Abstract
The cervical microbiota composition and diversity of HIV-positive women in the postpartum period is unknown. Using a high-throughput bacterial 16S rRNA gene sequencing, we identified four community state types (CSTs). CST III (Lactobacillusdominant) and CST IV (IV-A, IV-B.1, IV-B.2; high-diversity) were found in 41% and 59% of samples, respectively. We did not find association of any CST to postpartum period (six or twelve months), HPV infection or cytology (normal or lesion). However, five bacterial genera were associated with cervical lesions (Gardnerella, Aerococcus, Schlegelella, Moryella and Bifidobacterium), with significant odds ratio (OR) of 40 (2.28–706) for the presence of Moryella and 3.5 (1.36–8.9) for Schlegelella. Longitudinal analysis of samples at postpartum that regressed (lesion to normal), progressed (normal to lesion) and maintained the cytology (lesion or normal) evidenced Gardnerella with a significantly higher abundance in regressing lesions. In the current study, we report the first data on the cervical microbiota of HIV-positive women in the postpartum period. Consistent with previous studies of HIV-negative cohorts, HIV-positive women present a stable cervical microbiota of high-diversity in the postpartum period. Our results highlight that specific microbiota species may serve as sensors for changes in the cervical microenvironment associated with cervical lesions.
Introduction
Cervical cancer is the fourth most common cancer among women worldwide, and over 500,000 new cases are diagnosed each year, leading to more than 200,000 deaths1. The primary factor associated with the development of cervical cancer is the persistent infection by the human papillomavirus (HPV). Additional factors correlated with HPV persistence include immunodeficiency caused by HIV, smoking, use of oral contraceptives and, more recently reported, cervicovaginal dysbiosis2.
The classically-defined normal cervicovaginal microbiota is dominated by one or more Lactobacillus sp. (Lactobacillus crispatus, L. gasseri, L. iners or L. jensenii). However, in a state of dysbiosis, there is a marked reduction of Lactobacillus and a high diversity of bacteria, with increased abundance of anaerobic bacterial species3–5. Cervicovaginal dysbiosis is asymptomatic in some cases, turning the definition of normal/dysbiotic microbiota a point of current discussion. However, many women with cervicovaginal dysbiosis develop symptomatic bacterial vaginosis (BV), characterized by vaginal discomfort and homogeneous malodorous vaginal discharge6,7. Both symptomatic and asymptomatic dysbiosis have been associated with the risk to preterm birth, acquisition of sexually transmitted infections (STIs) and increased risk of pelvic inflammatory disease7–9. In general, previous studies on cervical microbiome have described five community state types (CSTs), I to V3. Whereas CSTs I, II, III and V are dominated by Lactobacillus species (L. crispatus, L. gasseri, L. iners or L. jensenii, respectively), CST IV is characterized by a high diversity and predominance of anaerobic bacteria as Gardnerella vaginalis, Atopobium vaginae, Prevotella sp. and other anaerobic bacterial species3.
It has been shown that reduction of Lactobacillus sp., combined with increased diversity of cervicovaginal microbiota, are risk factors for HPV acquisition, persistence, development of cervical intraepithelial neoplasia (CIN) and cervical cancer2. A study conducted with Korean women with and without CIN showed that those carrying Atopobium vaginae, Garderella vaginalis and L. iners in the absence of L. crispatus had an almost 6x higher risk for CIN. That study also showed the synergistic effect of this microbial pattern and oncogenic HPV infection on a very high risk (odds ratio of 34.1) of CIN10. In a longitudinal study with samples collected over a 16-week period, women with highly diverse or L. iners-dominated cervicovaginal microbiota were more likely to be HPV-positive, while a microbiota dominated by L. gasseri was associated with more rapid clearance of HPV infection11.
The high diversity of cervicovaginal microbiota is strongly correlated with local inflammation and it is also thought to increase the risk for HIV acquisition12. It is known that HIV-positive women more often present with high-risk HPV infection and development of cervical disease13. However, little is known about the influence of the cervicovaginal dysbiosis in HIV/HPV coinfected women. Comparison of HIV-infected and HIV-uninfected women showed that microbiota dominated by L. crispatus are associated with a lower prevalence of HIV/STIs14 and with decreased prevalence of HPV in HIV-infected woman15. In contrast increased microbiome diversity has been demonstrated in high frequency among HIV-positive woman14. The cervicovaginal dysbiosis may also generally affect maternal health, leading to postpartum complications16,17. A longitudinal study of woman followed-up during pregnancy and postpartum showed that the cervicovaginal microbiota composition is modified at six weeks postpartum, with increased bacterial diversity and decrease in L. crispatus moieties18.
Based on this information, we hypothesize that postpartum HIV-positive women have a highly diverse cervicovaginal microbiota, which could be associated with an increased risk for developing persistent HPV infection and consequently CIN. Therefore, our study aimed to evaluate the postpartum cervical microbiota profiles of HIV-positive women displaying diverse cervical intraepithelial neoplasia.
Results
Sociodemographic and clinical baseline characteristics
The median age of participants was 28 years-old. Approximately 60% of the women never smoked, the median age of first sexual intercourse was 15 years-old, 70% had 3 or more sexual partners and 48% used hormonal contraceptives before pregnancy (Table 1). The median CD4+ T-cell count and HIV viral load values of the studied subjects were of 579 cells/μl and 344 copies/ml at 6 months after labor, and of 548 cells/μl and 409 copies/ml at 12 months after labor, respectively, and those differences were not significant. The frequency of HPV-negative women was significantly higher at 12 months than at 6 months postpartum (8% versus 37%). However, the frequency of HPV-positive women with lesions (LSIL/HSIL) was higher at 12 months compared to six months postpartum (Table 2).
Table 1.
Sociodemographic characteristics of the studied participants.
| Age (median, range) | 28 (17–44) |
|---|---|
| Smoking (%, N/Total) | |
| Current smoker | 15 (12/80) |
| Former smoker | 26 (21/80) |
| Never smoker | 58 (46/80) |
| N/A1 | 1 (1/80) |
| Median age of first sexual intercourse (range) | 15 (9–25) |
| Number of sexual partners (%, N/Total) | |
| 1 to 3 sexual partners | 30 (24/80) |
| >3 sexual partners | 70 (56/80) |
| Contraceptive methods (%, N/Total) | |
| Hormonal | 48 (38/80) |
| Condom | 33 (27/80) |
| Other | 2 (2/80) |
| None | 16 (13/80) |
1N/A, not available.
Table 2.
Frequency of cervical cytology and HPV status at six and 12 months postpartum.
| 06 months % (n = 26) | 12 months % (n = 54) | % N/Total | p-value1 | ||
|---|---|---|---|---|---|
| Cervical Cytology | Normal | 65 (17/26) | 46 (25/54) | 52 (42/80) | 0.109 |
| Lesion | 35 (9/26) | 54 (29/54) | 48 (38/80) | ||
| HPV status | Positive | 92 (24/26) | 63 (34/54) | 72 (58/80) | 0.006 |
| Negative | 8 (2/26) | 37 (20/54) | 28 (22/80) | ||
| Cytology-HPV positive | Normal | 58 (15/26) | 15 (8/54) | 29 (23/80) | 0.003 |
| Lesion | 38 (9/26) | 48 (26/54) | 43 (35/80) | ||
| Cytology-HPV negative | Normal | 8 (2/26) | 31 (17/54) | 24 (19/80) | 0.556 |
| Lesion | 0 (0/26) | 6 (3/54) | 4 (3/80) |
1Pearson’s chi-square test.
Microbiome community diversity
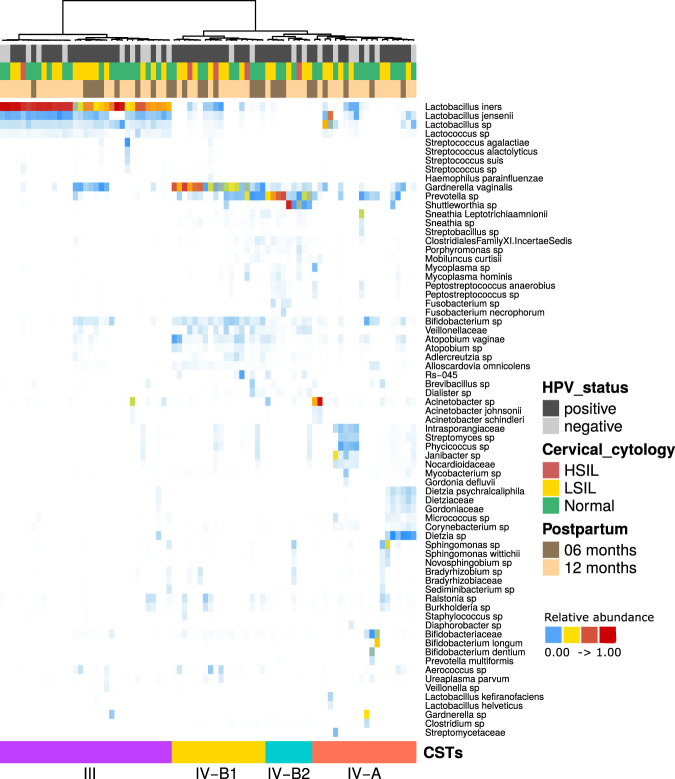
After an initial unsupervised clustering analysis, four distinct CSTs were found and were named according to previous designations11,19,20 (Fig. 1 and Supplementary Figure 1A).
Figure 1.
Heatmap generated by unsupervised hierarchical clustering analysis of cervical microbiomes of the studied participants. CSTs were defined using clustering based on Bray-Curtis dissimilarity and average linkage and are shown in color-coded groups at the bottom and also by the dendrogram at the top of the Figure. HPV status, cervical cytology and postpartum period are also color-coded according to the legend at the right of the Figure. CST III is L. iners-dominant; CST IV-A has a low proportion of Lactobacillus and a high diversity; CST IV-B.1 is G. vaginalis-dominant and CST IV-B.2 is Prevotella-dominant. All taxa shown in the graph presented relative abundance >1%.
CST III is dominated by L. iners. CST IV was observed in a distinct split between IV-A and IV-B. CST IV-A showed a high bacterial diversity and a low abundance of Lactobacillus. However, we noticed a clear split within CST IV-B in two groups, one dominated by Gardnerella (which we named CST IV-B.1) and another dominated by Prevotella (CST IV-B.2) (Fig. 1 and Supplementary Figure 1B).
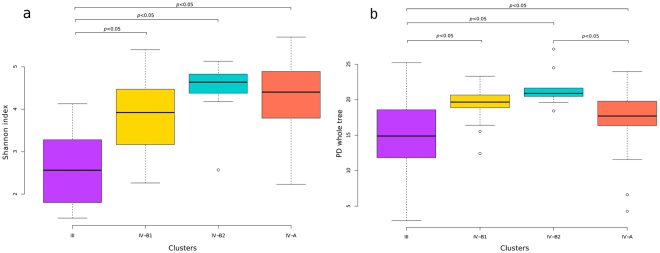
The Shannon’s diversity analysis showed a significantly lower diversity of CST III compared to the remaining CSTs (IV-A, IV-B.1, IV-B.2) (Fig. 2a). The Phylogenetic Diversity (PD) analysis corroborated those differences, and also suggested differences between CST IV-B2 and CST IV-A (Fig. 2b). These data show that our population under study predominantly carries a microbiota of high diversity and lacks Lactobacillus-dominated CSTs (except for L. iners), generally seen in other studies. Additionally, we performed bacterial profile analysis by reconstruction of the 16S gene to confirm the results obtained in the read analysis with QIIME, and the two methods showed a high degree of similarity, ranging from 61 to 86% (Supplementary Figure 2).
Figure 2.
Box plot analysis of alpha diversity using the (a) Shannon index and (b) phylogenetic diversity. Analyses in (a) and (b) were performed for each CST defined. The Mann-Whitney test was carried out to compare diversities between each CST, and the significant p-values at the 0.05 confidence level are depicted at the horizontal lines above the graphs.
We next analyzed the distribution of the samples classified as CSTs III and IV (IV-A, IV-B.1, IV-B.2) with respect to the postpartum period, HPV status and cytology (normal vs lesion). However, no significant differences were observed in any of the comparisons (Supplementary Tables 1 and 2).
Analysis of lesion biomarkers
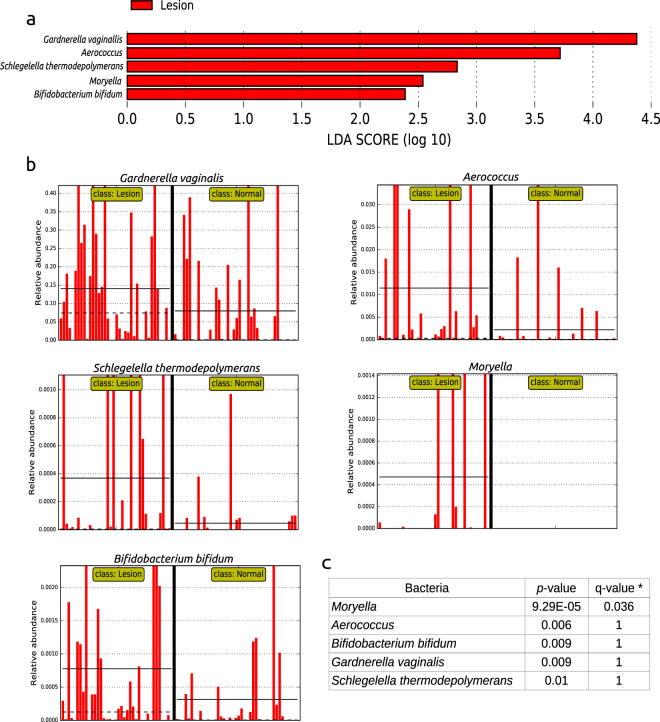
We carried out analyses to identify differences in the microbiota composition, independent of the CST classification, through the linear discriminant analysis (LDA) effect size (LEfSe) algorithm as a way to discover potential biomarkers linked to a lesion status. The LEfSe analysis comparing the microbiota between normal and lesion samples showed significant differences in the abundance of Gardnerella vaginallis, Aerococcus, Schlegelella thermodepolymerans, Moryella and Bifidobacterium bifidium, all present at higher abundance in lesions (Fig. 3). When we performed multiple testing correction to the LEfSe analysis (Fig. 3c), only Moryella remained significant (q-value = 0.036). However, due to the fact that the LEfSe algorithm still suggests significant associations (albeit uncorrected for multiple comparisons), we decided to keep all five bacterial taxa in further analysis for confirming their association with cervical lesions.
Figure 3.
Analysis of cervical lesion putative biomarkers using LEfSe. (a) Histogram of the LDA scores (log 10) showing bacteria that presented higher relative abundance in cervical lesions (red) when compared to normal cytology. Only statistically significant differences are shown. (b) Histograms showing the relative abundance of the five distinct taxa for each sample in the lesion and normal groups, separated by a thick black line. The solid and dotted black lines in the graphs indicate the mean and median relative abundance values for each group, respectively. The relative abundance (y-axis) is represented as a fraction of 1. (c) The p-values obtained by the LfESe analysis and the corrected p-values (q-values) are shown.
We calculated odds ratios (OR) to estimate the risk of carrying the bacterial taxa found in the previous analysis in cytological lesions. Schlegelella thermodepolymerans and Moryella presented ORs of 3.5 (1.4–8.9) and 40.1 (2.28–706), respectively, in lesions compared to normal samples (Table 3). These associations were confirmed as significant with the Chi-square test (Table 3). Overall, our results suggested that specific bacteria, albeit at low abundance, may to be significantly associated with cytological lesions.
Table 3.
Odds ratio (OR) for the occurrence of specific bacteria in cervical lesions.
| Bacteria | Normal % (N/Total) | Lesion % (N/Total) | p-value1 | q-value2 | OR (CI95%)3 | p-value4 | Adjusted OR (CI95%)5 | Adjusted p-value5 |
|---|---|---|---|---|---|---|---|---|
| Gardnerella vaginalis | ||||||||
| Presence | 46 (37/80) | 48 (38/80) | 0.028 | 0.14 | 11.3 (0.6–211.46) | 0.10 | NA | NA |
| Absence | 6 (5/80) | 0 (0/80) | ||||||
| Bifidobacterium bifidum | ||||||||
| Presence | 35 (28/80) | 39 (31/80) | 0.13 | 0.65 | 2.2 (0.8–6.3) | 0.13 | 2.88 (0.9–9.1) | 0.07 |
| Absence | 18 (14/80) | 9 (7/80) | ||||||
| Moryella | ||||||||
| Presence | 0 (0/80) | 15 (12/80) | <0.01 | <0.01 | 40.1 (2.3–705.8) | 0.01 | NA | NA |
| Absence | 53 (42/80) | 33 (26/80) | ||||||
| Schlegelella thermodepolymerans | ||||||||
| Presence | 14 (11/80) | 26 (21/80) | 0.01 | 0.04 | 3.5 (1.4–8.9) | 0.01 | 3.44 (1.3–8.9) | 0.01 |
| Absence | 39 (31/80) | 21 (17/80) | ||||||
| Aerococcus | ||||||||
| Presence | 50 (40/80) | 48 (38/80) | 0.17 | 0.85 | 4.8 (0.2–102.2) | 0.32 | NA | NA |
| Absence | 3 (2/80) | 0 (0/80) | ||||||
1Pearson’s chi-square test. 2Pearson’s chi-square test after Bonferroni’s correction. 3Odds ratios were calculated with Haldane’s modification, which adds 0.5 to all cells to accommodate possible zero counts. 4Calculated according to Sheskin, 2004. 5Calculated by logistic regression. The OR were corrected by age, smoking, contraceptive methods and number of sexual partners. NA (not available) depicts variables for which calculation of logistic regression is not possible due to the existence of zero counts in one or more categories.
We further analyzed the bacterial taxa that originally displayed significance in the LEfSe analysis in paired samples of the same individuals (6 and 12 months postpartum) for their association with the lesion status. A total of 25 women had longitudinal samples available to analyze; 16 maintained status (normal or lesion at both time points), 5 had lesions that regressed (lesion at the first time point and normal at the second), and 4 had lesions that progressed (normal at the first time point to lesion at the second). Only Gardnerella vaginalis presented significant differences in its relative abundance in samples that regressed the cytological status, showing a higher abundance in lesions compared to normal cervices of the same subjects (Fig. 4, Supplementary Figure 3).
Figure 4.
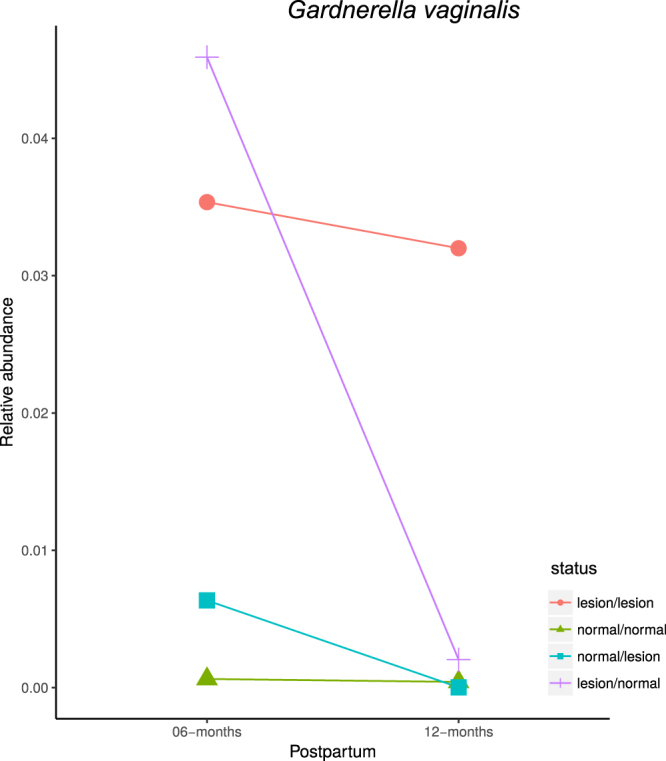
Gardnerella vaginalis abundance longitudinal analysis at six and twelve months postpartum in paired samples. The median relative abundance at six and twelve months postpartum of the paired samples that regressed (purple), progressed (blue), or maintained normal (green) or lesion (pink) cytology results is shown. The Wilcoxon test was performed with 95% confidence interval, and the p-value was only significant in the regression group (p = 0.043).
Discussion
HIV-infected women present a higher risk of HPV infection, persistence and development of cervical cancer compared to HIV-negative counterparts21. In the present study, we showed that HIV-positive women with HPV infection at 12 months postpartum presented a higher frequency of cervical premalignant lesions than at 6 months postpartum. The persistence of HPV infection is an essential but not sufficient factor for the development of CIN and cervical cancer, and recently the cervical microbiota dysbiosis has been proposed as a key player in the development of cervical diseases2,10. Several studies showed an association of high-diversity cervical microbiota with HPV infection, CIN, cervical cancer and HIV acquisition14,22,23. However, the profile of such microbiota in HIV-infected women in the postpartum period remained largely unknown.
In this study, we identified four CSTs in postpartum HIV-positive women, which for the sake of definition in previous reports have been named CSTs III, IV-A, IV-B.1 and IV-B.2. CST III is dominated by L. iners, while CSTs IV (A and B) are high-diversity communities dominated by anaerobic bacteria. We did not find CSTs I, II and V described in previous studies which are dominated by L. crispatus, L. gasseri and L. jensenii, respectively3. L. crispatus (CST I) is present in high frequency in the vaginal microbiota of healthy white (European) and Asian women3,24. The absence of this CST among our samples is congruent with the observation that L. crispatus is associated with reduction in HIV acquisition and is generally absent in HIV-infected women22. Furthermore, the composition of CSTs is likely different in distinct ethnic groups. Studies showed that women of African ancestry have a higher prevalence of CST IV when compared to those of Asian and European ancestries20,24. The Brazilian population is highly mixed due to its colonization history, with large admixing of European and African descendants25,26. Therefore, the ethnic background of the participants in this study may also explain the high proportion of CST IV (A and B) found in the samples.
CSTs IV-A and B are characterized by a high diversity of bacteria and modest to absent proportion of Lactobacillus sp., while CST IV-B shows a specific high proportion of Prevotella, Gardnerella, Atopobium, Mobiluncus and other bacterial taxa11,19,20. Herein, we describe a further stratification of CST IV-B into IV-B.1 (Gardnerella-dominant) and IV-B.2 (Prevotella-dominant). These two CSTs formed two distinct clusters, but no significant differences were observed in their diversity by Shannon index and PD whole tree analysis. Additionally, CST IV has been associated with HPV infection, CIN and HIV acquisition14,22,23. In this study, we showed that CST IV comprised a high proportion (59%) of the samples, but distinct CSTs were not associated with HPV status, the presence of CIN or the postpartum period (6 versus 12 months). A previous study reported a high frequency of CST IV among HIV- and HPV-positive women27. Additionally, HIV-positive women do not show significant variation in their microbiota irrespective of their HPV status27. Our data are in agreement with previous reports12,22,27 and suggest that HIV-positive women present a stable community of high-diversity bacteria in the postpartum period that is not altered by CIN status, HPV infection or time postpartum. Addionally, our results are consistent with a study that analyzed the microbiota from an HIV-negative women cohort with normal cytology, which showed a persistent and highly diverse microbiota in the postpartum period of samples collected for up to one year after delivery28. On the other hand, another study of vaginal microbiome among European HIV-negative women showed an increased prevalence of CST IV with increasing severity of cervicovaginal lesions, irrespective of HPV status29. Our findings prompt further investigation on the stability of microbiome in HIV-negative women with cervical lesions at different time points postpartum compared to HIV-positive women.
Since the CSTs found herein were stable over time, we performed an association analysis of individual bacteria with cervical lesions. We found four bacterial genera in low abundance (Bifidobacterium, Moryella, Schlegella and Aerococcus) and one in high abundance (Gardnerella) associated with cervical lesions in initial analyses. The Moryella and Aerococcus genera belong to the Firmicutes phylum. Recent studies associated this phylum with colorectal cancer30,31, but the role of these bacteria in cervical cancer remains unknown. The Aerococcus genus modulates the immune response by the production of proinflammatory cytokines12. Moryella was first identified in abscesses of clinical samples32, suggesting it may also play a role in immune modulation. Additionally, Gardnerella vaginalis is associated with high-diversity microbiome and is also able to modulate the production of proinflammatory cytokines in the cervical region12,22. Whereas inflammatory responses are required to eliminate sexually transmitted infections effectively, a hyperactivated chronic inflammation in the cervix has been correlated with increased risk of CIN and HIV acquisition12,22,33.
Bifidobacterium has been previously reported in vaginal samples of healthy women34. Recent studies described this genus with an antitumor activity, preventing tumor grown and present only in low frequency in colorectal cancer35,36. We found herein a positive association of this bacterium with cervical lesions, but its role in the cervical microbiota remains to be elucidated. The Schlegella genus was additionally shown in a positive association with lesions, however their role in the cervix is also unknown, and no additional reports are available for comparison. Studies showed the presence of bacterial DNA during sample preparation with DNA extraction kits and other laboratory reagents37–39. The presence of Schlegella has been previously reported in negative controls of experiments, and it has been suggested that this bacterium is present as contaminant DNA in nucleic acid extraction kit reagents40. However, all samples this study were treated with the same reagents, and the presence of Schlegella was only observed in samples with cervical lesions. Further studies are therefore necessary to evaluate the association of this bacterium with premalignant cervical lesions.
The five genera originally associated with cervical lesions in the analysis depicted above had their OR estimated for their presence in comparison with normal cytology. The OR of the Moryella and Schlegella genera were highly significant, of 40x (2.28–706) and 3.5x (1.36–8.9) respectively, whereas the three other genera were not significant. These results and the Chi-square statistics reinforce the strength of the association of these two bacteria with cervical lesions.
The availability of longitudinal samples from many study participants (at 6 and 12 months postpartum) allowed us to evaluate the robustness of particular bacteria as biomarkers for cervical lesions. Upon longitudinal analysis of the taxa that were significantly associated with lesions in the former tests, none of them varied significantly among women that remained with the same status (normal or lesion) over the two time points analyzed (Fig. 4). On the other hand, for those women that progressed from normal to lesion (n = 4) or regressed from lesion to normal (n = 5), only Gardnerella vaginalis significantly changed its relative abundance in women in which the lesion regressed. However, among women who changed from normal cytology to cervical lesions, G. vaginalis levels remained statistically unchanged. These data may suggest that G.vaginalis may either induce the developmnent of cervical lesions or may colonize the cervix after lesions develop, and our data cannot discriminate between these two possibilities. The lack of additional associations may be due, at least in part, to the small number of paired samples analyzed in each category.
The cervical microbiota displays a complex interaction with the local environment and with the immune system, playing a central role in the cervical homeostasis. Here we demonstrated that the cervical microbiota communities of HIV-positive women at postpartum are stable, generally presenting a high diversity of bacteria and a lack of L. crispatus dominant types. Moreover, we found three bacterial genera associated with cervical lesions (Moryella, Schlegella and Gardnerella) through distinct analyses. Little is known about the function of these bacteria on cervical microbiota homeostasis, and it is not clear how these bacteria modulate the development of cervical cancer, whether they arise before or after cervical lesions appear and whether they are a necessary component for the development of malignancy. We propose that distinct microbiota species may serve as sensors for changes in the cervical microenvironment, being able to modulating it or being modified by it towards health or disease.
Methods
Sample collection
Eighty women enrolled at the Program for HIV-infected Pregnant Women at the Federal University of Rio Janeiro (UFRJ), Brazil, participated in this study. HIV-positive serological status was confirmed by an HIV rapid or ELISA test and subsequent Western blot following recommendations for HIV diagnosis by the Brazilian Ministry of Health. All participants were under antiretroviral treatment at the time of sample collection. Cervical smear collection was carried out from 2009 to 2010, and two time points were collected for each patient, at six and 12 months postpartum, respectively. However, both time points have been retrieved only for 25 subjects, totaling 50 (25 × 2) paired samples, that were used in the longitudinal analyses. As selection criteria for the longitudinal analyses, we selected samples of subjects with cytology information in the two time points collected. Individuals with ASCUS cytology at any one of the time points were excluded from the analyses. For 80 subjects, unpaired (single timepoint) samples were used in the analysis; the 6-month postpartum time point was available for 26 women, and 12-month timepoint was available for 54 women. Overall, a total of 105 individual samples have been analyzed.
Cytological Pap smear results were obtained from patients’ medical charts and were classified according to the 2001 Bethesda reporting guidelines41. Clinical, obstetrical and sociodemographic variables were also retrieved from the medical records and from a questionnaire answered by the participants. All participants of this study were mixed-race people of European and African ancestries. The study was approved by the Ethical Committees of UFRJ (protocols #029/08 to Clementino Fraga Filho University Hospital and #18/10 to Martagão Gesteira Childcare and Pediatrics Institute) and of the Brazilian National Cancer Institute (INCA) (protocol #142/10). All participants signed an informed consent before enrollment in the study. All experimental procedures involving sample collection were performed in accordance with the Brazilian National Ethics Committee guidelines and regulations.
DNA extraction and HPV typing
Sample collection was performed with two endocervical cytobrushes; one was placed in 100% ethanol for Pap smear diagnosis, while the other was placed into 1 ml of phosphate-buffered saline and stored at −80 °C for DNA extraction. Total genomic DNA was extracted from cervical samples with the RBC Genomic DNA Extraction Kit (Realbiotech, Taiwan), according to the manufacture’s protocol, and was stored at −20 °C until use. HPV DNA was detected by PCR amplification of an L1 gene fragment using consensus primers MY09/MY11 and GP5/GP6, and reaction conditions previously described42. All PCR products were analyzed by electrophoresis on 1.5% agarose gels, and positive samples were purified with the Illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare Life Sciences, Chicago, IL) and sequenced by Sanger. The sequences obtained were analyzed through BLAST search, and HPV types were assigned based on the HPV type that best matched the query samples.
Bacterial 16S PCR and analysis
The V3-V6 region of the bacterial 16S rRNA gene was PCR-amplified using the primers 338F and 1061R, which have been reported to amplify over 90% of the bacterial sequences present in the Greengenes database43. PCR reactions were carried out using 5 μl Taq 10X buffer, 1.5 μl 50 mM MgCl2, 0.4 μl 25 mM dNTP mix, 25 pmol/μl each primer and 0.3 μl Taq Platinum polymerase (5U/μl). The final volume was adjusted to 50 μl with RNase/DNase free water (Life Technologies, Carlsbad, CA). An initial denaturation step of 95 °C for 5 min was carried out, followed by 35 cycles of denaturation (95 °C, 30 s), annealing (59 °C, 30 s) and extension (72 °C, 1 min), and a final elongation step of 6 min at 72 °C. PCR products were visualized by electrophoresis in 1.5% agarose gels and bands of the expected size of 700–1,000 bp were purified with the Illustra GFX PCR DNA and Gel Band Purification Kit. Purified products were quantified in a NanoDrop apparatus (Thermo Fisher Scientific, Waltham, MA), and 0.4 ng of DNA was used to prepare each library with the Nextera XT DNA Sample Preparation kit (Illumina Inc., San Diego, CA). Library concentrations were assessed by qPCR with the KAPA Library Quantification kit (KAPA Biosystems, Wilmington, MA). Libraries were diluted to a final concentration of 10 pM, loaded onto the flow cell and clonal clustering with cBot (Illumina Inc.) was carried out. Sequencing by synthesis was performed on an Illumina HiSeq. 2500 system (Illumina Inc.).
The BCL2FastQ2 Conversion Software (version 2.18, Illumina Inc.) was used to demultiplex data and convert BCL files to FASTQ file formats. Formatted reads were subjected to the FastQC (Babraham Bioinformatics, Cambridge, CBE) for quality filtering using the split_libraries_fastq.py script of QIIME44. Operational taxonomic units (OTUs) were assigned to the reads using QIIME’s pick_closed_reference_otus.py script against the Greengenes Database, and the Uclust algorithm was used to group and assign taxa45. Sequences were clustered into OTUs using a 97% similarity threshold. OTUs with a number of sequences lower than 0.005% of the total number of sequences generated were discarded and those remaining were summarized at the species level. A rarefaction curve was constructed for each sample (Supplementary Figure 4). The reconstruction of 16S consensus sequence was performed for a partial set of samples using EMIRGE46,47 and taxa were assigned to consensus sequences using Blastn against the Greengenes Database. The resultant Blastn top hit (according to sorting criteria of the lowest e-value, highest bit-score, highest percent identity and longest alignment length) was used for classification. The taxa presented and their relative abundances as estimated in EMIRGE were reported and compared to the results obtained in QIIME for a subset of the samples to investigate possible divergence in taxonomic assignment between the two methods.
Unsupervised hierarchical clustering based on Bray-Curtis dissimilarity and average linkage was applied to define clusters according to abundance and taxa diversity of each sample. The clusters found were associated with vaginal microbiome CSTs classified in previous studies11,19,20. The alpha diversity of each defined CST was estimated using the Shannon index and the Phylogenetic Diversity (PD) whole tree methods. Clusterization, diversity analyses and plots were carried out using the R environment.
Statistical analyses
Patients’ sociodemographic and clinical data have been summarized with frequency and medians. Frequencies of cervical cytology results and HPV status were compared between the two timepoints studied (6 and 12 months postpartum) using the Pearson’s chi-square test in the SPSS environment (IBM Corporation, Chicago, IL). The same test was used to compare the distribution of CSTs between different cervical cytology results (normal and LSIL/HSIL), between different HPV statuses (HPV+ and HPV−) and between the two time points postpartum (6 and 12 months). Shannon indexes of the different CSTs were compared using the Mann-Whitney test in SPSS. The Linear Discriminative Analysis (LDA) Effect Size (LEfSe) program of the Galaxy environment48, which uses Kruskal-Wallis and estimates the effect size of the comparisons, was used for evidencing potential biomarkers that distinguish normal from lesion samples. A 99% confidence interval has been used in this analysis. We corrected the p-value obtained with LEfEe for multiple testing using the Bonferroni’s correction in the R package. The odds ratio (OR) and 95% confidence interval values for the presence of specific taxa associated with risk of lesions were estimated using Microsoft Excel for Windows, and p-values were calculated according to Sheskin49. We calculated the unadjusted OR using the Haldane’s modification, which adds 0.5 to all cells containing zero counts to allow statistical calculations. The OR was adjusted to confounder variables (smoking, sexual partners, ages and contraceptive methods) using logistic regression. The relative abundance of specific bacterial taxa was compared in groups of paired samples that maintained the lesion status (normal or lesion in both samples) or changed the status between samples (normal at 6 months and lesion at 12 months and vice-versa) with the Wilcoxon test in SPSS.
Data availability
All raw data files are available on Sequence Read Archive (SRA) upon accession number PRJNA392046.
Electronic supplementary material
Acknowledgements
We thank Carolina Furtado for help and advice to sequencing in the HiSeq 2500 Illumina platform and Anke Bergmann for support in statistical analyses. We are also indebted to Dr. Héctor Seuànez for providing the sequencing platform and the infrastructure where the samples of the study were run. This work was conducted at the Brazilian National Cancer Institute (INCA) and was supported by grants of the Rio de Janeiro State Science Foundation (FAPERJ) E26/170.026/2008 and of the Brazilian Research Council (CNPq) 573806/2008-0, both under the auspice of the Brazilian Institute of Science and Technology (INCT) for Cancer Control. GC received an MSc scholarship from the Brazilian Ministry of Health while performing this work.
Author Contributions
G.C. and M.A.S. conceived the study. E.S.M. and A.I.M. collected the samples and associated sociodemographic and clinical data. E.A.S., J.D.S. and G.C. performed DNA extraction and HPV typing, while G.C. generated all bacterial 16S data. G.C. and R.L.C. performed all bioinformatics analyses. G.C., R.L.C. and M.A.S. analyzed and interpreted the data. G.C., R.L.C. and M.A.S. wrote the paper. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17351-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bruni, L. et al. ICO Information Center on HPV and Cancer (HPV Information Center). Tech. Rep., Human Papillomavirus and Related Diseases in the World (2015).
- 2.Mitra A, et al. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4:58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravel J, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu MB, et al. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS ONE. 2013;8:e79812. doi: 10.1371/journal.pone.0079812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgdorff H, et al. Unique insights in the cervicovaginalLactobacillus iners and L. crispatus proteomes and their associations with microbiota dysbiosis. PLoS ONE. 2016;11:e0150767. doi: 10.1371/journal.pone.0150767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biagi E, et al. Quantitative variations in the vaginal bacterial population associated with asymptomatic infections: A real-time polymerase chain reaction study. European Journal of Clinical Microbiology and Infectious Diseases. 2009;28:281–285. doi: 10.1007/s10096-008-0617-0. [DOI] [PubMed] [Google Scholar]
- 7.van de Wijgert, J. H. H. M. & Jespers, V. The global health impact of vaginal dysbiosis. Research in Microbiology S0923–2508 (2017). [DOI] [PubMed]
- 8.Wijgert VD, J. H. H M, et al. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE. 2014;9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brotman RM. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. Journal of Clinical Investigation. 2011;121:4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh H, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clinical Microbiology and Infection. 2015;21:1–674. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Brotman RM, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. Journal of Infectious Diseases. 2014;210:1723–1733. doi: 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anahtar MN, et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham AG, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. Journal of acquired immune deficiency syndromes (1999) 2013;62:405–13. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgdorff H, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. The ISME journal. 2014;8:1781–93. doi: 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimers LL, et al. The Cervicovaginal Microbiota and Its Associations With Human Papillomavirus Detection in HIV-Infected and HIV-Uninfected Women. Journal of Infectious Diseases. 2016;214:1361–1369. doi: 10.1093/infdis/jiw374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eschenbach DA, Gravett MG, Chen KC, Hoyme UB, Holmes KK. Bacterial vaginosis during pregnancy. An association with prematurity and postpartum complications. Scandinavian Journal Of Urology And Nephrology. Supplementum. 1984;86:213–222. [PubMed] [Google Scholar]
- 17.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstetrics and gynecology. 1990;75:52–58. [PubMed] [Google Scholar]
- 18.MacIntyre DA, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Scientific reports. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajer P, et al. Temporal dynamics of the human vaginal microbiota. Science translational medicine. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forney, L. J. Microbial Ecology in States of Health and Disease (National Academies Press, Washington, D.C., 2014). [PubMed]
- 21.Aoki Y, Tosato G. Neoplastic Conditions in the Context of HIV-1 Infection. Current HIV Research. 2004;2:343–349. doi: 10.2174/1570162043351002. [DOI] [PubMed] [Google Scholar]
- 22.Gosmann C, et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity. 2017;46:29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J. E. et al. Association of the Vaginal Microbiota with Human Papillomavirus Infection in a Korean Twin Cohort. PLoS ONE8 (2013). [DOI] [PMC free article] [PubMed]
- 24.Fettweis JM, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (United Kingdom) 2014;160:2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos HC, et al. A minimum set of ancestry informative markers for determining admixture proportions in a mixed American population: the Brazilian set. European journal of human genetics: EJHG. 2015;24:1–7. doi: 10.1038/ejhg.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callegari-Jacques SM, et al. Historical genetics: Spatiotemporal analysis of the formation of the Brazilian population. American Journal of Human Biology. 2003;15:824–834. doi: 10.1002/ajhb.10217. [DOI] [PubMed] [Google Scholar]
- 27.Dareng EO, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiology and Infection. 2016;144:123–137. doi: 10.1017/S0950268815000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiGiulio DB, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proceedings of the National Academy of Sciences. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitra A, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Scientific Reports. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cipe G, Idiz U, Firat D, Bektasoglu H. Relationship between intestinal microbiota and colorectal cancer. World J Gastrointest Oncol. 2015;7:233–240. doi: 10.4251/wjgo.v7.i10.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Frontiers in Microbiology. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlier J-P, K’ouas G, Han XY. Moryella indoligenes gen. nov., sp. nov., an anaerobic bacterium isolated from clinical specimens. International journal of systematic and evolutionary microbiology. 2007;57:725–9. doi: 10.1099/ijs.0.64705-0. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes JV, et al. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review) Oncology Letters. 2015;9:1015–1026. doi: 10.3892/ol.2015.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korshunov VM, et al. The vaginal Bifidobacterium flora in women of reproductive age. Zhurnal mikrobiologii, epidemiologii, i immunobiologii. 1999;(4):74–78. [PubMed] [Google Scholar]
- 35.Sivan A, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes, K. R. et al. Bifidobacterium breve reduces apoptotic epithelial cell shedding in an exopolysaccharide and MyD88-dependent manner. Open Biology7 (2017). [DOI] [PMC free article] [PubMed]
- 37.Mohammadi T, Reesink HW, Vandenbroucke-Grauls CM, Savelkoul PH. Removal of contaminating DNA from commercial nucleic acid extraction kit reagents. Journal of Microbiological Methods. 2005;61:285–288. doi: 10.1016/j.mimet.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Newsome T, Li B-J, Zou N, Lo S-C. Presence of bacterial phage-like DNA sequences in commercial Taq DNA polymerase reagents. Journal of clinical microbiology. 2004;42:2264–7. doi: 10.1128/JCM.42.5.2264-2267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen H, Rogelj S, Kieft TL. Sensitive, real-time PCR detects low-levels of contamination by Legionella pneumophila in commercial reagents. Molecular and Cellular Probes. 2006;20:147–153. doi: 10.1016/j.mcp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Salter SJ, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biology. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon D, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Jama. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 42.Meyrelles ARI, et al. HIV/HPV co-infection during pregnancy in southeastern Brazil: Prevalence, HPV types, cytological abnormalities and risk factors. Gynecologic Oncology. 2013;128:107–112. doi: 10.1016/j.ygyno.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Ong SH, et al. Species Identification and Profiling of Complex Microbial Communities Using Shotgun Illumina Sequencing of 16S rRNA Amplicon Sequences. PLoS ONE. 2013;8:e60811. doi: 10.1371/journal.pone.0060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 46.Miller CS, et al. EMIRGE: reconstruction of full-length ribosomal genes from microbial community short read sequencing data. Genome Biology 2011 12:5. 2011;75:4599–4615. doi: 10.1186/gb-2011-12-5-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller CS, et al. Short-Read Assembly of Full-Length 16S Amplicons Reveals Bacterial Diversity in Subsurface Sediments. PLoS ONE. 2013;8:e56018. doi: 10.1371/journal.pone.0056018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biology. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheskin, D. J. Handbook of parametric and nonparametric statistical procedures, vol. 23 (Chapman & Hall, Boca Raton, FL, 2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data files are available on Sequence Read Archive (SRA) upon accession number PRJNA392046.