Abstract
Dickkopf 1 (DKK1) is a secreted protein and antagonizes oncogenic Wnt signalling by binding to the Wnt co‐receptor, low‐density lipoprotein receptor‐related protein 6. DKK1 has also been suggested to regulate its own signalling, associated with tumour aggressiveness. However, the underlying mechanism by which DKK1 promotes cancer cell proliferation has remained to be clarified for a long time. The cytoskeleton‐associated protein 4 (CKAP4), originally identified as an endoplasmic reticulum membrane protein, was recently found to act as a novel DKK1 receptor. DKK1 stimulates cancer cell proliferation when CKAP4 is expressed on the cell surface membrane. Although there are no tyrosine residues in the intracellular region of CKAP4, CKAP4 forms a complex with PI3K upon the binding of DKK1, leading to the activation of Akt. Both DKK1 and CKAP4 are frequently expressed in pancreatic and lung tumours, and their simultaneous expression is negatively correlated with prognosis. Knockdown of CKAP4 in cancer cells and treatment of mice with the anti‐CKAP4 antibody inhibit Akt activity in cancer cells and suppress xenograft tumour formation, suggesting that CKAP4 may represent a therapeutic target for cancers expressing both DKK1 and CKAP4. This review will provide details of the novel DKK1‐CKAP4 signalling axis that promotes cancer proliferation and discuss the possibility of targeting this pathway in future cancer drug development.
Linked Articles
This article is part of a themed section on WNT Signalling: Mechanisms and Therapeutic Opportunities. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.24/issuetoc
Abbreviations
- AAH
atypical adenomatous hyperplasia
- APC
adenomatous polyposis coli
- APF
anti‐proliferating factor
- CKAP4
cytoskeleton‐associated protein 4
- CRD
cysteine‐rich domain
- DKK
Dickkopf
- ER
endoplasmic reticulum
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- LRP6
low‐density lipoprotein receptor‐related protein 6
- MM
multiple myeloma
- SP‐A
surfactant protein A
- TCF
T‐cell factor
- tPA
tissue plasminogen activator
- VSMC
vascular smooth muscle cell
- WIF‐1
Wnt inhibitory factor‐1
Introduction
Mutations and aberrant activities of the Wnt pathway are frequently observed in a wide variety of diseases (Moon et al., 2004; Clevers and Nusse, 2012), which is not surprising given the importance of Wnt signalling in various cellular processes, including cell proliferation, differentiation, motility and polarization. The diseases associated with abnormalties of Wnt signalling occur in tissues that normally depend on Wnt, most notably in the intestine. Germline mutations in the adenomatous polyposis coli (APC) gene, a Wnt signalling component, cause familial adenomatous polyposis coli and additional mutations in kras, p53 and smad4 lead to the progression of polyps to malignant tumours, that is, colorectal cancer (Kinzler and Vogelstein, 1996). Loss of APC functions leads to the stabilization of β‐catenin and the constitutive complexes between β‐catenin and T‐cell factor 4 (TCF4) by disrupting the Axin complex function, which degrades cytoplasmic β‐catenin, resulting in the expression of various Wnt target genes, some of which are critical for cell proliferation and migration (Kikuchi, 2003; Polakis, 2007; Kikuchi et al., 2011). More than 75% of sporadic colorectal cancer patients carry the APC loss of function mutations and about 15% of patients have β‐catenin oncogenic mutations (Kinzler and Vogelstein, 1996). Mutations in the β‐catenin and Axin1 genes are also observed in a variety of solid tumours, and these mutations cause the stabilization of β‐catenin (Walther et al., 2009).
The synthetic compounds that disrupt the interaction of β‐catenin and TCF4, including PKF115‐854, CPG049090 and ICG‐001 (Emami et al., 2004; Lepourcelet et al., 2004), and inhibit β‐catenin stabilization, such as IWR1, XAV939, JW67 and JW74 (Chen et al., 2009; Huang et al., 2009; Waaler et al., 2011), may be of potential in the development of new anti‐cancer drugs. However, although some analogues of these compounds have been advanced to clinical testing, these approaches do not always guarantee future success. Therefore, in addition to the development of the inhibitors that target Wnt signal molecules directly, other target molecules involved in Wnt signalling need to be identified.
Dickkopf1 (DKK1), a secreted protein, is a direct target molecule whose expression is induced by a β‐catenin/TCF4 complex and essential for animal development (Niehrs, 2006). As DKK1 inhibits Wnt signalling, DKK1 basically acts as a tumour suppressor (Table 1). Interestingly, DKK1 is frequently and highly expressed in certain types of human cancers, and its expression is associated with tumour aggressiveness, suggesting that DKK1 can also function as an oncogenic protein (Table 1). Therefore, DKK1‐dependent cancer signalling may be a novel target, but how DKK1 promotes tumour formation has remained to be clarified for a long time. In this review, we describe details of a novel receptor of DKK1 in cancer cells and discuss a possible cancer therapy that would target the new DKK1 signalling pathway.
Table 1.
Functions of DKK1 in various cancer cells
| Cancer types (References) | Cell lines | Phenotypes | Clinical samples | Functions of DKK1 |
|---|---|---|---|---|
| Renal cell carcinoma (Hirata et al., 2011) | A498 | DKK1 overexpression inhibited cell proliferation and migration and induced apoptosis. | – | Tumour suppressive |
| Colon cancer (Aguilera et al., 2006; Qi et al., 2012) | HCT116 | DKK1 overexpression inhibited cell proliferation, migration and invasion. | Among 217 cases, 131 cases (60.4%) showed positive for DKK1. | Tumour suppressive |
| DLD‐1 | DKK1 overexpression inhibited cell proliferation. | Among 54 cases, DKK1 was hypermethylated in nine cases (17%). | Tumour suppressive | |
| Breast cancer (Mikheev et al., 2008, Qiao et al., 2008, DiMeo et al., 2009) | MCF‐7 | DKK1 knockdown promoted cell proliferation. | – | Tumour suppressive |
| SUM1315 and MDA‐MB‐231 | DKK1 overexpression inhibited cell proliferation. | – | Tumour suppressive | |
| Melanoma (Mikheev et al., 2007) | MDA‐MB435 | DKK1 overexpression inhibited cell proliferation and induced apoptosis. | – | Tumour suppressive |
| Placental choriocarcinoma (Peng et al., 2006) | JAR | DKK1 overexpression inhibited cell proliferation and induced apoptosis. | – | Tumour suppressive |
| Mesothelioma (Lee et al., 2004) | H28, MS‐1 | DKK1 overexpression inhibited cell proliferation and induced apoptosis. | – | Tumour suppressive |
| Cervical carcinoma (Mikheev et al., 2004) | HeLa | DKK1 overexpression inhibited cell proliferation and induced apoptosis. | – | Tumour suppressive |
| Gliobalastoma (Shou et al., 2002) | U87MG | DKK1 induced apoptosis. | – | Tumour suppressive |
| Hepatcellular carcinoma (Chen et al., 2013) | Bel7402 | DKK1 knockdown inhibited cell migration and invasion. | Metastatic tumours showed much higher DKK1 expression in comparison with that in the primary tumours. | Oncogenic |
| Lung cancer (Sato et al., 2010a; Kimura et al., 2016) | A549 | Anti‐DKK1 Ab or DKK1 knockdown inhibited cell proliferation, migration and invasion. | Among 128 cases, 98 cases (76.6%) showed positive for DKK1. | Oncogenic |
| Pancreas cancer (Takahashi et al., 2010; Kimura et al., 2016) | S2‐CP8 | DKK1 knockdown inhibited cell proliferation, migration and invasion. |
Among 23 cases, 17 cases (73.9%) showed positive for DKK1. Among 59 cases, 45 cases (76.3%) showed positive for DKK1. |
Oncogenic |
| SUIT‐2 | DKK1 knockdown inhibited cell migration. | – | Oncogenic | |
| Oesophageal cancer (Makino et al., 2009; Li et al., 2011) | EC9706 | DKK1 overexpression promoted cell proliferation and invasion. | Among 138 cases, 115 cases (83.4%) showed positive for DKK1. | Oncogenic |
| – | – | Among 170 cases, 72 cases (42.4%) showed positive for DKK1. Patients with DKK1‐positive tumours had poorer DFS than those with negative oesophageal cancer (squamous cell carcinoma type). | Oncogenic | |
| Multiple myeloma (Yaccoby et al., 2007; Fulciniti et al., 2009) | Primary multiple myeloma cell | Anti‐DKK1 Ab inhibited cell proliferation. | – | Oncogenic |
| Prostate cancer (Hall et al., 2008; Hall et al., 2010; Rachner et al., 2014) | PC‐3 | DKK1 knockdown inhibited tumour formation. |
High DKK1 levels in tumours were associated with shorter overall survival. High DKK1 levels in the serum were associated with a significantly shorter overall and disease specific survival. |
Oncogenic |
DKK1 as a negative regulator of Wnt signalling
There are at least six secretory antagonistic proteins that modulate Wnt signalling. Soluble frizzled‐related proteins (Kawano and Kypta, 2003), frizzled‐related protein (also known as Crescent) (Pera and De Robertis, 2000), Cerberus (Piccolo et al., 1999) and Wnt inhibitory factor‐1 (WIF‐1) (Hsieh et al., 1999) bind to Wnt proteins and sequester them from Wnt cell surface receptors, thereby inhibiting the Wnt pathway. Wnt modulator in surface ectoderm (also known as sclerostin domain containing 1) causes Wnt signalling inhibition by competing with Wnts for the binding to the Wnt co‐receptor low‐density lipoprotein receptor‐related proteins 6 (LRP6) (Itasaki et al., 2003). DKK1 also binds to LRP6 and sequesters it from the cell surface membrane, resulting in the inhibition of the Wnt pathway (Mao et al., 2001; Semënov et al., 2001; Brott and Sokol, 2002; Yamamoto et al., 2008; Sakane et al., 2010).
DKK1 was originally identified as an embryonic head inducer in Xenopus embryos and shown to be a secreted protein that antagonizes Wnt signalling (Glinka et al., 1998; Niehrs, 2006). Subsequently, the DKK protein family was shown to be comprised of four members (DKK1, DKK2, DKK3 and DKK4), and DKKs were identified in other vertebrates including humans and in invertebrates. From the phenotypes of DKK1 knockout mice, DKK1 is essential for various developmental processes, including anterior–posterior patterning, limb development, somatogenesis and eye formation (Mukhopadhyay et al., 2001; Niehrs, 2006). Heterozygous DKK1 mutant mice are variable but show a high bone mass due to increased bone formation (Morvan et al., 2006), whereas transgenic expression of DKK1 causes osteopenia and suppresses cell proliferation in the intestines with architectural degeneration (Pinto et al., 2003; Li et al., 2006). Thus, DKK1 is involved in many biological phenomena in the development and adult life of animals.
Of the multiple Wnt signalling pathways including the β‐catenin‐dependent pathway (β‐catenin pathway) and the β‐catenin‐independent pathway, DKK1 has been thought to modulate mainly the former pathway. DKK1 contains two characteristic cysteine‐rich domains (CRD‐1 and CRD‐2) (Glinka et al., 1998) and binds to LRP6 through CRD‐2, thereby suppressing the β‐catenin pathway. In the ‘two endocytic routes and two outputs model’, LRP6 is predominantly present in the lipid‐raft (detergent‐resistant) membrane fractions, and Ror2, a Wnt5a receptor, is found in the non‐lipid raft membrane fractions (Yamamoto et al., 2006; Kikuchi et al., 2009; Sato et al., 2010a) (Figure 1). Wnt3a induces LRP6 internalization in a caveolin‐dependent manner, which is required for the activation of the β‐catenin pathway, and Wnt5a induces the internalization of Ror2 with frizzled in a clathrin‐dependent manner, resulting in the activation of the β‐catenin‐independent pathway (Yamamoto et al., 2006; Sato et al., 2010a; Jiang et al., 2012; Demir et al., 2013), whereas DKK1 induces LRP6 internalization through a clathrin‐mediated route, resulting in removal of LRP6 from the plasma membrane (Mao et al., 2001; Yamamoto et al., 2008; Sakane et al., 2010) (Figure 1). Kremen1/2 has also been suggested to be a cell surface receptor of DKK1 (Mao et al., 2002), but the role of Kremen1/2 may be marginal in DKK1‐dependent Wnt signal inhibition (Ellwanger et al., 2008; Wang et al., 2008). As DKK1 is one of the direct target molecules expressed by the β‐catenin pathway (Gonzalez‐Sancho et al., 2005), DKK1 creates a negative feedback loop for Wnt signalling.
Figure 1.
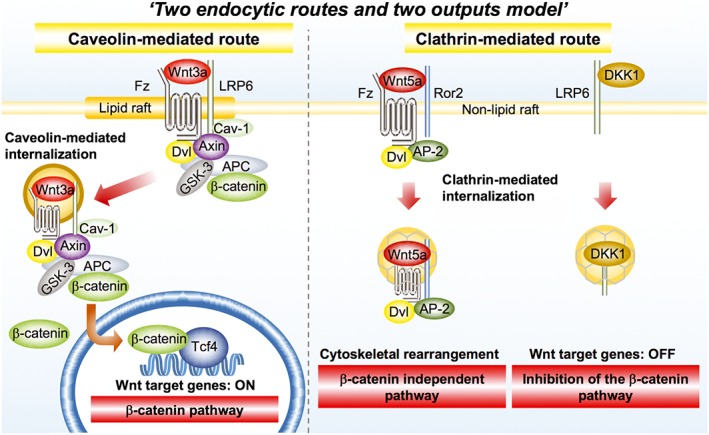
‘Two endocytic routes and two outputs model’ in the Wnt signalling pathways. See details in the text. AP‐2, adaptor protein complex 2; Cav‐1, caveolin‐1; Fz, frizzled; Ror2, receptor tyrosine kinase‐like orphan receptor 2; Dvl, dishevelled; GSK‐3, glycogen synthase kinase 3.
DKK1 has dual actions on tumour formation
Given that DKK1 acts as a negative regulator of Wnt signalling that potentially functions in oncogenic signalling, it would be reasonable that DKK1 has tumour‐growth inhibitory activity (Table 1). Indeed, DKK1 is down‐regulated in colorectal cancer and melanoma probably due to DNA hypermethylation and this observation is associated with advanced stages of tumourigenesis (Gonzalez‐Sancho et al., 2005; Aguilera et al., 2006; Kuphal et al., 2006). DKK1 has shown growth inhibition of various cancer cell lines in vitro and in vivo, including MCF‐7, MDA‐MB‐231 and SUM‐1315 breast cancer, HCT116 and DLD‐1 colon cancer and Hela cervical cancer cells (Mikheev et al., 2008; Qiao et al., 2008; DiMeo et al., 2009; Qi et al., 2012). In addition, DKK1 has been reported to induce or increase sensitivity to apoptosis in H28 and MS‐1 mesothelioma, U87MG glioblastoma, A498 renal cell carcinoma, MDA‐MB435 melanoma, JAR placental choriocarcinoma and HeLa cells (Shou et al., 2002; Lee et al., 2004; Mikheev et al., 2004; Peng et al., 2006; Mikheev et al., 2007; Hirata et al., 2011), but it is not clear if the apoptosis‐inducing effects of DKK1 are through the inhibition of Wnt signalling.
In parallel with reports that DKK1 functions as a tumour suppressor, it has been shown that DKK1 expression levels are increased in a variety of cancers, including oesophageal, lung, pancreas, prostate and breast cancers, multiple myeloma, hepatoblastoma, hepatocellular carcinoma (HCC) and Wilms' tumour and that its high expression in tumour tissue is associated with cancer progression and aggressiveness (Tian et al., 2003; Wirths et al., 2003; Forget et al., 2007; Yaccoby et al., 2007; Yamabuki et al., 2007; Hall et al., 2008; Makino et al., 2009; Takahashi et al., 2010; Li et al., 2011; Chen et al., 2013; Rachner et al., 2014; Kimura et al., 2016) (Table 1). Serum DKK1 levels are also significantly higher in lung, oesophageal and prostate cancer patients than in healthy controls (Yamabuki et al., 2007; Rachner et al., 2014). By ELISA, more than 70% of DKK1‐positive cases were diagnosed as lung and oesophageal cancers and less than 5% of healthy controls were DKK1 positive, suggesting that DKK1 may be useful as a diagnostic and prognostic marker (Yamabuki et al., 2007). It is not surprising that DKK1 is increased in multiple myeloma with osteolytic lesions, because DKK1 inhibits osteoblast proliferation and induces osteopenia by antagonizing Wnt signalling (Krishnan et al., 2006). Interestingly, DKK1 supports prostate cancer growth independent of Wnt signalling through the reduction in p21 expression (Hall et al., 2010).
DKK1 elevation in cancers may be explained as a result of aberrant activation of Wnt signalling, because DKK1 is a direct target molecule of Wnt signalling. However, as there are cancers where Wnt signalling is not activated aberrantly, it is hard to explain why DKK1 expression increases in these cancers in the context of Wnt signalling. The mechanism of Wnt signal‐independent expression of DKK1 needs to be clarified. In addition, the molecular mechanism underlying DKK1‐dependent cancer progression remains unclear. It could be hypothesized that DKK1 binds to an unknown cell surface receptor to stimulate cell proliferation and that DKK1 has distinct functions, independent of Wnt signalling. To support this hypothesis, a novel receptor of DKK1 had to be identified in cancer cells.
Identification of CKAP4 as a novel DKK1 receptor
Wnt proteins are secreted apically and basolaterally and in both ways in polarized epithelial cells (Figure 2). For instance, Wnt3a, Wnt5a and Wnt5b are secreted basolaterally in polarized MDCK cells and rat intestinal epithelial IEC6 cells, whereas Wnt11 is secreted apically (Gon et al., 2013; Yamamoto et al., 2013; Yamamoto et al., 2015). Wnt1 and Wingless (Wnt1 homologue in Drosophilla) are secreted both apically and basolaterally in MDCK cell and Drosophilla wing disc cells (Yamazaki et al., 2016; Yamamoto et al., in press). Wnt receptor Frizzled2 is localized to the basolateral membrane, and frizzled7 is localized to both apical and basolateral membranes (Yamamoto et al., 2015). Wnt co‐receptors, such as LRP6 for Wnt3a and Ror2 for Wnt5a, are localized to the basolateral membrane, whereas receptor‐like tryosine kinase (Ryk) for Wnt11 is present in the apical membrane (Gon et al., 2013; Yamamoto et al., 2015). Thus, it is likely that ligands and receptors in Wnt signalling trafficked to the same direction for efficient signalling.
Figure 2.
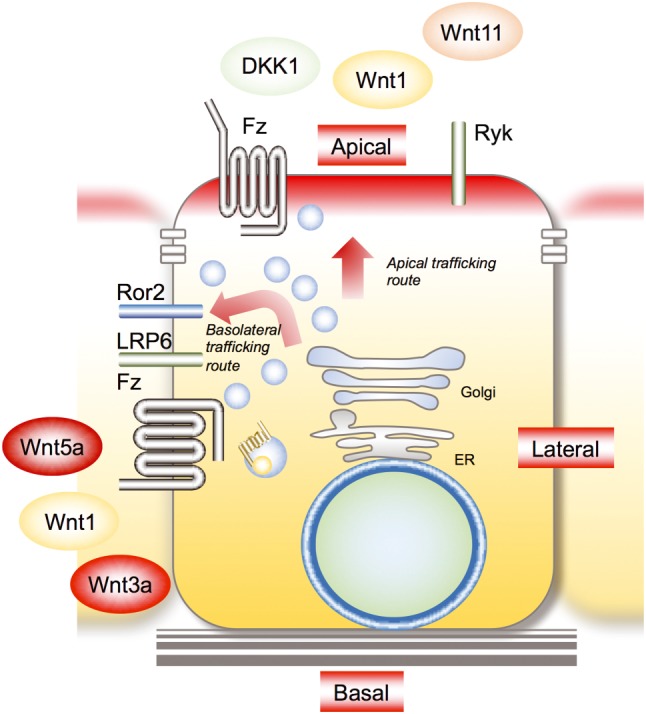
Polarized trafficking of Wnt proteins and receptors. Wnt3a, Wnt5a and Wnt5b are secreted basolaterally, and Wnt11 is secreted apically. Wnt1 is secreted in both ways. Wnt receptor frizzled2 is localized to the basolateral membrane, and Frizzled7 is localized to both apical and basolateral membranes. Wnt co‐receptors are localized to specific membrane domains (e.g. basolateral membrane, LRP6 and Ror2; apical membrane, Ryk). DKK1 is secreted apically. Fz, Frizzled; Ryk, receptor‐like tyrosine kinase.
DKK1 is predominantly secreted apically in polarized MDCK cells, and the addition of recombinant DKK1 to the apical side results in cell proliferation, but addition to the basolateral side shows no effect (Kimura et al., 2016). Because LRP6 is localized to the basolateral membrane of MDCK cells, it is thought that DKK1 acts on the putative receptor that is localized to the apical membrane and stimulates cell proliferation. To identify the receptor that mediates DKK1‐dependent cell proliferation, biotinylated cell surface proteins were precipitated with DKK1 and the precipitated proteins analysed by mass spectrometry (Kimura et al., 2016). Among the DKK1‐binding proteins, a 63‐kDa protein was identified as cytoskeleton‐associated protein 4 (CKAP4, also known as P63, CLIMP‐63 and ERGIC‐63).
Structure and functions of CKAP4
CKAP4 is a type II transmembrane protein that is not glycosylated but is reversibly palmitoylated (Schweizer et al., 1993a; Schweizer et al., 1994; Klopfenstein et al., 1998; Vedrenne and Hauri, 2006). CKAP4 was originally discovered as a protein largely confined to the endoplasmic reticulum (ER). CKAP4 has an intracellular region (aa 1–105), a transmembrane region (aa 106–127) and an ER luminal region containing stretches of heptad repeats (aa 128–602) (Klopfenstein et al., 2001) (Figure 3A). CKAP4's luminal region consists of predicted coiled‐coil domains (aa 130–214 and aa 256–460) with a leucine zipper (LZ) (aa 468–503) followed by a coiled‐coil domain (aa 533–602). The coiled‐coil domain has the propensity to oligomerize and tends to be involved in intermolecular interactions. Indeed, CKAP4 runs as bands of 63 and 120 kDa and a poorly resolved smear higher than at 310 kDa in SDS‐PAGE under non‐reducing conditions (Schweizer et al., 1993a). In addition, the recombinant luminal region forms a helical 91 nm long rod‐like structures, and its sedimentation equilibrium is 25.7 S (Klopfenstein et al., 2001). Fluorescence recovery after photobleaching analysis revealed that wild‐type CKAP4 has a very low recovery rate and that the CKAP4 mutant with deletion of the luminal region shows a rapid rate (Klopfenstein et al., 2001). Thus, the CKAP4 luminal region seems to form higher‐order oligomers. These structural characteristics may explain the specific localization of CKAP4 to the rough ER, where it is excluded from high‐curvature regions (Shibata et al., 2010).
Figure 3.
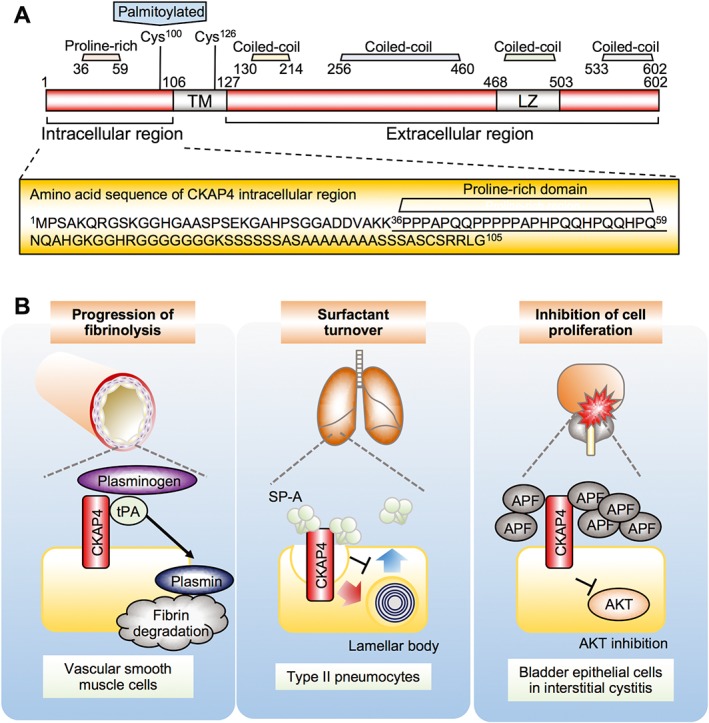
Structure and functions of CKAP4 in the cell surface membrane. (A) Schematic diagram of CKAP4. Amino acid residues in the CKAP4 intracellular region are enlarged, showing no tyrosine residues. The proline‐rich motif that interacts with the p85 subunit of PI3K is underlined. Cys, cysteine; TM, transmembrane. (B) Possible functions of CKAP4 in the cell surface membrane. Left panel, tPA binds to CKAP4 and regulates plasmin production and progression of fibrinolysis in vascular smooth muscle cells. Middle panel, SP‐A binds to CKAP4 and induces CKAP4 internalization and regulates secretion of lamellar body. Right panel, APF binds to CKAP4 and inhibits cell proliferation through the suppression of Akt in bladder epithelial cells.
Another approach to clarify the mechanism by which ER sheets are formed identified CKAP4 as an abundant integral membrane protein enriched in ER sheets (Shibata et al., 2010). Knockdown of CKAP4 in COS cells did not affect ER morphology, but the ER sheets were spread throughout the cytoplasm. In addition, the luminal width was reduced in CKAP4‐depeleted cells. Therefore, CKAP4 could function in segregating ER sheets close to the nucleus and maintaining the luminal width by the binding of CKAP4 localizing to opposing cisternal membranes in an antiparallel or parallel manner.
In the transmembrane and intracellular regions, there are two cysteine residues (Cys100 and Cys126) (Figure 3A). CKAP4 with Mr of 120 kDa in non‐reducing SDS‐PAGE may be a dimer of CKAP4 through a disulfide bond. Although Cys126 is a target of the disulfide bond, it is reported that Cys100 is modified with palmitate by palmitoyl acyltransferase DHHC2 (Schweizer et al., 1995; Zhang et al., 2008). Palmitoylation of CKAP4 is detected in the mitotic phase but minimally or not in interphase of CHO cells (Mundy and Warren, 1992), and palmitoylation is enhanced by brefeldin A, which blocks transport of proteins out of the ER and results in the disassembly of the Golgi apparatus (Misumi et al., 1986), in interphase of Vero cells (Schweizer et al., 1993b). Wild‐type CKAP4 is present on the cell surface and perinuclear membranes in addition to the ER, whereas CKAP4 C100S, in which Cys100 is mutated to Ser, is confined to the ER (Planey et al., 2009). Furthermore, anti‐proliferating factor (APF) induces nuclear translocation of CKAP4 in wild‐type HeLaS3 cells but not in DHHC2 knockdown cells (Planey et al., 2009). Thus, palmitoylation may be required for the trafficking of CKAP4 from the ER to the cell surface membrane and nucleus.
CKAP4 functions as a cell surface membrane protein
Although the predominant localization of CKAP4 is to the ER, CKAP4 has been shown to be present on the cell surface membrane of vascular smooth muscle cells (VSMCs), type II pneumocytes and bladder epithelial cells, where it functions as a receptor for tissue plasminogen activator (tPA), surfactant protein A (SP‐A) and APF, respectively (Razzaq et al., 2003; Conrads et al., 2006; Gupta et al., 2006) (Figure 3B). The ER luminal region of CKAP4 is equivalent to its extracellular region and functions to bind ligands when CKAP4 is localized to the cell surface membrane.
tPA catalyses the conversion of plasminogen to plasmin, a major enzyme responsible for clot degradation (Clowes et al., 1990). tPA binds to CKAP4 on VSMC surface membrane and anti‐CKAP4 antibody inhibits the binding of tPA to VSMC (Razzaq et al., 2003). Expression of an N‐terminally truncated CKAP4 mutant that is localized to the plasma membrane in COS cells results in more than sevenfold increase in plasminogen activity, which is reversed with an anti‐CKAP4 antibody (Schweizer et al., 1994; Razzaq et al., 2003).
SP‐A binds to type II pneumocytes in the lung via calcium‐dependent (specific) binding and calcium‐independent (non‐specific) binding. Knockdown of CKAP4 results in an inhibition of SP‐A specific binding (Bates et al., 2008) and anti‐CKAP4 antibody blocks SP‐A's specific binding and inhibits SP‐A's ability to inhibit surfactant secretion (Gupta et al., 2006; Bates, 2010), supporting CKAP4's ability to bind to SP‐A. Although the mechanism is not clear, it is reported that SP‐A induces CKAP4 translocation from the ER to cell surface membrane through Akt activation (Kazi et al., 2010).
APF is a sialoglycopeptide elevated in the urine of patients with interstitial cystitis, a chronic, painful bladder disease (Keay et al., 2000). APF inhibits the proliferation of normal bladder epithelial cells and bladder cancer cells through binding to CKAP4. Knockdown of CKAP4 or treatment with anti‐CKAP4 antibody inhibits APF's effects on cell proliferation and gene expression (Conrads et al., 2006). It has also been shown that changes in MMP2, p53 and CCN2 protein expression and Akt phosphorylation in response to APF are abrogated following CKAP4 knockdown in T24 bladder carcinoma cells (Shahjee et al., 2010; Matika et al., 2012). These results indicate that CKAP4 is essential for mediating the signalling from three ligands, tPA, SP‐A and APF.
CKAP4 functions as a receptor of DKK1 and regulates cell proliferation
CKAP4 is localized to the apical membrane of polarized MDCK cells (Kimura et al., 2016). Therefore, the addition of DKK1 to the apical region stimulates MDCK cell proliferation. DKK1 induces the internalization of CKAP4 from the cell surface membrane through a clathrin‐mediated route. DKK1 directly binds to the extracellular region of CKAP4 with a Kd of 0.42 nM which is comparable with the Kd (0.34–0.5 nM) of DKK1 binding to LRP6 (Mao et al., 2001; Semënov et al., 2001). The binding of DKK1 to CKAP4 activates Akt through PI3K (Figure 4).
Figure 4.
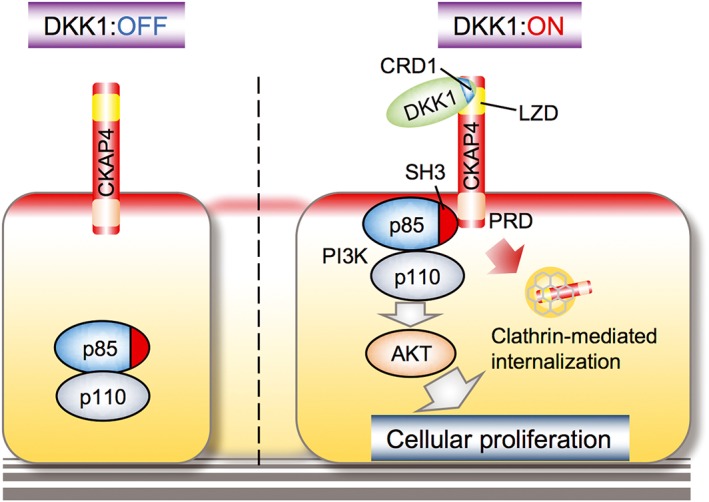
The DKK1 and CKAP4 signalling axis. DKK1 binds to the extracellular region of CKAP4. CRD‐1 of DKK1 and LZD of CKAP4 are required for their interaction. PRD of CKAP4 binds to the SH3 domain of the p85 subunit upon DKK1 binding to CKAP4, resulting in the activation of the PI3K and Akt signalling pathway and the promotion of cell proliferation. CRD, cysteine‐rich domain; LZD, leucine‐rich domain; PRD, proline‐rich domain.
It is well known that as soon as growth factor receptors, such as EGF receptor and PDGF receptor, are tyrosine phosphorylated in the intracellular region by ligand binding, the SH2 domain of the p85 regulatory subunit of PI3K is recruited to the receptor, resulting in the production of phosphatidylinositol‐3,4,5‐triphosphate and then activating Akt (Wymann and Pirola, 1998). However, as there are no tyrosine residues in the cytoplasmic region of CKAP4 (Figure 3A), it is theoretically impossible to bind to PI3K by the same mechanism as growth factor receptors. Instead, the intracellular region of CKAP4 has the proline‐rich motif to which the SH3 domain of p85 binds, depending on DKK1. The proline‐rich motif of CKAP4 may be masked in the absence of DKK1, and the closed region would be opened by the binding of CKAP4 and DKK1, which results in the recruitment of p85. This is the novel mechanism by which a cell surface receptor activates Akt through PI3K in response to an extracellular ligand. Thus, when CKAP4 is present on the cell surface membrane, DKK1 can promote cell proliferation through the activation of the PI3K‐Akt signal cascade although it remains to be clarified whether DKK1‐dependent CKAP internalization is involved in the regulation of signalling.
DKK1 and CKAP4 are required for cancer cell proliferation
Because CKAP4 was discovered as an ER residual protein (Schweizer et al., 1993a), it is reasonable that CKAP4 is expressed in many cancer and non‐cancer cells lines. However, whether CKAP4 is localized to the cell surface membrane depends on cell type. For instance, CKAP4 is detected on the cell surface membrane of A549 lung, S2‐CP8 pancreatic, HeLaS3 cervical and TE‐8 oesophageal cancer cells and HepG2 hepatoblastoma cells (unpublished observations). DKK1 expression levels in cultured cell lines vary depending on cell type: DKK1 is well expressed in some cancer cell lines, including A549, S2‐CP8, TE‐8, HepG2 and KKLS gastric cancer cells; DKK1 is less expressed in HeLaS3 and AGS gastric cancer cells; and DKK1 is minimally detected in non‐tumour cell lines, including MDCK, X293T kidney epithelial and Eph4 mammary cells.
p85 forms a complex with CKAP4 in S2‐CP8 cells, and complex formation is suppressed by DKK1 knockdown (Kimura et al., 2016). Akt activity and cell proliferation are suppressed in DKK1‐ or CKAP4‐depleted S2‐CP8 and A549 cells, and inhibition is rescued by expression of DKK1 or CKAP4. In contrast, CKAP4 knockdown in HeLaS3 cells does not affect cell proliferation, because HeLaS3 cells express CKAP4 but little express DKK1. Thus, expression of both DKK1 and CKAP4 is necessary for cancer cell proliferation, and CKAP4 acts as an oncogene product when it is expressed on the cell surface membrane. The formation of the xenograft tumours derived from CKAP4‐depeleted S2‐CP8 and A549 cells is less than that of control tumours (Kimura et al., 2016). Under the conditions where expression of wild‐type DKK1 rescues the phenotypes induced by DKK1 knockdown, DKK1ΔCRD‐1, which fails to bind to CKAP4, does not rescue tumour formation, supporting the notion that DKK1 functionally interacts with CKAP4 in tumour formation in vivo. Therefore, CKAP4 may be a potential molecular target for cancer therapy.
APF inhibits Akt activity and proliferation in bladder cells through CKAP4 (Shahjee et al., 2010). In HCC cells, CKAP4 associates with EGF receptors and inhibits EGFR signalling, resulting in decreased proliferation and invasion capabilities (Li et al., 2014a). It is unknown whether other CKAP4 ligands, such as SP‐A and tPA, are involved in the proliferation of these cancer cells. Thus, the downstream signalling and binding proteins of CKAP4 in cancer cells may vary depending on cell type.
CKAP4 expression is related with prognosis of cancer patients
In line with the observations that high expression levels of DKK1 in tumour tissue are associated with aggressiveness in various types of cancers, CKAP4 is also expressed in pancreatic, lung and oesophageal tumours and is minimally detected in non‐tumour tissues (Kimura et al., 2016) (unpublished observations) (Figure 5A). Importantly, patients positive for both DKK1 and CKAP4 show poor prognosis and reduced relapse‐free survival than do patients positive for either DKK1 or CKAP4 or negative for both. Thus, simultaneous expression of DKK1 and CKAP4, a ligand and a receptor, plays critical roles in cancer aggressiveness. It is notable that DKK1 but not CKAP4 is detected in lung atypical adenomatous hyperplasia (AAH). AAH cells proliferate along the pre‐existing alveolar epithelium and show increased cell size and prominent nucleoli. The grade of atypia in lung AAH is usually milder than that in adenocarcinoma (Mori et al., 2001). Therefore, CKAP4 expression may be involved in the transition from the precancerous state to the cancerous state.
Figure 5.
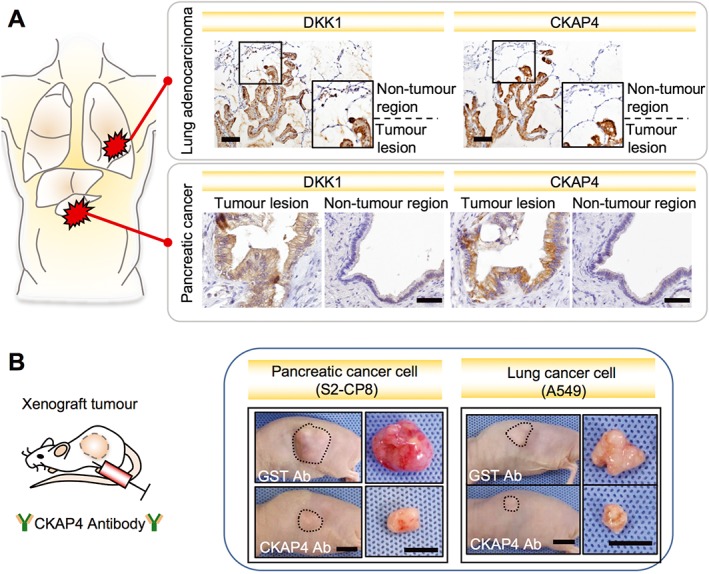
Simultaneous expression of DKK1 and CKAP4 in cancers and inhibition of cancer cell proliferation by anti‐CKAP4 antibody. (A) In lung adenocarcinoma and pancreatic cancer tissues, DKK1 and CKAP4 are expressed in tumour lesions and minimally detected in non‐tumour regions. Scale bars, 100 μm in lung adenocarcinoma and 50 μm in pancreatic cancer. (B) In the xenograft tumour model using nude mice subcutaneously implanted with pancreatic cancer cells (S2‐CP8 cells, left panels) and lung cancer cells (A549 cells, right panels), anti‐CKAP4 antibody suppressed tumour formation. Anti‐Glutathione S‐transferase (GST) antibody was used as a control. Representative appearance of one mouse (left pictures) and extirpated xenograft tumours (right pictures) are shown. Scale bars, 10 mm (Kimura et al., 2016).
There are opposing reports for intrahepatic cholangiocarcinoma (ICC) and HCC (Li et al., 2013; Li et al., 2014b). CKAP4 expression is associated with tumour size and lymph node metastasis of ICC, but CKAP4 is expressed less in lymph node metastatic lesions rather than in primary lesions. ICC patients with low CKAP4 expression have a shorter overall survival and higher recurrence, suggesting that CKAP4 acts as tumour suppressor and is a favourable prognostic marker. HCC patients with high expression of CKAP4 show a favourable overall survival and a longer disease‐free survival compared with low expression. Since DKK1 expression levels in ICC and HCC have not been examined in these studies, whether the tumour suppressor activity of CKAP4 in ICC and HCC is related to DKK1 remains unclear.
DKK1‐CKAP signalling axis is a molecular target for cancer therapy
A series of experiments concerning the roles of DKK1 and CKAP4 in cancers suggested that both proteins are molecular targets for cancer therapy. A SCID‐hu mouse model, where DKK1‐producing multiple myeloma (MM) cells are injected in the implanted human bone, developed osteoporosis and bone lesions with growth of MM cells and increased DKK1 in murine blood (Fulciniti et al., 2009). Injection with a humanized anti‐DKK1 monoclonal antibody (BHQ880) increased and decreased the numbers of osteoblast and MM cells, respectively, in human bone. Since BHQ880 has no direct effect on MM cells but inhibits MM cell growth in the presence of bone marrow stromal cells, the inhibition by BHQ880 would not affect Wnt signalling directly in MM cells. Anti‐DKK1 polyclonal antibody also suppressed xenograft tumour formation by DKK1‐overexpressing A549 cells (Sato et al., 2010b). This antibody was generated by immunizing with the N‐terminal 120 amino acids of DKK1, including CRD‐1 which binds CKAP4 but not LRP6. Therefore, it is possible that growth inhibition by the antibody is due to the inhibition of the binding of DKK1 and CKAP4.
A humanized anti‐DKK1 monoclonal therapeutic antibody (DKN‐01) underwent phase I evaluation in relapsed oesophageal squamous carcinoma (five patients) and gastro‐oesophageal junctional cancer (four patients) in combination with paclitaxel (Bendell et al., 2016). Three patients had a partial response, and three other patients showed stable disease. Another phase I evaluation was done in advanced biliary cancer (21 patients) in combination with gemcitabine and cisplatin. Among 18 patients who were evaluable for responses, three patients had a partial response, five showed stable disease and one had progressive disease (see http://www.leaptx.com/news/). Further investigation for both evaluations is ongoing. However, the underlying mechanism of DKN‐01 effects on cancer growth is not known at present.
As an anti‐DKK1 antibody has possible side effects, including effects on bone homeostasis and cell proliferation by activating Wnt signalling, the anti‐CKAP4 antibody may be more specific for anti‐proliferative effects by blocking the DKK1‐CKAP4 signalling axis. Anti‐CKAP4 polyclonal antibody inhibits the in vitro binding of DKK1 to CKAP4, DKK1‐induced Akt activation in MDCK, S2‐CP8 and A549 cells and xenograft tumour formation caused by S2‐CP8 and A549 cells (Kimura et al., 2016) (Figure 5B). In contrast, the antibody did not affect tumour formation caused by HeLaS3 cells. In the cells, CKAP4 is expressed on the cell surface membrane but DKK1 expression is marginal, confirming that simultaneous expression of DKK1 and CKAP4 plays a role in cancer promotion. Thus, CKAP4 could be a molecular target for cancers expressing both DKK1 and cell surface CKAP4.
Future perspectives
In this article, the novel DKK1‐CKAP4 signalling axis in cancer proliferation and a possible cancer drug using anti‐CKAP4 antibody have been reviewed (Figure 6). Identification of CKAP4 as a DKK1 receptor expands the understanding of the DKK1 signalling mechanism and defines a previously unrecognized input to the PI3K and Akt pathway. There are several questions to be addressed in further studies.
Figure 6.
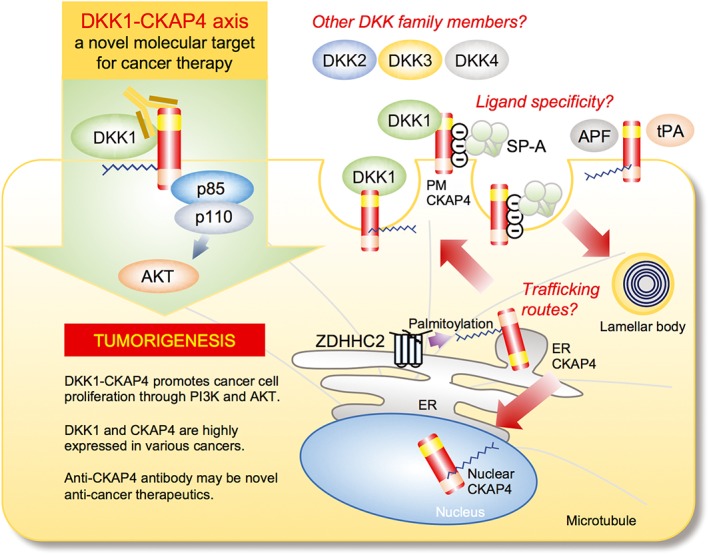
The DKK1–CKAP4 axis as a novel molecular target for cancer therapy and future perspectives. Upon binding to CKAP4, DKK1 promotes cancer cell proliferation through the activation of PI3K and Akt. Anti‐CKAP4 antibody inhibits the binding of DKK1 to CKAP4 and xenograft tumour formation. There are questions to be addressed. (1) ‘Trafficking routes’: it is unclear how subcellular localization of CKAP4 is regulated. Although CKAP4 functions as a receptor, CKAP4 is mainly localized to the ER. Post‐translational modification, such as palmitoylation at Cys100 by DHHC2, could be involved in the subcellular localization of CKAP4. (2) ‘Other Dkk family members’: it is unknown whether other DKK family members also act as a ligand for CKAP4. Since CRD‐1 is conserved among the four DKK genes, DKK2, 3 and 4 would interact with CKAP4. (3) ‘Ligand specificity’: Although several ligands for CKAP4 other than DKK1 have been identified, the specificity of the binding between CKAP4 and ligands has not been addressed. See details in the text.
The first question is how subcellular localization of CKAP4 is regulated. CKAP4 is largely confined to the ER, and only a marginal part (a few percent) is present on the cell surface membrane. Clarifying the trafficking mechanism of CKAP4 is important because the DKK1‐CKAP4 signalling axis is operational only when CKAP4 is localized to the cell surface membrane. When CKAP4 is overexpressed in the cell surface membrane of cancer cells, DKK1‐dependent inhibition of Wnt signalling may be suppressed by competing with LRP6 for the binding to DKK1. In addition, it has been reported that CKAP4 is palmitoylated at Cys100 by DHHC2 (Schweizer et al., 1995; Zhang et al., 2008) and APF‐mediated nuclear localization of CKAP4 requires the Cys100 residue. Thus, palmitoylation could be important for subcellular localization of CKAP4. Palmitoylated transmembrane proteins are recruited to the lipid raft microdomains of the cell surface membrane (Levental et al., 2010). LRP6 has been shown to be palmitoylated at the juxtamembrane cysteine residues (Abrami et al., 2008) and localized to both the detergent‐resistant membrane (DRM) and non‐DRM of the cell surface (Yamamoto et al., 2006). DKK1 induces the translocation of LRP6 from DRM to non‐DRM and internalizes LRP6 in a clathrin‐dependent manner, thereby suppressing Wnt signalling (Yamamoto et al., 2008). Therefore, it is tempting to speculate that DKK1 regulates the localization of CKAP4 in the microdomains of the cell surface membrane, as well as that of LRP6.
The second issue is whether other DKK family members also act as a ligand for CKAP4. The DKK family comprises four conserved proteins, DKK1, 2, 3 and 4. However, the functions of DKK family members other than DKK1 are less well characterized. DKK2 and DKK4 antagonize Wnt signalling, similar to DKK1, but DKK2 can also activate Wnt signalling depending on the cell (Niehrs, 2006). Physiological role of DKK3 is controversial and pleiotropic. While DKK3 positively regulates Wnt signalling in Müller glia and HEK293 cells (Nakamura et al., 2007), it is also reported that DKK3 has no effect on Wnt signalling (Veeck and Dahl, 2012). However, CRD‐1 is highly conserved among the four DKK genes, raising the possibility that DKK2, 3 and 4 could also interact with CKAP4.
The third one is specificity of the binding between CKAP4 and ligands. The LZ domain of CKAP4 is required for binding to DKK1. Although the region of CKAP4 that interacts with other ligands is not known, it has been hypothesized that the negatively charged amino acid cluster region of CKAP4 (the region of amino acid 318–328 containing five negatively charged amino acids) binds to the positively charged region of SP‐A (Bates, 2010). There are 165 charged residues over the entire 474‐amino acid extracellular region of CKAP4. These charged residues may be involved in the binding to other ligands through hydrophilic interactions. It is important to clarify whether these ligands share a common 3D structural domain that binds to CKAP4.
Lastly, humanized anti‐CKAP4 monoclonal antibody absolutely needs to be generated for use in human cancer therapy, and the antibody must be tested in mouse cancer models other than xenograft tumours derived from cancer cell lines. To examine the adverse effects of anti‐CKAP4 antibody in vivo, the analyses of phenotypes of CKAP4 knockout mice would provide information on expected side effects and contribute to the understanding of CKAP4 functions. It is also important to develop diagnostic methods to pick out cancer patients who would respond effectively to treatment with anti‐CAKP4 antibody. As an ELISA to detect serum DKK1 is already available, it would be necessary to determine the presence of cell surface CKAP4 in cancer patients. If CKAP4 was secreted with microvesicles from cancer cells into the serum, exosomes could be a good candidate to examine. Thus, much further work is needed to understand the whole picture of the novel DKK1‐CKAP4 signalling axis in tumourigenesis.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Conflict of interest
The authors declare no conflicts of interest.
Kikuchi, A. , Fumoto, K. , and Kimura, H. (2017) The Dickkopf1‐cytoskeleton‐associated protein 4 axis creates a novel signalling pathway and may represent a molecular target for cancer therapy. British Journal of Pharmacology, 174: 4651–4665. doi: 10.1111/bph.13863.
References
- Abrami L, Kunz B, Iacovache I, van der Goot FG (2008). Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A 105: 5384–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J et al. (2006). Epigenetic inactivation of the Wnt antagonist DICKKOPF‐1 (DKK‐1) gene in human colorectal cancer. Oncogene 25: 4116–4121. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SR (2010). P63 (CKAP4) as an SP‐A receptor: implications for surfactant turnover. Cell Physiol Biochem 25: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SR, Kazi AS, Tao JQ, Yu KJ, Gonder DS, Feinstein SI et al. (2008). Role of P63 (CKAP4) in binding of surfactant protein‐A to type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 295: L658–L669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell JC, Murphy JE, Mahalingam D, Halmos B, Sirard CA, Landau SB et al. (2016). Phase I study of DKN‐01, an anti‐DKK1 antibody, in combination with paclitaxel (pac) in patients (pts) with DKK1+ relapsed or refractory esophageal cancer (EC) or gastro‐esophageal junction tumors (GEJ). J Clin Oncol 34 (suppl 4S) abstract 111. [Google Scholar]
- Brott BK, Sokol SY (2002). Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol 22: 6100–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW et al. (2009). Small molecule‐mediated disruption of Wnt‐dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li M, Li Q, Wang CJ, Xie SQ (2013). DKK1 promotes hepatocellular carcinoma cell migration and invasion through b‐catenin/MMP7 signaling pathway. Mol Cancer 12: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R (2012). Wnt/b‐catenin signaling and disease. Cell 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Clowes MM, Au YP, Reidy MA, Belin D (1990). Smooth muscle cells express urokinase during mitogenesis and tissue‐type plasminogen activator during migration in injured rat carotid artery. Circ Res 67: 61–67. [DOI] [PubMed] [Google Scholar]
- Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR et al. (2006). CKAP4/p63 is a receptor for the frizzled‐8 protein‐related antiproliferative factor from interstitial cystitis patients. J Biol Chem 281: 37836–37843. [DOI] [PubMed] [Google Scholar]
- Demir K, Kirsch N, Beretta CA, Erdmann G, Ingelfinger D, Moro E et al. (2013). RAB8B is required for activity and caveolar endocytosis of LRP6. Cell Rep 4: 1224–1234. [DOI] [PubMed] [Google Scholar]
- DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S et al. (2009). A novel lung metastasis signature links Wnt signaling with cancer cell self‐renewal and epithelial‐mesenchymal transition in basal‐like breast cancer. Cancer Res 69: 5364–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger K, Saito H, Clement‐Lacroix P, Maltry N, Niedermeyer J, Lee WK et al. (2008). Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol 28: 4875–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M et al. (2004). A small molecule inhibitor of b‐catenin/CREB‐binding protein transcription [corrected]. Proc Natl Acad Sci U S A 101: 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget MA, Turcotte S, Beauseigle D, Godin‐Ethier J, Pelletier S, Martin J et al. (2007). The Wnt pathway regulator DKK1 is preferentially expressed in hormone‐resistant breast tumours and in some common cancer types. Br J Cancer 96: 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA et al. (2009). Anti‐DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998). Dickkopf‐1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362. [DOI] [PubMed] [Google Scholar]
- Gon H, Fumoto K, Ku Y, Matsumoto S, Kikuchi A (2013). Wnt5a signaling promotes apical and basolateral polarization of single epithelial cells. Mol Biol Cell 24: 3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Sancho JM, Aguilera O, Garcia JM, Pendas‐Franco N, Pena C, Cal S et al. (2005). The Wnt antagonist DICKKOPF‐1 gene is a downstream target of b‐catenin/TCF and is downregulated in human colon cancer. Oncogene 24: 1098–1103. [DOI] [PubMed] [Google Scholar]
- Gupta N, Manevich Y, Kazi AS, Tao JQ, Fisher AB, Bates SR (2006). Identification and characterization of p63 (CKAP4/ERGIC‐63/CLIMP‐63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 291: L436–L446. [DOI] [PubMed] [Google Scholar]
- Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET (2008). Dickkopf‐1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 68: 1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CL, Zhang H, Baile S, Ljungman M, Kuhstoss S, Keller ET (2010). p21CIP‐1/WAF‐1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf‐1. Cancer Res 70: 9916–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Ueno K et al. (2011). Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int J Cancer 128: 1793–1803. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH et al. (1999). A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398: 431–436. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al. (2009). Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC et al. (2003). Wise, a context‐dependent activator and inhibitor of Wnt signalling. Development 130: 4295–4305. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He X, Howe PH (2012). Disabled‐2 (Dab2) inhibits Wnt/b‐catenin signalling by binding LRP6 and promoting its internalization through clathrin. EMBO J 31: 2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R (2003). Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627–2634. [DOI] [PubMed] [Google Scholar]
- Kazi AS, Tao JQ, Feinstein SI, Zhang L, Fisher AB, Bates SR (2010). Role of the PI3‐kinase signaling pathway in trafficking of the surfactant protein A receptor P63 (CKAP4) on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 299: L794–L807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW (2000). Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin‐binding epidermal growth factor‐like growth factor production. J Urol 164: 2112–2128. [PubMed] [Google Scholar]
- Kikuchi A (2003). Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci 94: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A (2009). Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol 19: 119–129. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S (2011). New insights into the mechanism of wnt signaling pathway activation. Int Rev Cell Mol Biol 291: 21–71. [DOI] [PubMed] [Google Scholar]
- Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H et al. (2016). CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Invest 126: 2689–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996). Lessons from hereditary colorectal cancer. Cell 87: 159–170. [DOI] [PubMed] [Google Scholar]
- Klopfenstein DR, Kappeler F, Hauri HP (1998). A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J 17: 6168–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Klumperman J, Lustig A, Kammerer RA, Oorschot V, Hauri HP (2001). Subdomain‐specific localization of CLIMP‐63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha‐helical segment. J Cell Biol 153: 1287–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA (2006). Regulation of bone mass by Wnt signaling. J Clin Invest 116: 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK (2006). Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene 25: 5027–5036. [DOI] [PubMed] [Google Scholar]
- Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N et al. (2004). Dickkopf‐1 antagonizes Wnt signaling independent of b‐catenin in human mesothelioma. Biochem Biophys Res Commun 323: 1246–1250. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F et al. (2004). Small‐molecule antagonists of the oncogenic Tcf/b‐catenin protein complex. Cancer Cell 5: 91–102. [DOI] [PubMed] [Google Scholar]
- Levental I, Lingwood D, Grzybek M, Coskun U, Simons K (2010). Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A 107: 22050–22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M et al. (2006). Dkk1‐mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39: 754–766. [DOI] [PubMed] [Google Scholar]
- Li MH, Dong LW, Li SX, Tang GS, Pan YF, Zhang J et al. (2013). Expression of cytoskeleton‐associated protein 4 is related to lymphatic metastasis and indicates prognosis of intrahepatic cholangiocarcinoma patients after surgery resection. Cancer Lett 337: 248–253. [DOI] [PubMed] [Google Scholar]
- Li S, Qin X, Liu B, Sun L, Zhang X, Li Z et al. (2011). Dickkopf‐1 is involved in invasive growth of esophageal cancer cells. J Mol Histol 42: 491–498. [DOI] [PubMed] [Google Scholar]
- Li SX, Liu LJ, Dong LW, Shi HG, Pan YF, Tan YX et al. (2014a). CKAP4 inhibited growth and metastasis of hepatocellular carcinoma through regulating EGFR signaling. Tumour Biol 35: 7999–8005. [DOI] [PubMed] [Google Scholar]
- Li SX, Tang GS, Zhou DX, Pan YF, Tan YX, Zhang J et al. (2014b). Prognostic significance of cytoskeleton‐associated membrane protein 4 and its palmitoyl acyltransferase DHHC2 in hepatocellular carcinoma. Cancer 120: 1520–1531. [DOI] [PubMed] [Google Scholar]
- Makino T, Yamasaki M, Takemasa I, Takeno A, Nakamura Y, Miyata H et al. (2009). Dickkopf‐1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol 16: 2058–2064. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM et al. (2002). Kremen proteins are Dickkopf receptors that regulate Wnt/b‐catenin signalling. Nature 417: 664–667. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A et al. (2001). LDL‐receptor‐related protein 6 is a receptor for Dickkopf proteins. Nature 411: 321–325. [DOI] [PubMed] [Google Scholar]
- Matika CA, Wasilewski M, Arnott JA, Planey SL (2012). Antiproliferative factor regulates connective tissue growth factor (CTGF/CCN2) expression in T24 bladder carcinoma cells. Mol Biol Cell 23: 1976–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev AM, Mikheeva SA, Liu B, Cohen P, Zarbl H (2004). A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf‐1 as suppressor of HeLa cell transformation. Carcinogenesis 25: 47–59. [DOI] [PubMed] [Google Scholar]
- Mikheev AM, Mikheeva SA, Maxwell JP, Rivo JV, Rostomily R, Swisshelm K et al. (2008). Dickkopf‐1 mediated tumor suppression in human breast carcinoma cells. Breast Cancer Res Treat 112: 263–273. [DOI] [PubMed] [Google Scholar]
- Mikheev AM, Mikheeva SA, Rostomily R, Zarbl H (2007). Dickkopf‐1 activates cell death in MDA‐MB435 melanoma cells. Biochem Biophys Res Commun 352: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y (1986). Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem 261: 11398–11403. [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004). WNT and b‐catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701. [DOI] [PubMed] [Google Scholar]
- Mori M, Rao SK, Popper HH, Cagle PT, Fraire AE (2001). A typical adenomatous hyperplasia of the lung: a probable forerunner in the development of adenocarcinoma of the lung. Mod Pathol 14: 72–84. [DOI] [PubMed] [Google Scholar]
- Morvan F, Boulukos K, Clement‐Lacroix P, Roman Roman S, Suc‐Royer I, Vayssiere B et al. (2006). Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21: 934–945. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez‐Esteban C, Chen L, Tsukui T, Gomer L et al. (2001). Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1: 423–434. [DOI] [PubMed] [Google Scholar]
- Mundy DI, Warren G (1992). Mitosis and inhibition of intracellular transport stimulate palmitoylation of a 62‐kD protein. J Cell Biol 116: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RE, Hunter DD, Yi H, Brunken WJ, Hackam AS (2007). Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C (2006). Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25: 7469–7481. [DOI] [PubMed] [Google Scholar]
- Peng S, Miao C, Li J, Fan X, Cao Y, Duan E (2006). Dickkopf‐1 induced apoptosis in human placental choriocarcinoma is independent of canonical Wnt signaling. Biochem Biophys Res Commun 350: 641–647. [DOI] [PubMed] [Google Scholar]
- Pera EM, De Robertis EM (2000). A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb‐1. Mech Dev 96: 183–195. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T et al. (1999). The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397: 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H (2003). Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planey SL, Keay SK, Zhang CO, Zacharias DA (2009). Palmitoylation of cytoskeleton associated protein 4 by DHHC2 regulates antiproliferative factor‐mediated signaling. Mol Biol Cell 20: 1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2007). The many ways of Wnt in cancer. Curr Opin Genet Dev 17: 45–51. [DOI] [PubMed] [Google Scholar]
- Qi L, Sun B, Liu Z, Li H, Gao J, Leng X (2012). Dickkopf‐1 inhibits epithelial‐mesenchymal transition of colon cancer cells and contributes to colon cancer suppression. Cancer Sci 103: 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD (2008). Dkk‐1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett 269: 67–77. [DOI] [PubMed] [Google Scholar]
- Rachner TD, Thiele S, Gobel A, Browne A, Fuessel S, Erdmann K et al. (2014). High serum levels of Dickkopf‐1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer 14: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq TM, Bass R, Vines DJ, Werner F, Whawell SA, Ellis V (2003). Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type‐II transmembrane protein p63 (CKAP4). J Biol Chem 278: 42679–42685. [DOI] [PubMed] [Google Scholar]
- Sakane H, Yamamoto H, Kikuchi A (2010). LRP6 is internalized by Dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J Cell Sci 123: 360–368. [DOI] [PubMed] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A (2010a). Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 29: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K et al. (2010b). Wnt inhibitor Dickkopf‐1 as a target for passive cancer immunotherapy. Cancer Res 70: 5326–5336. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri HP (1993a). Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER‐to‐Golgi pathway. J Cell Sci 104: 671–683. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Hauri HP, Kornfeld S (1994). Retention of p63 in an ER‐Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J Cell Biol 126: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Jeno P, DeMaio A, Buchman TG, Hauri HP (1993b). A reversibly palmitoylated resident protein (p63) of an ER‐Golgi intermediate compartment is related to a circulatory shock resuscitation protein. J Cell Sci 104 (Pt 3): 685–694. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Kornfeld S (1995). Determination of the structural requirements for palmitoylation of p63. J Biol Chem 270: 9638–9644. [DOI] [PubMed] [Google Scholar]
- Semënov M, Tamai K, Brott BK, Kuhl M, Sokol S, He X (2001). Head inducer Dickkopf‐1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11: 951–961. [DOI] [PubMed] [Google Scholar]
- Shahjee HM, Koch KR, Guo L, Zhang CO, Keay SK (2010). Antiproliferative factor decreases Akt phosphorylation and alters gene expression via CKAP4 in T24 bladder carcinoma cells. J Exp Clin Cancer Res 29: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA (2010). Mechanisms determining the morphology of the peripheral ER. Cell 143: 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Ali‐Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS (2002). Human Dkk‐1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 21: 878–889. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Fukushima T, Yorita K, Tanaka H, Chijiiwa K, Kataoka H (2010). Dickkopf‐1 is overexpressed in human pancreatic ductal adenocarcinoma cells and is involved in invasive growth. Int J Cancer 126: 1611–1620. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B et al. (2003). The role of the Wnt‐signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494. [DOI] [PubMed] [Google Scholar]
- Vedrenne C, Hauri HP (2006). Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic 7: 639–646. [DOI] [PubMed] [Google Scholar]
- Veeck J, Dahl E (2012). Targeting the Wnt pathway in cancer: the emerging role of Dickkopf‐3. Biochim Biophys Acta 1825: 18–28. [DOI] [PubMed] [Google Scholar]
- Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D et al. (2011). Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res 71: 197–205. [DOI] [PubMed] [Google Scholar]
- Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D (2009). Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer 9: 489–499. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J et al. (2008). Characterization of the Kremen‐binding site on Dkk1 and elucidation of the role of Kremen in Dkk‐mediated Wnt antagonism. J Biol Chem 283: 23371–23375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG et al. (2003). Overexpression of human Dickkopf‐1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms' tumors. Lab Invest 83: 429–434. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Pirola L (1998). Structure and function of phosphoinositide 3‐kinases. Biochim Biophys Acta 1436: 127–150. [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr (2007). Antibody‐based inhibition of DKK1 suppresses tumor‐induced bone resorption and multiple myeloma growth in vivo. Blood 109: 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M et al. (2007). Dikkopf‐1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res 67: 2517–2525. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Awada C, Hanaki H, Sakane H, Tsujimoto I, Takahashi Y et al. (2013). The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J Cell Sci 126: 2931–2943. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Awada C, Matsumoto S, Kaneiwa T, Sugimoto T, Takao T et al. (2015). Basolateral secretion of Wnt5a in polarized epithelial cells is required for apical lumen formation. J Cell Sci 128: 1015–1063. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A (2006). Caveolin is necessary for Wnt‐3a‐dependent internalization of LRP6 and accumulation of b‐catenin. Dev Cell 11: 213–223. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A (2008). Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of b‐catenin signaling. Dev Cell 15: 37–48. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sato A, Kikuchi A (in press). The apical secretion of Wnt1 in polarized epithelia cells is regulated by exocyst‐mediated trafficking. J Biochem. In press. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Palmer L, Alexandre C, Kakugawa S, Beckett K, Gaugue I et al. (2016). Godzilla‐dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs. Nat Cell Biol 18: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Planey SL, Ceballos C, Stevens SM Jr, Keay SK, Zacharias DA (2008). Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Mol Cell Proteomics 7: 1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]


