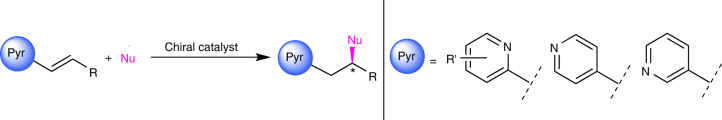
Abstract
General methods to prepare chiral pyridine derivatives are greatly sought after due to their significance in medicinal chemistry. Here, we report highly enantioselective catalytic transformations of poorly reactive β-substituted alkenyl pyridines to access a wide range of alkylated chiral pyridines. The simple methodology involves reactivity enhancement via Lewis acid (LA) activation, the use of readily available and highly reactive Grignard reagents, and a copper-chiral diphosphine ligand catalyst. Apart from allowing the introduction of different linear, branched, cyclic, and functionalised alkyl chains at the β-position of alkenyl pyridines, the catalytic system also shows high functional group tolerance.
Chiral pyridines are valuable building blocks in medicinal chemistry applications. Here, the authors report the copper-catalysed Lewis acid-assisted asymmetric alkylation of β-substituted alkenyl pyridines with Grignard reagents affording chiral pyridines with excellent enantioselectivity.
Introduction
Pyridines are among the most important classes of heterocyclic moieties, and occur in many bioactive molecules, such as natural products, pharmaceuticals, and agrochemicals1–4. Furthermore, pyridines are also useful structural motifs in various chemical transformations and they are widely employed as ligands5–10. Moreover, pyridine is the single most commonly found nitrogen-containing aromatic heterocycle among all U.S. FDA approved pharmaceuticals11, 12 and is present in more than 60 currently marketed drugs. As a result of their immense importance, methods to access different pyridine derivatives have been pursued for decades13. Although elaboration of the pyridine ring remains one of the most common and straightforward approaches, exploitation of the pyridine moiety to activate adjacent olefins towards enantioselective conjugate addition (CA) of nucleophiles has recently gained prominence (Fig. 1)14, 15.
Fig. 1.
Enantioselective nucleophilic addition to alkenyl pyridines
Highly enantioselective copper catalysed CA of hydride nucleophiles to β,β-disubstituted alkenyl pyridines was reported by Lam and co-workers16. However, when carbon nucleophiles are considered, non-enantioselective CAs of carbon nucleophiles to unsubstituted vinylpyridines are well known17, whereas examples of general methodologies for enantioselective additions to β-substituted alkenyl pyridines are rare18, 19. The diminished reactivity of these β-substituted alkenyl pyridines that lies at the heart of this deficiency is a persistent issue, because of the low activation of the conjugated alkene by the pyridine ring16. Consequently, only a few examples of enantioselective functionalisation of an alkenyl pyridine have been reported, and these all require the use of activated pyridines, higher temperatures, and a Rh-catalyst18, 19. Moreover, these methodologies focus on arylation and do not allow alkylation. As the poor electrophilicity of alkenyl pyridines is one of the major problems for nucleophilic additions, we anticipated that the use of highly reactive Grignard reagents might be helpful to overcome the low reactivity of these substrates. This idea was further supported by the nickel-catalysed CA of aryl Grignard reagents to alkenyl pyridine reported in 1998, providing good yields although nearly racemic products20.
Here we present a Cu-catalysed, highly enantio- and chemo-selective alkylation protocol of poorly reactive alkenyl pyridines using Lewis acid (LA) activation and Grignard reagents as nucleophiles. Various pyridine derivatives can undergo CA of a wide range of Grignard reagents, both linear and branched. The catalytic system shows a remarkable functional group tolerance, providing a handle for further product transformations. Finally, mechanistic studies that allow to disclose the reaction pathway are presented as well.
Results
Optimisation of reaction conditions
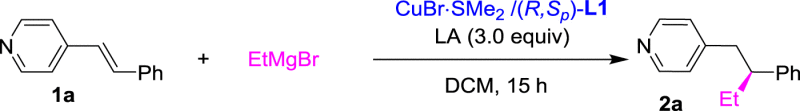
We started out our investigations by evaluating copper catalysed conjugate alkylation of alkenyl pyridines with Grignard reagents. 4-Styryl pyridine 1a and EtMgBr served as model reaction for the initial screening of suitable conditions (Table 1). Substrate 1a was tested in the presence of a catalytic system derived from CuBr·SMe2 salt and diphosphine ferrocenyl ligand L1 within the temperature range of −78 °C to ambient temperature. Analysis of the crude reaction mixture by 1H NMR revealed no conversion of the starting material, confirming the markedly lower reactivity of β-substituted alkenyl pyridines towards nucleophilic addition compared to vinylpyridines. Recently, our group developed a protocol that employs BF3⋅Et2O as a LA to activate poorly reactive heteroaromatic compounds towards nucleophilic addition of Grignard reagents21. We applied a similar protocol to 4-styryl pyridine 1a, but, once again, no product was detected (Table 1, entry 1). We envisioned that stronger activation of pyridine substrates could be achieved by using TMSOTf as LA to tackle the reactivity issue.
Table 1.
Optimisation of reaction conditions
| |||||
|---|---|---|---|---|---|
| Entry | Solvent | L, mol%a | Lewis acid, equiv.b | Conv. (%)c | ee (%)d |
| 1 | DCM | L1, 6 | BF3⋅Et2O, 1.1 | 0 | — |
| 2 | DCM | L1, 6 | B[PhF5]3, 1.1 | 0 | — |
| 3 | DCM | L1, 6 | TMSOTf, 1.5 | 89 | 89 |
| 4 | DCM | L1, 6 | TMSOTf, 2.0 | 85 | 85 |
| 5 | DCM | L1, 6 | TMSOTf, 3.0 | 91 | 89 |
| 6 | DCM | L1, 12 | TMSOTf, 1.5 | 91 | 92 |
| 7 | DCM | L1, 12 | TMSOTf, 2.0 | 95 | 93 |
| 8 | DCM | L1, 12 | TMSOTf, 3.0 | 100 | 93 |
| 9 | Et2O | L1, 12 | TMSOTf, 3.0 | 62 | 95 |
| 10 | MTBE | L1, 12 | TMSOTf, 3.0 | 67 | 96 |
| 11 | Toluene | L1, 12 | TMSOTf, 3.0 | 79 | 87 |
| 12 | DCM | L2, 12 | TMSOTf, 3.0 | 76 | 45 |
| 13 | DCM | L3, 12 | TMSOTf, 3.0 | 93 | 81 |
| 14 | DCM | L4, 12 | TMSOTf, 3.0 | 95 | 90 |
a6 or 12 mol% of L was used in combination with 5 or 10 mol% of CuBr·SMe2 respectively
bEquivalents of EtMgBr used correspond to that of the Lewis acid
cDetermined by 1H NMR spectroscopy
dDetermined by chiral HPLC
Indeed, screening of various LAs revealed that stronger activation of alkenyl pyridine does allow Grignard addition (Table 1, entries 1–5), with TMSOTf the optimal choice, providing alkylated pyridine product 2a with 89% conversion and 89% ee (Table 1, entry 3). Importantly, TMSOTf can promote the addition reaction in the absence of the copper catalyst as well. To reach full conversion in the catalytic reaction and to minimise the effect of the non-catalytic TMSOTf-promoted pathway on the final enantioselectivity of the reaction, we studied the effect of the stoichiometry of the copper catalyst, TMSOTf and Grignard reagents (Table 1, entries 4–8). Although the enantioselectivity remained largely unchanged, full conversion was reached only when 3 equiv. of EtMgBr and TMSOTf was used (Table 1, entry 5). On the other hand, carrying out the reaction with a two-fold increase in the Cu-catalyst loading, resulted in an increase of the enantioselectivity to 93% (Table 1, entries 6–8).
Having established the optimal LA and catalyst loading for the reaction, we investigated the effect of different solvents next. All solvents tested (DCM, Et2O, MTBE, and toluene) were well tolerated by the alkylation protocol, providing product 2a with excellent ee’s (Table 1, entry 8 vs entries 9–11). On the other hand, full conversion towards the addition product was reached only in DCM, which prompted us to carry out chiral ligand optimisation in this solvent.
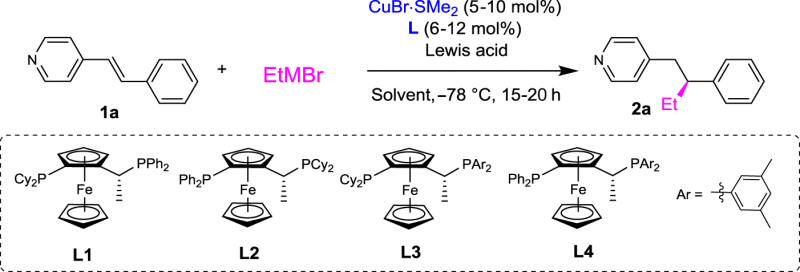
Among the different chiral ligands studied (for detailed ligand screening, see Supplementary Table 1) all the diphosphine ferrocenyl ligands (L1–L4) were able to promote the reaction to a different extent. Ligands L1, L3 and L4 showed superior reactivity, delivering the desired product in excellent conversion and ee (Table 1, entries 8, 13–14). As ligand L1 performed slightly better than L3 and L4, we adopted the following optimised conditions for further substrate scope study: CuBr·SMe2 (10 mol%), (R,S p)-L1 (12 mol%), Grignard reagent (3 equiv.), TMSOTf (3 equiv.), in DCM solvent for 16 h at −78 °C.
Pyridine substrate scope
For studying the substrate scope we chose the reaction between 4-alkenyl pyridines (1a–1d) and 2-alkenyl pyridines (3a–3g) with EtMgBr (Table 2). 4-Alkenyl pyridines 1a–1d readily underwent the nucleophilic addition reaction, irrespective of the β-substituent on the double bond (Table 2, entries 1–4). Alkyl and aryl substituents, both electron rich and electron poor, at the β-position were well tolerated, affording the alkylated products 2a–2d in excellent isolated yields and enantiopurities (Table 2, entries 1–4). When 3-octenyl pyridine, 2-styryl pyridine or 2-octenyl pyridine were subjected to our optimised reaction conditions, no conversion towards the addition product was noted (Table 2 scheme). Although the poor reactivity of 3-octenyl pyridine can be attributed to the lack of resonance activation of the adjacent olefin moiety upon coordination of the substrate to the LA, the lack of reactivity of the latter two is more surprising. Interestingly, introducing a tert-butyldimethylsilyl ether (OTBDS) group at the δ-position enhanced the reactivity sufficiently, thus providing corresponding product 4a with high yield and enantioselectivity (Table 2, entry 5).
Table 2.
Substrate scope
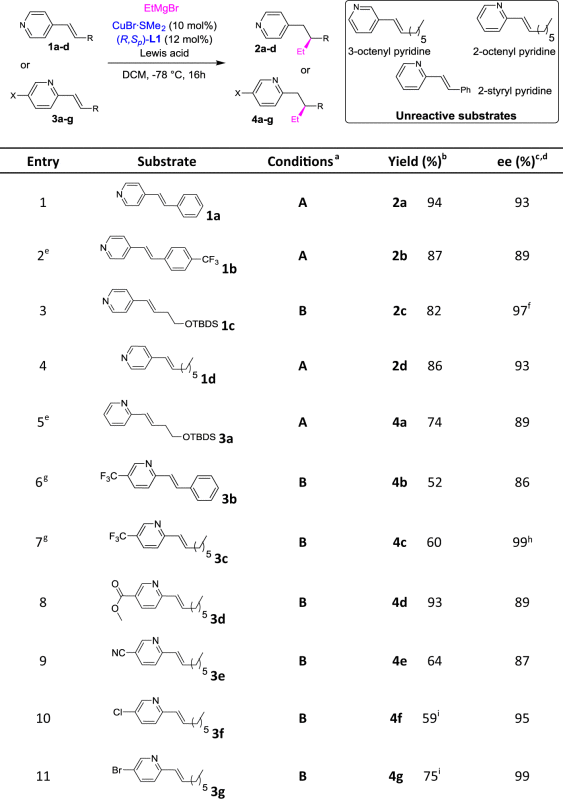
|
aConditions A: TMSOTf (3 equiv.) and EtMgBr (3 equiv.); conditions B: BF3⋅Et2O (1.5 equiv) and EtMgBr (1.5 equiv.)
bReported yields are for isolated products
cDetermined by chiral HPLC
dThe absolute configurations of products were determined on the basis of single-crystal X-ray diffraction analysis of compound 2a′ (see Supplementary Information)
eEtMgBr diluted in toluene and added dropwise over 2 h
fee of corresponding alcohol 2c' after deprotection
g5 mol% of catalyst used
hee of the corresponding ether 10
iSmall amount of unreacted substrate (≤9%) is not separable from product by chromatography, yields are calculated by purity of product in the mixed fraction by 1H NMR
As expected, alkenyl pyridines with an electron-withdrawing group in the aromatic ring (Tables 2, 3b and 3c) required milder activation. In this case, the use of 1.5 equiv. of BF3⋅OEt2 instead of 3.0 equiv. of TMSOTf provided the corresponding products 4b and 4c with good yields and excellent enantioselectivities (Table 2, entries 6, 7).
We were particularly interested in performing these reactions with functionalised pyridines, which can provide a handle for further modification after Grignard addition. For this purpose, several alkenyl pyridine substrates with various functional groups were prepared (Tables 2, 3d–3g) and subjected to the reaction protocol using BF3⋅OEt2.
Grignard reagents are often considered incompatible with reactive functional groups. Nevertheless, the excellent chemoselectivity of our protocol allowed us to perform addition reactions not only to alkenyl pyridines that contain halogens (Tables 2, 3f, 3g), but also to those containing highly reactive ester and cyano groups (Tables 2, 3d, 3e) in the aromatic ring. The corresponding alkylated products 4d–4g were obtained with good to excellent yields and ee’s (Table 2, entries 8–11).
Grignard reagent scope
To assess the nucleophile scope, substrates 1a and 1d were chosen as model compounds (Table 3). Addition of all tested linear Grignard reagents afforded the products 2a, 5a and 5b with good to excellent yields (75–94%) and ee’s (93–95%), independent of the chain length (Table 3, entries 1–3). The sterically demanding α-, β-, and γ-branched Grignard reagents were also tolerated, providing products 5c–5e with moderate to good yields (54–65%) (Table 3, entries 4–6). Particularly the γ- and α-branched Grignard reagents delivered products 5c and 5e with high ee’s of 97 and 89%, respectively (Table 3, entries 4 and 6), whereas the β-branched Grignard provided product 5d with moderate ee (64%; Table 3, entry 5). Grignard reagents bearing olefinic or aromatic substituents were also well tolerated, affording the corresponding products 5 f, 5 g and 5 h with good yields and excellent ee’s (Table 3, entries 7–9).
Table 3.
Grignard scope
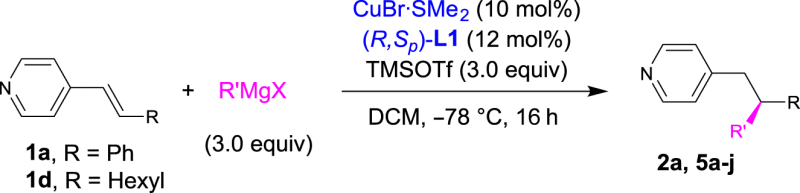
| |||||
|---|---|---|---|---|---|
| Entry | R′MgX | R | Product | Yield (%)a | ee (%)b |
| 1 | EtMgBr | Ph | 2a | 94 | 93 |
| 2 | PrMgCl | Ph | 5a | 75 | 93 |
| 3 | HexMgBr | Ph | 5b | 81 | 95 |
| 4 | i-PentMgBr | Ph | 5c | 65 | 97 |
| 5 | i-BuMgBr | Hexyl | 5d | 56 | 64 |
| 6c | c-PentMgBr | Hexyl | 5e | 54 | 89 |
| 7 | CH2=CH(CH2)2MgBr | Hexyl | 5f | 91 | 93 |
| 8 | CH2=CH(CH2)3MgBr | Ph | 5g | 66 | 90 |
| 9 | Ph(CH2)2MgBr | Hexyl | 5 h | 89 | 97 |
| 10d | MeMgBr | Hexyl | 5i | 50 | 93 |
| 11e | PhMgBr | Hexyl | 5j | 84 | 0 |
aReported yields are for isolated products
bDetermined by chiral HPLC
c4. equiv. of Grignard was used
dReaction was carried out at 0 °C for 5 h
eLigand L10 was used (see Supplementary Table 1 for L10 structure)
Next, we investigated the reaction between 4-alkenyl pyridine and MeMgBr, the least reactive Grignard reagent, but the most interesting for pharmaceutical applications.
We were pleased to find that, thanks to the substantial activation provided by TMSOTf, the reaction proceeded with 87% conversion within 5 h at 0 °C. The product was isolated with excellent ee (93%) and a moderate yield of 50% (Table 3, entry 10).
On the other hand, addition of PhMgBr resulted in a complex reaction mixture. Changing from ligand L1 to L10 allowed to obtain the addition product 5j with good yield, but unfortunately as a racemate (Table 3, entry 11). This result is in striking contrast with the 89% enantioselectivity obtained in our previous work for the addition of PhMgBr to alkenyl benzoxazole21 and could be attributed to an inability of the Cu-catalysed addition of PhMgBr to outcompete the LA promoted non-catalysed addition.
Practical aspects and products functionalisation
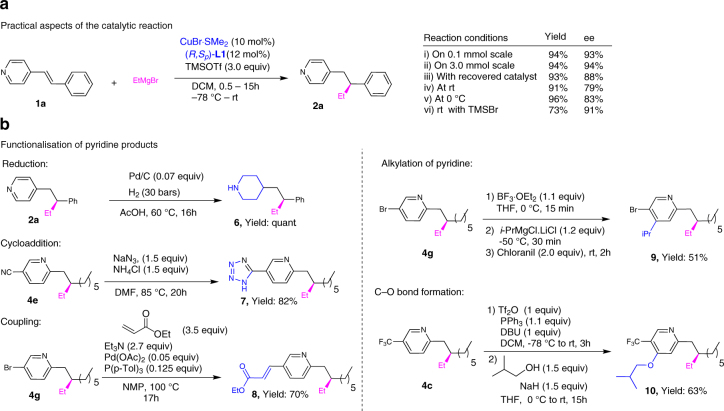
To evaluate the robustness of our methodology, a series of experiments was carried out for the addition of EtMgBr to 1a (Fig. 2a). Performing the reaction on a 30-fold larger scale (3 mmol vs 0.1 mmol) did not affect the outcome of the reaction and product 2a was isolated with the same excellent yield (94%) and ee (94%) as for the 0.1 mmol reaction. Remarkably, the catalyst recovered (as a copper complex) from this reaction could be recycled with only minor deviations from the original results. Moreover, to assess the effect of temperature on the reaction and on the compatibility of the LA and the Grignard, the reaction was carried out at room temperature. To our surprise, the reaction proceeded to completion in 35 min and the product 2a was isolated with 91% yield, although a significant drop in ee from 94 to 79% was noted. Assuming that the drop in enantioselectivity is due to the competing non-catalysed blank reaction, we performed the reaction at 0 °C, which improved the ee to 83%. Finally, we found that TMSBr is also an effective LA for this transformation, allowing the reaction to be carried out at room temperature and affording the final product with 73% yield and 91% ee (Fig. 2a).
Fig. 2.
Practical aspects of CA to alkenyl pyridines and functionalisation of chiral pyridine products. a Scaling up, raising the reaction temperature, as well as use of recovered Cu-catalyst, are well tolerated by the present catalytic system. b The presence of a reactive substituent on the pyridine ring allowed the addition products to be further functionalised
Owing to the high functional group tolerance of our catalytic system, we were able to perform further synthetic elaborations of our chiral pyridine products (Fig. 2b). Piperidine derivative 6 was prepared quantitatively by selectively reducing the pyridine ring in 2a in the presence of Pd catalyst and molecular hydrogen. The tetrazole ring is another heterocycle often encountered in the structures of approved pharmaceuticals11. To access tetrazole derivative 7 in good yield, we functionalised the cyano-substituted chiral pyridine 4e using [3 + 2] cycloaddition with NaN3. Product 4 g, which has a bromo substituent on the pyridine ring, can be employed in several transformations.
Upon treatment of 4 g with ethyl acrylate in the presence of Pd catalyst, the corresponding Heck coupling product 8 was obtained with good yield. Moreover, using 4 g it is possible to achieve direct functionalisation of the pyridine ring (product 9) using the protocol developed by Knochel and co-workers22. Another direct functionalisation of the pyridine ring of 4c, leading to the formation of pyridyl-ether 10 through pyridyl-phosphonium salt, could be achieved using a very recent methodology developed by McNally and co-workers23.
Mechanistic studies
The mechanism of this LA promoted Cu-catalysed addition of Grignard reagents to alkenyl pyridines might follow the same pathway as has been proposed for non-catalytic CA of organocuprates to α,β-unsaturated carbonyl compounds24–26. That implies formation of a reversible copper-alkene π-complex, followed by oxidative addition to form a Cu(III)-species, and reductive elimination to form the addition product enolate. Our catalytic system employs reagents and reactions conditions similar to those used for CAs to carbonyl based Michael acceptors27, 28, but contrary to these, alkenyl pyridines are unreactive towards organometallics in the absence of LA, even at non-cryogenic conditions. Furthermore, if the addition to alkenyl pyridines follows the same pathway, the aromaticity of the pyridines will be altered in several intermediate species. The necessity of LA to accomplish enantioselective CA to alkenyl pyridines adds another level of mechanistic complexity. To gain insight into the reaction mechanism, a series of experiments and NMR spectroscopic studies were carried out.
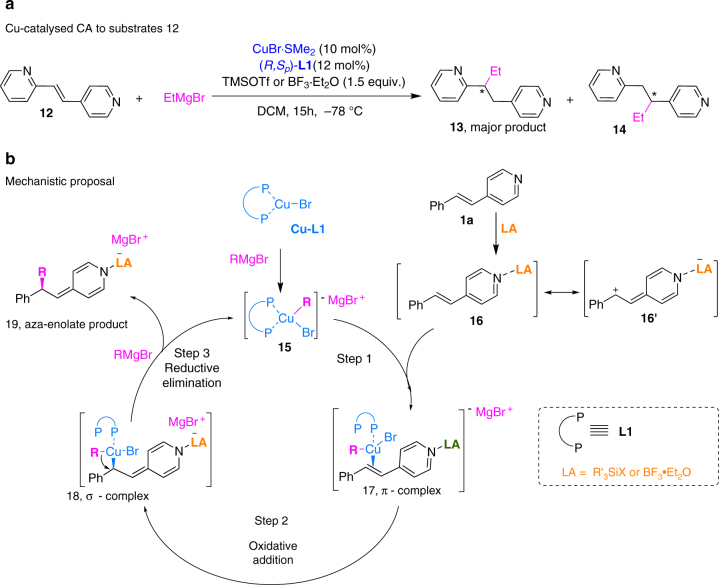
The experimental data discussed so far demonstrate that TMSOTf is the best LA for 4-substituted alkenyl pyridines, whereas BF3·Et2O is the most suitable LA for 2-substituted alkenyl pyridines. In addition, we found that no reaction occurs with non-activated 2-substituted alkenyl pyridines in the presence of either LA. Only after introducing an electron-withdrawing group (EWG) in the pyridine ring, CA occurs in the presence of BF3·Et2O (Table 2, entries 6–11). In contrast, using TMSOTf as LA, full conversion to side products (predominantly derived from the attack to the pyridine ring) occurs. On the other hand, non-activated 4-substituted alkenyl pyridine can only be converted to the CA product using TMSOTf (Table 2, entries 1, 2 and 4). This trend was further supported by the results obtained upon subjecting substrate 12, which contains both a 4- and a 2-substituted pyridine ring, to the CA reaction (Fig. 3a). In the presence of TMSOTf the major product (13) corresponds to CA with respect to the 4-pyridine site, whereas in the presence of BF3·Et2O the products resulting from CA with respect to both the 4- and 2-pyridine sites (13 and 14, respectively) were observed in a 10:1 ratio. These results clearly indicate that 4-substituted pyridine is intrinsically more reactive towards CA than its 2-substituted analogue.
Fig. 3.
Cu-catalysed CA to substrates 12 and proposed catalytic cycle. a These experiments clarify the difference in reactivity between 4-substituted and 2-substituted pyridines. b Proposed tentative catalytic cycle for LA promoted Cu-catalysed CA of Grignard reagents to alkenyl pyridines 1a
Further confirmation of the reactivity of pyridine substrates and the effect of LAs was obtained from 1H NMR spectroscopic studies (for details see Supplementary Methods). We investigated the interaction between various reagents (LAs and EtMgBr) and alkenyl pyridine substrates 1a and 3b at −60 °C (Supplementary Figs. 98 and 99). In all the mixed samples the signals corresponding to the pyridine are shifted with respect to the pure substrate sample. On the basis of the shifts of olefinic protons for substrate 1a upon mixing with either TMSOTf, BF3·Et2O, TMSCl or EtMgBr (1–2 equiv.) we can rank these reagents in terms of their activating Lewis acidic strength as: . Similar trends were observed for substrate 3b.
To study the effect of the strength and bulkiness of LAs on the CA of EtMgBr to substrate 1a, two sets of experiments were carried out, either in the presence or the absence of the chiral Cu-catalyst (Table 4). As disclosed above, in either case no conversion occurs when using BF3·Et2O as LA, whereas full conversion is observed for TMSOTf (Table 4, compare entries 1–4). The enantioselectivity of 93% obtained with TMSOTf is particularly remarkable, as the LA promoted background reaction in the absence of a copper catalyst is complete at −78 oC in 4 h. Varying the counterion of the silicon based LA allowed a closer look at the LA strength and confirmed that with the very weak TMSCl and somewhat stronger TMSBr no conversion was observed after 15 h without catalyst (Table 4, entries 5 and 6). Remarkably though, in the presence of the Cu-catalyst full conversion to the CA product was observed with TMSBr (Table 4, entry 8) and 60% conversion with TMSCl (Table 4, entry 7) and in both cases only one enantiomer was found. These results were unexpected, especially for the rather weak TMSCl.
Table 4.
Effect of different LAs in Cu(I)-catalysed addition of EtMgBr to 1a
| ||||||
|---|---|---|---|---|---|---|
| Entry | LA | T (°C) | Cu/L1 (%) | Conv. (%)a | Yield (%)b | ee (%)c |
| 1 | BF3·OEt2 | −78 | 10 | 0 | NA | NA |
| 2 | BF3·OEt2 | −78 | — | 0 | NA | NA |
| 3 | TMSOTf | −78 | 10 | 100 | 94 | 93 |
| 4 | TMSOTf | −78 | — | 100 (in 4 h) | ND | Rac |
| 5 | TMSCl | −78 | — | 0 | NA | NA |
| 6 | TMSBr | −78 | — | 0 | NA | NA |
| 7 | TMSCl | −78 | 10 | 60 | 47 | >99.9 |
| 8 | TMSBr | −78 | 10 | 100 | 73 | >99.9 |
| 9 | TESOTf | −78 | 10 | 95 | 85 | 88 |
| 10 | TBSOTf | −78 | 10 | 87 | 75 | 77 |
| 11 | TBDPSOTf | −78 | 10 | 96 | 47 | 62 |
| 12 | TBDPSOTf | −78 | — | 40 | ND | Rac |
| 13 | TBSCl, TBDPSCl, TBDMSCl | (−78)–0 | 10–25 | 0 | NA | NA |
| 14 | TESCl | 0 | 10 | 40 | ND | 82 |
| 15 | TESCl | 0 | — | 0 | NA | NA |
| 16 | TMSCl | 0 | 10 | 91 | ND | 91 |
aConversion was determined by 1H NMR
bIsolated yields
cDetermined by chiral HPLC
The effect of the bulkiness of the LA was investigated by testing a number of different steric variations of silyl triflates (Table 4, entries 9–11). To our surprise, an increase of steric bulk at the silicon atom resulted in decreased enantioselectivity: when moving from TMS- to triethylsilyl (TES-), tert-butyldimethylsilyl (TBS-) and finally to tert-butyldiphenylsilyl (TBDPS) substituted triflates, the ee dropped from 93 to 62% (Table 4, entries 3, 9–11). However, from these experiments it is not clear whether these differences in enantioselectivity are caused by steric bulk alone. The 40% conversion observed with TBDPSOTf without catalyst (Table 4, entry 12) shows that the background reaction is relevant, and thus that differences in the relative rates of the racemic non-catalysed with respect to the enantioselective Cu-catalysed CA reactions might also affect the enantioselectivity. Interestingly, in contrast to the result with TMSCl, carrying out the Cu-catalysed reactions using TBS-, TBDMS- and TBDPS-chlorides at both −78 oC and 0 oC did not give any substrate conversion (Table 4, entry 13). However, after screening of various LAs and conditions we found a set of experiments that settles this matter (Table 4, entries 14–16). At 0 oC the catalysed reaction with TMSCl gives an ee of 91% (Table 4, entry 16), whereas the same reaction with TESCl yields an ee of 82% (Table 4, entry 14). Importantly, without the catalyst no conversion is observed with TESCl, which unambiguously shows that the decreased enantioselectivity is not due to a background reaction and thus must be due to the larger bulk of the LA. Taken together, these results prove that the bulkiness of the LA affects both the enantioselectivity and the reactivity adversely and thus imply that the LAs are involved in both the stereo- and rate-determining step.
The geometry of alkenes is another important parameter, both for their reactivity and selectivity in addition reactions. Furthermore, when starting from less stable (Z)-alkenes, isomerisation to the (E)-stereoisomer might occur during the course of the reaction, thus affecting the overall reaction outcome while providing useful hints to understand the mechanism of the reaction27. Therefore, the influence of the alkene ((Z)/(E)) geometry on the enantioselective CA of EtMgBr to 1a and 3b in the presence of TMSOTf and BF3·Et2O, respectively, was studied (Supplementary Table 2). When subjecting (Z)-1a and (Z)-3b to the optimised reaction conditions, the enantioselectivities obtained in both reactions were lower than with (E)-1a and (E)-3b. Although the reduction in ee in the catalytic reaction could be related to the partial isomerisation of the corresponding (Z)-substrates’ double bond during the reaction, subsequent additional control experiments proved that the decreased enantioselectivity is intrinsic to the (Z)-geometry of the substrate and not due to (Z)–(E) isomerisation. This is at odds with our previous findings for Cu-catalysed CA to other N-containing alkenyl heteroarenes21, where we clearly observed isomerisation of alkenyl benzothiazole caused by all reaction components in combination, thus supporting CuI/CuIII redox chemistry with reversible formation of π- to σ-Cu-complexes responsible for substrate isomerisation21. The lack of Cu-mediated isomerisation in the present case does not rule out this mechanism, but might be suggestive of other possibilities.
Finally, we wanted to elucidate the structure of the initial product of these CA reactions before the reaction mixture is quenched. In particular, the question is whether a non-aromatic enol-like structure is formed and if so, whether it is a Mg-enolate or a LA-derived enolate (silyl enol ether for TMSOTf or boron enolate for BF3·OEt2). In the case of Cu-catalysed CA of EtMgBr to substrate 1a in the presence of TMSOTf, we only observed the formation of silyl-enolate (Supplementary Fig. 104). In the case of CA to 2-alkenyl pyridine substrate 3b using BF3·OEt2, our efforts to characterise the product structure before quenching of the reaction were hampered by severely broad signals observed in 1H NMR.
The information gathered during these investigations allows several inferences to be made regarding the mechanism of our catalytic system as well as a rationalisation of its behaviour.
The presence of LA is a necessary condition to accomplish enantioselective CA to alkenyl pyridines. The fact that LA promotes not only Cu-catalysed, but in several instances also non-catalytic background addition, makes it difficult to elucidate its role precisely. LA additives have been known to accelerate CA of organocuprates to various α,β-unsaturated carbonyl derivatives24. However, strong LAs, such as BF3·OEt2, were only used in combination with stoichiometric amounts of organocopper reagent (Yamamoto reagent) to avoid a compatibility problem commonly encountered between Grignard reagents and LAs24, 29–31. In contrast, for Cu-catalysis (as well as stoichiometric reactions) the use of TMSCl, which is more a silylating reagent than a LA, became common practice in CA24, 32–37. In our case, we use catalytic amounts of chiral copper complex in combination with strong LAs, which do not only activate the pyridine substrate but also interfere with the CA by reacting with the Grignard reagent or decomposing the chiral Cu-complex catalyst. As a result, the outcome of the reaction depends critically on the relative rates of the desired catalysed vs. the undesired background pathway, as well as on these competing reactions.
The proposed reaction pathway, based on experimental and spectroscopic data and taking into account the previous proposals on Cu-catalysed CA, is presented in Fig. 3. We expect that the first step in the catalytic cycle is initiated by the formation of the catalytically active complex 15 38, formed from precatalyst Cu-L1 upon transmetallation with Grignard reagent in a similar manner as has been proposed for Cu-catalysed CA to carbonyl based Michael acceptors employing similar solvent and diphosphine ligand27, 28. The first intermediate in the proposed cycle (17) is formed via π-complexation between activated alkenyl pyridine 16 and transmetallated copper complex 15. This is then followed by the formation of a σ-complex intermediate, 18. We anticipated the activated alkenyl pyridine to be an LA-pyridine complex, the formation of which was indeed observed by 1H NMR spectroscopy when using either BF3·OEt2, TMSOTf or TMSCl (Supplementary Figs. 98 and 99). Interestingly, although theoretically TMSCl is the weakest silicon based LA, especially in comparison with BF3·OEt2, it nevertheless is able to catalyse the reaction with non-activated substrate 1a, in contrast to BF3·OEt2 (Table 4, compare entries 1 and 7). This finding indicates that the role of the LA is not limited to the initial Lewis acidic substrate activation. Rather, it is also involved in the acceleration of the reaction via silylation of the reaction intermediates, leading to the formation of the more stable, non-aromatic intermediate 18 and the silyl aza-enolate 19 (Fig. 3). The structure of 19 was confirmed by NMR spectroscopic experiments (Supplementary Fig. 104).
Another aspect of the critical function of LA in the catalytic cycle is evident from the observation that its bulkiness has an important role in defining the enantioselectivity (Table 4, entries 9–16). The decrease in enantioselectivity from 91 to 82% when moving from TMSCl to TESCl can only be attributed to the bulkiness of the LA and not to its capability of promoting non-catalysed CA, as there is no background TESCl promoted reaction in the absence of a Cu catalyst (Table 4, entries 4–16). The origin of this effect in our system is unclear, as well as which are the rate- and enantio-determining steps.
The mechanistic pathway for Cu-catalysed CA, as well the origin of the accelerations observed for reactions with TMSCl, have been the subject of considerable debate. However, so far the mechanism has been studied only for non-catalytic reactions with organocopper reagents. The current view is that the oxidative addition (π- to σ-complex) is reversible and the following reductive elimination is the rate-limiting step. This is supported by (Z)–(E) isomerisations observed in CA of organocopper reagents using (Z)-enones and (Z)-enoates, as well as by kinetic isotope effect studies. With respect to the role of TMSCl several hypotheses were raised, namely silylation of π-complex to form silyl enol ether of σ-complex, by Corey32, 33, LA activation of the enone substrate, by Kuwajima35, and stabilisation of the σ-complex by the chloride of TMSCl, by Snyder and Bertz36. Kinetic isotope experiments by Singleton and co-workers37 resolved this question for the systems studied, with the data consistent with rate-limiting silylation of an intermediate π-complex. This also explains the lack of (Z)–(E)-isomerisations observed in the presence of TMSCl, which is then due to the rate-limiting step occurring earlier than in the system without TMSCl.
Our experiments differ from these mechanistic studies in two aspects, namely the fact that we use a catalytic system and a more Lewis basic substrate. In this case any LA we use is involved in the catalytic cycle from the start of the reaction, through complexation with the pyridine substrate (Supplementary Figs. 98 and 99). In principle steps 1–3 (Fig. 3) all lead to the formation of a chiral intermediate, and the fact that the bulkiness of the LA influences the ee indicates its involvement in the stereo-determining step. Similarly, any of these steps can be rate-determining, and the lack of isomerisation caused by LA/Cu/RMgBr does not distinguish between the steps. On the other hand, if the reductive elimination (Fig. 3, step 3) is the rate-limiting step, acceleration by the LA could prevent accumulation of σ-complex 18, thus quickly converting it to the final aza-enolate product 19 and preventing any Cu-mediated isomerisation to occur. Although steps 1 and 2 cannot be excluded, we currently believe that the reductive elimination is the rate- and stereo-determining step, facilitated by the presence of LA in the σ-complex 18 and leading to the formation of a stable aza-enolate 19. It is likely that the bulkiness of the LA affects the stability of non-aromatic intermediates 18 and product enolate 19 with the smallest LA being the most stable and in this manner contributes in both the reactivity and enantioselection. If the reductive elimination is the rate-limiting step, the rate of the reaction should also be dependent on the rate of re-forming the active catalyst 15. If this process occurs simultaneously with the reductive elimination process it will also be affected by the bulkiness of the LA in the intermediate 18 with the smallest LA allowing the fastest reaction.
Discussion
We have demonstrated that less reactive alkenyl pyridines can be used as Michael acceptors for enantioselective nucleophilic addition of Grignard reagents. The process exhibits a high functional group tolerance, a broad substrate scope including 4-alkenyl and 2-alkenyl pyridines with various substituents both on the aromatic ring and the β-position, as well as a broad Grignard scope including linear, branched, and functionalised examples. Importantly, enantioselective methylation of conjugated alkenyl pyridines is possible by using the least reactive Grignard reagent (MeMgBr), which is generally considered very difficult. Finally, these reactions can be carried out in the most common solvents and on comparatively large scales, while cryogenic conditions can be avoided. Several of the obtained chiral pyridine products could be transformed straightforwardly into diverse products, due to the remarkable functional group tolerance exhibited by this catalytic system.
Using NMR-guided spectroscopic studies, as well as variation of reaction parameters, we were able to identify that Cu-catalysed addition to alkenyl pyridines proceeds through a CA mechanism leading to aza-enolate products. We established that LA is involved in the catalytic cycle from the start of the reaction, and that its strength and bulkiness affect both the reactivity and the enantioselectivity of the CA, respectively. Furthermore, studies on the olefin geometry showed that Cu-induced (Z)–(E)-isomerisation does not occur in this catalytic system.
All together, these results prove that the reaction follows the common pathway of Cu-catalysed CA of organometallics and that the bulkiness of LAs affects both the enantioselectivity and the reactivity adversely, thus implying that the LAs are involved in both the stereo- and rate-determining step of the reaction.
Methods
General procedure A addition to 4-alkenyl pyridines
In a heat dried Schlenk tube equipped with septum and magnetic stirring bar, CuBr·SMe2 (0.10 equiv), and ligand (R,S p)-L1 (0.12 equiv) were dissolved in CH2Cl2 (1 mL/0.1 mmol of substrate) and stirred under nitrogen atmosphere for 15 min. The substrate (1.0 equiv) was added at once. After stirring for 5 min. at RT the reaction mixture was cooled to −78 °C and TMSOTf or BF3·OEt2 (1.5–3.0 equiv, see Supplementary Note 1) was added followed by RMgX (3.0 equiv). After stirring at −78 °C for 16 h, the reaction was quenched with MeOH (1 mL) followed by saturated aqueous solution of NH4Cl and warmed to RT. Reaction mixture was extracted with CH2Cl2 (3 × 10 mL). Combined organic phases were dried over MgSO4, filtered and solvents were evaporated on rotary evaporator. The oily crude was purified by flash chromatography on silica using mixture of pentane and EtOAc as eluent.
General procedure B for the addition to 2-alkenyl pyridines
In a heat dried Schlenk tube equipped with septum and magnetic stirring bar, CuBr·SMe2 (0.10 equiv), and ligand (R,S p)-L1 (0.12 equiv) were dissolved in Et2O or CH2Cl2 (1 mL/0.1 mmol of substrate) and stirred under nitrogen atmosphere for 15 min. The substrate (1.0 equiv) was added at once. After stirring for 5 min. at RT the reaction mixture was cooled to −78 °C and BF3·OEt2 (1.5 equiv) was added followed by RMgX (1.5 equiv.). After stirring at −78 °C for 16 h, the reaction was quenched with MeOH (1 mL) followed by saturated aqueous solution of NH4Cl and warmed to RT. Reaction mixture was extracted with CH2Cl2 (3 × 10 mL). Combined organic phases were dried over MgSO4, filtered and solvents were evaporated on rotary evaporator. The oily crude was purified by flash chromatography on silica using mixture of pentane and EtOAc as eluent.
General procedure C for the synthesis of racemic products
In a heat dried Schlenk tube equipped with septum and magnetic stirring bar, CuBr·SMe2 (0.10 equiv), and (±)-BINAP (0.12 equiv) were dissolved in CH2Cl2 (1 mL/0.1 mmol of substrate) and stirred under nitrogen atmosphere for 15 min. The substrate (1.0 equiv) was added at once. After stirring for 5 min. at RT the reaction mixture was cooled to −78 °C and TMSOTf (3.0 equiv) or BF3·OEt2 (1.5 equiv) was added followed by RMgX (1.5–3.0 equiv). After stirring at −78 °C for 16 h, the reaction was quenched with MeOH (1 mL) followed by saturated aqueous solution of NH4Cl and warmed to RT. Reaction mixture was extracted with CH2Cl2 (3 × 10 mL). Combined organic phases were dried over MgSO4, filtered and solvents were evaporated on rotary evaporator. The oily crude was purified by flash chromatography on silica using mixture of pentane and EtOAc as eluent. Unless otherwise noted all products were isolated as pale-yellow oil; for liquid/oily substrates, CuBr·SMe2 and ligand (R,S p)-L1 were dissolved in 0.7 mL of Et2O/CH2Cl2, whereas the remaining 0.3 mL of Et2O/CH2Cl2 was employed to transfer the substrate in the reaction Schlenk tube.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures, additional mechanistic studies, spectroscopic and physical data of compounds, see Supplementary Methods. For NMR and HPLC analysis of the compounds in this article, see Supplementary Figs. 1–156. For ligands screening and isomerisation studies see Supplementary Tables 1 and 2 respectively. The CCDC 1532252 (2a′ prepared from 2a, see Supplementary Note 2) contains the supplementary crystallographic data for this paper (Supplementary file). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
Financial support from NWO (Vidi and ECHO to S.R.H.) and the Ministry of Education, Culture and Science (Gravity programme 024.001.035 to S.R.H.) is acknowledged. We thank P. Ortiz for synthesis of ligands L7 and L8, Solvias for a generous gift of Josiphos ligands, T.D. Tiemersma-Wegman for HRMS support and Pieter van der Meulen for NMR support.
Author contributions
R.P.J. and F.L. designed and performed the experiments. T.P. performed additional experiments with respect to mechanistic studies. All authors contributed to editing the manuscript. S.R.H. guided the research.
Competing interests
The authors declare no competing financial interests.
Footnotes
Ravindra P. Jumde and Francesco Lanza contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-017-01966-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu YJ. Heterocycles and medicine: a survey of the heterocyclic drugs approved by the U.S. FDA from 2000 to present. Prog. Heterocycl. Chem. 2012;24:1–53. [Google Scholar]
- 2.Akhtar, J., Khan, A. A., Ali, Z., Haider, R. & Shahar Yar, M. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med.125, 143–149. [DOI] [PubMed]
- 3.Kiuru, P. & Yli-Kauhaluoma, J. P. in Heterocycles in Natural Product Synthesis (eds Majumdar, K. C. & Chattopadhyay, S. K.) 63–95 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011).
- 4.Guan AY, Liu CL, Sun XF, Xie Y, Wang MA. Discovery of pyridine-based agrochemicals by using intermediate derivatization methods. Bioorg. Med. Chem. 2016;24:342–353. doi: 10.1016/j.bmc.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Glorius F, Spielkamp N, Holle S, Goddard R, Lehmann CW. Efficient asymmetric hydrogenation of pyridines. Angew. Chem. Int. Ed. 2004;43:2850–2852. doi: 10.1002/anie.200453942. [DOI] [PubMed] [Google Scholar]
- 6.Henry GD. De novo synthesis of substituted pyridines. Tetrahedron. 2004;60:6043–6061. doi: 10.1016/j.tet.2004.04.043. [DOI] [Google Scholar]
- 7.Bagley MC, Glover C, Merritt EA. The Bohlmann-Rahtz pyridine synthesis: from discovery to applications. Synlett. 2007;16:2459–2482. doi: 10.1055/s-2007-986674. [DOI] [Google Scholar]
- 8.Hill MD. Recent strategies for the synthesis of pyridine derivatives. Chem. Eur. J. 2010;16:12052–12062. doi: 10.1002/chem.201001100. [DOI] [PubMed] [Google Scholar]
- 9.Kilah NL, Beer PD. Pyridine and pyridinium-based anion receptors. Top Heterocycl. Chem. 2010;24:301–340. [Google Scholar]
- 10.Chelucci G. Metal-complexes of optically active amino- and imino-based pyridine ligands in asymmetric catalysis. Coord. Chem. Rev. 2013;257:1887–1932. doi: 10.1016/j.ccr.2012.12.002. [DOI] [Google Scholar]
- 11.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 12.Goetz AE, Garg NK. Regioselective reactions of 3,4-pyridynes enabled by the aryne distortion model. Nat. Chem. 2013;5:54–60. doi: 10.1038/nchem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pozharskii, A. F., Soldatenkov, A. & Katritzky, A. R. (eds) Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, 2nd edn (Wiley, New York, 2011).
- 14.Best D, Lam HW. C ═ N-containing azaarenes as activating groups in enantioselective catalysis. J. Org. Chem. 2014;79:831–845. doi: 10.1021/jo402414k. [DOI] [PubMed] [Google Scholar]
- 15.Hepburn HB, Melchiorre P. Bronsted acid-catalyzed CA of photochemically generated α-amino radicals to alkenylpyridines. Chem. Commun. 2016;52:3520–3523. doi: 10.1039/C5CC10401G. [DOI] [PubMed] [Google Scholar]
- 16.Rupnicki L, Saxena A, Lam HW. Aromatic heterocycles as activating groups for asymmetric CA reactions. Enantioselective copper-catalyzed reduction of 2-alkenylheteroarenes. J. Am. Chem. Soc. 2009;131:10386–10387. doi: 10.1021/ja904365h. [DOI] [PubMed] [Google Scholar]
- 17.Klumpp DA. CAs to vinyl-substituted aromatic N-heterocycles. Synlett. 2012;23:1590–1604. doi: 10.1055/s-0031-1290984. [DOI] [Google Scholar]
- 18.Saxena A, Lam HW. Enantioselective rhodium-catalyzed arylation of electron-deficient alkenylarenes. Chem. Sci. 2011;2:2326–2331. doi: 10.1039/c1sc00521a. [DOI] [Google Scholar]
- 19.Friedman AA, Panteleev J, Tsoung J, Huynh V, Lautens M. Rh/Pd catalysis with chiral and achiral ligands: domino synthesis of aza-dihydrodibenzoxepines. Angew. Chem. Int. Ed. 2013;52:9755–9758. doi: 10.1002/anie.201303659. [DOI] [PubMed] [Google Scholar]
- 20.Houpis IN, et al. Ni-catalyzed nucleophilic CAs of Grignard and organozincate reagents to substituted 4-vinylpyridines. General synthesis of phosphodiesterase IV inhibitors. Tetrahedron. 1998;54:1185–1195. doi: 10.1016/S0040-4020(97)10217-4. [DOI] [Google Scholar]
- 21.Jumde RP, Lanza F, Veenstra MJ, Harutyunyan SR. Catalytic asymmetric addition of Grignard reagents to alkenyl-substituted aromatic N-heterocycles. Science. 2016;352:433–437. doi: 10.1126/science.aaf1983. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Mollat du Jourdin X, Knochel P. Transition-metal-free BF3-mediated regioselective direct alkylation and arylation of functionalized pyridines using Grignard or organozinc reagents. J. Am. Chem. Soc. 2013;135:4958–4961. doi: 10.1021/ja401146v. [DOI] [PubMed] [Google Scholar]
- 23.Hilton MC, Dolewski RD, McNally A. Selective functionalization of pyridines via heterocyclic phosphonium salts. J. Am. Chem. Soc. 2016;138:13806–13809. doi: 10.1021/jacs.6b08662. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikai N, Nakamura E. Mechanisms of nucleophilic organocopper(I) reactions. Chem. Rev. 2012;112:2339–2372. doi: 10.1021/cr200241f. [DOI] [PubMed] [Google Scholar]
- 25.Bertz SH, et al. Complexes of the Gilman reagent with double bonds across the π−σ continuum. Organometallics. 2012;31:7827–7838. doi: 10.1021/om300622c. [DOI] [Google Scholar]
- 26.Bertz SH, Cope SK, Murphy M, Ogle CA, Taylor BJ. Rapid injection NMR in mechanistic organocopper chemistry. Preparation of the elusive copper(III) intermediate. J. Am. Chem. Soc. 2007;129:7208–7209. doi: 10.1021/ja067533d. [DOI] [PubMed] [Google Scholar]
- 27.Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Catalytic asymmetric conjugate addition and allylic alkylation with grignard reagents. Chem. Rev. 2008;108:2824–2852. doi: 10.1021/cr068424k. [DOI] [PubMed] [Google Scholar]
- 28.Alexakis, A., Krause, N., Woodward, S. (eds.) Copper-Catalyzed Asymmetric Synthesis (Wiley-VCH, Weinheim, Germany, 2014).
- 29.Yamamoto Y, Yamamoto S, Yatagai H, Ishihara Y, Maruyama K. Lewis acid mediated reactions of organocopper reagents. Entrainment in the conjugate addition to alpha, beta-unsaturated ketones, esters, and acids via the RCu.cntdot.BF3 system. J. Org. Chem. 1982;47:119–126. doi: 10.1021/jo00340a026. [DOI] [Google Scholar]
- 30.Nakamura E, Yamanaka M, Mori S. Complexation of lewis acid with trialkylcopper(III): on the origin of BF3-acceleration of cuprate conjugate addition. J. Am. Chem. Soc. 2000;122:1826–1827. doi: 10.1021/ja993124o. [DOI] [Google Scholar]
- 31.Lipshutz BH, Ellsworth EL, Dimock SH. On the composition of Yamamoto’s reagent: “RCu.cntdot.BF3”. J. Am. Chem. Soc. 1990;112:5869–5871. doi: 10.1021/ja00171a031. [DOI] [Google Scholar]
- 32.Corey EJ, Boaz NW. The reactions of combined organocuprate-chlorotrimethylsilane reagents with conjugated carbonyl compounds. Tetrahedron Lett. 1985;26:6019–6022. doi: 10.1016/S0040-4039(00)95114-1. [DOI] [Google Scholar]
- 33.Corey EJ, Boaz NW. Evidence for a reversible d, π-complexation, β-cupration sequence in the conjugate addition reaction of Gilman reagents with α,β-enones. Tetrahedron Lett. 1985;26:6015–6018. doi: 10.1016/S0040-4039(00)95113-X. [DOI] [Google Scholar]
- 34.Alexakis A, Berlan J, Besace Y. Organocopper conjugate addition reaction in the presence of trimethylchlorosilane. Tetrahedron Lett. 1986;27:1047–1050. doi: 10.1016/S0040-4039(86)80044-2. [DOI] [Google Scholar]
- 35.Matsuzawa S, Horiguchi Y, Nakamura E, Kuwajima I. Chlorosilane-accelerated conjugate addition of catalytic and stoichiometric organocopper reagents. Tetrahedron. 1989;45:349–362. doi: 10.1016/0040-4020(89)80064-X. [DOI] [Google Scholar]
- 36.Bertz SH, Miao G, Rossiter BE, Snyder JP. New copper chemistry. 25. Effect of TMSCl on the conjugate addition of organocuprates to alpha enones: a new mechanism. J. Am. Chem. Soc. 1995;117:11023–11024. doi: 10.1021/ja00149a032. [DOI] [Google Scholar]
- 37.Frantz DE, Singleton DA. Isotope effects and the mechanism of chlorotrimethylsilane-mediated addition of cuprates to enones. J. Am. Chem. Soc. 2000;122:3288–3295. doi: 10.1021/ja993373c. [DOI] [Google Scholar]
- 38.Harutyunyan SR, et al. On the mechanism of the copper-catalyzed enantioselective 1,4-addition of Grignard reagents to α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 2006;128:9103–9118. doi: 10.1021/ja0585634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures, additional mechanistic studies, spectroscopic and physical data of compounds, see Supplementary Methods. For NMR and HPLC analysis of the compounds in this article, see Supplementary Figs. 1–156. For ligands screening and isomerisation studies see Supplementary Tables 1 and 2 respectively. The CCDC 1532252 (2a′ prepared from 2a, see Supplementary Note 2) contains the supplementary crystallographic data for this paper (Supplementary file). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.