Abstract
Mutations in components of the Wnt pathways are a frequent cause of many human diseases, particularly cancer. Despite the fact that a causative link between aberrant Wnt signalling and many types of human cancers was established more than a decade ago, no Wnt signalling inhibitors have made it into the clinic so far. One reason for this is that no pathway‐specific kinase is known. Additionally, targeting the protein–protein interactions needed to transduce the signal has not met with success so far. Complicating the search for and use of inhibitors is the complexity of the cascades triggered by the Wnts and their paramount biological importance. Wnt/β‐catenin signalling is involved in virtually all aspects of embryonic development and in the control of the homeostasis of adult tissues. Encouragingly, however, in recent years, first successes with Wnt‐pathway inhibitors have been reported in mouse models of disease. In this review, we summarize possible roads to follow during the quest to pharmacologically modulate the Wnt signalling pathway in cancer.
Linked Articles
This article is part of a themed section on WNT Signalling: Mechanisms and Therapeutic Opportunities. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.24/issuetoc
Abbreviations
- APC
adenomatous polyposis coli
- Bcl9/9l
Bcl9 and Bcl9l
- CK1
casein kinase
- DKK
Dickkopf
- FZD
Frizzled
- GSK3β
glycogen synthase kinase
- LRP5
low‐density lipoprotein 5
- PORCN
Porcupine
- Pygo
Pygopus
- SFRPs
secreted FZD‐related proteins
- WLS
Wntless
Introduction
Wnts activate diverse signalling cascades
Mammalian genomes encode for 19 different Wnt molecules, which can bind to 10 different Frizzled (FZD) receptors (Koike et al., 1999; Langton et al., 2016). FZDs belong to the family of seven‐pass transmembrane GPCRs. When bound by Wnt proteins on their extracellular cysteine rich domain, they activate the cytosolic protein Dishevelled to transduce the signal inside the cell (reviewed in Dijksterhuis et al., 2014). Several independent Wnt signalling cascades are activated in response to Wnts binding to their cognate receptors. The best studied and perhaps the most important is the β‐catenin‐dependent signalling cascade, mediated by β‐catenin (Figure 1). The β‐catenin‐dependent cascade is of foremost importance for normal development and tissue homeostasis. When deregulated, it causes the initiation and progression of a myriad of different tumour types. Besides the β‐catenin‐mediated cascade, there are other β‐catenin‐independent outputs, such as the planar cell polarity and the Wnt/Ca2+ signalling pathway. The nature of the pathway transduced depends on the receptors/co‐receptors present (He et al., 1997; van Amerongen et al., 2008). To transduce the β‐catenin‐dependent signal, FZD proteins bind the co‐receptors low‐density lipoprotein 5 (LRP5) or LRP6. Why in each specific context a particular Wnt/receptor combination activates one cascade or another is not entirely clear. However, some Wnts are thought to be preferentially β‐catenin dependent (e.g. Wnt3a) or independent (e.g. Wnt5a). Wnt5a normally binds to FZD receptors and Ror/Ryk instead of Lrp5/6 and activates, among others, JNK signalling (Yamanaka, 2002). β‐Catenin‐independent signalling is often associated with the regulation of cell adhesion, migration and polarity (reviewed in Veeman et al., 2003). Furthermore, it is also thought to suppress β‐catenin‐dependent signalling (Yuzugullu et al., 2009). The β‐catenin‐independent cascade has received increasing attention in recent years due to its role in melanoma formation and metastasis (Weeraratna et al., 2002; Chien et al., 2009).
Figure 1.
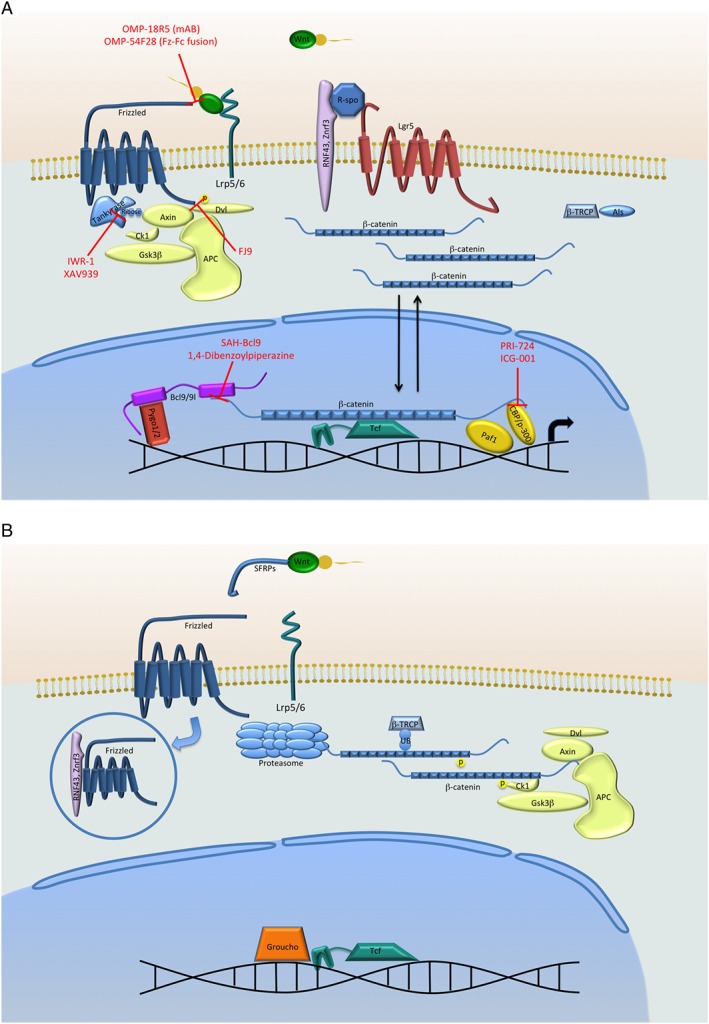
(A) The β‐catenin‐dependent Wnt signalling cascade in the ON‐state. Upon binding of the Wnts to the receptors of the FZD family and the co‐receptors LRP5/6, Dishevelled (Dvl) is recruited to the membrane, thus disassembling the destruction complex consisting of Axin, GSK3β, APC and CK1, preventing phosphorylation and thus protecting β‐catenin from proteasomal degradation. This allows β‐catenin to accumulate and translocate to the nucleus to initiate target gene transcription. Tankyrases can further increase the signal by marking Axin for degradation. Furthermore, when ZNRF3 and RNF43 are bound by R‐spondin and LGR5 and, therefore, unable to target FZD receptors for degradation, Wnt signalling is enhanced in the Wnt‐ON state. A further step protecting β‐catenin from degradation is the inhibition of E3 ubiquitin ligases such as βTRCP by Armless, at least in D. melanogaster. A selection of Wnt‐pathway inhibitors currently used in research are shown in red; red bars indicate the interaction they inhibit. (B) The Wnt signalling cascade in the OFF‐state. Without Wnts binding to the FZD and LRP receptors, the destruction complex is active and phosphorylates β‐catenin, thus marking it for proteasomal degradation. In the absence of LGR5, a Wnt target gene, the FZD receptor is also targeted for degradation by ZNRF3 and RNF43. Furthermore, E3 ubiquitin ligases, like βTRCP, promote proteasomal turnover of β‐catenin.
While the search for therapeutic targets has long focused on the transduction of the signal in the receiving cell, it is increasingly evident that an alternative strategy to modulate the Wnt signalling cascade is at the level of the ligands, for example, inhibiting their secretion.
Wnt secretion is dependent on Porcupine and Wntless
To be fully active, Wnts must undergo glycosylation and lipid modification (Figure 2). Whereas Wnt glycosylation enhances but is not essential for secretion and signalling, the lipid modifications are necessary for both functions. Wnts are acylated on two conserved residues, corresponding to cysteine 77 and serine 209 in mouse Wnt3a (Harterink and Korswagen, 2012). The enzyme responsible for these lipid modifications is the O‐acyl‐transferase Porcupine (PORCN). This is demonstrated by the fact that the genetic loss of PORCN, or the impairment of its activity, leads to Wnt molecules being retained in the endoplasmic reticulum (ER). The acylation of Drosophila Wnts in position Ser209 (or the mammalian homologue position) is required for the interaction of Wnts with Wntless (Wls), which is another protein critical for Wnt secretion (Herr and Basler, 2012). Wls is a multipass transmembrane protein that is an absolute requirement for the secretion of all Wnts (Bänziger et al., 2006; Bartscherer et al., 2006). The puzzle of how Wls promotes Wnt secretion remains unresolved; however, many pieces have already been put together. These include the unearthing of the role of the retromer complex in the retrieval of Wls, which establishes a trafficking loop from the ER to the plasma membrane via the Golgi (Herr et al., 2012). While our understanding of Wls function is not sufficient to generate small molecule inhibitors, suitable inhibitors for the enzyme PORCN have been discovered. Porcupine is an attractive target because it seems to be exclusively required for Wnt secretion. Moreover, we have also found that PORCN, which is the sole enzyme known to be specific to the Wnt cascade, is up‐regulated in murine cancer models. Additionally, elevated PORCN expression is an indicator for a bad prognosis in head and neck squamous cell carcinomas (the cancer genome atlas, unpublished observations by Dario Zimmerli).
Figure 2.
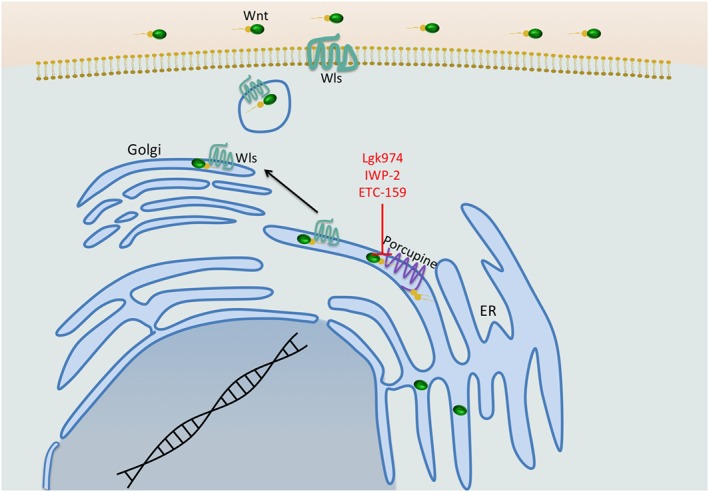
The secretory pathway of the ligands of the Wnt pathway. Wnts need to be coupled to fatty acids to be secreted. This happens in the ER by the acyltransferase Porcupine, which is a prime target for a small molecule inhibitor, since it is the only known enzyme specific to the pathway. Acylation of Wnts allows them to bind to Wntless (Wls) in the Golgi apparatus, which in turn facilitates secretion of the mature Wnts. Wls is a transmembrane protein required for the secretion of all Wnts.
Wnt signalling initiated by the Wnt–FZD interaction is highly regulated
Wnt signalling transduction is tightly regulated at the level of the ligand–receptor interaction. This is achieved by titration of the ligands and/or of the receptors.
Ligand availability can be modulated by the production of secreted FZD‐related proteins (SFRPs). SFRPs are secreted molecules with no direct signalling activity, but they possess a Wnt‐binding domain through which they sequester extracellular Wnts (Leyns et al., 1997; Wang et al., 1997). Another way to modulate Wnt signalling is to alter the level and/or availability of the receptors or co‐receptors. The four secreted Dickkopf (DKK) proteins are a well‐studied class of molecules that act in this way. In the Wnt cascade, DKKs act by binding to the FZD co‐receptors LRP5/6, thereby inhibiting the binding of the Wnts (Mao et al., 2001). Three of the DKK proteins (DKK1, 2 and 4) appear to be specific for the Wnt pathway and act by binding to LRP5/6 (Mao et al., 2001). Interestingly, DKK2 and DKK4 can act as either activators or as repressors of the pathway, depending on the abundance of the cofactor Kremen 2 (Mao and Niehrs, 2003). In contrast to the three other members of the DKK family, DKK3 acts in the TGF‐β signalling cascade (Pinho and Niehrs, 2007; Nakamura and Hackam, 2010). In addition to above‐mentioned mechanisms, there is a variety of other transmembrane or secreted inhibitors with various modes of action, such as WIF, WISE/SOST, CERBERUS, IGFBP, TIKI1, SHISA, WAIF1 and APCDD1 (reviewed in Cruciat and Niehrs, 2013).
Besides LRP proteins, there are other receptor‐co‐receptor pairs such as Ryk, which can enhance Wnt signalling (Lu et al., 2004). Additionally, there are ancillary receptor complexes, which regulate the levels of available Wnt receptors. Most prominent among them are LGR4/5/6. Those proteins came to fame as Wnt target genes expressed in the intestine and were found to mark various stem cell populations (Barker et al., 2007). Later on, it was revealed that they greatly increase Wnt signal transduction when they are bound by the extracellular R‐spondin. They act by inhibiting the ubiquitination of FZD receptors and their subsequent degradation by ZNRF3 and RNF43 (de Lau et al., 2014).
The diversity of mechanisms by which Wnt signalling is initiated by the Wnt‐FZD receptor interaction is regulated is both a bane and a boon. There are many potential targets, but their diversity also means that redundancy could affect the efficacy of any intervention.
β‐catenin is the central scaffold transducing the β‐catenin‐dependent Wnt signal
The central node of the β‐catenin‐dependent pathway is β‐catenin. β‐Catenin was discovered as a membrane‐associated protein that binds E‐cadherin (Kemler, 1993). Later, it was found that it regulates Wnt‐dependent transcription via the recruitment of different transcriptional cofactors to the regulatory regions of Wnt target genes. In a signalling ‘off state’, the so‐called ‘destruction complex’ [consisting of adenomatous polyposis coli (APC), Axin and the kinases responsible for the phosphorylation of β‐catenin – glycogen synthase kinase (GSK3β) and casein kinase (CK1)] marks cytosolic β‐catenin for proteosomal degradation (Stamos and Weis, 2013). Upon activation of this pathway, the rate limiting factor of the destruction complex, Axin, together with GSK3β, is recruited to the so‐called Wnt signalosome – consisting of WNT/FZD/LRP and multimer Dishevelled (Bilic et al., 2007). This destabilizes the destruction complex, leaving β‐catenin free to accumulate and to translocate into the nucleus, where it binds to the transcription factors of the TCF/LEF family. Acting together with a plethora of N‐ and C‐ terminal binding transcriptional co‐activators, β‐catenin and TCF/LEF facilitate target gene expression.
In the pathway, ‘off‐state’ TCFs are thought to silence target genes by recruiting co‐repressors such as Groucho. These co‐repressors are displaced by β‐catenin and its cohort of transcriptional activators (Clevers, 2006; Städeli et al., 2006; Mosimann et al., 2009; Valenta et al., 2012).
Recently, it was discovered that even if β‐catenin escapes degradation by the destruction complex, it can still be degraded by the proteasome unless rescued by Armless, a pathway component recently identified in Drosophila melanogaster. Armless protects Arm/β‐catenin from degradation by inhibiting the function of Ter94 in facilitating protein turnover (Reim et al., 2014). This discovery is interesting in light of this review, as it might represent a so far overlooked mechanism for therapeutic intervention.
The Wnt/β‐catenin transcriptional pathway is executed by N‐ and C‐ terminal co‐activators
β‐catenin facilitates transcription by recruiting several N‐ and C‐ terminal binding co‐activators.
The factors directly binding the N‐terminus of β‐catenin are Bcl9 and Bcl9l (the two mammalian paralogues of the Drosophila Legless); they in turn recruit Pygopus (Pygo1 and 2 in mammals). Bcl9/9l and Pygo are thought to form a ‘chain of adaptors’ extending from β‐catenin. The simple model arising from Drosophila is that Legless and Pygo are essential for the Wnt transcriptional output (Kramps et al., 2002; Thompson et al., 2002; reviewed in Mosimann et al., 2009 and Valenta et al., 2012). In mammals, although also required for a maximal Wnt output, the relative importance of Bcl9/9l and Pygo seems to be context‐dependent. In the mouse loss of function mutations in these genes do not replicate loss of β‐catenin‐dependent Wnt signalling (i.e. by mutations in β‐catenin). For example, β‐catenin signalling mutants die at E6.5, whereas Bcl9/9l knockout (KO) animals die at E10.5, while Pygo KO animals survive at least to E13.5. Moreover, recent work has demonstrated that the Pygo‐Bcl9 complex can also act independently of β‐catenin (Cantù et al., 2014; Cantù et al., 2017). We therefore speculate that the role of Bcl9 as well as Pygo is to act as a booster of the signal and facilitate transcription of specific target genes in a subset of cells with active Wnt signalling. As described later, the context‐dependent requirement of the so‐called N‐terminal chain of adaptors for facilitating the Wnt transcriptional output presents an exciting therapeutic target.
Another series of cofactors bind to β‐catenin's C‐terminus. This ensemble of cofactors comprises a diverse group of proteins, which have a more general role in transcription initiation and progression (reviewed in MacDonald et al., 2009; Mosimann et al., 2009). The most prominent among them are p300 and CBP, members of the basal transcriptional machinery, which were thought to have redundant modes of action in transcriptional activation (Hecht, 2000; Takemaru and Moon, 2000). However, recent studies suggest that although they are redundant in certain tissues, p300 and CBP can play critical roles and determine the nature of the transduced Wnt transcriptional programme. In lung fibrosis, the differential utilization of CBP or p300 seems to determine whether to execute alveolar repair or promote the fibroproliferation associated with fibrosis (Gottardi and Königshoff, 2013; Kahn, 2014).
Wnt signalling in cancer
Since Wnt signalling plays a role in nearly all developmental processes, it does not come as a surprise that it is also implicated in many cancers. There are several possibilities for a cancer cell to hijack this pathway. It can either inactivate/decrease the expression of an inhibitory component or activate/increase the expression of an activating factor. When, in 1991, mutations in the APC gene were discovered in 80% of colorectal cancers, efforts to find a drug acting on this protein were initiated, so far with limited success (Groden et al., 1991; Powell et al., 1992). In addition to mutations in APC, which is a fundamental component of the β‐catenin destruction complex, other Wnt pathway mutations have been found: rarely inactivating mutations in Axin and activating mutations in the gene encoding for β‐catenin (10%). Whereas in colon cancer APC mutations and β‐catenin are prevalent, in other cancer types, such as hepatocellular carcinoma, mutations in Axin predominate. Oncogenic ctnnb mutations occur in melanoma and in solid tumours such as thyroid tumours (Kahn, 2014; Mazzoni and Fearon, 2014). The fact that in different tumours, alternate Wnt signalling activating mutations occur means that distinctive strategies may need to be employed in each case. This will be further discussed in the specific sections for the different targets.
As shown in colon cancers, the Wnt pathway is also activated in some tumours through epigenetic silencing of inhibitors of the cascade (Suzuki et al., 2004). Some of these epigenetic changes affect the secreted inhibitors that regulate transduction at the level of the Wnt pathway ligand–receptor interaction: for instance, methylation of SFRP genes has been reported in colon, breast, lung, prostate and other cancers (Caldwell et al., 2004; Suzuki et al., 2004; Fukui et al., 2005). Mutations in inhibitory factors like ZNRF43 and RNF43 have also been reported. These proteins act as negative regulators by decreasing FZD protein abundance at the membrane. When they are lost, receptor levels increase, thereby increasing signalling. In fact, mutations in these proteins were found to be common in the extremely aggressive pancreatic ductal adenocarcinomas (Jiang et al., 2013). Another way by which Wnt signalling can be increased is by boosting ligand expression: the discovery of Int1 as a murine mammary tumour oncogene, as well as being a participant of the Wnt signalling field, is a prime example of this (Nusse and Varmus, 1982; Kahn, 2014).
While activating Wnt signalling is often a driver of tumour initiation, the evolution of a tumour to a fully malignant form seems in some cases to correlate with mutations that shut down the β‐catenin‐dependent Wnt cascade. A prime example of this is melanoma, where increased β‐catenin‐dependent Wnt signalling actually correlates with a better prognosis (Chien et al., 2009). Therefore, blocking β‐catenin‐dependent Wnt signalling should not be considered a cure‐all and different strategies will have to be applied in different diseases.
Therapeutic inhibitors of the Wnt pathway
Despite the challenges, especially the pivotal role of the pathway in tissue homeostasis, the Wnt pathway can be therapeutically targeted. An example is the targeting of the bone‐derived Wnt inhibitor Sclerostin to treat osteoporosis. The use of a humanized, anti‐sclerostin antibody is currently in phase III clinical trials (Appelman‐Dijkstra and Papapoulos, 2016). The approach is successful because of the tissue (bone)‐specific function of sclerostin. Discovering and exploiting the tissue‐ and disease‐specific features is likely to be the key to the wider application of Wnt pathway modulators. Below, we describe different targets and their potential usefulness.
Porcupine: a promising target for effective Wnt pathway inhibition
One of the most promising ways for targeting Wnt signalling is to block ligand production. Although, as noted above, many cancers, especially colon carcinomas, have activating mutations in components of the Wnt cascade in the receiving cell, there is a growing body of evidence that additional signalling induced by the presence of Wnts is critical for tumour progression (Lavergne et al., 2011; Koo et al., 2015). Currently, the best way to interfere with Wnt secretion is to inhibit the acyl‐transferase Porcupine (Figure 2).
One such inhibitor – Lgk974 – was identified in a high‐throughput screen performed on living cells. To achieve this, 2.4 million compounds were tested for their ability to suppress the activity of a transcriptional Wnt reporter in a cell line co‐cultured with another cell line overexpressing Wnt3a. Lgk974 binds directly to and inhibits Porcupine (Liu et al., 2013). Currently, it is being tested in a stage 1 dose escalation clinical trial (Lum and Clevers, 2012). Another small‐molecule inhibitor of Porcupine, ETC‐159, has also just entered the phase of clinical trials (Madan et al., 2016; Nile and Hannoush, 2016).
In mouse tumour models, Porcupine inhibitors (Table 1) showed very promising results in treating various types of cancer. The primary candidates for these studies were cancers known to be dependent on Wnt secretion, for example, due to RNF43 mutations (Liu et al., 2013). Studies were conducted in murine models of mammary carcinomas, basal cell carcinoma, keratoacanthomas, colon cancer and head and neck squamous cell carcinomas (Liu et al., 2013; Proffitt et al., 2013; Zito et al., 2014; Larsimont et al., 2015; Madan et al., 2016). Another scenario where inhibiting the secretion of ligands might be beneficial is when a cancer exploits them to influence the surrounding tissue to create its own niche.
Table 1.
Selected Wnt pathway inhibitors and their use in mouse tumour models
| Compound name | Mode of action | Tested applications | Publications for in vivo inhibitor use if applicable |
|---|---|---|---|
| Lgk974 | Inhibits Porcupine | Cell lines, div. murine cancer models, phase 1 clinical trial | Liu et al., 2013 Clinical trial identifier: NCT01351103 |
| ETC‐159 | Inhibits Porcupine | Rspo3 translocations in CRC xenografts | Madan et al., 2016 |
| Wnt‐C59 | Inhibits Porcupine | Cell lines, murine cancer models | Proffitt et al., 2013 |
| IWP‐2 | Inhibits Porcupine | Murine keratoacanthoma model, cell lines | Zito et al., 2014 |
| Xav939 | Tankyrase 1 + 2 | Cell lines, xenografts | Huang et al., 2009, Arques et al., 2016 |
| ICG‐001 | Inhibits β‐catenin‐ CBP interaction | Diverse murine tumour models | Emami et al., 2004 |
| PRI‐724 (2nd generation of ICG‐ 001) | Inhibits β‐catenin‐ CBP interaction | Clinical trial phase 1 | Clinical trials identifier:NCT01764477, NCT01606579 |
| OMP‐18R5 (mAB) | Antibody against FZD receptors | Various xenograft models, clinical trial phase 1 | Gurney et al., 2012 Clinical trials identifiers: NCT01973309, NCT01345201 |
| OMP‐54F28 (Fzd8‐Fc fusion) | Competes with FZDs for Wnts | Various xenograft models, clinical trial phase 1 | Wei et al. 2011 Clinical trial identifier: NCT02092363 |
| FJ9 | Inhibits Dishevelled PDZ domain interaction with FZD | Cell lines, xenograft models | Fujii et al. 2007 |
| SAH‐BCL9 | Inhibits Bcl9‐β‐catenin interaction | Cell lines, xenograft models | Takada et al., 2012 |
| 1,4‐Dibenzoylpiperazines | Inhibits Bcl9‐β‐catenin interaction | Cell lines | Wisniewski et al., 2016 |
There are several aspects that need to be considered when evaluating the therapeutic potential of globally blocking the secretion of all Wnts. The first is that systemic abolishion of Wnt secretion will result in defects in gut homeostasis (Valenta et al., 2016). It is, therefore, essential to either target inhibitors directly to their site of action or to use smaller doses that do not attenuate Wnt signalling to the extent that tissue homeostasis is affected. The existence of a useful therapeutic window is demonstrated by studies showing that treatment with the Porcupine inhibitor Lgk974 resulted in cancer regression, but gut homeostasis was unaffected (Liu et al., 2013).
The possibility that blocking Wnt secretion will affect both β‐catenin‐dependent and ‐independent Wnt signalling also needs to be taken into account. The consequences of applying Porcupine inhibitors will therefore depend on what Wnts are present and what pathways are activated. Since β‐catenin‐dependent and ‐independent Wnt signalling seem to influence each other, predicting the outcome is not easy (Yuzugullu et al., 2009; Grumolato et al., 2010). An illustrative example is melanoma, where the relative contribution of β‐catenin‐dependent and ‐independent signalling is unclear, particularly in the later stages such as metastases formation. The loss of the β‐catenin‐independent Wnt5a has been found to inhibit tumour growth and metastasis (Weeraratna et al., 2002; Anastas et al., 2014); however, it also seems to lead to activation of β‐catenin‐dependent signalling, which in different studies has positive or negative effects on tumour progression (Damsky et al., 2011; Yang et al., 2012; Caramel et al., 2013). While this might seem to restrict the utility of Porcupine inhibitors, it may be an advantage to block all Wnt‐dependent outputs, β‐catenin‐dependent and ‐independent, and thereby simplify the playing field.
A further critical open question with regard to the therapeutic application of Porcupine inhibitors is their effect on the immune system and how those effects will impinge on the efficacy of treatments. Since the inhibitors are typically applied orally, the possibility that the loss of Wnt secretion will affect the tumour micro‐environment or the proliferation and differentiation of the infiltrating immune cells cannot be excluded.
However, the above‐mentioned challenges necessitate further work to understand the consequences of globally blocking Wnt production and find solutions in order to circumvent challenges such as the paramount importance of Wnt signalling for tissue homeostasis.
Alternatives to small molecules are neutralizing antibodies or biologicals to inhibit the receptors
The development of small molecule inhibitors against components of the Wnt pathway is a challenging task, especially due to the lack of easily targetable enzymes specific for the pathway. An alternative is to use either antibodies against surface molecules like FZD or LRP, or more simply exploit ‘natural’ inhibitors of the cascade.
The difficulty of targeting FZDs with antibodies is the sheer number of them with poorly defined roles in transducing the signal. This raises the problem of the specificity of the antibody to specific receptors, as well as possible alternative routes for the cell to transduce the signal if only one specific receptor is blocked. Despite these challenges, promising results have been reported with the use of FZD antibodies: one example is the antibody OMP‐18R5 that, even though it targets five different FZDs, appears to specifically hamper tumour growth without affecting normal tissues (Gurney et al., 2012).
Another approach, shown to be promising in mice, is the use of biologically occurring inhibitors. A prime example for this is the injection of SFRP proteins, which are ‘natural’ inhibitors of the pathway (Polesskaya et al., 2003). Instead of using existing SFRPs, it is also possible to engineer new ones by simply removing the transmembrane domain of an FZD of interest, thus rendering the Wnt binding part soluble. These engineered proteins can then act as an artificial soluble SFRP (Wei et al., 2011). An additional possibility is to use other soluble inhibitors like DKK (Aicher et al., 2008). Surprisingly, antibodies against DKK1 have anti‐tumourigenic effects in cancer cell lines and xenograft models, which are thought to be Wnt signalling‐dependent (Sato et al., 2010). This has to be carefully evaluated, as it points to a broader role for DKK1 in cancer than simply being a negative feedback regulator of the Wnt pathway. With these results in mind, it might not be advisable to increase the DKK1 dose to inhibit the Wnt pathway, since this might have unexpected effects.
Tankyrase inhibitors
Tankyrase is a member of the PARP superfamily of enzymes that add ADP‐ribose onto target proteins. With respect to Wnt signalling, tankyrase PARylates Axin and targets it for proteasomal degradation. Inhibition of tankyrase thus leads to an increased abundance of Axin and consequently to an overactivated destruction complex, the final effect being inhibition of the pathway (Huang et al., 2009).
Initial results with tankyrase inhibitors seemed to be promising: in particular, the combined administration of Akt, PI3K and tankyrase inhibitors to human colon carcinoma cell lines xenografted into mice and rats induced apoptosis in cells escaping the therapy targeting only Akt and PI3K. This combined therapy was particularly effective in those cases where the accumulation of nuclear β‐catenin was observed in the tumours (Arques et al., 2016).
An impasse of this strategy is that tankyrase has multiple substrates and is critical for many basic cellular processes, for example, in telomere maintenance, mitosis and insulin‐mediated glucose uptake; inhibiting it may therefore lead to severe side effects (Riffell et al., 2012).
The β‐catenin – TCF interaction is an attractive but elusive target
Another possibility for modulating the β‐catenin‐dependent Wnt signalling cascade very downstream is to target the nuclear function of β‐catenin, more specifically by inhibiting the TCF‐β‐catenin interaction (Valenta et al., 2012). This would be especially efficacious in colon carcinoma, where the majority of the mutations affect the destruction complex. However, there are a number of hurdles that to date have proven insurmountable. Firstly, β‐catenin plays an important role in cell adhesion where, in association with E‐cadherin, it forms the adherens junctions, and the interaction sites of TCF and E‐cadherin overlap. Secondly, the binding affinity of β‐catenin to TCF is quite high (ca. 20 nM). Nevertheless, several screens have been performed with the aim of disrupting this interaction. Although several compounds were identified that reduced Wnt signalling in reporter assays and inhibited the growth of colon cancer cell lines, the mechanisms of action of the molecules remained unclear and their specificity was limited (Kahn, 2014).
However, as mentioned above, β‐catenin interacts with various transcriptional cofactors via its C‐ and N‐terminus. Targeting these interactions represents an interesting alternative strategy.
Targeting the interaction between β‐catenin and its C‐terminal cofactors – a difficult case
Various screens have been conducted in order to find suitable inhibitors of β‐catenin's interaction with C‐terminal cofactors like CBP and p300. Even though some of these screens yielded efficacious inhibitors, none of them seem to specifically inhibit the interaction with β‐catenin. ICG‐001, which does inhibit Wnt signalling, generally interferes with CBP's activity and does not inhibit the binding of CBP to β‐catenin. Interestingly, ICG‐001 does not inhibit the very closely related p300. Since the inhibitor is effective in colon cancer mouse xenograft models, there may be a tissue‐specific requirement for CBP in the colon (Emami et al., 2004; McMillan and Kahn, 2005). However, because ICG‐001 inhibits CBP, which is part of the general transcriptional machinery, administering this compound could result in severe side effects. Several phase 1 clinical trials are currently being conducted to study the efficacy and side effects of this inhibitor in patients.
Targeting β‐catenin's N‐terminal interaction partners is the promising alternative
The only known N‐terminal binding partners of β‐catenin are the paralog proteins Bcl9and Bcl19l and indirectly Pygo1/Pygo2 (Figure 1) (Kramps et al., 2002). While in D. melanogaster these proteins seem to be mandatory for all Wnt signalling outputs, in the mouse this appears not to be the case and their function seems to be more restricted (Kramps et al., 2002; Song et al., 2007; Cantù et al., 2014). For example, in the intestinal epithelia, N‐terminal co‐activators are not needed for normal maintenance of homeostasis, but only during inflammation‐induced regeneration. Moreover, colon carcinomas heavily depend on Bcl9/9l to become malignant (Deka et al., 2010). Importantly, preventing the binding between β‐catenin and Bcl9/9l has exactly the same effect as the complete deletion of Bcl9/9l; therefore, this genetically very well‐defined interaction represents an ideal target for the development of small molecule inhibitors (Moor et al., 2015). In terms of specificity, the context‐dependent requirement of the N‐terminal activators makes it an exciting therapeutic target. Several studies exploring this possibility have been recently published (Hoggard et al., 2015; Wisniewski et al., 2016).
Also promising seems to be the use of stapled peptides. This technology exploits the fact that the Bcl9‐β‐catenin interaction is mediated by a helical segment of Bcl9, which binds a large groove of β‐catenin's structure. Metabolically stable triazole‐stapled Bcl9 α‐helical peptides seem to be an effective approach for inhibiting this interaction (Kawamoto et al., 2012; Takada et al., 2012). These stapled peptides appear to be good inhibitors in vitro and in mouse xenograft models, but the efficacy of such molecules in the clinic has not yet been tested. A possible drawback of inhibiting Bcl9/Bcl9l functions is suggested by recent findings, which show that a dysfunctional Bcl9l impairs caspase 2 expression, thus permitting higher aneuploidy tolerance in colorectal cancer cells (López‐García et al., 2017). Whether this is also the case when Bcl9l‐β‐catenin binding is inhibited will have to be investigated carefully.
Another attractive target is the Bcl9/9l partner, Pygo2. From a developmental viewpoint, the requirement for Pygo2 seems to be even more restricted than that of Bcl9/9l: for example, mouse embryos lacking Pygo2 die at E13.5, while Bcl9/9l loss of function is lethal at earlier stages, between E9.5 and E10.5 (Cantù et al., 2014). Pygo1 seems to be negligible; so far, no phenotype could be observed upon its loss. Interestingly, Pygo2 plays crucial roles in mammary gland outgrowth as well as in mammary cancer stem cells. Furthermore, it may also play a role in some models of intestinal tumour initiation and progression (Talla and Brembeck, 2016). Additionally, there is evidence that Pygo's chromatin binding ability is required for mammary gland outgrowth (Watanabe et al., 2014). Chromatin binding is not essential for Wnt signal transduction in the development and normal homeostasis of mice, suggesting that targeting this interaction will have few side effects (Cantu et al., 2013). Therefore, Pygo's chromatin binding capability is a promising target for drug development. The therapeutic potential of targeting the binding of Pygo to Bcl9/91 requires further exploration of when and where this interaction is required; the interaction is also relevant in Wnt‐independent contexts (Cantù et al., 2014).
Delivering inhibitors directly to malignant cells via carrier molecules
In the adult organism Wnt signalling is critical for stem cell maintenance and tissue homeostasis, systemically blocking Wnt signalling will therefore be problematic (Valenta et al., 2016). One way of circumventing this is to use the inhibitors at sub‐lethal doses, where only the Wnt signalling‐dependent cancer cells are affected. An alternative is to develop strategies to deliver the inhibitors directly and exclusively to the tumour tissue. This could be done by linking an inhibitor/toxin to a compound, which is attracted by the tumour, as for example was described by Krall et al., 2014 (Wichert et al., 2015) for acetazolamide, a ligand with specific receptors in cancerous lesions in clear‐cell renal cell carcinomas. If such molecules also exist in Wnt driven tumours, these would be needed to be established. Other strategies to deliver the drug via nanoparticles to a specific tissue/cancer have also been proposed: one method would be to exploit chemical gradients, such as differences in pH or in oxygen concentration. Tumours are often hypoxic, thus the redox potential in the vicinity of the tumours is altered. Additionally, liposomes could be used to deliver the inhibitors (Muller and Keck, 2004; Allen and Cullis, 2013). All these methods have potential, but testing their practical implementation will be an important step in modulating the Wnt‐pathway in disease.
Round up and looking forward
Wnt signalling is of paramount importance both in disease and in tissue maintenance; therefore, any therapeutic intervention involving Wnt signalling must solve this conundrum. Targeting inhibitors to the afflicted tissue is a promising but underexplored option. Other possible solutions have emerged and will continue to emerge, as our understanding of the complexities of Wnt signalling in cancer improves.
An exciting target is the Bcl9/9l‐Pygo branch of β‐catenin‐dependent Wnt signalling, since it is not essential for adult tissue homeostasis but, in the case of colorectal cancer, is required for tumour progression. Blocking the β‐catenin‐Bcl9/9l interaction is one targetable interface. Although targeting a protein–protein interaction is challenging, recently, there have been some very promising results using stapled peptides as well as small molecule inhibitors. Further work is needed to determine other cancer types where impinging on the chain of adaptors could be harnessed. Therefore, further scrutiny of the developmental and disease relevance of the chain of adaptors is important. Basic research that refines our understanding of the intricacies of β‐catenin‐dependent Wnt signalling will reveal other opportunities – the identification of additional context‐ and tissue‐specific factors is critical.
Another promising avenue is to target Wnt production by inhibiting Porcupine, which is specifically required for the production and secretion of active Wnts. In cancers where Wnt secretion or receptor turnover is over‐activated, Porcupine inhibition can be effective, for example, in head and neck squamous cell carcinoma with Notch mutations (Liu et al., 2013). One future challenge to this is to gauge the consequences of the combined effect of blocking β‐catenin‐dependent and ‐independent Wnt signalling. More work is needed to understand the interplay of these signalling cascades. In tumours with downstream mutations in the Wnt cascade, the utility of treatment with Porcupine inhibitors is less obvious; however, as mentioned earlier in such cases, ligand‐mediated augmentation of the signalling also probably plays a role in the later stages of tumour progression. Furthermore, β‐catenin‐independent Wnt signalling, which is also blocked by Porcupine inhibition, is known to play a role in the later stages of tumourigenesis, for example, Wnt5a signalling in melanoma metastases. It is critical to get a better understanding of the biology and the genetics of the tumours that we aim to treat. Given the diversity of the mutational landscapes found in different tumours, a therapy, which does not work in colorectal cancer or melanoma, might well work in mammary tumours.
The practical solution for effective inhibition of Wnt signalling is probably going to be to combine the above‐mentioned approaches and, thereby, gain a decisive advantage over the tumour. One would need to carefully weigh the effect of a multifarious approach on homeostasis against the potential enhanced efficacy in killing cancer cells. An additional advantage of a varied strategy would be that it takes away alternative routes for the cancer to escape therapy. Further efficacy from a treatment point of view could also be achieved by using Wnt signalling inhibition as an adjunct therapy to other molecular medicine approaches, chemotherapy, radiology and/or surgery.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b).
Conflict of interest
The authors declare no conflicts of interest.
Zimmerli, D. , Hausmann, G. , Cantù, C. , and Basler, K. (2017) Pharmacological interventions in the Wnt pathway: inhibition of Wnt secretion versus disrupting the protein–protein interfaces of nuclear factors. British Journal of Pharmacology, 174: 4600–4610. doi: 10.1111/bph.13864.
References
- Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C et al. (2008). The Wnt antagonist Dickkopf‐1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res 103: 796–803. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, Cullis PR (2013). Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65: 36–48. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R (2008). Alternative Wnt signaling is initiated by distinct receptors. Sci Signal 1: re9–re9. [DOI] [PubMed] [Google Scholar]
- Anastas JN, Kulikauskas RM, Tamir T, Rizos H, Long GV, von Euw EM et al. (2014). WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J Clin Invest 124: 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelman‐Dijkstra NM, Papapoulos SE (2016). Sclerostin Inhibition in the Management of Osteoporosis. Calcif Tissue Int 98: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arques O, Chicote I, Puig I, Tenbaum SP, Argiles G, Dienstmann R et al. (2016). Tankyrase inhibition blocks Wnt/ ‐catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res 22: 644–656. [DOI] [PubMed] [Google Scholar]
- Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K (2006). Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006). Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang Y‐L, Davidson G, Zimmermann T, Cruciat C‐M, Bienz M et al. (2007). Wnt induces LRP6 signalosomes and promotes dishevelled‐dependent LRP6 phosphorylation. Science 316: 1619–1622. [DOI] [PubMed] [Google Scholar]
- Caldwell GM, Jones C, Gensberg K, Jan S, Hardy RG, Byrd P et al. (2004). The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res 64: 883–888. [DOI] [PubMed] [Google Scholar]
- Cantu C, Valenta T, Hausmann G, Vilain N, Aguet M, Basler K (2013). The Pygo2‐H3K4me2/3 interaction is dispensable for mouse development and Wnt signaling‐dependent transcription. Development 140: 2377–2386. [DOI] [PubMed] [Google Scholar]
- Cantù C, Zimmerli D, Hausmann G, Valenta T, Moor A, Aguet M et al. (2014). Pax6‐dependent, but β‐catenin‐independent, function of Bcl9 proteins in mouse lens development. Genes Dev 28: 1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantù C, Pagella P, Shajiei TD, Zimmerli D, Valenta T, Hausmann G et al. (2017). A cytoplasmic role of Wnt/β‐catenin transcriptional cofactors Bcl9, Bcl9l, and Pygopus in tooth enamel formation. Sci Signal 10 eaah4598. [DOI] [PubMed] [Google Scholar]
- Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G, Wierinckx A et al. (2013). A switch in the expression of embryonic EMT‐inducers drives the development of malignant melanoma. Cancer Cell 24: 466–480. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ et al. (2009). Activated Wnt/ss‐catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci 106: 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006). Wnt/beta‐catenin signaling in development and disease. Cell 127: 469–480. [DOI] [PubMed] [Google Scholar]
- Cruciat C‐M, Niehrs C (2013). Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5: a015081–a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE et al. (2011). β‐catenin signaling controls metastasis in Braf‐activated Pten‐deficient melanomas. Cancer Cell 20: 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka J, Wiedemann N, Anderle P, Murphy‐Seiler F, Bultinck J, Eyckerman S et al. (2010). Bcl9/Bcl9l are critical for Wnt‐mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res 70: 6619–6628. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis JP, Petersen J, Schulte G (2014). WNT/Frizzled signalling: receptor‐ligand selectivity with focus on FZD‐G protein signalling and its physiological relevance: IUPHAR Review 3: Frizzleds as GPCRs. Br J Pharmacol 171: 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M et al. (2004). A small molecule inhibitor of ‐catenin/CREB‐binding protein transcription. Proc Natl Acad Sci 101: 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, You L, Xu Z, Uematsu K, Shan J, He B et al. (2007). An antagonist of dishevelled protein‐protein interaction suppresses ‐catenin‐dependent tumor cell growth. Cancer Res 67: 573–579. [DOI] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H et al. (2005). Transcriptional silencing of secreted frizzled related protein 1 (SFRP1) by promoter hypermethylation in non‐small‐cell lung cancer. Oncogene 24: 6323–6327. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Königshoff M (2013). Considerations for targeting β‐catenin signaling in fibrosis. Am J Respir Crit Care Med 187: 566–568. [DOI] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H et al. (1991). Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66: 589–600. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R et al. (2010). Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 24: 2517–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L et al. (2012). Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci 109: 11717–11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harterink M, Korswagen HC (2012). Dissecting the Wnt secretion pathway: key questions on the modification and intracellular trafficking of Wnt proteins. Acta Physiol 204: 8–16. [DOI] [PubMed] [Google Scholar]
- He X, Saint‐Jeannet J‐P, Wang Y, Nathans J, Dawid I, Varmus H (1997). A member of the Frizzled protein family mediating axis induction by Wnt‐5A. Science 275: 1652–1654. [DOI] [PubMed] [Google Scholar]
- Hecht A (2000). The p300/CBP acetyltransferases function as transcriptional coactivators of beta‐catenin in vertebrates. EMBO J 19: 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr P, Basler K (2012). Porcupine‐mediated lipidation is required for Wnt recognition by Wls. Dev Biol 361: 392–402. [DOI] [PubMed] [Google Scholar]
- Herr P, Hausmann G, Basler K (2012). WNT secretion and signalling in human disease. Trends Mol Med 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Hoggard LR, Zhang Y, Zhang M, Panic V, Wisniewski JA, Ji H (2015). Rational design of selective small‐molecule inhibitors for β‐catenin/B‐cell lymphoma 9 protein–protein interactions. J Am Chem Soc 137: 12249–12260. [DOI] [PubMed] [Google Scholar]
- Huang S‐MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al. (2009). Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620. [DOI] [PubMed] [Google Scholar]
- Jiang X, Hao H‐X, Growney JD, Woolfenden S, Bottiglio C, Ng N et al. (2013). Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci 110: 12649–12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M (2014). Can we safely target the WNT pathway? Nat Rev Drug Discov 13: 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto SA, Coleska A, Ran X, Yi H, Yang C‐Y, Wang S (2012). Design of triazole‐stapled BCL9 α‐helical peptides to target the β‐catenin/B‐cell CLL/lymphoma 9 (BCL9) protein–protein interaction. J Med Chem 55: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R (1993). From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9: 317–321. [DOI] [PubMed] [Google Scholar]
- Koike J, Takagi A, Miwa T, Hirai M, Terada M, Katoh M (1999). Molecular cloning of Frizzled‐10, a novel member of the Frizzled gene family. Biochem Biophys Res Commun 262: 39–43. [DOI] [PubMed] [Google Scholar]
- Koo B‐K, van Es JH, van den Born M, Clevers H (2015). Porcupine inhibitor suppresses paracrine Wnt‐driven growth of Rnf43;Znrf3 ‐mutant neoplasia. Proc Natl Acad Sci 112: 7548–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall N, Pretto F, Decurtins W, Bernardes GJL, Supuran CT, Neri D (2014). A small‐molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed 53: 4231–4235. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S et al. (2002). Wnt/wingless signaling requires BCL9/legless‐mediated recruitment of pygopus to the nuclear beta‐catenin‐TCF complex. Cell 109: 47–60. [DOI] [PubMed] [Google Scholar]
- Langton PF, Kakugawa S, Vincent J‐P (2016). Making, exporting, and modulating Wnts. Trends Cell Biol 26: 756–765. [DOI] [PubMed] [Google Scholar]
- Larsimont J‐C, Youssef KK, Sánchez‐Danés A, Sukumaran V, Defrance M, Delatte B et al. (2015). Sox9 controls self‐renewal of oncogene targeted cells and links tumor initiation and invasion. Cell Stem Cell 17: 60–73. [DOI] [PubMed] [Google Scholar]
- de Lau W, Peng WC, Gros P, Clevers H (2014). The R‐spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 28: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne E, Hendaoui I, Coulouarn C, Ribault C, Leseur J, Eliat P‐A et al. (2011). Blocking Wnt signaling by SFRP‐like molecules inhibits in vivo cell proliferation and tumor growth in cells carrying active β‐catenin. Oncogene 30: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim S‐H, Piccolo S, Robertis EMD (1997). Frzb‐1 is a secreted antagonist of Wnt signaling expressed in the spemann organizer. Cell 88: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T et al. (2013). Targeting Wnt‐driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci 110: 20224–20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐García C, Sansregret L, Domingo E, McGranahan N, Hobor S, Birkbak NJ et al. (2017). BCL9L dysfunction impairs caspase‐2 expression permitting aneuploidy tolerance in colorectal cancer. Cancer Cell 31: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D (2004). Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119: 97–108. [DOI] [PubMed] [Google Scholar]
- Lum L, Clevers H (2012). The unusual case of Porcupine. Science 337: 922–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009). Wnt/β‐catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J et al. (2016). Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene 35: 2197–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Niehrs C (2003). Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 302: 179–183. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A et al. (2001). LDL‐receptor‐related protein 6 is a receptor for Dickkopf proteins. Nature 411: 321–325. [DOI] [PubMed] [Google Scholar]
- Mazzoni SM, Fearon ER (2014). AXIN1 and AXIN2 variants in gastrointestinal cancers. Cancer Lett 355: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan M, Kahn M (2005). Investigating Wnt signaling: a chemogenomic safari. Drug Discov Today 10: 1467–1474. [DOI] [PubMed] [Google Scholar]
- Moor AE, Anderle P, Cantù C, Rodriguez P, Wiedemann N, Baruthio F et al. (2015). BCL9/9L‐β‐catenin signaling is associated with poor outcome in colorectal cancer. EBioMedicine 2: 1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K (2009). Beta‐catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10: 276–286. [DOI] [PubMed] [Google Scholar]
- Muller RH, Keck CM (2004). Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol 113: 151–170. [DOI] [PubMed] [Google Scholar]
- Nakamura REI, Hackam AS (2010). Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 28: 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile AH, Hannoush RN (2016). Fatty acylation of Wnt proteins. Nat Chem Biol 12: 60–69. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE (1982). Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109. [DOI] [PubMed] [Google Scholar]
- Pinho S, Niehrs C (2007). Dkk3 is required for TGF‐β signaling during Xenopus mesoderm induction. Differentiation 75: 957–967. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA (2003). Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113: 841–852. [DOI] [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer‐Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN et al. (1992). APC mutations occur early during colorectal tumorigenesis. Nature 359: 235–237. [DOI] [PubMed] [Google Scholar]
- Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA et al. (2013). Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT‐driven mammary cancer. Cancer Res 73: 502–507. [DOI] [PubMed] [Google Scholar]
- Reim G, Hruzova M, Goetze S, Basler K (2014). Protection of armadillo/β‐catenin by armless, a novel positive regulator of wingless signaling. PLoS Biol 12: e1001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell JL, Lord CJ, Ashworth A (2012). Tankyrase‐targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov 11: 923–936. [DOI] [PubMed] [Google Scholar]
- Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K et al. (2010). Wnt inhibitor Dickkopf‐1 as a target for passive cancer immunotherapy. Cancer Res 70: 5326–5336. [DOI] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS et al. (2007). Pygopus 2 has a crucial, Wnt pathway‐independent function in lens induction. Development 134: 1873–1885. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Städeli R, Hoffmans R, Basler K (2006). Transcription under the control of nuclear Arm/beta‐catenin. Curr Biol 16: R378–R385. [DOI] [PubMed] [Google Scholar]
- Stamos JL, Weis WI (2013). The ‐catenin destruction complex. Cold Spring Harb Perspect Biol 5: a007898–a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair K‐W, Schuebel KE, Markowitz SD, Dong Chen W et al. (2004). Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36: 417–422. [DOI] [PubMed] [Google Scholar]
- Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao J‐J, Mani M et al. (2012). Targeted disruption of the BCL9/β‐catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med 4 148ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT (2000). The transcriptional coactivator CBP interacts with beta‐catenin to activate gene expression. J Cell Biol 149: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talla SB, Brembeck FH (2016). The role of Pygo2 for Wnt/ß‐catenin signaling activity during intestinal tumor initiation and progression. Oncotarget 7: 80612–80632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin‐Arbesfeld R, Musisi H, Bienz M (2002). A new nuclear component of the Wnt signalling pathway. Nat Cell Biol 4: 367–373. [DOI] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K (2012). The many faces and functions of β‐catenin. EMBO J 31: 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB et al. (2016). Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep 15: 911–918. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003). A second canon. Functions and mechanisms of beta‐catenin‐independent Wnt signaling. Dev Cell 5: 367–377. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M (1997). Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt‐8. Cell 88: 757–766. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Fallahi M, Dai X (2014). Chromatin effector Pygo2 regulates mammary tumor initiation and heterogeneity in MMTV‐Wnt1 mice. Oncogene 33: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M et al. (2002). Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1: 279–288. [DOI] [PubMed] [Google Scholar]
- Wei W, Chua M‐S, Grepper S, So SK (2011). Soluble Frizzled‐7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichert M, Krall N, Decurtins W, Franzini RM, Pretto F, Schneider P et al. (2015). Dual‐display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat Chem 7: 241–249. [DOI] [PubMed] [Google Scholar]
- Wisniewski JA, Yin J, Teuscher KB, Zhang M, Ji H (2016). Structure‐based design of 1,4‐dibenzoylpiperazines as β‐catenin/B‐cell lymphoma 9 protein–protein interaction inhibitors. ACS Med Chem Lett 7: 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H (2002). JNK functions in the non‐canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P‐T, Anastas JN, Toroni RA, Shinohara MM, Goodson JM, Bosserhoff AK et al. (2012). WLS inhibits melanoma cell proliferation through the β‐catenin signalling pathway and induces spontaneous metastasis: WNTLESS/WLS in melanoma tumourigenesis. EMBO Mol Med 4: 1294–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E, Toylu A et al. (2009). Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito G, Saotome I, Liu Z, Ferro EG, Sun TY, Nguyen DX et al. (2014). Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross‐talk. Nat Commun 5: 3543. [DOI] [PMC free article] [PubMed] [Google Scholar]


