Abstract
Wnt signalling is a fundamental pathway involved in embryonic development and adult tissue homeostasis. Mutations in the pathway frequently lead to developmental defects and cancer. As such, therapeutic intervention of this pathway has generated tremendous interest. Dickkopf‐1 (DKK1) is a secreted inhibitor of β‐catenin‐dependent Wnt signalling and was originally characterized as a tumour suppressor based on the prevailing view that Wnt signalling promotes cancer pathogenesis. However, DKK1 appears to increase tumour growth and metastasis in preclinical models and its elevated expression correlates with a poor prognosis in a range of cancers, indicating that DKK1 has more complex cellular and biological functions than originally appreciated. Here, we review current evidence for the cancer‐promoting activity of DKK1 and recent insights into the effects of DKK1 on signalling pathways in both cancer and immune cells. We discuss the rationale and promise of targeting DKK1 for oncology.
Linked Articles
This article is part of a themed section on WNT Signalling: Mechanisms and Therapeutic Opportunities. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.24/issuetoc
Abbreviations
- APC
adenomatous polyposis coli
- CK1
casein kinase 1
- CKAP4
cytoskeleton‐associated protein 4
- Cys
cysteine‐rich
- DKK1
Dickkopf‐1
- FZD
Frizzled
- GSK3
glycogen synthase kinase 3
- LCC
latency competent cancer
- LRP5/6
low‐density lipoprotein receptor‐related proteins 5 and 6
- MDSCs
myeloid‐derived suppressor cells
- NK
natural killer
- PCP
planar cell polarity
- Rac
Ras‐related C3 botulinum toxin substrate
- RNF43
ring finger protein 43
- ROR
receptor tyrosine kinase‐like orphan receptor
- ZNRF3
zinc and ring finger 3
Introduction – an overview of Wnt signalling and cancer
Wnt signalling is a multifaceted pathway that regulates stem cell maintenance, cell fate decisions, cell proliferation, survival, migration and polarity determination during development and adult tissue homeostasis (Logan and Nusse, 2004; MacDonald et al., 2009; Clevers and Nusse, 2012; Clevers et al., 2014; Sedgwick and D'Souza‐Schorey, 2016). Given the diverse cellular outcomes mediated by Wnt signalling, it is not surprising that it is exceedingly complex, involving 19 Wnts, 10 Frizzled (FZD) Wnt receptors, other classes of receptors including low‐density lipoprotein receptor‐related proteins 5 and 6 (LRP5/6), agonists and antagonists. Wnt signalling is classified into two main branches: β‐catenin‐dependent and β‐catenin‐independent. The β‐catenin‐dependent Wnt pathway is better characterized and understood and is mediated by the tight regulation of β‐catenin stability (MacDonald et al., 2009). In the absence of Wnt, signalling is kept off through β‐catenin degradation by the action of the ‘destruction’ complex which consists of axin, adenomatous polyposis coli (APC), casein kinase Iα and glycogen synthase kinase 3 (Figure 1A). Through phosphorylation of β‐catenin, the ‘destruction’ complex targets β‐catenin for ubiquitin‐mediated proteasomal degradation. The β‐catenin‐dependent Wnt signalling pathway is initiated by Wnt binding to FZD receptors and the LRP5/6 co‐receptor (Figure 1B). This begins a signalling cascade that inhibits the ‘destruction’ complex and leads to the stabilization of β‐catenin. The β‐catenin protein then translocates to the nucleus and interacts with DNA‐binding T‐cell factor/lymphoid enhancer factor family members, thereby activating a Wnt‐responsive transcriptional programme. Mutations in β‐catenin‐dependent Wnt signalling components occur frequently in cancer and result in constitutive β‐catenin accumulation and signalling (Polakis, 2012; Zhan et al., 2017). For example, loss‐of‐function APC mutations are prevalent in colorectal cancer, and CTNNB1 (β‐catenin) stabilizing mutations have been identified in colorectal cancer and a high percentage of liver and endometrioid tumours (Kwong and Dove, 2009; McConechy et al., 2014; Zucman‐Rossi et al., 2015). Additionally, loss‐of‐function alterations in zinc and ring finger 3 (ZNRF3)/ring finger protein 43 (RNF43), which are ubiquitin ligases promoting FZD degradation, or translocations involving R‐spondin proteins, which are secreted Wnt agonists by inhibiting ZNRF3/RNF43, are also found in colorectal cancer and other malignancies (Seshagiri et al., 2012; Assie et al., 2014; Giannakis et al., 2014; Hao et al., 2016). As such, targeting/inhibiting β‐catenin‐dependent Wnt signalling has garnered much attention and there are multiple oncology candidates in preclinical and clinical development (Anastas and Moon, 2013; Lu et al., 2016). Although targeting the β‐catenin‐dependent Wnt pathway is attractive, caution is warranted due to the ubiquitous nature of the pathway and the possibility for serious side effects (Kahn, 2014).
Figure 1.
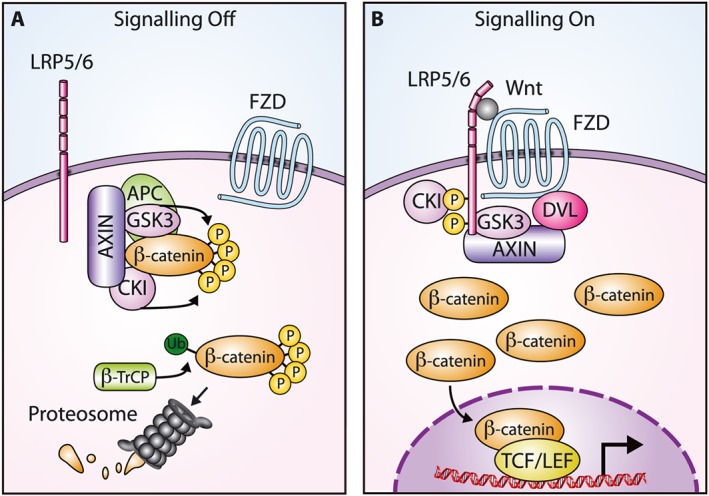
Overview of β‐catenin‐dependent Wnt signalling. (A) In the absence of Wnt, β‐catenin is bound by the ‘destruction’ complex and phosphorylated by GSK3 and casein kinase I (CKI). Phosphorylation results in targeting for ubiquitin‐mediated degradation. (B) Wnt binding to a FZD receptor and the LRP5/6 co‐receptor disrupts the ‘destruction’ complex and stabilizes β‐catenin. The β‐catenin protein translocates to the nucleus, interacts with T‐cell factor/lymphoid enhancer factor (TCF/LEF) family transcription factors and activates a Wnt‐responsive transcriptional programme. β‐TrCP, β‐transducin repeat containing protein; DVL, Dishevelled.
The β‐catenin‐independent Wnt signalling pathway regulates cell motility and polarity throughout development (Wang, 2009; Sedgwick and D'Souza‐Schorey, 2016). It is not nearly as well characterized as β‐catenin‐dependent Wnt signalling and involves multiple overlapping pathways, of which the Wnt/planar cell polarity (PCP) pathway is best understood and others, such as a Wnt/Ca2+ pathway, have been proposed (Semenov et al., 2007; Liu et al., 2016a). β‐catenin‐independent Wnt signalling does not require LRP5/6 and is instead initiated through Wnt interaction with FZD or additional receptors, such as receptor tyrosine kinase‐like orphan receptor (ROR) and receptor‐like tyrosine kinase (RYK) (Green et al., 2014). Many downstream mediators are utilized adding further complexity (Sugimura and Li, 2010; van Amerongen, 2012). For example, heterotrimeric G‐proteins, Rho‐family small GTPases, JNK and calcium/calmodulin‐dependent protein kinase II have all been implicated as downstream components. Some investigators have suggested that, similar to β‐catenin‐dependent Wnt signalling, these β‐catenin‐independent pathways may directly tie into gene regulation through nuclear factor of activated T‐cells (NFAT), ATF2 and c‐Jun transcription factors, although this requires further substantiation (Saneyoshi et al., 2002; Schambony and Wedlich, 2007; Rao and Kuhl, 2010; Bengoa‐Vergniory et al., 2014). Not surprisingly, given that β‐catenin‐independent Wnt signalling regulates cell motility, the pathway has been implicated in promoting cancer (Katoh, 2005; Wang, 2009; Sedgwick and D'Souza‐Schorey, 2016). For example, Wnt‐5a activates β‐catenin‐independent Wnt pathways, leading to invasion, metastasis and proliferation of some cancers (Asem et al., 2016; Kumawat and Gosens, 2016). ROR1, a receptor for Wnt‐5a, is also involved in cancer progression and is overexpressed in both haematological and solid malignancies (Borcherding et al., 2014). The therapeutic intervention of β‐catenin‐independent Wnt signalling has promise; however, a better understanding of this pathway will be necessary to fully exploit targeting it for oncology.
Discovery and characterization of DKK1
Dickkopf‐1 (DKK1) is a member of the Dickkopf family and has most extensively been characterized as a secreted protein that is an inhibitor of β‐catenin‐dependent Wnt signalling. The Dickkopf family consists of four members (DKK1–4), which contain two conserved cysteine‐rich (Cys) domains involved in protein–protein interactions (Niehrs, 2006). The Cys domains define the family and there is not a high degree of sequence similarity outside of these regions. DKK1 is essential for development, and homozygous null mice die at birth with severe head defects and limb dysmorphogenesis (Mukhopadhyay et al., 2001). Mice with reduced DKK1 expression levels are viable but have increased bone mass, indicating a role for DKK1 in bone development and homeostasis (MacDonald et al., 2007; Pinzone et al., 2009). DKK1 expression in adult tissues does not appear to be as ubiquitous; however, DKK1 has been detected in various tissues including bone, placenta, intestine, colon and prostate (Glinka et al., 1998; Fedi et al., 1999; Monaghan et al., 1999; Zhang et al., 2004; Forget et al., 2007; Aguilera et al., 2015). Of the Dickkopf family members, DKK1 is the best understood.
DKK1 was originally identified in Xenopus as an inhibitor of β‐catenin‐dependent Wnt signalling and an inducer of head formation during embryogenesis, a phenotype that coined the Dickkopf (German for ‘big head, stubborn’) nomenclature (Glinka et al., 1998). Its human homologue was also characterized as a potent Wnt inhibitor (Fedi et al., 1999). Thereafter, multiple labs demonstrated that DKK1 impeded β‐catenin‐dependent Wnt signalling by binding to the LRP6 co‐receptor with high affinity and blocking signalling (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001). More recent structural studies have supported this model and expanded our understanding of DKK1‐mediated inhibition of β‐catenin‐dependent Wnt signalling. The crystal structures of DKK1 and LRP6 along with binding data suggest that DKK1 occupies multiple Wnt domains on LRP6 and that this presumably prevents virtually all Wnt binding to the co‐receptor (Ahn et al., 2011; Bourhis et al., 2011; Chen et al., 2011; Cheng et al., 2011; Bao et al., 2012). Furthermore, DKK1 binding to LRP6 can induce a conformational change that may allosterically impede Wnt binding (Matoba et al., 2017). DKK1 can also form a ternary complex with the kremen 1 co‐receptor and LRP6, possibly leading to depletion of LRP6 from the cell surface and decreased signalling; however, this model remains controversial (Mao et al., 2002; Semenov et al., 2008; Wang et al., 2008; Zebisch et al., 2016). Although DKK1 clearly regulates Wnt signalling through inhibition of the β‐catenin‐dependent pathway, this may be an oversimplification because DKK1 has also been linked to the activation of β‐catenin‐independent Wnt signalling. For example, DKK1 has been implicated in promoting β‐catenin‐independent Wnt signalling during Xenopus and zebrafish development, neurite outgrowth, in Alzheimer's disease pathogenesis, as well as in oncology models (Pandur et al., 2002; Caneparo et al., 2007; Endo et al., 2008; Thudi et al., 2011; Wang and Zhang, 2011; Tao et al., 2013; Killick et al., 2014; Krause et al., 2014; Marzo et al., 2016). DKK1 activation of β‐catenin‐independent Wnt signalling is not well understood but is probably indirect and involves DKK1 shifting the Wnt signalling balance from the β‐catenin‐dependent pathway to β‐catenin‐independent pathways (discussed in a later section). Thus, the effect of DKK1 on cellular function presumably involves the interrogation of outputs from both β‐catenin‐dependent and independent Wnt pathways, adding further complexity to its regulation of Wnt signalling.
Based on the ability of DKK1 to inhibit β‐catenin‐dependent Wnt signalling, a pathway that is frequently overactivated in cancer, it is not surprising that DKK1 was initially characterized as a tumour suppressor. Early studies in gastrointestinal cancer showed that DKK1 expression was decreased in tumours and that the gene was frequently methylated and silenced (Gonzalez‐Sancho et al., 2005; Aguilera et al., 2006; Sato et al., 2007). Additional studies indicated DKK1 could suppress tumours by inducing apoptosis and inhibiting tumour growth, proliferation, invasion and angiogenesis (Lee et al., 2004; Maehata et al., 2008; Mikheev et al., 2008; Qiao et al., 2008; Hirata et al., 2011; Kim et al., 2012; Menezes et al., 2012; Qi et al., 2012). However, paradoxically, many correlative and functional studies have linked DKK1 to the promotion of cancer (Tables 1 and 2) (Mazon et al., 2016). The ability of DKK1 to function as a tumour suppressor or promoter is probably dependent on numerous contextual factors such as the type of cancer, heterogeneity within the tumour, Wnt signalling pathway wiring and the tumour micro‐environment. Deciphering this will advance the development of DKK1‐targeted therapies for oncology currently undergoing clinical development (Lu et al., 2016).
Table 1.
Cancers with tumours that express DKK1 or induce elevated patient serum levels
| Cancer | Reference |
|---|---|
| Bladder | (Sun et al., 2015) |
| Breast | (Forget et al., 2007; Voorzanger‐Rousselot et al., 2007; Bu et al., 2008; Sato et al., 2010; Smadja et al., 2010; Xu et al., 2012; Zhou et al., 2014; Rachner et al., 2014a) |
| Chondrosarcoma | (Chen et al., 2014; Zarea et al., 2016) |
| Cholangiocarcinoma | (Sato et al., 2010; Shi et al., 2013; Shi et al., 2016) |
| Cervical | (Jiang et al., 2009; Sato et al., 2010; Jiang et al., 2013) |
| Colon/rectal | (Kemik et al., 2011; Gurluler et al., 2014; Aguilera et al., 2015) |
| Endometrial | (Jiang et al., 2009) |
| Oesophageal | (Yamabuki et al., 2007; Darlavoix et al., 2009; Makino et al., 2009; Li et al., 2011; Begenik et al., 2014; Lyros et al., 2015) |
| Gastric | (Sato et al., 2010; Gao et al., 2012; Gomceli et al., 2012; Lee et al., 2012; Liu et al., 2016b) |
| Glioblastoma | (Zhou et al., 2010) |
| Kidney | (Wirths et al., 2003; Forget et al., 2007) |
| Liver | (Wirths et al., 2003; Patil et al., 2005; Yu et al., 2009; Sato et al., 2010; Tung et al., 2011; Shen et al., 2012; Chen et al., 2013; Tao et al., 2013; Yang et al., 2013; Huang et al., 2014; Zhang et al., 2014; Kim et al., 2015; Desert et al., 2016) |
| Laryngeal | (Shi et al., 2014) |
| Lung | (Forget et al., 2007; Yamabuki et al., 2007; Sheng et al., 2009; Sato et al., 2010; Li et al., 2013; Chu et al., 2014; Dong et al., 2014; Xiang et al., 2015; Kimura et al., 2016; Yao et al., 2016) |
| Malignant fibrous histiocytoma | (Matushansky et al., 2007) |
| Multiple myeloma | (Tian et al., 2003; Politou et al., 2006; Qian et al., 2007) |
| Osteosarcoma | (Lee et al., 2007) |
| Ovarian | (Chamorro et al., 2005; Shizhuo et al., 2009; Wang and Zhang, 2011) |
| Pancreatic | (Sato et al., 2010; Takahashi et al., 2010; Han et al., 2015; Kimura et al., 2016) |
| Prostate | (Hall et al., 2008; Rachner et al., 2014b) |
| Solid tumours, meta‐analysis | (Liu et al., 2014) |
| Urothelial | (Shen et al., 2010) |
Table 2.
Preclinical evidence for DKK1 promoting cancer pathogenesis
| Cancer | Selected evidence for DKK1 cancer‐promoting activity | Reference |
|---|---|---|
| Breast |
|
(Voorzanger‐Rousselot et al., 2007; Smadja et al., 2010; Malladi et al., 2016) |
| Cholangiocarcinoma |
|
(Shi et al., 2013; Shi et al., 2016) |
| Colorectal |
|
(Aguilera et al., 2015) |
| Oesophageal |
|
(Li et al., 2011) |
| Liver |
|
(Yu et al., 2009; Tung et al., 2011; Chen et al., 2013; Tao et al., 2013; Huang et al., 2014; Kim et al., 2015; Chen et al., 2016) |
| Laryngeal |
|
(Shi et al., 2014) |
| Lung |
|
(Sato et al., 2010; Li et al., 2013; Salim et al., 2015; D'Amico et al., 2016; Kimura et al., 2016; Malladi et al., 2016; Yao et al., 2016; Pang et al., 2017) |
| Melanoma |
|
(D'Amico et al., 2016) |
| MFH |
|
(Matushansky et al., 2007) |
| Multiple myeloma |
|
(Yaccoby et al., 2007; Fulciniti et al., 2009; Heath et al., 2009; Pozzi et al., 2013) |
| Osteosarcoma |
|
(Gregory et al., 2003; Krause et al., 2014; Goldstein et al., 2016) |
| Ovarian |
|
(Wang and Zhang, 2011) |
| Pancreatic |
|
(Takahashi et al., 2010; Kimura et al., 2016) |
| Prostate |
|
(Hall et al., 2010; Thudi et al., 2011) |
MFH, malignant fibrous histiocytoma; hMSCs, human mesenchymal stem cells; PDX, patient‐derived xenograft.
DKK1 overexpression in cancer
Clinical studies in a range of cancers have detected elevated levels of DKK1 in patient serum or tumours and this was frequently associated with a poor prognosis, such as advanced stage, decreased overall survival, vascular invasion and metastasis (Table 1). For example, DKK1 staining has been detected in a high percentage of oesophageal and cholangiocarcinoma tumours and this correlated with a decrease in overall survival (Yamabuki et al., 2007; Shi et al., 2013). In tumours from breast, cholangiocarcinoma, laryngeal squamous cell carcinoma, liver, rectal and gastric cancers, elevated levels of DKK1 have been observed with vascular invasion, lymphatic invasion or VEGF‐C expression, implicating DKK1 in promoting cancer cell migration and metastasis (Smadja et al., 2010; Kemik et al., 2011; Tung et al., 2011; Shi et al., 2013, 2014; Tao et al., 2013; Liu et al., 2016b). In support of this, DKK1 positivity has been associated with lymph node metastasis in cancers (Kemik et al., 2011; Li et al., 2013; Shi et al., 2013; Shi et al., 2014; 2016). DKK1 staining in tumours has also been co‐detected with β‐catenin, and in some instances, patients with dual staining had a worse prognosis, including decreased overall survival (Yu et al., 2009; Xu et al., 2012; Chen et al., 2013, 2014; Shi et al., 2014, 2016). It is puzzling that DKK1, an inhibitor of β‐catenin‐dependent Wnt signalling, is detected in tumours with β‐catenin, an indicator of activated Wnt/β‐catenin signalling. However, activated β‐catenin‐dependent Wnt signalling can result in the up‐regulation of DKK1, potentially as a negative feedback mechanism under physiological conditions (Niida et al., 2004; Chamorro et al., 2005; Gonzalez‐Sancho et al., 2005; Bu et al., 2008; Chen et al., 2016). In tumours that stain for β‐catenin and DKK1, the negative feedback may have been disrupted by, for example, stabilizing mutations in β‐catenin that would render the inhibitory activity of DKK1 inoperative. It is interesting to speculate that in the context of constitutively activated β‐catenin‐dependent Wnt signalling, the increased expression of DKK1 is contributing to tumour growth and poor prognosis. Further research is required to address this issue.
DKK1 promotes proliferation, invasion and tumour growth in preclinical models
In addition to clinical data, direct evidence for DKK1 cancer‐promoting activity exists for preclinical cancer models (Table 2). For example, DKK1 promoted migration and/or invasion in cholangiocarcinoma, oesophageal, liver, laryngeal, lung and pancreatic cancer cell lines (Table 2). For some of these cancer cell lines, this may have occurred through DKK1 regulation of MMP expression, a family of proteases with well characterized roles in cancer cell migration (Kessenbrock et al., 2010; Chen et al., 2013; Shi et al., 2013; Shi et al., 2016). Furthermore, DKK1 knockdown reduced the expression of VEGF‐C, a protein associated with promoting metastasis to lymph nodes, in cholangiocarcinoma (Park et al., 2006; Shi et al., 2013). Along with migration and invasion, DKK1 also stimulated proliferation in cell culture experiments (Table 2). However, this was not a universal feature, and for certain cancers, DKK1 promoted migration and/or invasion without having a detectable effect on proliferation (Tung et al., 2011; Wang and Zhang, 2011; Chen et al., 2013; Li et al., 2013). It is currently not well understood why there was this difference, but it may be due to variations in Wnt signalling pathways across cancer cell lines or different cell culture conditions. Elevated DKK1 expression also occurred in liver, breast and lung cancer cells that had stem cell‐like characteristics, suggesting that it may contribute to the development of an undifferentiated phenotype (Chen et al., 2016; Malladi et al., 2016). Supporting this, DKK1 overexpression prevented the differentiation of osteosarcoma cells and increased the level of the cancer stem cell marker aldehyde dehydrogenase 1 in these cells (Krause et al., 2014). Taken together, there is ample evidence from cell culture model systems that DKK1 may contribute to cancer progression by promoting migration, invasion, proliferation and cancer stem cell‐like properties.
DKK1 has been documented to affect tumour growth in in vivo models representing a range of cancers. For example, tumour models for breast cancer, cholangiocarcinoma, liver cancer, lung cancer, melanoma, multiple myeloma, osteosarcoma, ovarian cancer and prostate cancer all responded to changes in DKK1 levels (Table 2). In multiple myeloma, treatment with anti‐DKK1 antibodies reduced disease burden and improved bone health in mouse models (Yaccoby et al., 2007; Fulciniti et al., 2009; Pozzi et al., 2013). Lung cancer, melanoma, osteosarcoma and prostate cancer also responded to anti‐DKK1 antibody treatment in vivo (Hall et al., 2010; Sato et al., 2010; D'Amico et al., 2016; Goldstein et al., 2016). In both breast cancer and hepatocellular carcinoma xenograft models, DKK1 increased tumour growth and promoted angiogenesis, suggesting that DKK1 has pro‐angiogenic activity (Smadja et al., 2010; Tung et al., 2011). In support of this, knockdown of DKK1 decreased tumour growth and angiogenesis in hepatocellular carcinoma (Huang et al., 2014). In addition to affecting primary tumour growth, DKK1 has also been linked to the development of metastasis in bone, breast, liver, lung and prostate cancer models, possibly related to the prominent role of DKK1 in stimulating migration and invasion observed in vitro (Thudi et al., 2011; Tao et al., 2013; Huang et al., 2014; Goldstein et al., 2016; Malladi et al., 2016; Pang et al., 2017). Taken together, these data demonstrate that DKK1 has tumour‐promoting activity in animal models via effects on tumour growth, metastasis and angiogenesis.
DKK1 modulation of signalling pathways in cancer cells
Emerging evidence is improving our understanding of how DKK1 can promote tumour growth and metastasis through the modulation of signalling pathways in cancer cells. For example, an elegant study has demonstrated that DKK1 contributes to metastasis in an in vivo model through inhibition of β‐catenin‐dependent Wnt signalling (Figure 2A) (Malladi et al., 2016). The authors initially identified and characterized latency competent cancer (LCC) cells that had a stem cell‐like phenotype and tumour‐initiating capability by avoiding immune clearance. Impeding DKK1 expression re‐sensitized these LCC cells to β‐catenin‐dependent Wnt signalling and up‐regulated the expression of activating ligands for natural killer (NK) cells, leading to NK cell‐mediated clearance of the LCC cells and reduced metastasis. These results suggest the intriguing possibility that the reactivation of β‐catenin‐dependent Wnt signalling could be an effective way to eliminate tumour‐initiating cells with metastatic potential through immune surveillance. However, caution and further studies are warranted, since the reactivation of β‐catenin‐dependent Wnt signalling may also induce proliferation of LCC cells and in principle increase tumour growth. An important issue to address is how to enhance tumour immune surveillance without promoting tumour growth.
Figure 2.
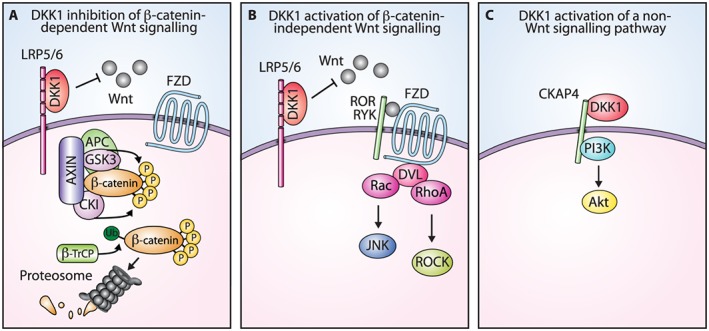
DKK1 regulation of signalling pathways. (A) DKK1 inhibition of β‐catenin‐dependent Wnt signalling. DKK1 inhibits β‐catenin‐dependent Wnt signalling by binding to the LRP5/6 co‐receptor and blocking Wnt binding, which results in β‐catenin degradation. (B) Model of DKK1 activation of β‐catenin‐independent Wnt signalling. DKK1 binding to the LRP5/6 co‐receptor shifts Wnt and the FZD receptor to β‐catenin‐independent signalling pathways. A simplified version of the β‐catenin‐independent Wnt/PCP pathway is shown as an example. (C) DKK1 activation of a non‐Wnt signalling pathway. DKK1 binds to the CKAP4 receptor and activates PI3K/Akt signalling. GSK3, glycogen synthase kinase 3; CKI, casein kinase I; β‐TrCP, β‐transducin repeat containing protein; RYK, receptor‐like tyrosine kinase; DVL, Dishevelled; ROCK, Rho‐associated protein kinase.
For certain cancer cells, inhibition of β‐catenin‐dependent Wnt signalling by DKK1 can favour the formation of an undifferentiated phenotype, which in general is more malignant. For example, DKK1 has been implicated in having a role in limiting the ability of malignant fibrous histiocytoma cells to differentiate by blocking β‐catenin‐dependent Wnt signalling (Matushansky et al., 2007). In osteosarcoma, DKK1 inhibited β‐catenin‐dependent Wnt signalling and impeded differentiation (Goldstein et al., 2016). Treatment with an anti‐DKK1 antibody reduced tumour growth in patient‐derived xenograft models, increased nuclear β‐catenin staining and increased the expression of osteopontin, a bone differentiation marker. Taken together, these results indicate that for some cancers DKK1 can contribute to tumour growth by impeding β‐catenin‐dependent Wnt signalling.
DKK1 can also promote cancer pathogenesis by activating β‐catenin‐independent Wnt signalling. This finding is not unexpected, given the role of β‐catenin‐independent Wnt signalling in cell migration and polarity during development (Sedgwick and D'Souza‐Schorey, 2016). In liver cancer cells, knockdown of DKK1 decreased metastasis and reduced the levels of phosphorylated JNK, a downstream mediator of the Wnt/PCP pathway, suggesting that signalling was occurring through β‐catenin‐independent Wnt pathways (Tao et al., 2013). The overexpression of DKK1 in prostate cancer cells increased metastatic potential and resulted in JNK activation without affecting β‐catenin levels, suggesting that DKK1 was acting primarily through a β‐catenin‐independent Wnt pathway (Thudi et al., 2011). A similar result has been observed in ovarian cancer cells where DKK1 promoted cell invasion and increased phosphorylated JNK without affecting β‐catenin levels (Wang and Zhang, 2011). Furthermore, DKK1 staining in ovarian tumours correlated with that of phosphorylated JNK. In osteosarcoma, DKK1 overexpression increased tumour growth, RhoA expression and JNK phosphorylation, further supporting its role in activating β‐catenin‐independent Wnt signalling (Krause et al., 2014). Taken together, these results implicate DKK1 activation of β‐catenin‐independent Wnt signalling in cancer cells as a potential driver of tumour growth and metastasis. Even though evidence exists for DKK1 activation of β‐catenin‐independent Wnt signalling in developmental and cancer models, the mechanistic details have not been elucidated. It has been hypothesized that DKK1 shifts the Wnt signalling balance from the β‐catenin‐dependent pathway to β‐catenin‐independent pathways (Figure 2B) (Endo et al., 2005, 2008; Caneparo et al., 2007; Wang and Zhang, 2011; Krause et al., 2014). This potentially occurs through DKK1 binding to the LRP5/6 co‐receptor blocking Wnt and increasing the availability of a Wnt pool to activate β‐catenin‐independent signalling pathways. Likewise, DKK1 could shift FZD receptors to β‐catenin‐independent pathways since there would be fewer Wnt bound LRP5/6 co‐receptors to interact with. Going forward, it will be important to increase our understanding of how DKK1 promotes the activation of β‐catenin‐independent Wnt signalling and the extent that this contributes to tumour growth and metastasis.
DKK1 modulates β‐catenin‐dependent and ‐independent Wnt signalling in cancer cells, but is it limited to these pathways? For instance, even though DKK1 showed a clear phenotypic response in lung, pancreatic and prostate cancer cell lines in culture, attempts to detect measurable changes in either β‐catenin‐dependent or independent Wnt signalling pathways were largely unsuccessful, leading the authors to speculate that the effects of DKK1 occurred through an undefined signalling pathway (Hall et al., 2010; Sato et al., 2010; Takahashi et al., 2010). This question has begun to be addressed by a screening approach that has identified the cytoskeleton‐associated protein 4 (CKAP4) as a novel DKK1 receptor (Kimura et al., 2016). Further characterization indicated that CKAP4 interacted with PI3K and that DKK1 binding resulted in the activation of Akt signalling and increased the proliferation of the cancer cells (Figure 2C). Disrupting DKK1–CKAP4 signalling with either a shRNA‐targeting DKK1 or a CKAP4 antibody impeded tumour growth in pancreatic and lung xenograft models. These data suggest a scenario in which DKK1 can signal through a Wnt receptor‐independent pathway to promote tumour growth.
DKK1 modulation of signalling pathways in immune cells
Immune modulation has revolutionized the treatment paradigm for cancer and delivered significant clinical benefit (Topalian et al., 2015). With the approval of immune checkpoint inhibitors, such as therapeutic antibodies targeting cytotoxic t‐lymphocyte‐associated protein 4 (CTLA‐4), programmed cell death 1 (PD‐1) and programmed cell death ligand 1 (PD‐L1), it has become possible to harness the immune system for an anti‐tumour response. However, only a subset of patients responds to these therapies, and many novel immune mediated strategies are being pursued to overcome this limitation. For example, the presence of myeloid‐derived suppressor cells (MDSCs) in the tumour micro‐environment is associated with a poor prognosis, and blocking the function of these cells may have therapeutic benefits (Draghiciu et al., 2015). Recently, DKK1 was shown to signal to MDSCs through the inhibition of β‐catenin‐dependent Wnt signalling and thereby promote tumour growth in murine syngeneic models (D'Amico et al., 2016). An anti‐DKK1 antibody resulted in tumour regression and a shift in the tumour micro‐environment from anti‐inflammatory to pro‐inflammatory, suggesting that the anti‐DKK1 antibody was having immune modulatory activity. This was supported by linking DKK1 to MDSC immunosuppressive activities, such as the production of ROS and suppression of T‐cell proliferation. Interestingly, the primary source of DKK1 was not from the tumour, but rather from bone, suggesting that high levels of DKK1 in tumours may not necessarily be a prerequisite for efficacy from a DKK1‐targeted therapy. Mechanistically, this study gives a clear example of DKK1 signalling to immune cells and the benefit of blocking DKK1 in order to promote an anti‐tumour immune response.
DKK1 may also contribute to an immunosuppressive tumour micro‐environment by modulating signalling in additional immune cells besides MDSCs. DKK1 from activated platelets signalled to CD4+ T‐cells and promoted a pathogenic CD4+ T‐helper 2 response as a result of environmental challenges (Chae et al., 2016). Intriguingly, this may not occur through β‐catenin‐dependent Wnt signalling. Even though this study was not conducted in a cancer model, DKK1 antagonized T‐helper 1 polarization and suppressed the secretion of the pro‐inflammatory cytokine interferon γ, which are both cellular events that are usually not favourable for an anti‐tumour immune response (Fridman et al., 2012). Given these clinical implications, it will be important to elucidate whether DKK1 signals to CD4+ T‐cells and additional immune cells in the tumour micro‐environment and the extent this contributes to immunosuppression.
A model for DKK1 cancer‐promoting activity and clinical implications
DKK1 has diverse functional consequences on cancer and immune cells that contribute to cancer progression. Here, we propose a model highlighting the multitude of potential mechanisms involving DKK1 (Figure 3). DKK1 from the tumour or a host tissue source, such as bone, signals to both tumour cells and immune cells to promote tumour growth. The modulation of Wnt signalling in immune cells by DKK1 results in an immunosuppressive tumour micro‐environment. In addition, DKK1 regulation of Wnt signalling and PI3K/Akt signalling in cancer cells contributes to tumour growth and immune evasion and may favour a cancer stem cell phenotype.
Figure 3.
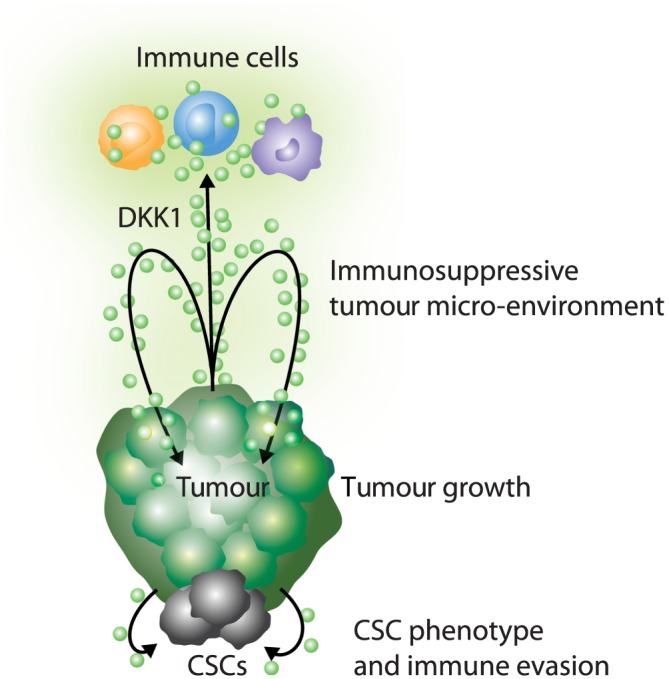
Model for DKK1 cancer‐promoting activity. DKK1 signals to tumour cells and immune cells, resulting in an immunosuppressive tumour micro‐environment, tumour growth, metastasis, a cancer stem cell (CSC) phenotype and immune evasion.
Even though DKK1 can clearly promote tumour growth, it has also been hypothesized to function as a tumour suppressor by inhibiting β‐catenin‐dependent Wnt signalling in cancer cells. This apparent paradox can be explained in part by the diverse functional outcomes of Wnt signalling and thus DKK1, which can vary depending on cancer types. Here, we propose three non‐mutually exclusive mechanistic models to reconcile how DKK1, an inhibitor of β‐catenin‐dependent Wnt signalling, can have tumour promoting activity. (i) Depending on the Wnt signalling wiring in a cancer cell, inhibition of β‐catenin‐dependent Wnt signalling is not necessarily tumour suppressive. As discussed earlier, DKK1 and its inhibition of β‐catenin‐dependent Wnt signalling favoured an undifferentiated phenotype in osteosarcoma and the tumour‐initiating ability of LCC cells. (ii) The characterization of DKK1 as only an inhibitor of β‐catenin‐dependent Wnt signalling in cancer cells is an oversimplification. It is important to consider other potential regulatory outcomes of DKK1. For example, DKK1 activation of β‐catenin‐independent Wnt signalling and/or PI3K/Akt signalling in cancer cells or DKK1 signalling to immune cells may outweigh any tumour‐suppressive activity of DKK1 inhibition of β‐catenin‐dependent Wnt signalling in cancer cells. (iii) In some tumours, β‐catenin‐dependent Wnt signalling is constitutively activated downstream of DKK1. In this context, it can be hypothesized that DKK1 is unable to inhibit β‐catenin‐dependent Wnt signalling, thereby eradicating its potential tumour suppressor activity. Further mechanistic insights are crucial for understanding the cancer‐promoting activities of DKK1 and deciding which indications are most likely to respond to DKK1‐targeted therapies.
Two anti‐DKK1 neutralizing antibodies have been or are currently being evaluated clinically for potential use in oncology (Lu et al., 2016). BHQ880, an antibody developed by Novartis Pharmaceuticals, has completed phase 1B trials in multiple myeloma (NCT00741377, NCT01337752 and NCT01302886). Published data indicate that BHQ880 is well tolerated, and some clinical benefit was observed when it was given in combination with zoledronic acid and anti‐myeloma therapies (Iyer et al., 2014). Leap Therapeutics is developing DKN‐01, a humanized anti‐DKK1 monoclonal antibody. An initial dose finding study (NCT01457417) in patients with advanced malignancies demonstrated that DKN‐01 monotherapy was well tolerated, and clinical activity in patients with refractory non‐small cell lung cancer was observed (Edenfield et al., 2014). Currently, DKN‐01 is being evaluated in phase 1B trials for advanced cholangiocarcinoma and relapsed/refractory oesophageal/gastro‐oesophageal junction and gastric cancer in combination with standard of care chemotherapy (NCT02375880 and NCT02013154). Preliminary data indicate promising clinical activity in both diseases, and DKN‐01 continues to be well tolerated (Eads et al., 2016; Ryan et al., 2016). Based on these results and the increasing understanding of DKK1 tumour promoting activity, further clinical development is warranted.
Wnt signalling is exceedingly complex, and the role of DKK1 in modulating this pathway and additional signalling pathways in both cancer and immune cells to promote tumour growth and metastasis has not been fully elucidated. Even given the complexity, we believe that DKK1 is a promising oncology target for the following reasons. (i) Preliminary clinical data with anti‐DKK1 therapeutic antibodies are encouraging, demonstrating a good safety profile and potential benefit when used as a monotherapy or in combination. (ii) For many cancers, elevated tumour levels of DKK1 correlated with a poor prognosis. (iii) Emerging evidence suggests that DKK1 signals to both tumour and immune cells to promote cancer. Therefore, a DKK1‐neutralizing therapy could have the benefit of both a direct anti‐tumour effect and the stimulation of a pro‐inflammatory anti‐tumour response. (iv) DKK1 does not appear to be widely expressed in adult tissues, suggesting that on target toxicity from a DKK1‐directed therapy would be limited, possibly avoiding or ameliorating the immune‐mediated adverse events observed with approved checkpoint inhibitors.
Concluding remarks
Wnt signalling is a fundamental pathway involved in development and adult tissue homeostasis. It is frequently dysregulated in oncology, and multiple therapeutics are currently in preclinical and clinical development. DKK1 is an attractive therapeutic target for oncology given its potential for broad clinical applicability. Elevated levels of DKK1 are detected in the serum and tumours of many cancer patients, spanning a wide range of malignancies and frequently correlate with a poor prognosis. Preclinically blocking DKK1 activity decreases proliferation, migration and invasion in cancer cell lines and has efficacy in multiple mouse tumour models. Clinically, anti‐DKK1 neutralizing antibodies have shown promise and are well tolerated. Although the mechanisms of action of DKK1 are not fully characterized, it is becoming increasingly apparent that it can modulate signalling pathways on both cancer and immune cells to promote tumour growth and metastasis. Current and future clinical trials will address the promise of therapeutically targeting DKK1 for oncology.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Conflict of interest
M.H.K. is an employee and shareholder of Leap Therapeutics. X.H. is a member of the scientific advisory board of Leap Therapeutics.
Acknowledgements
The authors wish to thank Walter Newman, David Shapiro, Cyndi Sirard and Melissa Geddie for critical reading of this manuscript and Tom DiCesare for graphic support. X.H. acknowledges support by NIH (RO1‐GM057603 and RO1‐AR060359) and by Boston Children's Hospital Intellectual and Developmental Disabilities Research Center (P30 HD‐18655). X.H. is an American Cancer Society Research Professor.
Kagey, M. H. , and He, X. (2017) Rationale for targeting the Wnt signalling modulator Dickkopf‐1 for oncology. British Journal of Pharmacology, 174: 4637–4650. doi: 10.1111/bph.13894.
References
- Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J et al. (2006). Epigenetic inactivation of the Wnt antagonist DICKKOPF‐1 (DKK‐1) gene in human colorectal cancer. Oncogene 25: 4116–4121. [DOI] [PubMed] [Google Scholar]
- Aguilera O, Gonzalez‐Sancho JM, Zazo S, Rincon R, Fernandez AF, Tapia O et al. (2015). Nuclear DICKKOPF‐1 as a biomarker of chemoresistance and poor clinical outcome in colorectal cancer. Oncotarget 6: 5903–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn VE, Chu ML, Choi HJ, Tran D, Abo A, Weis WI (2011). Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev Cell 21: 862–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT (2013). WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13: 11–26. [DOI] [PubMed] [Google Scholar]
- Asem MS, Buechler S, Wates RB, Miller DL, Stack MS (2016). Wnt5a signaling in cancer. Cancers (Basel) 8: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O et al. (2014). Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 46: 607–612. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA (2001). Novel mechanism of Wnt signalling inhibition mediated by Dickkopf‐1 interaction with LRP6/Arrow. Nat Cell Biol 3: 683–686. [DOI] [PubMed] [Google Scholar]
- Bao J, Zheng JJ, Wu D (2012). The structural basis of DKK‐mediated inhibition of Wnt/LRP signaling. Sci Signal 5: pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenik H, Kemik AS, Emre H, Dulger AC, Demirkiran D, Ebinc S et al. (2014). The association between serum Dickkopf‐1 levels and esophageal squamous cell carcinoma. Hum Exp Toxicol 33: 785–788. [DOI] [PubMed] [Google Scholar]
- Bengoa‐Vergniory N, Gorrono‐Etxebarria I, Gonzalez‐Salazar I, Kypta RM (2014). A switch from canonical to noncanonical Wnt signaling mediates early differentiation of human neural stem cells. Stem Cells 32: 3196–3208. [DOI] [PubMed] [Google Scholar]
- Borcherding N, Kusner D, Liu GH, Zhang W (2014). ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell 5: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D et al. (2011). Wnt antagonists bind through a short peptide to the first beta‐propeller domain of LRP5/6. Structure 19: 1433–1442. [DOI] [PubMed] [Google Scholar]
- Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C et al. (2008). Breast cancer‐derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer 123: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caneparo L, Huang YL, Staudt N, Tada M, Ahrendt R, Kazanskaya O et al. (2007). Dickkopf‐1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally‐like homolog Knypek. Genes Dev 21: 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae WJ, Ehrlich AK, Chan PY, Teixeira AM, Henegariu O, Hao L et al. (2016). The Wnt antagonist Dickkopf‐1 promotes pathological type 2 cell‐mediated inflammation. Immunity 44: 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE (2005). FGF‐20 and DKK1 are transcriptional targets of beta‐catenin and FGF‐20 is implicated in cancer and development. EMBO J 24: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhou H, Zhang X, Ma X, Liu Z, Liu X (2014). Elevated levels of Dickkopf‐1 are associated with beta‐catenin accumulation and poor prognosis in patients with chondrosarcoma. PLoS One 9: e105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li M, Li Q, Wang CJ, Xie SQ (2013). DKK1 promotes hepatocellular carcinoma cell migration and invasion through beta‐catenin/MMP7 signaling pathway. Mol Cancer 12: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bubeck D, MacDonald BT, Liang WX, Mao JH, Malinauskas T et al. (2011). Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev Cell 21: 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang YW, Li Y, Zhang JW, Zhang T, Fu BS et al. (2016). Constitutive expression of Wnt/betacatenin target genes promotes proliferation and invasion of liver cancer stem cells. Mol Med Rep 13: 3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L et al. (2011). Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol 18: 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Teng J, Jiang L, Zhong H, Han B (2014). Lung cancer‐derived Dickkopf1 is associated with bone metastasis and the mechanism involves the inhibition of osteoblast differentiation. Biochem Biophys Res Commun 443: 962–968. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346 1248012. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R (2012). Wnt/beta‐catenin signaling and disease. Cell 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- D'Amico L, Mahajan S, Capietto AH, Yang Z, Zamani A, Ricci B et al. (2016). Dickkopf‐related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J Exp Med 213: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlavoix T, Seelentag W, Yan P, Bachmann A, Bosman FT (2009). Altered expression of CD44 and DKK1 in the progression of Barrett's esophagus to esophageal adenocarcinoma. Virchows Arch 454: 629–637. [DOI] [PubMed] [Google Scholar]
- Desert R, Mebarki S, Desille M, Sicard M, Lavergne E, Renaud S et al. (2016). “Fibrous nests” in human hepatocellular carcinoma express a Wnt‐induced gene signature associated with poor clinical outcome. Int J Biochem Cell Biol 81: 195–207. [DOI] [PubMed] [Google Scholar]
- Dong LL, Qu LY, Chu LY, Zhang XH, Liu YH (2014). Serum level of DKK‐1 and its prognostic potential in non‐small cell lung cancer. Diagn Pathol 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghiciu O, Lubbers J, Nijman HW, Daemen T (2015). Myeloid derived suppressor cells–an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology 4: e954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads J, El‐Khoueiry A, Manji G, Abrams T, Khorana AA, Miksad R et al. (2016). Phase I study of DKN‐01 (D), an anti‐DKK1 monoclonal antibody, in combination with gemcitabine (G) and cisplatin (C) in patients (pts) with advanced biliary cancer (ABC). Ann Oncol 27 (suppl_6): 698P. [Google Scholar]
- Edenfield WJ, Richards DA, Vukelja SJ, Weiss GJ, Sirard CA, Landau SB et al. (2014). A phase 1 study evaluating the safety and efficacy of DKN‐01, an investigational monoclonal antibody (Mab) in patients (pts) with advanced non‐small cell lung cancer. J Clin Oncol 32 5s (suppl; abstr 8068). [Google Scholar]
- Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A et al. (2008). Wnt‐3a and Dickkopf‐1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3‐ and c‐Jun N‐terminal kinase‐dependent mechanism. Mol Cell Biol 28: 2368–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A et al. (2005). Wnt‐3a‐dependent cell motility involves RhoA activation and is specifically regulated by dishevelled‐2. J Biol Chem 280: 777–786. [DOI] [PubMed] [Google Scholar]
- Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP et al. (1999). Isolation and biochemical characterization of the human Dkk‐1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem 274: 19465–19472. [DOI] [PubMed] [Google Scholar]
- Forget MA, Turcotte S, Beauseigle D, Godin‐Ethier J, Pelletier S, Martin J et al. (2007). The Wnt pathway regulator DKK1 is preferentially expressed in hormone‐resistant breast tumours and in some common cancer types. Br J Cancer 96: 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Pages F, Sautes‐Fridman C, Galon J (2012). The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12: 298–306. [DOI] [PubMed] [Google Scholar]
- Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA et al. (2009). Anti‐DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Xie R, Ren C, Yang X (2012). Dickkopf‐1 expression is a novel prognostic marker for gastric cancer. J Biomed Biotechnol 2012: 804592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis M, Hodis E, Jasmine Mu X, Yamauchi M, Rosenbluh J, Cibulskis K et al. (2014). RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 46: 1264–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998). Dickkopf‐1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362. [DOI] [PubMed] [Google Scholar]
- Goldstein SD, Trucco M, Bautista Guzman W, Hayashi M, Loeb DM (2016). A monoclonal antibody against the Wnt signaling inhibitor dickkopf‐1 inhibits osteosarcoma metastasis in a preclinical model. Oncotarget 7: 21114–21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomceli I, Bostanci EB, Ozer I, Kemik AS, Turhan N, Tez M et al. (2012). A novel screening biomarker in gastric cancer: serum Dickkopf‐1. Hepatogastroenterology 59: 1661–1664. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Sancho JM, Aguilera O, Garcia JM, Pendas‐Franco N, Pena C, Cal S et al. (2005). The Wnt antagonist DICKKOPF‐1 gene is a downstream target of beta‐catenin/TCF and is downregulated in human colon cancer. Oncogene 24: 1098–1103. [DOI] [PubMed] [Google Scholar]
- Green J, Nusse R, van Amerongen R (2014). The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CA, Singh H, Perry AS, Prockop DJ (2003). The Wnt signaling inhibitor dickkopf‐1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem 278: 28067–28078. [DOI] [PubMed] [Google Scholar]
- Gurluler E, Tumay LV, Guner OS, Kucukmetin NT, Hizli B, Zorluoglu A (2014). The role of preoperative serum levels for Dickkopf‐related protein 1 as a potential marker of tumor invasion in patients with stage II and III colon cancer. Eur Rev Med Pharmacol Sci 18: 1742–1747. [PubMed] [Google Scholar]
- Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET (2008). Dickkopf‐1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 68: 1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CL, Zhang H, Baile S, Ljungman M, Kuhstoss S, Keller ET (2010). p21CIP‐1/WAF‐1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf‐1. Cancer Res 70: 9916–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SX, Zhou X, Sui X, He CC, Cai MJ, Ma JL et al. (2015). Serum Dickkopf‐1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget 6: 19907–19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Jiang X, Cong F (2016). Control of Wnt receptor turnover by R‐spondin‐ZNRF3/RNF43 signaling Module and its dysregulation in cancer. Cancers (Basel) 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD Jr, Evans HR et al. (2009). Inhibiting Dickkopf‐1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res 24: 425–436. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Ueno K et al. (2011). Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int J Cancer 128: 1793–1803. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yang X, Zhao F, Shen Q, Wang Z, Lv X et al. (2014). Overexpression of Dickkopf‐1 predicts poor prognosis for patients with hepatocellular carcinoma after orthotopic liver transplantation by promoting cancer metastasis and recurrence. Med Oncol 31: 966. [DOI] [PubMed] [Google Scholar]
- Iyer SP, Beck JT, Stewart AK, Shah J, Kelly KR, Isaacs R et al. (2014). A Phase IB multicentre dose‐determination study of BHQ880 in combination with anti‐myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal‐related events. Br J Haematol 167: 366–375. [DOI] [PubMed] [Google Scholar]
- Jiang T, Huang L, Zhang S (2013). DKK‐1 in serum as a clinical and prognostic factor in patients with cervical cancer. Int J Biol Markers 28: 221–225. [DOI] [PubMed] [Google Scholar]
- Jiang T, Wang S, Huang L, Zhang S (2009). Clinical significance of serum DKK‐1 in patients with gynecological cancer. Int J Gynecol Cancer 19: 1177–1181. [DOI] [PubMed] [Google Scholar]
- Kahn M (2014). Can we safely target the WNT pathway? Nat Rev Drug Discov 13: 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M (2005). WNT/PCP signaling pathway and human cancer (review). Oncol Rep 14: 1583–1588. [PubMed] [Google Scholar]
- Kemik O, Kemik AS, Sumer A, Begenik H, Purisa S, Tuzun S et al. (2011). Relationship between clinicopathologic variables and serum and tissue levels of dickkopf‐1 in patients with rectal cancer. J Invest Med 59: 947–950. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z (2010). Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141: 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick R, Ribe EM, Al‐Shawi R, Malik B, Hooper C, Fernandes C et al. (2014). Clusterin regulates beta‐amyloid toxicity via Dickkopf‐1‐driven induction of the WNT–PCP–JNK pathway. Mol Psychiatry 19: 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BR, Shin HJ, Kim JY, Byun HJ, Lee JH, Sung YK et al. (2012). Dickkopf‐1 (DKK‐1) interrupts FAK/PI3K/mTOR pathway by interaction of carbonic anhydrase IX (CA9) in tumorigenesis. Cell Signal 24: 1406–1413. [DOI] [PubMed] [Google Scholar]
- Kim SU, Park JH, Kim HS, Lee JM, Lee HG, Kim H et al. (2015). Serum Dickkopf‐1 as a biomarker for the diagnosis of hepatocellular carcinoma. Yonsei Med J 56: 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H et al. (2016). CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Invest 126: 2689–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause U, Ryan DM, Clough BH, Gregory CA (2014). An unexpected role for a Wnt‐inhibitor: Dickkopf‐1 triggers a novel cancer survival mechanism through modulation of aldehyde‐dehydrogenase‐1 activity. Cell Death Dis 5: e1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumawat K, Gosens R (2016). WNT‐5A: signaling and functions in health and disease. Cell Mol Life Sci 73: 567–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LN, Dove WF (2009). APC and its modifiers in colon cancer. Adv Exp Med Biol 656: 85–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N et al. (2004). Dickkopf‐1 antagonizes Wnt signaling independent of beta‐catenin in human mesothelioma. Biochem Biophys Res Commun 323: 1246–1250. [DOI] [PubMed] [Google Scholar]
- Lee HS, Lee HE, Park DJ, Kim HH, Kim WH, Park KU (2012). Clinical significance of serum and tissue Dickkopf‐1 levels in patients with gastric cancer. Clin Chim Acta 413: 1753–1760. [DOI] [PubMed] [Google Scholar]
- Lee N, Smolarz AJ, Olson S, David O, Reiser J, Kutner R et al. (2007). A potential role for Dkk‐1 in the pathogenesis of osteosarcoma predicts novel diagnostic and treatment strategies. Br J Cancer 97: 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Qin X, Guo X, Cui A, He Y, Wei S et al. (2013). Dickkopf‐1 is oncogenic and involved in invasive growth in non small cell lung cancer. PLoS One 8: e84944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Qin X, Liu B, Sun L, Zhang X, Li Z et al. (2011). Dickkopf‐1 is involved in invasive growth of esophageal cancer cells. J Mol Histol 42: 491–498. [DOI] [PubMed] [Google Scholar]
- Liu LJ, Xie SX, Chen YT, Xue JL, Zhang CJ, Zhu F (2016a). Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J Gastroenterol 22: 7486–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Li YF, Deng ZQ, Cao JQ (2016b). Prognostic significance of Dickkopf‐1 in gastric cancer survival: a meta‐analysis. Genet Test Mol Biomarkers 20: 170–175. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tang W, Xie L, Wang J, Deng Y, Peng Q et al. (2014). Prognostic significance of Dickkopf‐1 overexpression in solid tumors: a meta‐analysis. Tumour Biol 35: 3145–3154. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004). The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810. [DOI] [PubMed] [Google Scholar]
- Lu B, Green BA, Farr JM, Lopes FC, Van Raay TJ (2016). Wnt drug discovery: weaving through the screens, patents and clinical trials. Cancers (Basel) 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyros O, Rafiee P, Nie L, Medda R, Jovanovic N, Otterson MF et al. (2015). Wnt/beta‐catenin signaling activation beyond robust nuclear beta‐catenin accumulation in nondysplastic Barrett's esophagus: regulation via Dickkopf‐1. Neoplasia 17: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X et al. (2007). Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009). Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehata T, Taniguchi H, Yamamoto H, Nosho K, Adachi Y, Miyamoto N et al. (2008). Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J Gastroenterol 14: 2702–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino T, Yamasaki M, Takemasa I, Takeno A, Nakamura Y, Miyata H et al. (2009). Dickkopf‐1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol 16: 2058–2064. [DOI] [PubMed] [Google Scholar]
- Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y et al. (2016). Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165: 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM et al. (2002). Kremen proteins are Dickkopf receptors that regulate Wnt/beta‐catenin signalling. Nature 417: 664–667. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A et al. (2001). LDL‐receptor‐related protein 6 is a receptor for Dickkopf proteins. Nature 411: 321–325. [DOI] [PubMed] [Google Scholar]
- Marzo A, Galli S, Lopes D, McLeod F, Podpolny M, Segovia‐Roldan M et al. (2016). Reversal of synapse degeneration by restoring Wnt signaling in the adult hippocampus. Curr Biol 26: 2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba K, Mihara E, Tamura‐Kawakami K, Miyazaki N, Maeda S, Hirai H et al. (2017). Conformational freedom of the LRP6 ectodomain Is regulated by N‐glycosylation and the binding of the Wnt antagonist Dkk1. Cell Rep 18: 32–40. [DOI] [PubMed] [Google Scholar]
- Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA et al. (2007). Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest 117: 3248–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazon M, Masi D, Carreau M (2016). Modulating Dickkopf‐1: a strategy to monitor or treat cancer? Cancers (Basel) 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConechy MK, Ding J, Senz J, Yang W, Melnyk N, Tone AA et al. (2014). Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol 27: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes ME, Devine DJ, Shevde LA, Samant RS (2012). Dickkopf1: a tumor suppressor or metastasis promoter? Int J Cancer 130: 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev AM, Mikheeva SA, Maxwell JP, Rivo JV, Rostomily R, Swisshelm K et al. (2008). Dickkopf‐1 mediated tumor suppression in human breast carcinoma cells. Breast Cancer Res Treat 112: 263–273. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A et al. (1999). Dickkopf genes are co‐ordinately expressed in mesodermal lineages. Mech Dev 87: 45–56. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez‐Esteban C, Chen L, Tsukui T, Gomer L et al. (2001). Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1: 423–434. [DOI] [PubMed] [Google Scholar]
- Niehrs C (2006). Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25: 7469–7481. [DOI] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y et al. (2004). DKK1, a negative regulator of Wnt signaling, is a target of the beta‐catenin/TCF pathway. Oncogene 23: 8520–8526. [DOI] [PubMed] [Google Scholar]
- Pandur P, Lasche M, Eisenberg LM, Kuhl M (2002). Wnt‐11 activation of a non‐canonical Wnt signalling pathway is required for cardiogenesis. Nature 418: 636–641. [DOI] [PubMed] [Google Scholar]
- Pang H, Ma N, Jiao M, Shen W, Xin B, Wang T et al. (2017). The biological effects of dickkopf1 on small cell lung cancer cells and bone metastasis. Oncol Res 25: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BK, Paik YH, Park JY, Park KH, Bang S, Park SW et al. (2006). The clinicopathologic significance of the expression of vascular endothelial growth factor‐C in intrahepatic cholangiocarcinoma. Am J Clin Oncol 29: 138–142. [DOI] [PubMed] [Google Scholar]
- Patil MA, Chua MS, Pan KH, Lin R, Lih CJ, Cheung ST et al. (2005). An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene 24: 3737–3747. [DOI] [PubMed] [Google Scholar]
- Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ et al. (2009). The role of Dickkopf‐1 in bone development, homeostasis, and disease. Blood 113: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2012). Wnt signaling in cancer. Cold Spring Harb Perspect Biol 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA et al. (2006). Serum concentrations of Dickkopf‐1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 119: 1728–1731. [DOI] [PubMed] [Google Scholar]
- Pozzi S, Fulciniti M, Yan H, Vallet S, Eda H, Patel K et al. (2013). In vivo and in vitro effects of a novel anti‐Dkk1 neutralizing antibody in multiple myeloma. Bone 53: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Sun B, Liu Z, Li H, Gao J, Leng X (2012). Dickkopf‐1 inhibits epithelial‐mesenchymal transition of colon cancer cells and contributes to colon cancer suppression. Cancer Sci 103: 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Xie J, Hong S, Yang J, Zhang L, Han X et al. (2007). Dickkopf‐1 (DKK1) is a widely expressed and potent tumor‐associated antigen in multiple myeloma. Blood 110: 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD (2008). Dkk‐1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett 269: 67–77. [DOI] [PubMed] [Google Scholar]
- Rachner TD, Gobel A, Thiele S, Rauner M, Benad‐Mehner P, Hadji P et al. (2014a). Dickkopf‐1 is regulated by the mevalonate pathway in breast cancer. Breast Cancer Res 16: R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner TD, Thiele S, Gobel A, Browne A, Fuessel S, Erdmann K et al. (2014b). High serum levels of Dickkopf‐1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer 14: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TP, Kuhl M (2010). An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 106: 1798–1806. [DOI] [PubMed] [Google Scholar]
- Ryan DP, Murphy J, Mahalingam D, Strickler J, Stein S, Sirard C et al. (2016). Current results of a phase I study of DKN‐01 in combination with paclitaxel (P) in patients (pts) with advanced DKK1+ esophageal cancer (EC) or gastro‐esophageal junction tumors (GEJ). Ann Oncol 27 (suppl_2) ii108. [Google Scholar]
- Salim H, Zong D, Haag P, Novak M, Mork B, Lewensohn R et al. (2015). DKK1 is a potential novel mediator of cisplatin‐refractoriness in non‐small cell lung cancer cell lines. BMC Cancer 15: 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K (2002). The Wnt/calcium pathway activates NF‐AT and promotes ventral cell fate in Xenopus embryos. Nature 417: 295–299. [DOI] [PubMed] [Google Scholar]
- Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S et al. (2007). Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis 28: 2459–2466. [DOI] [PubMed] [Google Scholar]
- Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K et al. (2010). Wnt inhibitor Dickkopf‐1 as a target for passive cancer immunotherapy. Cancer Res 70: 5326–5336. [DOI] [PubMed] [Google Scholar]
- Schambony A, Wedlich D (2007). Wnt‐5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 12: 779–792. [DOI] [PubMed] [Google Scholar]
- Sedgwick AE, D'Souza‐Schorey C (2016). Wnt signaling in cell motility and invasion: drawing parallels between development and cancer. Cancers (Basel) 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X (2007). SnapShot: noncanonical Wnt signaling pathways. Cell 131: 1378. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X (2001). Head inducer Dickkopf‐1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11: 951–961. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Zhang X, He X (2008). DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem 283: 21427–21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB et al. (2012). Recurrent R‐spondin fusions in colon cancer. Nature 488: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CH, Hsieh HY, Wang YH, Chen SY, Tung CL, Wu JD et al. (2010). High Dickkopf‐1 expression is associated with poor prognosis in patients with advanced urothelial carcinoma. Exp Ther Med 1: 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y et al. (2012). Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large‐scale, multicentre study. Lancet Oncol 13: 817–826. [DOI] [PubMed] [Google Scholar]
- Sheng SL, Huang G, Yu B, Qin WX (2009). Clinical significance and prognostic value of serum Dickkopf‐1 concentrations in patients with lung cancer. Clin Chem 55: 1656–1664. [DOI] [PubMed] [Google Scholar]
- Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y, Qiu SJ et al. (2013). High expression of Dickkopf‐related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer 119: 993–1003. [DOI] [PubMed] [Google Scholar]
- Shi XD, Yu XH, Wu WR, Xu XL, Wang JY, Xu LB et al. (2016). Dickkopf‐1 expression is associated with tumorigenity and lymphatic metastasis in human hilar cholangiocarcinoma. Oncotarget 7: 70378–70387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Gong HL, Zhou L, Tian J, Wang Y (2014). Dickkopf‐1 is a novel prognostic biomarker for laryngeal squamous cell carcinoma. Acta Otolaryngol 134: 753–759. [DOI] [PubMed] [Google Scholar]
- Shizhuo W, Tao J, Shulan Z, Bing Z (2009). The expression and significance of Dickkopf‐1 in epithelial ovarian carcinoma. Int J Biol Markers 24: 165–170. [DOI] [PubMed] [Google Scholar]
- Smadja DM, d'Audigier C, Weiswald LB, Badoual C, Dangles‐Marie V, Mauge L et al. (2010). The Wnt antagonist Dickkopf‐1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol 30: 2544–2552. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R, Li L (2010). Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res C Embryo Today 90: 243–256. [DOI] [PubMed] [Google Scholar]
- Sun DK, Wang L, Wang JM, Zhang P (2015). Serum Dickkopf‐1 levels as a clinical and prognostic factor in patients with bladder cancer. Genet Mol Res 14: 18181–18187. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Fukushima T, Yorita K, Tanaka H, Chijiiwa K, Kataoka H (2010). Dickkopf‐1 is overexpressed in human pancreatic ductal adenocarcinoma cells and is involved in invasive growth. Int J Cancer 126: 1611–1620. [DOI] [PubMed] [Google Scholar]
- Tao YM, Liu Z, Liu HL (2013). Dickkopf‐1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig Liver Dis 45: 251–257. [DOI] [PubMed] [Google Scholar]
- Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL et al. (2011). Dickkopf‐1 (DKK‐1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 71: 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B et al. (2003). The role of the Wnt‐signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung EK, Mak CK, Fatima S, Lo RC, Zhao H, Zhang C et al. (2011). Clinicopathological and prognostic significance of serum and tissue Dickkopf‐1 levels in human hepatocellular carcinoma. Liver Int 31: 1494–1504. [DOI] [PubMed] [Google Scholar]
- van Amerongen R (2012). Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol 4: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorzanger‐Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clezardin P et al. (2007). Increased Dickkopf‐1 expression in breast cancer bone metastases. Br J Cancer 97: 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J et al. (2008). Characterization of the Kremen‐binding site on Dkk1 and elucidation of the role of Kremen in Dkk‐mediated Wnt antagonism. J Biol Chem 283: 23371–23375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang S (2011). Dickkopf‐1 is frequently overexpressed in ovarian serous carcinoma and involved in tumor invasion. Clin Exp Metastasis 28: 581–591. [DOI] [PubMed] [Google Scholar]
- Wang Y (2009). Wnt/planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther 8: 2103–2109. [DOI] [PubMed] [Google Scholar]
- Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG et al. (2003). Overexpression of human Dickkopf‐1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms' tumors. Lab Invest 83: 429–434. [DOI] [PubMed] [Google Scholar]
- Xiang XJ, Liu YW, Chen DD, Yu S (2015). Differential expression of Dickkopf‐1 among non‐small cell lung cancer cells. Mol Med Rep 12: 1935–1940. [DOI] [PubMed] [Google Scholar]
- Xu WH, Liu ZB, Yang C, Qin W, Shao ZM (2012). Expression of Dickkopf‐1 and beta‐catenin related to the prognosis of breast cancer patients with triple negative phenotype. PLoS One 7: e37624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr (2007). Antibody‐based inhibition of DKK1 suppresses tumor‐induced bone resorption and multiple myeloma growth in vivo. Blood 109: 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M et al. (2007). Dikkopf‐1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res 67: 2517–2525. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen GD, Fang F, Liu Z, Lau SH, Zhang JF et al. (2013). Dickkopf‐1: as a diagnostic and prognostic serum marker for early hepatocellular carcinoma. Int J Biol Markers 28: 286–297. [DOI] [PubMed] [Google Scholar]
- Yao L, Zhang D, Zhao X, Sun B, Liu Y, Gu Q et al. (2016). Dickkopf‐1‐promoted vasculogenic mimicry in non‐small cell lung cancer is associated with EMT and development of a cancer stem‐like cell phenotype. J Cell Mol Med 20: 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B et al. (2009). Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta‐catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol 50: 948–957. [DOI] [PubMed] [Google Scholar]
- Zarea M, Mohammadian Bajgiran A, Sedaghati F, Hatami N, Taheriazam A, Yahaghi E et al. (2016). Diagnostic investigations of DKK‐1 and PDCD5 expression levels as independent prognostic markers of human chondrosarcoma. IUBMB Life 68: 597–601. [DOI] [PubMed] [Google Scholar]
- Zebisch M, Jackson VA, Zhao Y, Jones EY (2016). Structure of the dual‐mode Wnt regulator Kremen1 and insight into ternary complex formation with LRP6 and Dickkopf. Structure 24: 1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T, Rindtorff N, Boutros M (2017). Wnt signaling in cancer. Oncogene 36: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhao Y, Yang Q (2014). Sensitivity and specificity of Dickkopf‐1 protein in serum for diagnosing hepatocellular carcinoma: a meta‐analysis. Int J Biol Markers 29: e403–e410. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Li X, Zhang J, Mao J, Li Z et al. (2004). The LRP5 high‐bone‐mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol 24: 4677–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SJ, Zhuo SR, Yang XQ, Qin CX, Wang ZL (2014). Serum Dickkopf‐1 expression level positively correlates with a poor prognosis in breast cancer. Diagn Pathol 9: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu F, Xu Q, Wang X (2010). Analysis of the expression profile of Dickkopf‐1 gene in human glioma and the association with tumor malignancy. J Exp Clin Cancer Res 29: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman‐Rossi J, Villanueva A, Nault JC, Llovet JM (2015). Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149: 1226–1239 e1224. [DOI] [PubMed] [Google Scholar]


