Abstract
Percentage of body fat (%BF) is estimated in clinical practice using anthropometric equations, but little is known about their reliability in overweight/obese patients with type-2 diabetes. The aim of this study was to compare, in overweight/obese adults with type-2 diabetes, %BF estimated with several commonly used anthropometric equations and %BF measured with dual-energy X-ray absorptiometry (DXA, Hologic). The %BF was measured with DXA in 40 patients aged 40–68 years with type-2 diabetes (mean HbA1c, 7.3 ± 0.9%). Body density was estimated in the same patients by means of four anthropometric equations and converted to %BF using the Siri and Brozek equations. Paired-sample t-test and the mean signed difference procedure were used to compare anthropometric equation-derived %BF and DXA measurements. The coefficient of determination was computed. Bland-Altman analysis was used to test the agreement between methods. Among the four anthropometric equations, the Durnin-Womersley equation only showed close agreement with DXA in both female and male patients; the other equations significantly underestimated or overestimated %BF. Two new predictive equations were developed using DXA as the reference to predict total body and trunk %BF. Further comparative studies are required to confirm and refine the accuracy of practical, non-invasive methods for monitoring %BF in this population.
Introduction
Type-2 diabetes is a very common metabolic disorder characterized by hyperglycemia, which is related to insulin resistance and relative insulin deficiency1. Excess body fat (BF) contributes to impairing insulin action and is thought to be the primary factor underlying the global epidemic of type-2 diabetes which occurs worldwide. Consistently, rates of type-2 diabetes have increased markedly in parallel with those of obesity2. Moreover, excess BF is among the strongest risk factors for cardiovascular morbidity and mortality in type-2 diabetes patients3–5. Therefore, in clinical practice, an accurate assessment of body composition is a crucial point in the management of diabetic patients, and should be used for monitoring the effects of lifestyle interventions in these subjects.
BF can be assessed using different methods and tools, with variable costs, practicality and accuracy. Dual-energy X-ray absorptiometry (DXA) is considered to have high accuracy in estimating fat mass and fat-free mass6,7 but is not easily always available in the clinical setting. A common alternative is skinfold thicknesses, which is a simple and inexpensive approach to objectively assess BF8–10. In this regard, a number of different regression equations have been developed to estimate the percentage of BF (%BF) from skinfold thickness requiring measurements at three, four or seven sites, possibly in combination with body circumferences and age11–16.
The regression equations proposed by Jackson and Pollock (JP) are based on the assumption that fat is distributed subcutaneously and internally in a similar manner in all individuals17. However, it has been shown that fat distribution varies across sex, ethnicity, body type, and age18–20. Therefore, the predictive capability of these skinfold regression equations across different populations is limited. Similarly, the regression equations by Durnin and Womersley (DW), Visser (V), Kwok (K) and Gause-Nilsson (GN)11,14–16 have been used to predict %BF primarily in healthy older adults, so again caution should be used when extending use in patients.
It is clear that equations may work well on the samples from which they were derived, but there are many aspects of study cohorts that may influence the accuracy of the equation, such as age, obesity, sex, race, ethnicity, and clinical conditions. Several studies have found mixed results in terms of accuracy for several skinfold thickness equations when compared to DXA in different populations21–28. Skinfold thickness measurement proved to be an accurate method for determining %BF through the DW equation in overweight patients with chronic obstructive pulmonary disease29 and Chambers et al.30, when studying a sample of older overweight and obese Caucasian American females and males, showed good similarity between DXA and both DW and JP equations. The performance of GN, V and K regression equations in prediction of %BF was inadequate in this case.
These variations should be considered when selecting a skinfold thickness regression equation to estimate %BF. In the absence of studies demonstrating the validity of an equation within a specific population, results are not reliable.
Body composition has been evaluated in a number of studies in patients with diabetes, using different methods5,31–33. However, to the best of our knowledge only one study has compared DXA with the skinfold thickness method in the measurement of %BF in these patients31. Ingberg et al.31, showed that %BF was often overestimated through skinfold thickness measurements when compared with estimations by DXA in adolescent girls with type-1 diabetes. Until now, no studies have compared the %BF calculated through skinfold thickness regression equations with %BF measured by DXA in a sample of overweight/obese male and female adult patients with type-2 diabetes. Thus, to date, it is unclear which estimations by skinfold thickness equations may best replicate %BF measured by DXA in typical overweight and obese adult patients with diabetes.
The aim of the present study was to assess whether the equations most commonly used for predicting %BF skinfold thickness may fit with direct measures of %BF obtained by DXA in overweight/obese patients with type-2 diabetes. Further, two predictive equation were developed using DXA measurements as the reference for estimation of percentage total body and trunk fat.
Results
One participant in the study dropped out early and did not complete all evaluations. Accordingly, data from 39 participants, 12 females and 27 males, were included in the analysis with complete data for all body composition and skinfold thickness variables. Duration of diabetes, metabolic control and lipid profile were comparable in male and female participants (duration of diabetes 10.3 ± 7.5 vs 9.9 ± 5.7 years; HbA1c 7.3 ± 0.7 vs 7.2 ± 0.7%; fasting glucose 164 ± 31 vs 145 ± 27 mg/dL; total cholesterol 170 ± 30 vs 171 ± 33 mg/dL; LDL-cholesterol 94.3 ± 29.6 vs 91.0 ± 18.7; and triglycerides 152 ± 96 vs 126 ± 85 mg/dL, respectively; p = ns for all), whereas HDL-cholesterol was lower in males than in females (46.7 ± 11 vs 54.5 ± 9.4 mg/dL, p < 0.05). The demographic, anthropometric and body composition characteristics of participants are reported in Table 1. No sex differences were found in age, weight and BMI, while height was higher in males than in females.
Table 1.
Characteristics of the participants enrolled in the study by sex. Data are presented as mean (standard deviation).
| Variable | Female (n = 12) | Male (n = 27) |
|---|---|---|
| Age, years | 56.2 (8.1) | 56.6 (7.1) |
| Weight, kg | 77.2 (14.9) | 86.2 (13.3) |
| Height, cm | 158.1 (4.6)*** | 172.9 (4.8) |
| BMI, kg/m2 | 30.9 (5.4) | 28.9 (4.3) |
| Body fat (DXA), % | 37.95 (4.94)*** | 27.26 (4.95) |
| Triceps skinfold, mm | 22.3 (7.2)*** | 11.3 (3.5) |
| Subscapular skinfold, mm | 30.3 (11.0)* | 20.6 (6.9) |
| Biceps skinfold, mm | 15.6 (5.6)** | 8.3 (2.5) |
| Supra-iliac skinfold, mm | 26.7 (8.4)* | 20.1 (8.9) |
BMI = body mass index; DXA = dual-energy X-ray absorptiometry;
*P < 0.05, **P < 0.01; ***P < 0.001.
In the female group %BF-DXA and skinfold thickness at the triceps, biceps, subscapular, and suprailiac sites were significantly greater in females than in males (Table 1).
A summary of the results related to accuracy and bias in estimates of %BF, with anthropometric equations relative to DXA, is presented in Table 2. In both females and males, the estimate of %BF calculated from DWB was similar to %BF-DXA (1.08%, P = 0.79 and 0.17%, P = 0.23, respectively). However, %BF predicted by DWS was similar to %BF-DXA in males (0.87%, P = 0.17) but not in females (2.75%, P = 0.09). In both females and males, the DWB equation developed the smallest MSD (−0.89% and 0.03%, respectively), while the G equation the highest (10.24% and 6.26%, respectively).
Table 2.
Summary of the associations between % body fat (%BF) estimated with different anthropometric equations (see text for abbreviations) and the criterion value (obtained using dual-energy x-ray absorptiometry, DXA).
| Females (n = 12) | Mean ± SD | MSD | R2 | SEE | E | Males (n = 27) | MSD | R2 | SEE | E |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | ||||||||||
| %BF-DXA | 37.95 ± 4.94 | 27.26 ± 4.95 | ||||||||
| %BF-DWS | 40.70 ± 4.51** | −0.89 | 0.639 | 3.11 | 3.99 | 28.13 ± 4.42 | 0.03 | 0.598 | 3.20 | 3.25 |
| %BF-DWB | 39.03 ± 4.16 | −2.76 | 0.639 | 3.11 | 3.05 | 27.43 ± 4.08 | −0.87 | 0.598 | 3.20 | 3.21 |
| %BF-VS | 45.41 ± 2.66*** | −5.23 | 0.589 | 3.32 | 8.13 | 31.86 ± 2.20*** | −3.41 | 0.601 | 3.19 | 5.75 |
| %BF-VB | 43.38 ± 2.46*** | −7.46 | 0.589 | 3.32 | 6.35 | 30.87 ± 2.03*** | −4.60 | 0.601 | 3.19 | 5.05 |
| %BF-G | 27.71 ± 5.89*** | 10.24 | 0.691 | 2.88 | 10.71 | 21.00 ± 5.36*** | 6.26 | 0.708 | 2.72 | 6.89 |
| %BF-K | 46.61 ± 8.06*** | −8.66 | 0.724 | 2.72 | 9.74 | 33.38 ± 6.40*** | −6.12 | 0.754 | 2.50 | 6.90 |
MSD = mean signed difference; R2 = R Square; SEE = standard error of estimate; E = total error; *P < 0.05, **P < 0.01; ***P < 0.001.
The VS, VB, and K predictive equations significantly overestimated %BF, as compared to DXA, by 7.46%, 5.43% and 8.66% respectively, in females, and by 4.60%, 3.61% and 6.12% respectively, in males (P < 0.001 for all). Conversely, the G equation significantly underestimated %BF compared to DXA in both females and males, by 10.24% and 6.26% respectively, (P < 0.001 for both). As shown in Table 3, SEE ranged from 2.72% to 3.32% in females, and from 2.50 to 3.20 in males. In both sexes, E was lower for DWB compared with the other predictive equations. Overall, %BF estimates showed smaller E in males than females with most predictive equations.
Table 3.
Equations used to predict percent of body fat (%BF) in overweight/obese patients with type-2 diabetes.
| Reference | Equations |
|---|---|
| Durnin and Womersley11 | BD for F = 1.1339 - 0.0645 log10 (Sum 4) |
| BD for M = 1.1715 - 0.0779 log10 (Sum 4) | |
| %BF-DWS = (4.95/BD - 4.50) × 100 (Siri’s equation 37) | |
| %BF-DWB = (4.57/BD - 4.14) × 100 (Brozek’s equation 38) | |
| Visser et al.14 | BD = 1.0688 + 0.0212 (Sex A) - 0.0356 (log10 [Sum 4]) |
| %BF-VS = (4.95/BD - 4.50) × 100 (Siri’s equation 37) | |
| %BF-VB = (4.57/BD - 4.14) × 100 (Brozek’s equation 38) | |
| Gause-Nilsson and Dey16 | %BF-GN = 36.078 + 3.917 log10(Sum 4) - 5.114(Sex A) + 0.384(W) - 0.289(H) |
| Kwok et al.15 | %BF-K = −27.149 + 6.137(Sex B) + 1.120 (BMI) + 17.308 log10 (Sum 2) |
BD, body density; Sum 4, triceps + biceps + subscapular + suprailliac; Sex A, female = 0, male = 1; Sex B, female = 2, male = 1; Sum 2, triceps + biceps; W, weight (kg); H, Height (cm).
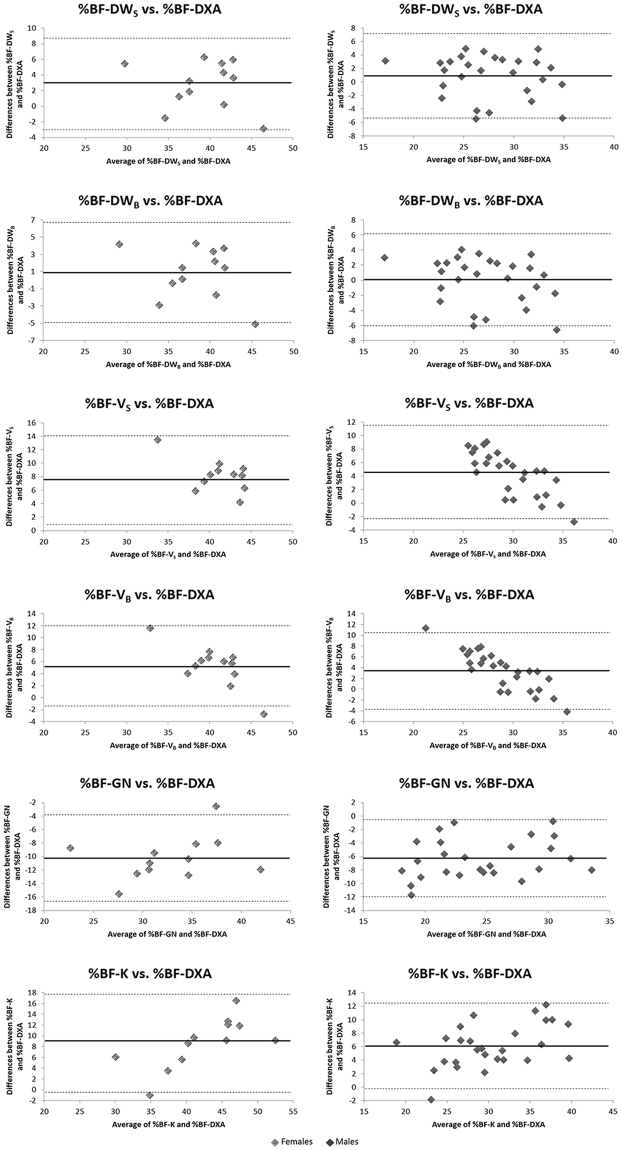
Figure 1 shows the agreement of anthropometric equations estimated and DXA-measured %BF by Bland-Altman plots. This analysis confirmed the correspondence between the %BF-DXA and DWB and DWS equations in males, and between the %BF-DXA and DWB in females (Fig. 1). In both groups, at least 91.7% of the data points felt within the 95% limits of agreement in each plots.
Figure 1.
Bland-Altman Plots. %BF, body fat percentage; DXA, dual-energy X-ray absorptiometry; DWB, Durnin-Womersley equation for body density and Brozek’s equation to convert body density to %BF; DWS, Durnin-Womersley equation for body density and Siri’s equation to convert body density to %BF; VB, Visser equation for body density and Brozek’s equation to convert body density to %BF; VS, Visser equation for body density and Siri’s equation to convert body density to %BF; G, Gause-Nilsson equation; K, Kwok equation. Solid lines indicate main error, dashed lines indicate ± 2 standard deviations.
In the total sample, entering sex, BMI and the sum of four skinfolds (i.e., triceps, biceps, subscapular and suprailiac skinfolds) in stepwise multiple regression analysis, a statistically significant model was yielded for both total body and trunk %BF (F = 72.73, P < 0.001; F = 47.95, P < 0.001, respectively). The model was:
Total body %BF = 3.071 + 0.211 (triceps skinfold) + 0.756 (BMI) + 6.861 (sex); female = 1; male = 0; adjusted R2 = 0.850, SEE = 2.71.
Trunk %BF = 9.334 + 0.168 (suprailiac skinfold) + 0.629 (BMI) + 6.536 (sex); female = 1; male = 0; adjusted R2 = 0.788, SEE = 3.01.
For the total body %BF and the trunk %BF equation, the Durbin-Watson was 2.23 and 2.17, respectively, indicating that there was no autocorrelation between the residuals and the variance inflation factor was <3.1 and <2.8, respectively, for all variables showing that each model was robust to collinearity.
Discussion
In this study, we assessed the reliability of %BF estimates obtained with several anthropometric equations using DXA-measured %BF as the criterion in overweight and obese adult patients with type-2 diabetes.
We used DXA as the reference technique to provide an accurate measure of body fat percentage. DXA measurements are greatly reproducible and the validity of this method has been previously demonstrated in different populations34–37.
The main finding of the study was the weakness of skinfold thickness anthropometric predictive equations in estimating %BF as compared to DXA in both sexes. In fact, the DW was the only regression equation that adequately predicted %BF in overweight and obese adult patients with type-2 diabetes.
In particular, the DWB equation in females, and both DWS and DWB equations in males gave results that were not significantly different from those obtained by DXA. These results were confirmed by Bland-Altman analysis (Fig. 1), which showed good agreement between %BF-DXA and %BF-DWB equation in females and with both %BF-DWB and %BF-DWS equations in males. This lack of predictive ability for %BF-DWS equation in females may be due to differences in the pattern of body fat distribution between sexes38. In this regard, skinfold thickness at the triceps, biceps, subscapular, and suprailiac sites was greater in females than in males (Table 1). In addition, %BF-DXA was also higher in females, although BMI was similar between sexes. The other anthropometric equations investigated in this study, i.e. VS, VB, and K equations, significantly overestimated, whereas G underestimated %BF. Interestingly, these differences between DXA and the predictive anthropometric equations used in our study we have found in type-2 diabetes patients were also reported in a study carried out in nondiabetic overweight and obese subjects30.
Recently, Chambers et al.30 showed that equations from DW and V accurately predicted %BF in obese females but overestimated %BF in overweight and obese males. Moreover, in this sample, the K equation overestimated fat mass in both males and females, and the G equation underestimated %BF in females, but overestimated it in males. Unfortunately, the study from Chambers et al.30 did not report the performance of the DW and V equations using the Brozek equation, thereby preventing full comparison with our findings.
Some authors reported a low accuracy of %BF predicted by DW equation in obese people21,22, using either bioelectrical impedance or underwater weighing as a reference. Reasons underlying these differences with our findings are not easily explained. In these latter studies, only obese and not overweight individuals were studied.
It appears obvious that obesity may affect the accuracy of skinfold thickness assessment, possibly due to differences in body fat distribution. Notably, some methodological aspects may contribute to limiting the use of skinfold thickness measurements in obese subjects. For example, the calipers currently used are not large enough to pinch and measure certain body sites such as the abdominal, suprailiac or thigh areas in obese subjects. Another important factor is that raising skinfolds for measurement is more difficult in obese people. Proper technique requires pinching a fold of skin and fat between the thumb and forefinger and separating them from the underlying muscle21, this process being subject to considerable variation between investigators. Thus, either over or underestimation by skinfold thickness measurement is possibly due not only to the characteristics of subjects investigated, but also to the technical ability of data collectors.
In order to offer a practical tool for %BF estimation in type-2 diabetic patients, a predictive equation was developed regressing DXA-measured %BF against easy-to-measure variables such as sex, BMI, and skinfold thicknesses. The resulting model included BMI, triceps skinfold, and sex, all showing positive contribution to %BF. The percentage of explained variance in the dependent variable was high (85%) and the SEE was small (2.71%) suggesting that the equation has good accuracy in predicting %BF in type-2 diabetic patients. Given the relevance of visceral fat as a health risk factor in overweight, obese and type-2 diabetes people, a predictive equation was also developed using DXA-measured trunk %BF, a proxy of visceral fat39 as the dependent variable and the same above anthropometric measurement as independent variables. The resulting model included BMI, suprailiac skinfold, and sex, all showing positive contribution to trunk %BF. The percentage of explained variance (78.8%) and the SEE (3.01) were such that the equation could be considered fairly good accuracy in predicting the percentage of centrally located fat. However, both models should be used with some caution in practice because of the small number of patients and the lack of a cross-validation sample.
Our study has some limitations that should be acknowledged; first, we did not investigate a non-diabetic control group. This aspect precluded us from assessing whether there may be specific differences between diabetic and non-diabetic subjects. Patients with type-2 diabetes are typically characterized by central fat distribution, with the presence of ectopic fat depots. Therefore, skinfold thickness might predict fat mass to different extents in people with or without diabetes. While this issue should be further explored in future studies, our data are important in giving information about the more appropriate equations for estimating fat mass in diabetic patients through simple skinfold thickness measurement. Another limitation is related to the homogeneous characteristics of the diabetic subjects recruited in this study, who were all older Caucasian adults, mostly overweight or obese. Thus, some caution should be taken when extrapolating data from the present study to diabetic patients with different characteristics. Nevertheless, our sample made up of overweight and obese adult patients does reflect the clinical features of most typical patients with type-2 diabetes.
Conversely, the main strengths of our study are the use of DXA as the reference standard, which is a well-accepted method providing an accurate assessment of fat mass, and the measurement of skinfold thickness by a trained operator.
In conclusion, estimating fat mass is important in the management of type-2 diabetes. While most of the equations derived from skinfold thickness over or underestimated %BF in a cohort of overweight/obese diabetic patients, DW equation gave reliable results. Further, a new well-performing predictive equation for %BF was developed in our sample of overweight and obese adult patients with type-2 diabetes using the accurate DXA measurements as the dependent variable. Hopefully, this information will assist clinicians and nutritionists in assessing fat mass in their patients through skinfold thickness equations. However, further research in a larger number of patients is needed in order to confirm the validity of anthropometric equations in this population.
Methods
According to Chow and colleagues40, the required sample size was estimated “a priori” using the formula: , where is the mean value of the differences between the %FM calculated with DXA and the %FM calculated from skinfolds equations and was the standard deviation of these differences. Preliminary investigation showed that was approximately 4% in each experimental group. Setting the minimum clinically meaningful difference at 2% (effect size = ) and the type I error at α = 0.05, the minimum sample size required for having an 80% power (i.e., β = 0.20) was 34 subjects.
Accordingly, the study was carried out on baseline data from a total of 40 (12 females and 28 males) sedentary patients with type-2 diabetes, aged 40–68 years, recruited to participate in the RAED2 Study, a randomized controlled trial aimed at comparing the metabolic effects of aerobic and resistance training in diabetic subjects41). Inclusion criteria were type-2 diabetes for at least 1 year, hemoglobin (Hb)A1c 6.5–9.0% (reference limit, <6.5%), baseline physical activity <1,000 MET min per week by the International Physical Activity Questionnaire (IPAQ). Allowed diabetes medications were oral hypoglycemic agents. Exclusion criteria were moderate/severe somatic or autonomic neuropathy, cardiovascular disease, preproliferative/proliferative retinopathy, chronic renal failure, as well as therapy with beta-blockers or current smoking. All female participants were postmenopausal and not on hormonal replacement therapy. All methods were carried out in accordance with relevant guidelines and regulations. This study was approved by the Ethical Committee of the Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy, which was according to the Helsinki Declaration (revised 2008). Written informed consent was obtained from all the participants.
Anthropometry and body composition analysis
Body mass was taken at the nearest 0.1 kg with an electronic scale (Tanita electronic scale BWB-800 MA); stature was measured with a Harpenden stadiometer (Holtain Ltd., Crymych, Pembs. UK) to the nearest 0.5 cm; body mass index (BMI) was calculated as weight (kg)/height (m)2. Whole body and regional body composition was evaluated using a total body scanner (QDR Explorer W, Hologic, MA, USA; fan-bean technology, software for Windows XP version 12.6.1), according to the manufacturer’s procedures. Instrument quality control (QC) of the DXA scanner was performed daily before actual use by means of the Hologic encapsulated spine phantom (Hologic Inc, Bedford, MA) provided by the manufacturer. Over the study period, the Hologic software always passed the daily QC calibration and the phantom measurements (i.e., bone mineral density, bone mineral content and area values) always fell within the control limit of upper and lower ranges set as a baseline ± 1.5%. Furthermore, the long-term DXA scanner performance was monitored over the entire study period during which the measurements from phantom scans remained stable without drifting and with a coefficient of variation lower than 1% for each of the phantom measurements. All scans were performed and analyzed by the same operator (CM), in order to ensure consistency. The DXA-derived percentage of body fat (%BF) at the total body level (%BF-DXA) as well as in the trunk region were included in the analysis. The Hologic software includes the neck, chest, abdominal and pelvic areas in what is defined as the trunk region. Its upper perimeter is the inferior edge of the chin and the lower boundary intersects the middle of the femoral necks without touching the brim of the pelvis. The trunk region is separated from the arm region at the glenohumeral joint.
A trained operator (CM) performed skinfold thickness measurements twice with a Harpenden caliper (Gima, Milan, Italy) on the subject’s right side at the triceps, biceps, subscapular, and suprailiac sites, according to standard procedures42,43. If the two measurements differed by more than 2 mm, a third measurement was taken, and the two closest were then averaged and recorded to the nearest 0.1 mm as the final value. Established age and sex-specific prediction equations, based on DW11 and V14, were used to estimate body density (BD), and BD values were then converted to %BF according to both Siri44 and Brozek45 equations (DWS, DWB, VS, and VB, respectively) (Table 1). In addition, %BF was also estimated using the prediction equations developed by Gause-Nilsson and Dey16 (G), and by Kwok et al.15 (K) (Table 3). Accordingly, %BF estimated from six predictive equations (%BF-DWS, %BF-DWB, %BF-VS, %BF-VB, %BF-G and %BF-K) were compared to %BF-DXA (Table 3). The anthropometric prediction equations to be compared with DXA were selected for the following reasons. Firstly, the selected equations are the most commonly used in the clinical setting to predict %BF in older adults. In particular, the age- and sex-specific DW equation11 is widely used in Europe46. Secondly, the selected equations use a small number of anthropometric measurements to assess %BF, which is saving clinicians and researchers time and resources. Thirdly, several skinfolds used in other predictive equations (e.g., the abdominal skinfold) are difficult to measure in overweight and obese subjects because of the inadequate size of most calipers (upper measurement limit of 45 to 55 mm) along with the difficulty of grasping and holding a large skinfold while reading the caliper dial.
Biochemical analyses
Hemoglobin A1c was measured by a Diabetes Control Complications Trial (DCCT)-aligned method, with an automated high-performance liquid chromatography analyzer (Bio-Rad Diamat, Milan, Italy). Serum total cholesterol, HDL-cholesterol and triglycerides were determined by standard laboratory procedures (DAX 96; Bayer Diagnostics, Milan, Italy). LDL-cholesterol was calculated using Friedewald’s equation47.
Statistical analysis
Descriptive statistics (mean and standard deviation) were computed by sex for all variables using standard procedures. Normality of data was assessed with the Kolmogorov-Smirnov test. Sex comparisons were made using independent-sample t tests. Paired-sample t tests were performed to assess whether %BF skinfold thickness values were accurate in comparison with DXA. The mean signed difference (MSD i.e., the average of the differences between the skinfold thickness estimated %BF and the criterion [DXA] measurement) was also computed to test the ability of anthropometric equations to accurately estimate the mean level of %BF48.
The coefficient of determination (R2) and the standard error of estimate (SEE) for %BF-DXA and equation-derived %FM values were computed to test agreement between methods. The total error (total error , where Y′ = the predicted %BF values from the skinfold regression equation, Y = the criterion %BF values from DXA, and n is the number of participants) was calculated according to Lohman49. The visual absolute agreement (bias and limits of agreement) between the %BF resulting from predicting equations (i.e. %BF-DWS, %BF-DWB, %BF-VS, %BF-VB, %BF-G and %BF-K) and %BF-DXA was evaluated assessing the Bland and Altman plots50.
A stepwise multiple regression analysis on the total sample with %BF-DXA as dependent variable and sex, BMI and the four skinfolds (i.e., triceps, biceps, subscapular and suprailiac skinfolds) as potential predictors was carried out. Adjusted R2 and SEE were used to represent the goodness of the predictor model. Homoscedasticity of data was assessed by plotting the residuals of multiple regression analysis against the predicted values. The presence of serial correlations among the residuals was tested using the Durbin-Watson statistic and the variance inflation factor was calculated to check for multicollinearity in our multiple linear regression model.
Statistical analyses were performed using SPSS v. 16.0 (IBM Corp., Armonk, NewYork, USA), alpha value being set at 0.05.
Data availability
The dataset generated and analyzed during the current study is not publicly available because it refers to personal and delicate data of our patients. It is available from the corresponding author on reasonable request.
Acknowledgements
We would like to thank all the participants in this study and the staff of the Division of Endocrinology, Diabetes and Metabolism, University and Azienda Ospedaliera Universitaria Integrata Verona.
Author Contributions
E.B., V.C., and C.M. designed and conducted the study. V.C., performed the statistical analysis. C.M., and C.Z., and P.M. wrote the paper. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Standards of Medical Care in Diabetes-2016: summary of revisions. Diabetes Care. January, 39, Suppl 1: S 4-5 (2016). [DOI] [PubMed]
- 2.Billington CJ, et al. Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 3.Rimm EB, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;12:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom R, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;3:516–525. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 5.Hariri AH, Oliver NS, Johnston DG, Stevenson JC, Godsland IF. Adiposity measurements by BMI, skinfolds and dual energy X-ray absorptiometry in relation to risk markers for cardiovascular disease and diabetes in adult males. Dis Markers. 2013;6:753–764. doi: 10.1155/2013/763907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prior BM, et al. In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol (1985). 1997;83:623–630. doi: 10.1152/jappl.1997.83.2.623. [DOI] [PubMed] [Google Scholar]
- 7.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol (1985). 1998;84:372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]
- 8.Tran ZV, Weltman A. Predicting body composition of men from girth measurements. Hum Biol. 1988;60:167–175. [PubMed] [Google Scholar]
- 9.Tran ZV, Weltman A. Generalized equation for predicting body density of women from girth measurements. Med Sci Sports Exerc. 1989;21:01–104. doi: 10.1249/00005768-198902000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/BJN19910073. [DOI] [PubMed] [Google Scholar]
- 11.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/BJN19740060. [DOI] [PubMed] [Google Scholar]
- 12.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/BJN19780152. [DOI] [PubMed] [Google Scholar]
- 13.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- 14.Visser M, van den Heuvel E, Deurenberg P. Prediction equations for the estimation of body composition in the elderly using anthropometric data. Br J Nutr. 1994;71:823–833. doi: 10.1079/BJN19940189. [DOI] [PubMed] [Google Scholar]
- 15.Kwok T, Woo J, Lau E. Prediction of body fat by anthropometry in older Chinese people. Obes Res. 2001;9:97–101. doi: 10.1038/oby.2001.12. [DOI] [PubMed] [Google Scholar]
- 16.Gause-Nilsson I, Dey DK. Percent body fat estimation from skin fold thickness in the elderly. Development of a population-based prediction equation and comparison with published equations in 75-year-olds. J Nutr Health Aging. 2005;9:19–24. [PubMed] [Google Scholar]
- 17.Heyward, V. H. & Wagner, D. R. Applied body composition assessment (2nd eds Human Kinetics, 2004).
- 18.Kuczmarski, R. J. Need for body composition information in elderly subjects: Am J Clin Nutr. 50 (5Suppl), 1150–1157; discussion 1231–1235 (1989). [DOI] [PubMed]
- 19.Jensen RK, Fletcher P. Distribution of mass to segments of elderly males and females. J Biomech. 1994;27:89–96. doi: 10.1016/0021-9290(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 20.Nevill AM, et al. Can we use the Jackson and Pollock equations to predict body density/fat of obese individuals in the 21st century? Int J Body Compos Res. 2008;2:114–121. [PMC free article] [PubMed] [Google Scholar]
- 21.Gray DS, et al. Skinfold thickness measurements in obese subjects. Am J Clin Nutr. 1990;51:571–577. doi: 10.1093/ajcn/51.4.571. [DOI] [PubMed] [Google Scholar]
- 22.Teran JC, et al. Percent body fat in obese white females predicted by anthropometric measurements. Am J Clin Nutr. 1991;53:7–13. doi: 10.1093/ajcn/53.1.7. [DOI] [PubMed] [Google Scholar]
- 23.De Lorenzo A, et al. Comparison of different techniques to measure body composition in moderately active adolescents. Br J Sports Med. 1998;32:215–219. doi: 10.1136/bjsm.32.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sartorio A, et al. Body composition analysis by dual-energy X-ray absorptiometry and anthropometry in adults with childhood-onset growth hormone (GH) deficiency before and after six months of recombinant GH therapy. J Endocrinol Invest. 1997;20:417–423. doi: 10.1007/BF03347994. [DOI] [PubMed] [Google Scholar]
- 25.Facchini M, et al. Changes of autonomic cardiac profile after a 3-week integrated body weight reduction program in severely obese patients. J Endocrinol Invest. 2003;26:138–142. doi: 10.1007/BF03345142. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez G, et al. Body fat measurement in adolescents: comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur J Clin Nutr. 2005;59:1158–1166. doi: 10.1038/sj.ejcn.1602226. [DOI] [PubMed] [Google Scholar]
- 27.Haas VK, et al. Body composition changes in female adolescents with anorexia nervosa. Am J Clin Nutr. 2009;89:1005–1010. doi: 10.3945/ajcn.2008.26958. [DOI] [PubMed] [Google Scholar]
- 28.El Ghoch M, et al. Comparison between dual-energy X-ray absorptiometry and skinfolds thickness in assessing body fat in anorexia nervosa before and after weight restoration. Clin Nutr. 2012;31:911–916. doi: 10.1016/j.clnu.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Hronek M, et al. Skinfold anthropometry–the accurate method for fat free mass measurement in COPD. COPD. 2013;10:597–603. doi: 10.3109/15412555.2013.781151. [DOI] [PubMed] [Google Scholar]
- 30.Chambers AJ, Parise E, McCrory JL, Cham R. A comparison of prediction equations for the estimation of body fat percentage in non-obese and obese older Caucasian adults in the United States. J Nutr Health Aging. 2014;18:586–590. doi: 10.1007/s12603-014-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingberg CM, et al. Body composition in adolescent girls with Type 1 diabetes. Diabet Med. 2003;20:1005–1011. doi: 10.1046/j.1464-5491.2003.01055.x. [DOI] [PubMed] [Google Scholar]
- 32.Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract. 2016;122:106–112. doi: 10.1016/j.diabres.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto T, Fukuda K, Oshima Y, Moritani T. Effects of non-exercise activity and daily exercise activity upon glucose and fat metabolism in type 2 diabetes. J Sports Med Phys Fitness. 2016;56:1214–1220. [PubMed] [Google Scholar]
- 34.Wang ZM, et al. Six-compartment body composition model: inter-method comparisons of total body fat measurement. Int J Obes Relat Metab Disord. 1998;22:329–337. doi: 10.1038/sj.ijo.0800590. [DOI] [PubMed] [Google Scholar]
- 35.Wells JC, et al. J. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–912. doi: 10.1093/ajcn/69.5.904. [DOI] [PubMed] [Google Scholar]
- 36.Barbosa AR, Santarem JM, Jacob Filho W, Meirelles E, Marucci JM. Comparison of body fat using anthropometry bioelectrical impedance and DEXA in elderly woman. Arch Latinoam Nutr. 2001;51:49–56. [PubMed] [Google Scholar]
- 37.Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring). 2012;20:30–39. doi: 10.1038/oby.2011.211. [DOI] [PubMed] [Google Scholar]
- 38.Cerqueira M, et al. Validity of body adiposity index in predicting body fat in a sample of Brazilian women. Obesity (Silver Spring). 2013;21:E696–699. doi: 10.1002/oby.20543. [DOI] [PubMed] [Google Scholar]
- 39.Clasey JL, et al. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res. 1999;7(3):256–264. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 40.Chow, S. C., Wang, H. & Shao, J. Sample Size Calculations in Clinical Research (2nd edition. Chapman and Hall/CRC, 2007).
- 41.Bacchi E, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35:676–682. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohman, T. G., Roche, A. F. & Martorell, R. Anthropometric Standardization Reference Manual. Champaign (IL: Human Kinetics Books; 1988).
- 43.Norton, K. & Olds, T. Anthropometrica (University of New South Wales Press, Sydney; 1996).
- 44.Siri, W. E. Body composition from fluid spaces and density: analysis of methods. Brozek J, Henschel A, (eds). Techniques for measuring body composition. (Washington DC: National Academy of Sciences) 223–244 (1961).
- 45.Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 46.Leahy S, O’Neill C, Sohun R, Toomey C, Jakeman P. Generalised equations for the prediction of percentage body fat by anthropometry in adult men and women aged 18-81 years. Br J Nutr. 2013;109(4):678–685. doi: 10.1017/S0007114512001870. [DOI] [PubMed] [Google Scholar]
- 47.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 48.Cui Z, Truesdale KP, Cai J, Stevens J. Evaluation of anthropometric equations to assess body fat in adults: NHANES 1999-2004. Med Sci Sports Exerc. 2014;46(6):1147–1158. doi: 10.1249/MSS.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 49.Lohman TG. Skinfolds and body density and their relation to body fatness: a review. Hum Biol. 1981;53:181–225. [PubMed] [Google Scholar]
- 50.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analyzed during the current study is not publicly available because it refers to personal and delicate data of our patients. It is available from the corresponding author on reasonable request.