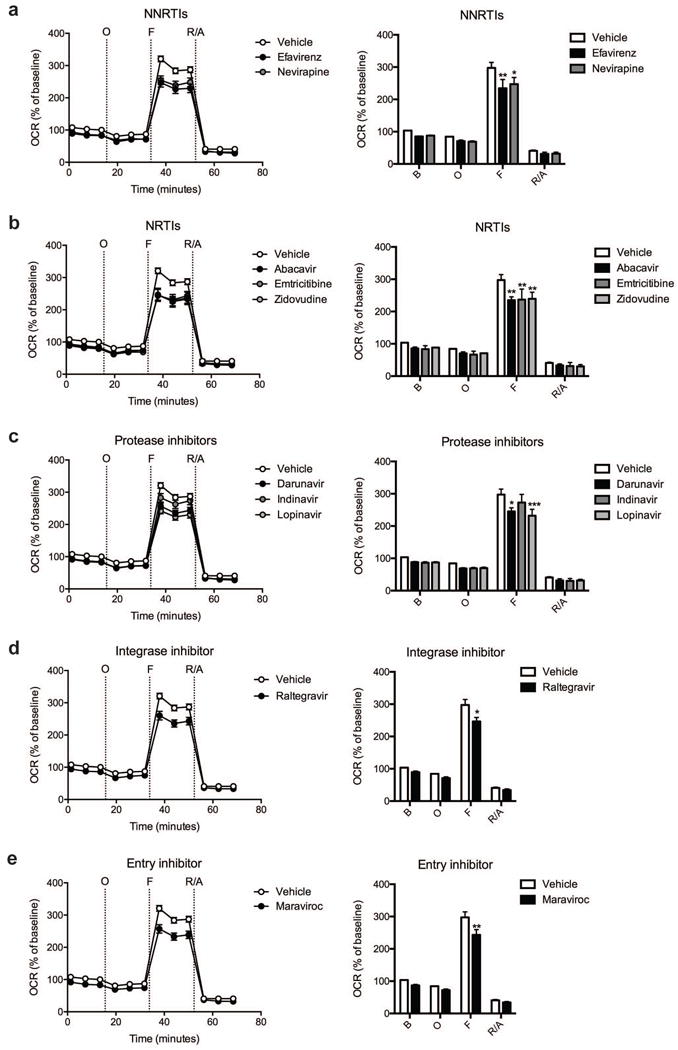
Effect of ARV drug treatment on striatal nerve terminal respiration. The OCR was measured in striatal nerve terminals exposed to vehicle or 25 μM ARV drug for 2 hours. Left: Graphical representation of the relative OCR responses over time expressed as a percent response from baseline (third measurement, before oligomycin injection); sequential additions are indicated as O (5 μM oligomycin), F (4 μM FCCP), and R/A (2 μM rotenone and 2 μM antimycin A). Right: Quantification of the mean OCR in striatal nerve terminals exposed to (a) NNRTIs (efavirenz or nevirapine), (b) NRTIs (abacavir, emtricitabine, or zidovudine), (c) protease inhibitors (darunavir, indinavir, or lopinavir), (d) integrase inhibitor (raltegravir), or (e) entry inhibitor (maraviroc) is shown for respiration under baseline conditions (B) and after the sequential additions (O, F, and R/A). Data (mean ± SEM; n = 3) were compared with those for vehicle control and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test; significance versus control: *p < 0.05, **p < 0.01, ***p < 0.001. For ease of visualization the data is presented with the vehicle control compared to each class of ARV drugs individually.
Fig. 4.
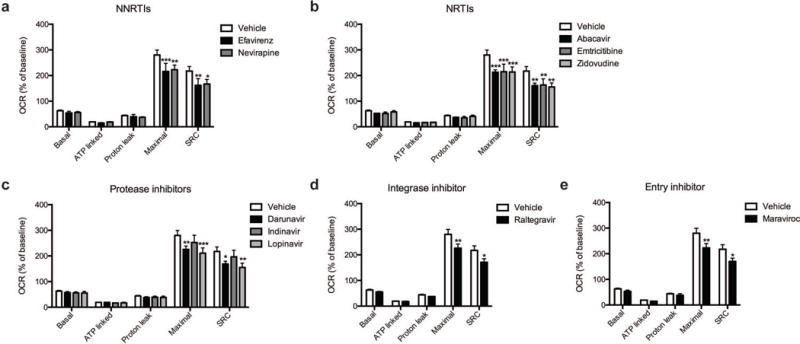
Effect of ARV drug treatment on mitochondrial respiration. Mitochondrial respiratory parameters calculated from the OCR (Figure 3) in striatal nerve terminals exposed for 2 hours to vehicle or 25 μM ARV drug (a) NNRTIs (efavirenz or nevirapine), (b) NRTIs (abacavir, emtricitabine, or zidovudine), (c) protease inhibitors (darunavir, indinavir, or lopinavir), (d) integrase inhibitor (raltegravir), or (e) entry inhibitor (maraviroc) is shown for basal mitochondrial respiration (B minus R/A), ATP linked respiration (B minus O), proton leak (O minus R/A), maximal mitochondrial respiration (F minus R/A), and SRC (F minus B). Data (mean ± SEM; n = 3) were compared with those for vehicle control and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test; significance versus control: p < 0.05, **p < 0.01, ***p < 0.001. For ease of visualization the data is presented with the vehicle control compared to each class of ARV drugs individually.
Cortical nerve terminals did not exhibit significant alterations in the assessed bioenergetic parameters upon treatment with any of the ten different ARV drugs versus vehicle control (Fig. 1 and 2). No significant difference in the level of baseline respiration was observed between striatal nerve terminals treated with any of the ten different ARV drugs versus vehicle control (Fig. 3) (Choi et al, 2009). Further, following the addition of oligomycin, which inhibits ATP synthase (complex V of the electron transport chain (ETC)), respiration decreased equally in all treatment conditions (Fig. 3). However, upon addition of the uncoupling agent FCCP, striatal nerve terminals treated with efavirenz or nevirapine (Fig. 3a), abacavir, emtricitabine, or zidovudine (Fig. 3b), darunavir or lopinavir (Fig. 3c), raltegravir (Fig. 3d), or maraviroc (Fig. 3e) exhibited a significantly lower level of induction of maximal respiration rate. No significant change was found with indinavir (Fig. 3c). The increase in mitochondrial respiration was inhibited equally, in all treatment conditions, after the addition of rotenone and antimycin A, which shut down oxidative phosphorylation by inhibiting ETC complexes I and III, respectively.
The basal mitochondrial respiration (baseline respiration minus non-mitochondrial respiration), which is composed of ATP-synthesis linked oxygen consumption (oligomycin sensitive mitochondrial respiration) and proton leak (oligomycin insensitive mitochondrial respiration) were calculated using established methodology (Brand, 1990; Jekabsons and Nicholls, 2004). ARV treated striatal nerve terminals show no significant difference in any of these parameters (Fig. 4). The addition of FCCP allows for determination of the maximal mitochondrial respiration (FCCP-induced respiration minus non-mitochondrial respiration) and, thereby, the spare respiratory capacity (SRC; maximal mitochondrial respiration minus basal mitochondrial respiration, a measure of the energetic reserve to respond to increased demand) under these conditions. The results show that treatment with the NNRTI drugs efavirenz and nevirapine (Fig. 4a), the NRTI drugs abacavir, emtricitabine, and zidovudine (Fig. 4b), the protease inhibitors darunavir and lopinavir (Fig. 4c), the integrase inhibitor raltegravir (Fig. 4d), and the entry inhibitor maraviroc (Fig. 4e) lead to a significant decrease in maximal mitochondrial respiration and impair SRC in the striatal nerve terminals. The nine different ARV drugs ranked in order of decreasing striatal nerve terminal SRC impairment is as follows: lopinavir > zidovudine > abacavir > efavirenz > emtricitabine > nevirapine > maraviroc > raltegravir > indinavir (with the latter not a significant change).
Compared with cortical nerve terminals, the striatal nerve terminals have a significantly higher spare respiratory capacity (Fig. 5, Supplementary Table S3), in the absence of alterations in any of the other mitochondrial parameters.
Fig. 5.
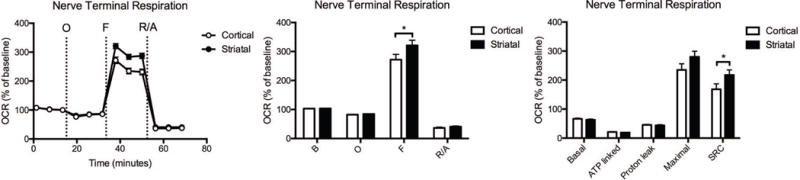
Cortical versus striatal nerve terminal respiration. The OCR was measured in cortical and striatal nerve terminals exposed to vehicle for 2 hours. Left: Graphical representation of the relative OCR responses over time expressed as a percent response from baseline (third measurement, before oligomycin injection); sequential additions are indicated as O (5 μM oligomycin), F (4 μM FCCP), and R/A (2 μM rotenone and 2 μM antimycin A). Middle: Quantification of the mean OCR is shown for respiration under baseline conditions (B) and after the sequential additions (O, F, and R/A). Right: Mitochondrial respiratory parameters calculated from the OCR is shown for basal mitochondrial respiration (B minus R/A), ATP linked respiration (B minus O), proton leak (O minus R/A), maximal mitochondrial respiration (F minus R/A), and SRC (F minus B). Striatal nerve terminal data (mean ± SEM; n = 3) were compared with those for cortical nerve terminals and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test; significance versus cortical: *p < 0.05.
Concentration-dependent decrease in striatal mitochondrial spare respiratory capacity
Two of the ARV drugs were then chosen for further study: efavirenz due to its known neurotoxic and neurocognitive effects, and maraviroc, as it has entered clinical trials for the treatment of HAND. Efavirenz provoked a significant and concentration-dependent decrease in mitochondrial SRC in striatal nerve terminals due to an impaired ability to induce maximal mitochondrial respiration (Fig. 6a, Supplementary Table S4). At 6.25 μM, SRC is decreased by 25%; at 12.5 μM, SRC is lowered by 29%; at 25 μM, SRC is reduced 45%. The reduction of maximal mitochondrial respiration and SRC due to maraviroc treatment, similar to efavirenz, was significant and concentration-dependent (Fig. 6b, Supplementary Table S4). At 6.25 μM, SRC is decreased by 26%; at 12.5 μM, SRC is lowered by 31%; at 25 μM, SRC is reduced 35%. Of note, neither efavirenz nor maraviroc significantly altered basal mitochondrial respiration, ATP linked respiration, or proton leakage at any of the tested concentrations.
Fig. 6.
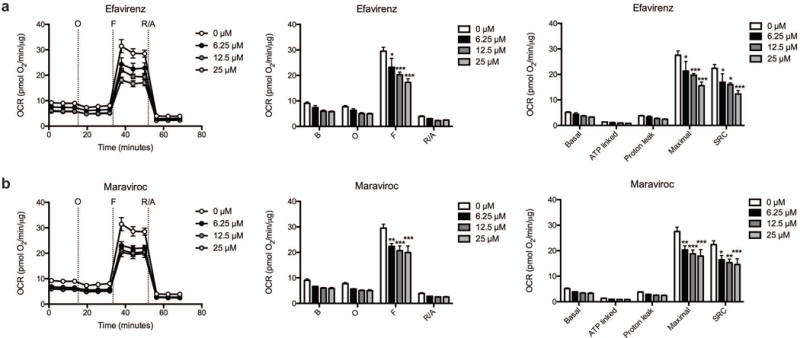
Dose-dependence of the efavirenz and maraviroc induced respiratory alterations. The OCR was measured in striatal nerve terminals exposed to vehicle or 6.25, 12.5, and 25 μM (a) efavirenz or (b) maraviroc for 2 hours. Left: Graphical representation of the relative OCR responses over time expressed as a percent response from baseline (third measurement, before oligomycin injection); sequential additions are indicated as O (5 μM oligomycin), F (4 μM FCCP), and R/A (2 μM rotenone and 2 μM antimycin A). Middle: Quantification of the mean OCR in striatal nerve terminals exposed to (a) efavirenz or (b) maraviroc is shown for respiration under baseline conditions (B) and after the sequential additions (O, F, and R/A). Right: Mitochondrial respiratory parameters calculated from the OCR in striatal nerve terminals exposed for 2 hours to vehicle or 25 μM (a) efavirenz or (b) maraviroc is shown for basal mitochondrial respiration (B minus R/A), ATP linked respiration (B minus O), proton leak (O minus R/A), maximal mitochondrial respiration (F minus R/A), and SRC (F minus B). Data (mean ± SEM; n = 3) were compared with those for vehicle control and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test; significance versus control: *p < 0.05, **p < 0.01, ***p < 0.001. For ease of visualization the data is presented with the vehicle control compared to each ARV drug individually.
Efavirenz and maraviroc reduce ATP production in striatal nerve terminals
Previously, we found that ATP levels are lowered in SH-SY5Y neuroblastoma cells and in primary rat striatal neurons after incubation with efavirenz for 2 hours (Purnell and Fox, 2014), thus we assessed levels of ATP in striatal nerve terminals after incubation with efavirenz or maraviroc (Fig. 7). Here, in striatal nerve terminals treated with 6.25 μM efavirenz, a 40% drop in ATP levels is seen after 2 hours. Increasing the efavirenz dose to 12.5 μM and 25 μM decreases the ATP production more than 45% and 57%, respectively. Similarly, doses of maraviroc above 6.25 μM lower ATP levels in these nerve terminals at 2 hours. At 12.5 μM, ATP levels are decreased by 24%; at 25 μM, ATP is lowered by 50%. In striatal nerve terminals treated with oligomycin, an inhibitor of mitochondrial ATP synthase, ATP levels were reduced 79% revealing that in striatal nerve terminals the majority of the ATP is produced through mitochondrial oxidative phosphorylation.
Fig. 7.
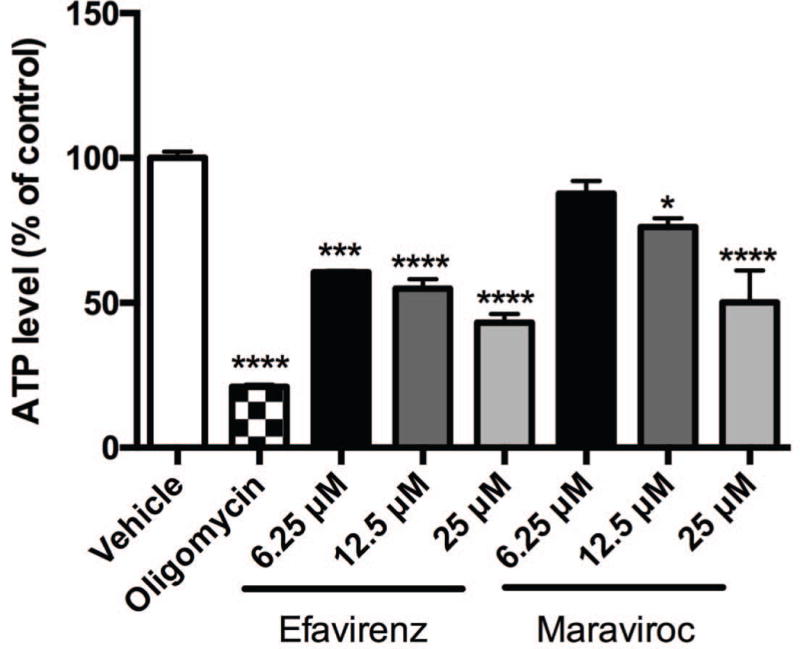
ATP production in striatal nerve terminals. ATP assay in striatal nerve terminals exposed to vehicle, 10 μM oligomycin, or 6.25, 12.5, and 25 μM efavirenz or maraviroc for 2 hours. Data (mean ± SEM; n = 3) were compared with those for vehicle control and was analyzed by ANOVA with Bonferroni post-hoc test; significance versus control: *p < 0.05, ***p < 0.001, ****p < 0.0001.
Discussion
Accumulating evidence suggests that commonly prescribed HIV ARV drugs have the potential to induce CNS adverse effects (Robertson et al, 2012; Treisman and Soudry, 2016), and several lines of clinical evidence link changes in mitochondria to neuropsychiatric abnormalities (Anglin et al, 2012). Mitochondrial function is closely related to synaptic function. Here, we investigated the acute effects of ten different ARV drugs with high CPE representative of five different drug classes on cortical (Fig. 1 and 2) and striatal (Fig. 3 and 4) presynaptic nerve terminal mitochondrial respiration. We found that exposure to nine of the ten ARVs studied here significantly reduced the maximal mitochondrial respiration, and thus SRC, of striatal (but not cortical) nerve terminals. Although the mechanisms underlying the selective vulnerability of striatal as compared to cortical nerve terminals to the ARV-induced SRC impairment remains to be elucidated, previous studies have revealed that declines in maximal mitochondrial respiratory capacity due to other stressors (i.e. aging) are region-specific (Pandya et al, 2016). Additionally, metabolic poisons acting at the mitochondrial ETC complex I (i.e. rotenone) and complex II (i.e. 3-nitropropionic acid) tend to selectively damage the striatum (Fern, 2003; Sauerbeck et al, 2011). Our data revealing a significantly higher intrinsic mitochondrial spare respiratory capacity in striatal compared to cortical nerve terminals (Fig. 5), suggests that differences in the bioenergetics capacity may underlie the selective vulnerability of striatal nerve terminals to ARV treatment.
Further investigation of striatal nerve terminals following acute treatment with two of the nine drugs identified to impair SRC: the NNRTI efavirenz and the entry inhibitor maraviroc revealed that the effects were concentration-dependent (Fig. 6). This loss of maximal respiratory capacity in response to FCCP uncoupling (i.e. reduced SRC) suggests that nerve terminals within the striatum are sensitive to ARV drug exposure and may contribute to CNS adverse effects. SRC, the ability of mitochondria to meet increased energy demand with increased respiration, is critical for synaptic activity to avoid an energetic crisis from lack of sufficient ATP and reduced SRC is associated with neuronal death (Yadava and Nicholls, 2007). Presynaptic nerve terminals require high levels of ATP for the maintenance of synaptic function. We found that in striatal nerve terminals, there is a drop in ATP levels after 2 hours of treatment with efavirenz and maraviroc (Fig. 7) indicating synaptic dysfunction may play a role in ARV drug neurotoxicity.
One limitation of our study is the acute treatment at relatively high concentrations, whereas those treated for HIV infection have chronic exposure (many years) at lower concentrations (although the levels of drug that are present in the brain itself are largely unknown). The structural and functional integrity of isolated nerve terminals is compromised within several hours after preparation (Dunkley et al, 2008; Whittaker, 1993; Xu et al, 2013), necessitating the acute (2 hour) treatment paradigm. Our previous work revealed that treatment of primary rat striatal neurons with 25 μM efavirenz for 2 hours reduced ATP levels by 66%, without inducing non-mitochondrial cytotoxicity (Purnell and Fox, 2014), thus, for our 2 hour treatment of nerve terminals we chose to compare the ten different ARV drugs using this dose. Although HIV patients are rarely treated with an individual ARV drug, we chose to examine the effects of the ten different ARV drugs separately to determine the relative toxicity of each individual drug on nerve terminal respiration.
We note that clinically CNS side effects are indeed found. The use of the NNRTI efavirenz is associated with both acute and chronic neurological, neuropsychiatric, and neurocognitive abnormalities (Apostolova et al, 2015; Ma et al, 2016). In addition, the use of the integrase inhibitor raltegravir has been associated with neuropsychiatric symptoms (Harris et al, 2008; Teppler et al, 2011). Several of these ARVs have also been shown to lead to CNS abnormalities in rodent models (Shah et al, 2016). Mitochondrial effects have been examined for a number of these ARVs, as discussed below.
NNRTIs
Previous studies in our laboratory (Purnell and Fox, 2014), and by others have demonstrated several toxic effects of the NNRTI efavirenz on primary neurons and cultured neuroblastoma cell lines (Funes et al, 2015), involving mitochondrial dysfunction. This study demonstrates that striatal (but not cortical) nerve terminals exhibit altered mitochondrial respiratory parameters after exposure to several different ARV drugs, including efavirenz. In striatal nerve terminals, efavirenz provoked a concentration-dependent decrease in maximal mitochondrial respiration and SRC (Fig. 4a). These results are consistent with findings in SH-SY5Y neuroblastoma and U-251MG glioblastoma cells (Funes et al, 2014; Funes et al, 2015), where acute (1 hour) treatment with efavirenz (10 and 25 μM) reduced maximal respiration and reserve capacity. Efavirenz also reduces oxygen consumption in mitochondria isolated from cultured astrocytes (60 μM but not 10 μM) (Brandmann et al, 2013), and in cultured Hep3B cells (4 hours; 10, 15, 25, 50, and 100 μM but not 5 μM) (Blas-Garcia et al, 2010). Consistent with our findings (Fig. 7), lower ATP levels have been reported previously upon efavirenz treatment (Blas-Garcia et al, 2010; Funes et al, 2014; Imaizumi et al, 2015; Jin et al, 2016; Purnell and Fox, 2014). Metabolites of efavirenz have also been implicated in dendritic spine injury in neurons (Tovar-y-Romo et al, 2012), but it is unlikely that such metabolites were present here. The effects on maximal mitochondrial respiration and SRC in the striatal nerve terminals were reproduced by nevirapine (Fig. 4a), another NNRTI, although the reduction of these mitochondrial parameters was less significant compared to efavirenz. The effect of nevirapine treatment on oxygen consumption has been studied in human Hep3B hepatoblastoma cells; however, these cells showed unaltered oxygen consumption when treated with nevirapine (4 hours; 10, 25, and 50 μM) (Blas-Garcia et al, 2010). Long-term exposure (7 days) of mixed neuronal-glial cerebrocortical cells to combination nevirapine (1 μM)/zidovudine (10 μM) reduced ATP levels; however, treatment with either drug alone did not (Sanchez et al, 2015).
NRTIs
The NRTI drugs abacavir, emtricitabine, and zidovudine significantly reduced maximal mitochondrial respiration and the associated SRC in striatal nerve terminals (Fig. 4b). The significance of the reductions caused by these NRTI drugs was similar to efavirenz. The purine analogue abacavir reduces oxygen consumption in Hep3B cells when treated acutely (Blas-Garcia et al, 2016). Acute treatment with the pyrimidine analogues emtricitabine and zidovudine also lowered Hep3B cell oxygen consumption (Blas-Garcia et al, 2016). Zidovudine treatment has also been shown to reduce the rate of oxygen consumption in human umbilical vein endothelial cells (HUVEC) (Jiang et al, 2007), in adipocytes (3T3-F442A white and T37i brown) (Viengchareun et al, 2007), and in vascular smooth muscle cells (VSMC) (Hebert et al, 2004). Further, zidovudine inhibits respiration of mitochondria from rat skeletal muscle and brain (Modica-Napolitano, 1993), and has been shown to lower cellular ATP levels (Nagiah et al, 2015; Viengchareun et al, 2007).
Protease inhibitors
The protease inhibitors darunavir and lopinavir, but not indinavir, were found to cause a significant loss of maximal mitochondrial respiration and SRC in striatal nerve terminals (Fig. 4c). The effect of darunavir on oxygen consumption has not been previously reported; however, no alterations in mitochondrial membrane potential or reactive oxygen species production were observed when Hep3B or primary rat cortical neurons were treated with darunavir (Blas-Garcia et al, 2014). The effect of lopinavir alone on oxygen consumption rate had not been previously reported; however, alterations in mouse hepatocyte mitochondrial respiration following treatment with the protease inhibitor combination lopinavir and ritonavir have been observed (El Hoss et al, 2015). Treatment of HUVEC (Jiang et al, 2007) and VSMC (Hebert et al, 2004) with indinavir significantly decreased the rate of oxygen consumption. Further, 3T3-F442A white and T37i brown adipocytes exposed to indinavir results in decreased mitochondrial respiration and reduced ATP levels (Viengchareun et al, 2007).
Integrase inhibitor
The effect of treatment with the integrase inhibitor raltegravir on mitochondrial respiration has not been studied. We found that treatment of striatal nerve terminals with raltegravir impaired maximal mitochondrial respiration and reduced SRC (Fig. 4d). A slight reduction in mitochondrial membrane potential was reported in Hep3B cells following raltegravir treatment, although the generation of reactive oxygen species (ROS) was unaltered (Blas-Garcia et al, 2014). Primary rat cortical neurons exhibited no changes in mitochondrial membrane potential or ROS levels after exposure to raltegravir (Blas-Garcia et al, 2014).
Entry inhibitor
There have been no reports of specific CNS toxicity with the CCR5 receptor antagonist maraviroc to date. Of note chemokines and their receptors (such as CCR5) are suggested to be involved in physiological neuroglial communication (Park et al, 2009), neuronal differentiation (Park et al, 2009), and neuronal survival under pro-apoptotic conditions (Meucci et al, 1998). Further, loss of dopaminergic neurons (Choi et al, 2013) and increased brain damage after ischemic stroke (Sorce et al, 2010) has been reported in CCR5-deficient mice. Thus, under certain conditions, loss of CCR5 function (i.e. maraviroc treatment) may be detrimental. Similar to darunavir and raltegravir, the effect of maraviroc treatment on mitochondrial respiration has not been reported. Here, we found that maraviroc reduced maximal mitochondrial respiration and SRC in striatal nerve terminals (Fig. 4e). Further examination of maraviroc revealed a concentration-dependent impairment of striatal nerve terminal maximal mitochondrial respiration and SRC (Fig. 6) as well as a reduction of intraterminal ATP levels (Fig. 7). Interestingly, maraviroc has shown preliminary beneficial effects on cognition in HAND (Gates et al, 2016; Ndhlovu et al, 2014), and clinical trials using maraviroc for HAND are underway (ClinicalTrials.gov Identifiers NCT02159027 and NCT02519777), under the hypothesis that such treatment will be beneficial to neuropsychological/neurocognitive performance. Such studies will help ascertain the true effects of maraviroc on the CNS in HAND.
It is interesting that drugs representing five completely different categories of ARV drugs led to similar alterations in striatal nerve terminal mitochondrial function. Specifically, when administered alone, nine drugs compromised maximal mitochondrial respiration and SRC in striatal (but not cortical) nerve terminals. Although the upstream mechanism(s) causing mitochondrial injury may not be the same, the downstream mitochondrial dysfunction identified here (i.e. impaired maximal mitochondrial respiration and SRC) represents a common pathway by which these ARV drugs may initiate neuronal dysfunction. Several of the ARV drugs tested here have been shown previously to dissipate the mitochondrial membrane potential, including efavirenz (Blas-Garcia et al, 2014; Funes et al, 2014; Jin et al, 2016; Purnell and Fox, 2014), emtricitabine (Groener et al, 2011), indinavir (Jiang et al, 2007), lopinavir (Groener et al, 2011), and zidovudine (Caron et al, 2004; Jiang et al, 2007; Nagiah et al, 2015). The mitochondrial membrane potential is a central regulator of cell health, it provides the charge gradient required for mitochondrial calcium sequestration and ATP generation, and also regulates ROS production (Perry et al, 2011). Mitochondrial ROS generation underlies oxidative damage in many diseases and several ARV drugs have been shown to increase ROS levels including efavirenz (Blas-Garcia et al, 2010; Imaizumi et al, 2015), abacavir (Wang et al, 2009a), zidovudine (Lagathu et al, 2007; Nagiah et al, 2015; Valenti et al, 2002; Wang et al, 2009a), indinavir (Lagathu et al, 2007; Wang et al, 2009a; Wang et al, 2009b), and lopinavir (Deng et al, 2010; Lagathu et al, 2007; Taura et al, 2013). Future studies will be required to investigate the upstream mechanism(s) underlying the ARV-induced SRC impairment and selective vulnerability of striatal nerve terminals.
Our results support the notion that alterations in mitochondrial function contribute to ARV neurotoxicity. The observation of impaired striatal nerve terminal SRC upon exposure to several different ARV drugs suggests that presynaptic mitochondrial dysfunction may underlie adverse CNS side effects seen in HIV patients undergoing combination ARV therapy.
Supplementary Material
Acknowledgments
Funding sources This work was supported by the National Institute of Health Grant Nos. P30 MH062261 and P01 DA028555.
Footnotes
Compliance with ethical standards All animal procedures were carried out under approved University of Nebraska Medical Center Institutional Animal Care and Use Committee protocols in AAALAC certified facilities following NIH guidelines.
Conflict of interest Kelly L. Stauch, Katy Emanuel, Benjamin G. Lamberty, Brenda Morsey, and Howard S. Fox report no conflict of interest.
References
- Anglin RE, Garside SL, Tarnopolsky MA, Mazurek MF, Rosebush PI. The psychiatric manifestations of mitochondrial disorders: a case and review of the literature. J Clin Psychiatry. 2012;73:506–12. doi: 10.4088/JCP.11r07237. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. 2015;70:2693–708. doi: 10.1093/jac/dkv183. [DOI] [PubMed] [Google Scholar]
- Blas-Garcia A, Apostolova N, Ballesteros D, Monleon D, Morales JM, Rocha M, Victor VM, Esplugues JV. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology. 2010;52:115–25. doi: 10.1002/hep.23647. [DOI] [PubMed] [Google Scholar]
- Blas-Garcia A, Marti-Rodrigo A, Victor VM, Polo M, Alegre F, Funes HA, Apostolova N, Esplugues JV. The purine analogues abacavir and didanosine increase acetaminophen-induced hepatotoxicity by enhancing mitochondrial dysfunction. J Antimicrob Chemother. 2016;71:916–26. doi: 10.1093/jac/dkv424. [DOI] [PubMed] [Google Scholar]
- Blas-Garcia A, Polo M, Alegre F, Funes HA, Martinez E, Apostolova N, Esplugues JV. Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: a comparison with efavirenz. J Antimicrob Chemother. 2014;69:2995–3000. doi: 10.1093/jac/dku262. [DOI] [PubMed] [Google Scholar]
- Brand MD. The contribution of the leak of protons across the mitochondrial inner membrane to standard metabolic rate. J Theor Biol. 1990;145:267–86. doi: 10.1016/s0022-5193(05)80131-6. [DOI] [PubMed] [Google Scholar]
- Brandmann M, Nehls U, Dringen R. 8-Hydroxy-efavirenz, the primary metabolite of the antiretroviral drug Efavirenz, stimulates the glycolytic flux in cultured rat astrocytes. Neurochem Res. 2013;38:2524–34. doi: 10.1007/s11064-013-1165-2. [DOI] [PubMed] [Google Scholar]
- Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, Winston A, van Sighem A, Miro JM, Podzamczer D, Olson A, Arribas JR, Moreno S, Meyer L, del Romero J, Dabis F, Bucher HC, Wandeler G, Vourli G, Skoutelis A, Lanoy E, Gasnault J, Costagliola D, Hernan MA, Collaboration H-C. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83:134–41. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M, Auclair M, Lagathu C, Lombes A, Walker UA, Kornprobst M, Capeau J. The HIV-1 nucleoside reverse transcriptase inhibitors stavudine and zidovudine alter adipocyte functions in vitro. AIDS. 2004;18:2127–36. doi: 10.1097/00002030-200411050-00004. [DOI] [PubMed] [Google Scholar]
- Choi DY, Lee MK, Hong JT. Lack of CCR5 modifies glial phenotypes and population of the nigral dopaminergic neurons, but not MPTP-induced dopaminergic neurodegeneration. Neurobiol Dis. 2013;49:159–68. doi: 10.1016/j.nbd.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Lee DW, Rajagopalan S, Nicholls DG, Andersen JK, Brand MD. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J Neurosci. 2011;31:4524–34. doi: 10.1523/JNEUROSCI.5817-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–91. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf. 2013;12:841–6. doi: 10.1517/14740338.2013.823396. [DOI] [PubMed] [Google Scholar]
- Deng W, Baki L, Yin J, Zhou H, Baumgarten CM. HIV protease inhibitors elicit volumesensitive Cl- current in cardiac myocytes via mitochondrial ROS. J Mol Cell Cardiol. 2010;49:746–52. doi: 10.1016/j.yjmcc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 2008;3:1718–28. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, Obermann M, Rosenkranz T, Schielke E, Straube E, German Association of Neuro AuN-I HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol. 2017 doi: 10.1007/s00415-017-8503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hoss S, Bahr GM, Echtay KS. Lopimune-induced mitochondrial toxicity is attenuated by increased uncoupling protein-2 level in treated mouse hepatocytes. Biochem J. 2015;468:401–7. doi: 10.1042/BJ20150195. [DOI] [PubMed] [Google Scholar]
- Fern R. Variations in spare electron transport chain capacity: The answer to an old riddle? J Neurosci Res. 2003;71:759–62. doi: 10.1002/jnr.10553. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Choi SW, Day NU, Gerencser AA, Hubbard A, Melov S. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free Radic Biol Med. 2011;50:866–73. doi: 10.1016/j.freeradbiomed.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes HA, Apostolova N, Alegre F, Blas-Garcia A, Alvarez A, Marti-Cabrera M, Esplugues JV. Neuronal bioenergetics and acute mitochondrial dysfunction: a clue to understanding the central nervous system side effects of efavirenz. J Infect Dis. 2014;210:1385–95. doi: 10.1093/infdis/jiu273. [DOI] [PubMed] [Google Scholar]
- Funes HA, Blas-Garcia A, Esplugues JV, Apostolova N. Efavirenz alters mitochondrial respiratory function in cultured neuron and glial cell lines. J Antimicrob Chemother. 2015;70:2249–54. doi: 10.1093/jac/dkv098. [DOI] [PubMed] [Google Scholar]
- Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016;30:591–600. doi: 10.1097/QAD.0000000000000951. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–78. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groener JB, Seybold U, Vollbrecht T, Bogner JR. Short communication: decrease in mitochondrial transmembrane potential in peripheral blood mononuclear cells of HIV-uninfected subjects undergoing HIV postexposure prophylaxis. AIDS Res Hum Retroviruses. 2011;27:969–72. doi: 10.1089/AID.2010.0348. [DOI] [PubMed] [Google Scholar]
- Harris M, Larsen G, Montaner JS. Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS. 2008;22:1890–2. doi: 10.1097/QAD.0b013e32830e0169. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert VY, Crenshaw BL, Romanoff RL, Ekshyyan VP, Dugas TR. Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovasc Toxicol. 2004;4:117–31. doi: 10.1385/ct:4:2:117. [DOI] [PubMed] [Google Scholar]
- Imaizumi N, Kwang Lee K, Zhang C, Boelsterli UA. Mechanisms of cell death pathway activation following drug-induced inhibition of mitochondrial complex I. Redox Biol. 2015;4:279–88. doi: 10.1016/j.redox.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J Biol Chem. 2004;279:32989–3000. doi: 10.1074/jbc.M401540200. [DOI] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol Appl Pharmacol. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Jin J, Grimmig B, Izzo J, Brown LA, Hudson C, Smith AJ, Tan J, Bickford PC, Giunta B. HIV non-nucleoside reverse transcriptase inhibitor efavirenz reduces neural stem cell proliferation in vitro and in vivo. Cell Transplant. 2016 doi: 10.3727/096368916X691457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagathu C, Eustace B, Prot M, Frantz D, Gu Y, Bastard JP, Maachi M, Azoulay S, Briggs M, Caron M, Capeau J. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- Lepeta K, Lourenco MV, Schweitzer BC, Martino Adami PV, Banerjee P, Catuara-Solarz S, de La Fuente Revenga M, Guillem AM, Haidar M, Ijomone OM, Nadorp B, Qi L, Perera ND, Refsgaard LK, Reid KM, Sabbar M, Sahoo A, Schaefer N, Sheean RK, Suska A, Verma R, Vicidomini C, Wright D, Zhang XD, Seidenbecher C. Synaptopathies: synaptic dysfunction in neurological disorders - A review from students to students. J Neurochem. 2016;138:785–805. doi: 10.1111/jnc.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19:137–42. [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, Group C Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, Grant I, Ellis RJ, Group H Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56:416–23. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, Morgello S, Simpson DM, Heaton RK, Grant I, Letendre SL, Group C Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. 2016;22:170–8. doi: 10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano JS. AZT causes tissue-specific inhibition of mitochondrial bioenergetic function. Biochem Biophys Res Commun. 1993;194:170–7. doi: 10.1006/bbrc.1993.1800. [DOI] [PubMed] [Google Scholar]
- Nagiah S, Phulukdaree A, Chuturgoon A. Mitochondrial and Oxidative Stress Response in HepG2 Cells Following Acute and Prolonged Exposure to Antiretroviral Drugs. J Cell Biochem. 2015;116:1939–46. doi: 10.1002/jcb.25149. [DOI] [PubMed] [Google Scholar]
- Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, Paul R, Zhang G, Ho E, Hanks N, Nakamoto B, Shiramizu BT, Shikuma CM. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND) J Neurovirol. 2014;20:571–82. doi: 10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–73. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Royland JE, MacPhail RC, Sullivan PG, Kodavanti PR. Age- and brain region-specific differences in mitochondrial bioenergetics in Brown Norway rats. Neurobiol Aging. 2016;42:25–34. doi: 10.1016/j.neurobiolaging.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Park MH, Lee YK, Lee YH, Kim YB, Yun YW, Nam SY, Hwang SJ, Han SB, Kim SU, Hong JT. Chemokines released from astrocytes promote chemokine receptor 5-mediated neuronal cell differentiation. Exp Cell Res. 2009;315:2715–26. doi: 10.1016/j.yexcr.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell PR, Fox HS. Efavirenz induces neuronal autophagy and mitochondrial alterations. J Pharmacol Exp Ther. 2014;351:250–8. doi: 10.1124/jpet.114.217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18:388–99. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salabei JK, Gibb AA, Hill BG. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc. 2014;9:421–38. doi: 10.1038/nprot.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AB, Varano GP, de Rozieres CM, Maung R, Catalan IC, Dowling CC, Sejbuk NE, Hoefer MM, Kaul M. Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob Agents Chemother. 2015;60:168–79. doi: 10.1128/AAC.01632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbeck A, Pandya J, Singh I, Bittman K, Readnower R, Bing G, Sullivan P. Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. J Neurosci Methods. 2011;198:36–43. doi: 10.1016/j.jneumeth.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12:309. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A. Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotox Res. 2016 doi: 10.1007/s12640-016-9646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Bonnefont J, Julien S, Marq-Lin N, Rodriguez I, Dubois-Dauphin M, Krause KH. Increased brain damage after ischaemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol. 2010;160:311–21. doi: 10.1111/j.1476-5381.2010.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura M, Kariya R, Kudo E, Goto H, Iwawaki T, Amano M, Suico MA, Kai H, Mitsuya H, Okada S. Comparative analysis of ER stress response into HIV protease inhibitors: lopinavir but not darunavir induces potent ER stress response via ROS/JNK pathway. Free Radic Biol Med. 2013;65:778–88. doi: 10.1016/j.freeradbiomed.2013.08.161. [DOI] [PubMed] [Google Scholar]
- Teppler H, Brown DD, Leavitt RY, Sklar P, Wan H, Xu X, Lievano F, Lehman HP, Mast TC, Nguyen BY. Long-term safety from the raltegravir clinical development program. Curr HIV Res. 2011;9:40–53. doi: 10.2174/157016211794582650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-y-Romo LB, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, McArthur JC, Haughey NJ. Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther. 2012;343:696–703. doi: 10.1124/jpet.112.195701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman GJ, Soudry O. Neuropsychiatric Effects of HIV Antiviral Medications. Drug Saf. 2016;39:945–57. doi: 10.1007/s40264-016-0440-y. [DOI] [PubMed] [Google Scholar]
- Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis. 2011;53:836–42. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti D, Atlante A, Barile M, Passarella S. Inhibition of phosphate transport in rat heart mitochondria by 3′-azido-3′-deoxythymidine due to stimulation of superoxide anion mitochondrial production. Biochem Pharmacol. 2002;64:201–6. doi: 10.1016/s0006-2952(02)01065-1. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10:207–14. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viengchareun S, Caron M, Auclair M, Kim MJ, Frachon P, Capeau J, Lombes M, Lombes A. Mitochondrial toxicity of indinavir, stavudine and zidovudine involves multiple cellular targets in white and brown adipocytes. Antivir Ther. 2007;12:919–29. [PubMed] [Google Scholar]
- Wang X, Chai H, Lin PH, Yao Q, Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009a;174:771–81. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liao D, Lin PH, Yao Q, Chen C. Highly active antiretroviral therapy drugs inhibit in vitro cholesterol efflux from human macrophage-derived foam cells. Lab Invest. 2009b;89:1355–63. doi: 10.1038/labinvest.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker VP. Thirty years of synaptosome research. J Neurocytol. 1993;22:735–42. doi: 10.1007/BF01181319. [DOI] [PubMed] [Google Scholar]
- Xu J, Chen Q, Zen K, Zhang C, Zhang Q. Synaptosomes secrete and uptake functionally active microRNAs via exocytosis and endocytosis pathways. J Neurochem. 2013;124:15–25. doi: 10.1111/jnc.12057. [DOI] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang NC, Ho WM, Chen YH, Hu ML. A convenient one-step extraction of cellular ATP using boiling water for the luciferin-luciferase assay of ATP. Anal Biochem. 2002;306:323–7. doi: 10.1006/abio.2002.5698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


