Abstract
Stachyflin, aureol, smenoqualone, strongylin A, and cyclosmenospongine belong to a family of tetracyclic meroterpenoids, which, by nature of their unique molecular structures and various biological properties, have attracted synthetic and medicinal chemists alike. Despite their obvious biosynthetic relationship, only scattered reports on the synthesis and biological investigation of individual meroterpenoids have appeared so far. Herein, we report a highly modular synthetic strategy that enabled the synthesis of each of these natural products and 15 non-natural derivatives. The route employs an auxiliary-controlled Diels–Alder reaction to enable the enantioselective construction of the decalin subunit, which is connected to variously substituted arenes by either carbonyl addition chemistry or sterically demanding sp2–sp3 cross-coupling reactions. The selective installation of either the cis- or trans-decalin stereochemistry is accomplished by an acid-mediated cyclization/isomerization reaction. Biological profiling reveals that strongylin A and a simplified derivative thereof have potent antibiotic activity against methicillin-resistant Staphylococcus aureus.
Polycyclic meroterpenoids show a wide range of biological activities. Here, the authors report a modular approach to synthesize a number of natural and non-natural tetracyclic meroterpenoids, which display antibiotic activity against methicillin-resistant Staphylococcus aureus.
Introduction
Meroterpenoids, which are derived from a mixed biosynthetic terpenoid pathway, display a broad spectrum of biological activities and are equipped with a wealth of structural complexity that originates from highly sophisticated biosynthetic pathways1–9. The structurally-related natural products stachyflin (1)10, aureol (2)8,11, smenoqualone (3)12, strongylin A (4)13, cyclosmenospongine (5)14, and mamanuthaquinone (6)15 constitute a unique subclass of polycyclic meroterpenoids that was previously harvested from marine and fungal sources. Since the first isolation of aureol in 1980, considerable interest has arisen to prepare these complex natural products by chemical synthesis and explore their biological activities. Despite the successful synthesis of individual members in a reasonable number of synthetic operations (10–27 linear steps), none of the reported routes16–24 has enabled a practical access to the whole family of these fascinating natural products (Fig. 1).
Fig. 1.
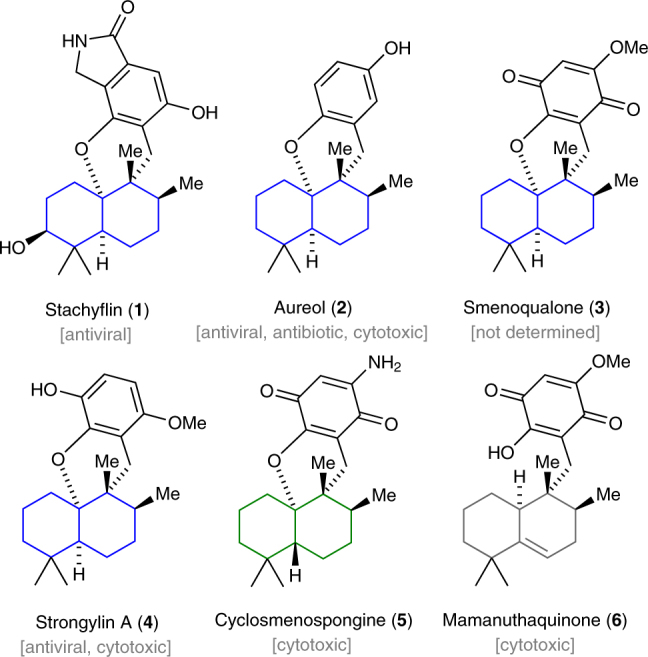
Selected members of polycyclic meroterpenoids and their reported biological activity. Within this subclass of natural products, two distinct stereochemistries with respect to the decalin subunit have been noted. While compounds 1–4 possess a cis-decalin, cyclosmenospongine (5) features a unique trans-decalin scaffold. Both stereochemistries might biosynthetically originate from a 5,6-dehydrodecalin framework as present in mamanuthaquione (6). Color coding: blue, cis-decalin; green, trans-decalin
Although scattered reports have revealed the antiviral (1, 2, and 4)10,13,25, anticancer (2, 4, 5, and 6)13–15,26, and antibiotic (2)27 activities of several members of these natural products, an exhaustive biological screen of 1–6 and fully synthetic derivatives is still unavailable. So far, only a preliminary structure–activity relationship (SAR) study of semi-synthetic analogs of stachyflin (1) has been reported, revealing that subtle modifications of the aromatic isoindolinone component have a drastic effect on the observed H1N1 activity28–32. Here, we address these limitations and describe a highly modular synthetic platform for the construction of six natural products and 15 fully synthetic molecules that were previously inaccessible using semi-synthesis. Biological profiling reveals that this class of meroterpenoids has potent antibiotic activity against methicillin-resistant Staphylococcus aureus.
Results
Total synthesis of (+)-stachyflin (1)
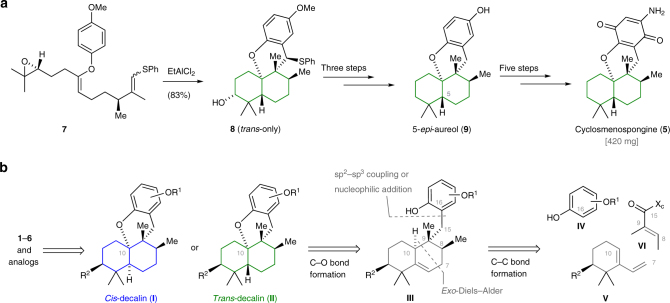
As illustrated in Fig. 2a, recent efforts by our group enabled a highly convergent and scalable route to the trans-decalin-containing natural product cyclosmenospongine (5). The developed synthesis proceeds via the intermediacy of 5-epi-aureol (9) and enabled production of 420 mg of 5 in a single batch33. However, all efforts to adapt this strategy for the construction of the cis-decalin subunit of 1–4 were unsuccessful.
Fig. 2.
Strategies for the construction of tetracyclic meroterpenoids. a The polyene cyclization reaction of aryl enol ether 7 enabled rapid assembly of the trans-decalin substructure of 5-epi-aureol (9) and cyclosmensopongine (5). However, this strategy could not be adapted for the preparation of meroterpenoids containing a cis-decalin subunit. b Synthesis of the 5,6-dehydrodecalin intermediate III should be accomplished by a highly modular, three-component coupling strategy of phenol IV, diene V, and dienophile VI. The ability to construct both cis- and trans-decalins from a common precursor would allow for the synthesis of 1–6 and a variety of non-natural derivatives for biological screening. Color coding: blue, cis-decalin, green, trans-decalin. R1 = alkyl, R2 = H or OBn, Xc = chiral auxiliary
We envisioned the synthesis of 1–6 by employing the highly convergent strategy depicted in Fig. 2b. For the retrosynthetic analysis, 1–6 were first traced back to their protected forms I and II. The carbon–oxygen bond disconnection at C10 leads to the 5,6-dehydrodecalin precursor III, which would enable the crucial late-stage assembly of either the cis- (kinetic product) or trans-decalin (thermodynamic product) by an acid-promoted isomerization/cyclization sequence. This event sets the remaining two of four consecutive stereocenters. To account for maximum modularity and convergence, we opted to break down III further into the simple building blocks phenol IV, diene V, and tiglic acid-derived dienophile VI using a sp2–sp3 cross-coupling (or nucleophilic addition) and an exo-selective, auxiliary-controlled Diels–Alder reaction that was described in seminal work by Danishefsky34 and Minnaard35.
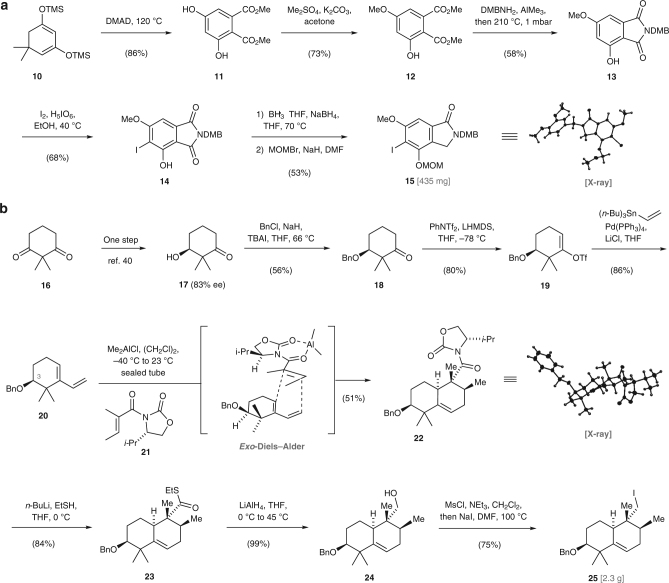
We began our investigations with the synthesis of stachyflin (1) which contains a rare isoindolinone subunit (Fig. 3a)36. As initial efforts to construct intermediate 15 according to a previously reported protocol37 were low yielding and not reproducible on a large scale, we set out to develop a more robust route. Our synthesis begins with a solvent-free Alder–Rickert reaction between the dimedone-derived bis-trimethylsilyl enol ether 10 and dimethyl 2-butynedioate (DMAD)38 to afford resorcinol 11 (86%). Careful monitoring of the reaction progress enables mono-methylation (Me2SO4, K2CO3, acetone) of 11 to furnish phenol 12 as a white crystalline solid in 73% yield. Exposure of 12 to a solution of 3,4-dimethoxybenzylamine (DMBNH2) and trimethylaluminum (Me3Al) effected clean conversion to the corresponding N,N-3,4-dimethoxydibenzyl phthalamide. The subsequent formation of imide 13 was induced by heating the neat phthalamide at 210 °C under reduced pressure (1 mbar) with simultaneous removal of the liberated N,N-3,4-dimethoxydibenzyl amine. While bromination (Br2, CH2Cl2 or Br2, AcOH) of 13 gave predominately the para-substituted phenol, exclusive formation of 14 occurred upon treatment with substoichiometric amounts of both iodine (0.6 equiv.) and periodic acid (0.2 equiv.)39. Treating a solution of 14 in tetrahydrofuran (1.0 M) in a sealed tube with borane tetrahydrofuran complex (3.0 equiv.) and substoichiometric quantities of sodium borohydride (0.05 equiv.) at elevated temperature (70 °C) effected regioselective reduction of the imide40. This protocol was crucial to obtain the product as a single regioisomer in high yield. Protection of the free phenol as its methoxymethyl ether completed the synthesis of isoindolinone 15 (435 mg). The overall sequence to 15 proceeds in six steps and involves only crystalline intermediates, thus making it practical on a large scale.
Fig. 3.
Synthesis of the isoindolinone component 15 and the dehydrodecalin 25. a The developed de novo synthesis of isoindolinone 15 proceeds in six linear steps and only involves crystalline intermediates. b For the construction of the 5,6-dehydrodecalin 25, an auxiliary-controlled exo-selective Diels–Alder cycloaddition was employed. This allowed the production of 25 in eight steps in gram quantities in a single batch. DMAD dimethyl acetylenedicarboxylate, n-Bu n-butyl, Bn benzyl, DMB 3,4-dimethoxybenzyl, DMF N,N-dimethylformamide, LHMDS lithium hexadimethylsilazide, MOM methoxymethyl, Ms methanesulfonyl, TBAI tetrabutylammonium iodide, Tf trifluoromethanesulfonyl, THF tetrahydrofuran
We then focused on the construction of the 5,6-dehydrodecalin component 25 by employing Fan auxilliary-controlled exo-selective Diels–Alder cycloadditon between diene 20 and tiglic acid-derived dienophile 21 (Fig. 3b).
Although the utility of 21 was previously demonstrated by Minnaard for the synthesis of 1-tuberculosinyl adenosine35, we were uncertain about the capability of 21 to override the inherent substrate selectivity of 20 and the extent of steric hindrance resulting from the benzyl ether at C3. Diene 20 was prepared from a known β-hydroxyketone 17 41 via a three-step sequence involving the formation of the benzyl-protected ketone 18, conversion to the vinyltriflate 19, and Stille coupling (vinylSn(n-Bu3), Pd(PPh3)4, LiCl, THF, 75 °C) to install the diene motif. Initial attempts to promote the reaction between 20 and 21 confirmed our concerns that 21 is reluctant to undergo cycloaddition under standard conditions (Me2AlCl, (CH2Cl)2, –40 °C to 23 °C). In order to overcome the low reactivity of 20, we first subjected the reactants solution to high pressure (14 kbar, 23 °C, Me2AlCl, CH2Cl2)42. Although the formation of the desired Diels–Alder product 22 was observed under these forcing conditions, the product yield was low due to competing decomposition to an intractable mixture of products. However, the relative stereochemistry of 22 could be validated by single-crystal structure analysis. After further optimization, we found that 22 could be reproducibly obtained in a good yield and excellent diastereoselectivity (dr = 13:1) by conducting the cycloaddition in 1,2-dichloroethane in a sealed tube and slowly warming the reaction mixture from –40 °C to 23 °C. In this context, it is important to note that the chiral auxiliary fully overrides the substrate selectivity and the observed diastereoselectivity corresponds to the optical purity of 20 (83% ee). Further conversion of 22 to iodide 25 via the intermediacy of thioester 23 and alcohol 24 proceeded smoothly to provide 2.3 g of 25 in a single batch.
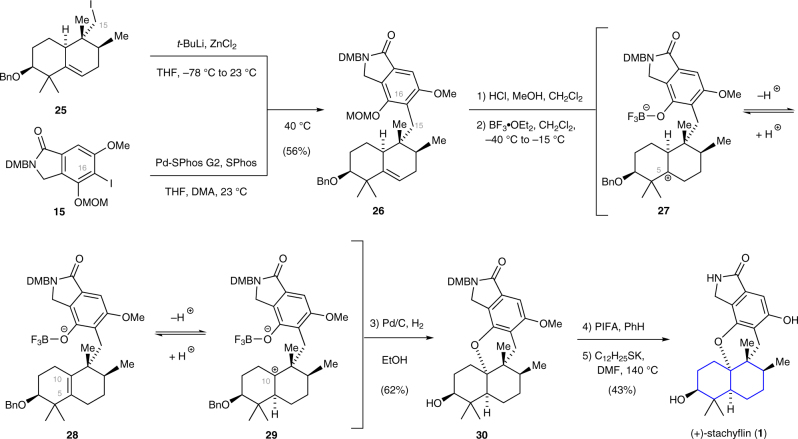
With both components in hand, we turned our attention to the critical linkage of 15 to 25 (Fig. 4). This process was expected to be exceptionally challenging as it requires carbon–carbon bond formation between C15, which resides at a sterically hindered neopentylic position and C16, itself flanked by two alkyl ether substituents of the arene. From an evaluation of different coupling strategies and based on our recent success to realize challenging carbon–carbon bond formations43, a sp2–sp3 Negishi cross-coupling reaction44 emerged as the method of choice. To this end, we subjected both coupling partners to an exhaustive screen of reactions conditions (see Supplementary Table 1). We found that the coupling could be efficiently mediated by treating a solution of 25, Pd-SPhos G2 (20 mol%), and SPhos (20 mol%)44 in tetrahydrofuran and N,N-dimethylacetamide (2:1) with the organozinc species derived from 15. The use of N,N-dimethylacetamide as a co-solvent and slightly elevated temperature (40 °C) were crucial to reproducibly observe full conversion, short reaction times, and acceptable yields (56%).
Fig. 4.
Component coupling and total synthesis of (+)-stachyflin (1). The sp2–sp3 Negishi cross-coupling reaction between arene 15 and iodide 25 provided the precursor for the intended cyclization reaction. Promotion of this step was accomplished by the addition of excess boron trifluoride etherate (10 equiv.) at low temperature (–40 °C) to exclusively produce the cis-decalin 30. At temperatures exceeding –15 °C, competing ionization of the C3 position and ring-contraction prevailed (byproduct not shown, see Supplementary Methods). t-BuLi tert-butyllithium, DMA N,N-dimethylacetamide, PIFA phenyliodine bis(trifluoroacetate), SPhos 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl, SPhos-Pd G2 chloro(2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II)
Having prepared the 5,6-dehydrodecalin intermediate 26 (330 mg), the stage was set to investigate the key-transformation for the installation of the cis-decalin framework. This step was inspired by a previous work on effect-related cascade cyclizations45 and was realized by first cleaving the methoxymethyl ether in 26. The so-formed phenol was unstable upon standing and therefore was directly exposed to boron trifluoride etherate at –40 °C. Slowly raising the temperature to –15 °C over a period of 1 h led to full consumption of the phenol. Although the mechanism of this cyclization reaction is still unclear, it may be that protonation of the alkene first affords the C5 carbocation 27. Whether this directly undergoes stereospecific 1,2-hydride shift to give the C10 carbocation 29 or involves the intermediacy of the C5–C10 alkene 28 is uncertain at this point. At temperatures below –15 °C, trapping of the cation by the phenol is kinetically controlled to exclusively provide the cis-decalin. The use of the secondary benzyl-protecting group and maintaining temperatures below –15 °C proved to be essential to minimize ionization of the C3 position and undesirable ring contraction (see Supplementary Methods and Supplementary Fig. 61). Hydrogenolysis of the cyclization product facilitated purification and afforded 30 in 62% yield. For the completion of the synthesis of 1, the DMB group was removed oxidatively using previously reported conditions (PIFA, benzene)46. Finally, cleavage of the methyl ether with potassium n-dodecanethiolate (n-C12H24SK) in N,N-dimethylformamide (DMF) at 140 °C proceeded smoothly to afford (+)-stachyflin (1). Spectroscopic data (1H NMR13,C NMR, optical rotation) were found to be identical with those reported for natural 1 10.
Total synthesis of natural products 2–6
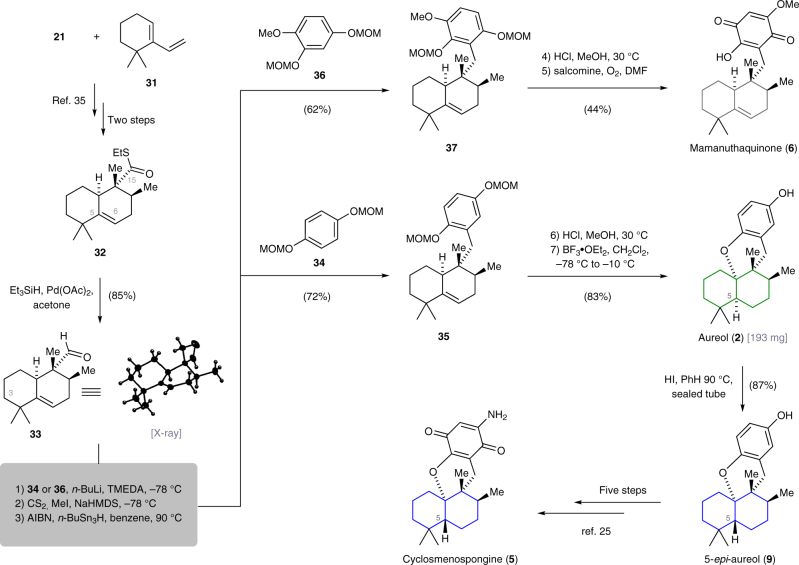
Having established a convergent and scalable synthesis of tetracyclic meroterpenoids bearing a cis-fused decalin system, we set out to modify the route in order to incorporate selective modifications and expand our library of natural and non-natural analogs. For the asymmetric synthesis of 2–6, which are derived from the common 3-deoxy-5,6-dehydrodecalin subunit 33, we utilized the previously reported two-step sequence by Minnaard (Fig. 5)35. Thioester 32 underwent smooth Fukuyama reduction47 to give aldehyde 33, whose relative stereochemistry was validated by single-crystal structure analysis (see Supplementary Fig. 62). Pleasingly, the use of the less sterically demanding arene component 34 48 enabled replacement of the previously required sp2–sp3 cross-coupling reaction and thus simplified the installation of the crucial C15–C16 carbon–carbon bond. The ortho-directed lithiation of 34 followed by 1,2-addition to 33 gave a mixture of diastereoisomeric benzyl alcohols. Application of the two-step Barton–McCombie deoxygenation protocol (CS2, MeI, NaHMDS, then AIBN, n-Bu3SnH, 90 °C)49 reproducibly provided 35 in good yield (72%). For the completion of the synthesis of aureol (2), both methoxymethyl ethers of 35 were first cleaved upon exposure to hydrochloric acid in methanol. The resulting hydroquinone was prone to oxidation and therefore was not purified but directly subjected to the optimized cyclization conditions (BF3·OEt2, CH2Cl2, –40 °C to –10 °C) to afford 193 mg of (+)-aureol (2) in a single batch. From there, the non-natural 5-epi-derivative 9 was prepared by thermal isomerization of the cis-decalin using hydroiodic acid in benzene at 90 °C (87%)50.
Fig. 5.
Chemical synthesis of (+)-aureol (2), (–)-cyclosmenospongine (5), (–)-mamanuthaquinone (6), and (+)-5-epi-aureol (9). The 3-deoxy-5,6-dehydrodecalin unit 33 was accessed in three steps from the known coupling of 21 and 31. Installation of the C15–C16 carbon–carbon bond was accomplished by ortho-directed lithiation of 34 or 36 and nucleophilic addition to 33. AIBN azobisisobutyronitrile, NaHMDS sodium hexadimethylsilazide, TMEDA N,N,N,N′-tetramethylethylenediamine
In addition, cyclosmenospongine (5) was accessible by subjecting 9 to our previously developed functionalization sequence33. In a similar vein, mamanuthaquinone (6) was synthesized by the coupling between 33 and arene 36 to give 37. Compound 37 was deprotected and then oxidized (salcomine, O2) to give 6, which slowly decomposed upon storage at –20 °C. Having demonstrated the generality and versatility of the developed modular synthetic platform, further structural modifications could be efficiently made by simple variation of the arene and decalin component, and adjustment of the cyclization conditions. While kinetic conditions (BF3·OEt2, CH2Cl2, –40 °C to –10 °C) gave the cis-decalin framework exclusively, equilibration under thermodynamic conditions (HI, benzene, 90 °C) afforded the trans-decalin as the only stereoisomer.
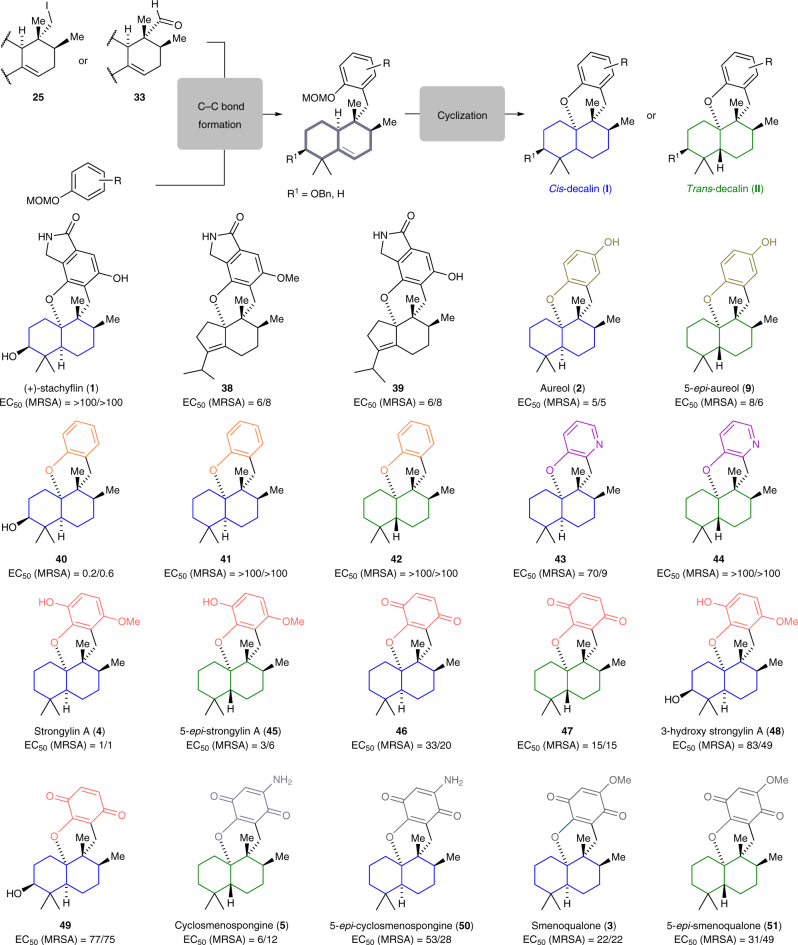
In this manner, (+)-smenoqualone (3) and (+)-strongylin A (4), and 15 fully synthetic tetracyclic analogs that were previously inaccessible via semi-synthesis could be prepared (Fig. 6 and Supplementary Methods). With the synthetic natural products 1–5 as well as the analogs 9 and 38–51 at hand, a basic phenotypic bioprofile of meroterpenoids was recorded in antibacterial and antiproliferative assays. Antimicrobial activities were tested against members of the ESKAPE panel51, consisting of the gram-positive bacteria methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcus faecium, the gram-negative bacteria Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumonia, and the yeast fungus Candida albicans. All compounds proved to be inactive against gram-negative pathogens and C. albicans. However, several analogs inhibited the growth of the MRSA-type strain DSM 11822 and the MRSA clinical isolate RKI 11-02670 with the following SARs (Fig. 6 and Supplementary Table 21): the highest activities were observed for 40, with EC50 values of 0.2 and 0.6 μM against DSM 11822 and RKI 11-02670, respectively, and for strongylin A (4), which was active with EC50 values of 1 and 1 μM. Surprisingly, the combination of the heteroatom functionalities of 4 and 40 led to a pronounced drop of activity, as demonstrated by 3-hydroxy-strongylin (48) (83/49 μM). Related compounds with 3-hydroxy function and a para-quinone unit like 49 or a heterocycle as found in stachyflin (1), 43 and 44 had also had little or no activity. In line with this, a non-hydroxylated, contracted cyclopentene ring, as present in the stachyflin analog 39, led to re-gained activity (6/8 μM). On the other hand, a lack of functionalities at the decalin and aromatic subunit also led to inactive compounds, as demonstrated by 41 and 42. While the mono-hydroxylated aureol 2 exhibited a potency (5/5 μM) comparable to 4, the oxidation of the methylated hydroquinone to a para-quinone as present in 46 was associated with a pronounced drop of anti-MRSA activity to 33/20 μM. A clear activity ranking for a cis- vs. trans-configuration of the decalin ring was not evident: the trans isomer was more potent in the 46 vs. 47, 3 vs. 51, and 5 vs. 50 pairs, while the opposite was true for the 4 vs. 45 and the 43 vs. 44 pairs. The antiproliferative activities of the compounds in the four mammalian cell lines L929, KB-3-1, MCF-7, and FS4-LTM were tested using a WST-1 assay that quantifies the metabolic activity of the cell population (Supplementary Table 22). The highest activities were observed for 40 (EC50 values of 7–14 μM) and 49 (EC50 values of 8–21 μM), both hydroxylated at the C3 position. The observation that the SAR did not parallel the antimicrobial activity suggests that a separation of antibiotic and cytotoxic activities is possible, and an even larger split may be obtained by further structural optimization.
Fig. 6.
Extension of the modular synthetic platform. Structural variation of the arene and decalin component enabled rapid extension of the natural products library and provided access to several non-natural analogs that were previously inaccessible via semi-synthesis. Each of the shown molecules depicted was prepared in seven or fewer steps starting from iodide 25 or aldehyde 33. Color coding was used to indicate the decalin stereochemistry (coding: blue, cis-decalin; green, trans-decalin) and to highlight the modified arene component. The effective concentrations (EC50 values) that inhibited the growth of two MRSA strains (DSM 11822/RKI 11-02670) are given in μM
Discussion
In summary, we established a highly modular and robust synthetic platform for the construction of variously substituted meroterpenoid scaffolds. Our efforts culminated in the enantioselective total syntheses of (+)-stachflin (1), (+)-aureol (2), (+)-smenoqualone (3), (+)-stronglin A (4), (–)-cyclosmenospongine (5), and (–)-mamanuthaquinone (6). Key steps include an asymmetric Diels–Alder reaction to install the 4,5-dehydrodecalin framework, either a highly efficient sp2–sp3-Negishi cross-coupling reaction or a nucleophilic addition reaction to forge the crucial C15–C16 carbon–carbon bond, and an acid-mediated cyclization to selectively generate either stereoisomers of the decalin subunit. Simultaneous structural variation of more than one coupling component leads to rapid expansion of the compound library. The library of natural and fully synthetic molecules obtained so far was screened against a panel of bacterial pathogens and mammalian cell lines. Notably, the pronounced antibiotic activity of 40, (+)-strongylin A (4), and (+)-aureol (2) against MRSA in the low-μM range appears promising. The reported SAR suggests that a further enhancement of activity is possible, e.g., through modifications at the aromatic ring, and such modifications enabled by the described synthetic platform.
Methods
NMR spectroscopy
NMR spectra were measured on a Bruker Avance III HD (400 MHz for proton nuclei, 100 MHz for carbon nuclei) spectrometer equipped with a CryoProbeTM, Bruker AXR300 (300 MHz for proton nuclei, 75 MHz for carbon nuclei), Varian VXR400 S (400 MHz for proton nuclei, 100 MHz for carbon nuclei), Bruker AMX600 (600 MHz for proton nuclei, 150 MHz for carbon nuclei), or Bruker Avance HD 800 (800 MHz for proton nuclei, 200 MHz for carbon nuclei). Proton chemical shifts are expressed in parts per million (ppm, δ scale) and the residual protons in the NMR solvent (CHCl3, δ = 7.26 ppm; C6D5H, δ = 7.16 ppm; DMSO-d6, δ = 2.50 ppm) were used as internal reference. Carbon chemical shifts are expressed in parts per million (δ scale, assigned carbon atom) and the residual solvent peaks (CDCl3, δ = 77.16 ppm; C6D6, δ = 128.06 ppm; DMSO-d6, δ = 39.52 ppm) were used as internal reference. The NMR spectroscopic data are reported as follows: chemical shift in ppm (multiplicity, coupling constants J (Hz), integration intensity) for 1H NMR spectra and chemical shift in ppm for 13C NMR spectra. Multiplicities are abbreviated as s (singlet), br s (broad singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Signals in the NMR spectra were assigned by the information obtained from 2D NMR experiments: homonuclear correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond coherence (HMBC). The software MestReNOVA 11.0 from Mestrelab Research S. L was used to analyze and process all raw fid files.
Mass spectrometry
High resolution mass spectra (HRMS) were measured at the Department of Chemistry, Ludwig-Maximilians-University Munich, on the following instruments by electron impact (EI) or electron spray (ESI) techniques: MAT 95 (EI) and MAT 90 (ESI) from Thermo Finnigan GmbH.
IR spectroscopy
Infrared spectra (IR) were recorded on a PerkinElmer Spectrum BX II FT-IR system from 4000 to 600 cm–1. Substances were directly applied on the ATR unit as a thin film or a thin powder layer. The data are represented as frequency of absorption (cm−1).
Optical rotation
Optical rotation values were recorded on a PerkinElmer 241 or Anton Paar MCP 200 polarimeter. The specific rotation is calculated as follows: . The wave length λ is reported in nm (sodium D line, λ = 589 nm), the measuring temperature ϕ in °C; α represents the recorded optical rotation, c the concentration of the analyte in g mL−1, and d the length of the cuvette in dm. Thus, the specific rotation is given in 10−1 deg cm2 g−1. The values for the specific rotation are reported as follows: specific rotation (concentration g 100 mL−1; solvent).
Melting points
Melting points were determined on a B-450 melting point apparatus from BÜCHI Labortechnik AG.
Data availability
CCDC 1534418 (15), 1534416 (22), and 1534618 (33) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre. The authors declare that all the data supporting the findings of this study are available within the article (and its supplementary information files).
Electronic supplementary material
Acknowledgements
T.M. acknowledges the German Research Foundation (Emmy Noether Project MA5999/2-1), the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement No 714049) and the Funds of the Chemical Industry (Sachkostenzuschuss and Dozentenpreis). We thank Dr. Kevin Mellem (Revolution Medicines) and Dr. Bryan Matsuura (LMU Munich) for helpful discussions.
Author contributions
R.W., K.S., F.-L.H., and T.M. conceived the synthetic route. R.W., K.S., F.-L.H., and B.K. conducted all experimental work and analyzed the results. R.W., M.B., and T.M. analyzed the data and wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-017-02061-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baunach M, Franke J, Hertweck C. Angew. Chem. Int. Ed. 2015. Terpenoid biosynthesis off the beaten track: unconventional cyclases and their impact on biomimetic synthesis; pp. 2604–2626. [DOI] [PubMed] [Google Scholar]
- 2.Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 2006;106:3412–3442. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]
- 3.Hertweck C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009;48:4688–4716. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- 4.Cornforth JW. Terpenoid biosynthesis. Chem. Ber. 1968;4:102–106. [PubMed] [Google Scholar]
- 5.Capon RJ. The acid-catalyzed rearrangement and absolute stereochemistry of isospongiaquinone. J. Nat. Prod. 1990;53:753–756. doi: 10.1021/np50069a042. [DOI] [Google Scholar]
- 6.Lakshmi V, Gunasekera SP, Schmitz FJ, Ji X, Van der Helm D. Acid-catalyzed rearrangement of arenerol. J. Org. Chem. 1990;55:4709–4711. doi: 10.1021/jo00302a042. [DOI] [Google Scholar]
- 7.Paul, M. D. Medicinal Natural Products: A Biosynthetic Approach 2nd edn (Wiley, Chichester, 2002).
- 8.Djura P, et al. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (polyfibrospongia) echina. J. Org. Chem. 1980;45:1435–1441. doi: 10.1021/jo01296a019. [DOI] [Google Scholar]
- 9.Urban S, Capon RJ. Marine sesquiterpene quinones and hydroquinones: acid-catalysed rearrangements and stereochemical investigations. Aust. J. Chem. 1994;47:1023–1029. doi: 10.1071/CH9941023. [DOI] [Google Scholar]
- 10.Minagawa K, et al. Stachyflin and acetylstachyflin, novel anti-influenza A virus substances, produced by stachybotrys sp. RF-7260. J. Antibiot. 2002;55:155–164. doi: 10.7164/antibiotics.55.155. [DOI] [PubMed] [Google Scholar]
- 11.Ciminiello P, Aversano CD, Fattorusso E, Magno S, Pansini M. Chemistry of verongida sponges. Secondary metabolite composition of the Caribbean sponge Verongula gigantea. J. Nat. Prod. 2000;63:263–266. doi: 10.1021/np990343e. [DOI] [PubMed] [Google Scholar]
- 12.Bourguet-Kondracki ML, Martin MT, Guyot M. Smenoqualone a novel sesquiterpenoid from the marine sponge Smenospongia sp. Tetrahedron Lett. 1992;33:8079–8080. doi: 10.1016/S0040-4039(00)74722-8. [DOI] [Google Scholar]
- 13.Wright AE, Rueth SA, Cross SS. An antiviral sesquiterpene hydroquinone from the marine sponge Strongylophora hartmani. J. Nat. Prod. 1991;54:1108–1111. doi: 10.1021/np50076a032. [DOI] [PubMed] [Google Scholar]
- 14.Utkina NK, Denisenko VA, Scholokova OV, Virovaya MV, Prokof’eva NG. Cyclosmenospongine, a new sesquiterpenoid aminoquinone from an Australian marine sponge Spongia sp. Tetrahedron Lett. 2003;44:101–102. doi: 10.1016/S0040-4039(02)02497-8. [DOI] [Google Scholar]
- 15.Swersey JC, Barrows LR, Ireland CM. Mamamuthaquinone: an antimicrobial and cytotoxic metabolite of Fasciospongia sp. Tetrahedron Lett. 1991;32:6687–6690. doi: 10.1016/S0040-4039(00)93575-5. [DOI] [Google Scholar]
- 16.Watanabe K, Sakurai J, Abe H, Katoh T. Total synthesis of (+)-stachyflin: a potential anti-influenza A virus agent. Chem. Commun. 2010;46:4055–4057. doi: 10.1039/c000193g. [DOI] [PubMed] [Google Scholar]
- 17.Taishi T, Takechi S, Mori S. First total synthesis of (±)-stachyflin. Tetrahedron Lett. 1998;39:4347–4350. doi: 10.1016/S0040-4039(98)00769-2. [DOI] [Google Scholar]
- 18.Sakurai J, Kikuchi T, Takahashi O, Watanabe K, Katoh T. Enantioselective total synthesis of (+)-stachyflin: a potential anti-influenza A virus agent isolated from a microorganism. Eur. J. Org. Chem. 2011;2011:2948–2957. doi: 10.1002/ejoc.201100173. [DOI] [Google Scholar]
- 19.Nakamura M, et al. An efficient synthesis of (+)-aureol via boron trifluoride etherate-promoted rearrangement of (+)-arenarol. Tetrahedron Lett. 2002;43:6929–6932. doi: 10.1016/S0040-4039(02)01627-1. [DOI] [Google Scholar]
- 20.Rosales A, et al. Synthesis of (±)-aureol by bioinspired rearrangements. J. Org. Chem. 2015;80:1866–1870. doi: 10.1021/jo502841u. [DOI] [PubMed] [Google Scholar]
- 21.Marcos IS, et al. Synthesis of quinone/hydroquinone sesquiterpenes. Tetrahedron. 2010;66:8280–8290. doi: 10.1016/j.tet.2010.08.038. [DOI] [Google Scholar]
- 22.Kuan KKW, Pepper HP, Bloch WM, George JH. Total synthesis of (+)-aureol. Org. Lett. 2012;14:4710–4713. doi: 10.1021/ol301715u. [DOI] [PubMed] [Google Scholar]
- 23.Kamishima T, Kikuchi T, Katoh T. Total synthesis of (+)-strongylin A, a rearranged sesquiterpenoid hydroquinone from a marine sponge. Eur. J. Org. Chem. 2013;2013:4558–4563. doi: 10.1002/ejoc.201300438. [DOI] [Google Scholar]
- 24.Katoh T, Atsumi S, Saito R, Narita K, Katoh T. Unified synthesis of the marine sesquiterpene quinones (+)-smenoqualone, (–)-Ilimaquinone, (+)-smenospongine, and (+)-sospongiaquinone. Eur. J. Org. Chem. 2017;2017:3837–3849. doi: 10.1002/ejoc.201700609. [DOI] [Google Scholar]
- 25.Wright, A. E., Cross, S. S., Burres, Neal, S. & Koehn, F. Novel antiviral and antitumor terpene hydroquinones and methods of use. US patent 5,051,519 (1990).
- 26.Mani L, et al. New antiplasmodial bromotyrosine derivatives from Suberea ianthelliformis Lendenfeld, 1888. Chem. Biodivers. 2012;9:1436–1451. doi: 10.1002/cbdv.201100309. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, et al. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 2002;65:476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- 28.Motohashi Y, et al. Antiviral activity of stachyflin on influenza A viruses of different hemagglutinin subtypes. Virol. J. 2013;10:118. doi: 10.1186/1743-422X-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi S, et al. Development of anti-influenza virus drugs I: improvement of oral absorption and in vivo anti-influenza activity of stachyflin and its derivatives. Pharm. Res. 1999;16:1041–1046. doi: 10.1023/A:1018983715982. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto J, et al. Development of anti-influenza drugs: II. Improvement of oral and intranasal absorption and the anti-influenza activity of stachyflin derivatives. J. Pharm. Pharmacol. 2000;52:1247–1255. doi: 10.1211/0022357001777225. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto J, et al. Identification of a novel HA conformational change inhibitor of human influenza virus. Arch. Virol. 1999;144:865–878. doi: 10.1007/s007050050552. [DOI] [PubMed] [Google Scholar]
- 32.Yanagita H, et al. Mechanism of drug resistance of hemagglutinin of influenza virus and potent scaffolds inhibiting its function. ACS Chem. Biol. 2012;7:552–562. doi: 10.1021/cb200332k. [DOI] [PubMed] [Google Scholar]
- 33.Speck K, Wildermuth R, Magauer T. Convergent assembly of the tetracyclic meroterpenoid (–)-cyclosmenospongine by a non-biomimetic polyene cyclization. Angew. Chem. Int. Ed. 2016;55:14131–14135. doi: 10.1002/anie.201608040. [DOI] [PubMed] [Google Scholar]
- 34.Yoon T, Danishefsky SJ, de Gala S. Angew. Chem. Int. Ed. 1994. A concise total synthesis of (±)-mamanuthaquinone by using an exo-Diels–Alder reaction; pp. 853–855. [Google Scholar]
- 35.Buter J, et al. Stereoselective synthesis of 1-tuberculosinyl adenosine; a virulence factor of Mycobacterium tuberculosis. J. Org. Chem. 2016;81:6686–6696. doi: 10.1021/acs.joc.6b01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speck K, Magauer T. The chemistry of isoindole natural products. Beilstein J. Org. Chem. 2013;9:2048–2078. doi: 10.3762/bjoc.9.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue S, Kim R, Hoshino Y, Honda K. Synthesis of tricyclic pyrano[2,3-e]isoindolin-3-ones as the core structure of stachybotrin A, B, and C. Chem. Commun. 2006;0:1974–1976. doi: 10.1039/b601433j. [DOI] [PubMed] [Google Scholar]
- 38.Quintiliano SAP, Silva LF. Practical synthesis of a functionalized 1-oxo-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid. Tetrahedron Lett. 2012;53:3808–3810. doi: 10.1016/j.tetlet.2012.05.060. [DOI] [Google Scholar]
- 39.Ende CW, Zhou Z, Parker KA. Total synthesis of (±)-bisabosqual A. J. Am. Chem. Soc. 2013;135:582–585. doi: 10.1021/ja3108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito S, Ishikawa T, Kuroda A, Koga K, Moriwake T. A revised mechanism for chemoselective reduction of esters with borane-dimethyl sulfide complex and catalytic sodium tetrahydroborate directed by adjacent hydroxyl group. Tetrahedron. 1992;48:4067–4086. doi: 10.1016/S0040-4020(01)92187-8. [DOI] [Google Scholar]
- 41.Yeung YY, Chein RJ, Corey EJ. Conversion of Torgov’s synthesis of estrone into a highly enantioselective and efficient process. J. Am. Chem. Soc. 2007;129:10346–10347. doi: 10.1021/ja0742434. [DOI] [PubMed] [Google Scholar]
- 42.Hugelshofer CL, Magauer T. High-pressure transformations in natural product synthesis. Synthesis. 2014;46:1279–1296. doi: 10.1055/s-0033-1341073. [DOI] [Google Scholar]
- 43.Speck K, Magauer T. Evolution of a polyene cyclization cascade for the total synthesis of (–)-cyclosmenospongine. Chem. Eur. J. 2016;23:1157–1165. doi: 10.1002/chem.201605029. [DOI] [PubMed] [Google Scholar]
- 44.Thaler T, et al. Highly diastereoselective Csp3–Csp2 Negishi cross-coupling with 1,2-, 1,3- and 1,4-substituted cycloalkylzinc compounds. Nat. Chem. 2010;2:125–130. doi: 10.1038/nchem.505. [DOI] [PubMed] [Google Scholar]
- 45.Katoh T. Total synthesis of decahydrobenzo[d]xanthene sesquiterpenoids aureol, strongylin A, and stachyflin: development of a new strategy for the construction of a common tetracyclic core structure. Heterocycles. 2013;87:2199–2223. doi: 10.3987/REV-13-779. [DOI] [Google Scholar]
- 46.Watanabe K, Shibata H, Imai Y, Katoh T. Deprotection of 3,4-dimethoxybenzyl (3,4DMB) group on γ-lactam nitrogen using phenyliodine(III) bis(trifluoroacetate) (PIFA): application to isoindolinone compounds. Heterocycles. 2012;84:1355–1361. doi: 10.3987/COM-11-S(P)77. [DOI] [Google Scholar]
- 47.Fukuyama T, Lin SC, Li L. Facile reduction of ethyl thiol esters to aldehydes: application to a total synthesis of (+)-neothramycin A methyl ether. J. Am. Chem. Soc. 1990;112:7050–7051. doi: 10.1021/ja00175a043. [DOI] [Google Scholar]
- 48.Shi ZF, Wang LJ, Wang H, Cao XP, Zhang HL. Synthesis of oligo(phenylene ethynylene)s with dendrimer ‘shells’ for molecular electronics. Org. Lett. 2007;9:595–598. doi: 10.1021/ol062793l. [DOI] [PubMed] [Google Scholar]
- 49.Barton DHR, McCombie SW. A new method for the deoxygenation of secondary alcohols. J. Chem. Soc. Perkin Trans. 1975;1:1574–1585. doi: 10.1039/p19750001574. [DOI] [Google Scholar]
- 50.Marcos I, et al. Chemistry of ent-halimic acid: synthesis of [4.3.3]propellanes. Synthesis. 2006;2006:3865–3873. doi: 10.1055/s-2006-950300. [DOI] [Google Scholar]
- 51.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CCDC 1534418 (15), 1534416 (22), and 1534618 (33) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre. The authors declare that all the data supporting the findings of this study are available within the article (and its supplementary information files).