Abstract
Wnt/β‐catenin signalling is initiated by a ternary Wnt‐Frizzled (FZD)‐LDL receptor‐related protein (LRP) 5/6 binding event. The resulting conformational changes in the FZD and LRP5/6 receptors promote the assembly of an intracellular signalosome driven by Dishevelled and Axin co‐polymerization. Recent evidence suggests that the FZD receptor and LRP5/6 participate in the assembly of this signalosome by forming regulatory scaffolds for stabilizing Dishevelled and Axin adapters. In this review, we focus on the contributions of Wnts and their receptors in the assembly of the signalosome. We present an emerging model, which unifies Wnt receptor oligomerization with intracellular signalosome formation, and then discuss how FZD receptors might be targeted to either disrupt or enhance their capacity as a dynamic sensor of Wnt binding.
Linked Articles
This article is part of a themed section on WNT Signalling: Mechanisms and Therapeutic Opportunities. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.24/issuetoc
Abbreviations
- CRD
cysteine‐rich domain
- DEP
Dishevelled EGL‐10 and pleckstrin
- DIX
Dishevelled and Axin
- DKK1
Dickkopf‐related protein 1
- FZD
Frizzled
- LRP
LDL receptor‐related protein
- PDZ
PSD‐95/Dlg1/ZO‐1
- TMD
transmembrane domain
- Wnt
wingless/int1
- βP
β‐propeller
Introduction
Wingless/int1 (Wnt) signal activation is tightly controlled by a dynamic signalosome consisting of Class Frizzled GPCRs (FZDs), LDL receptor‐related protein (LRP) 5/6 coreceptors and Dishevelled and Axin adapters (Cong et al., 2004; Kaykas et al., 2004; Bilic et al., 2007; Gammons et al., 2016a). In this review, we discuss emerging evidence for a model of FZD and LRP5/6 co‐oligomerization. In this model, oligomerization of the Wnt signalling complex is facilitated by domains with weak homo‐oligomerization propensities such as the FZD cysteine‐rich domain (CRD), the LRP5/6 β‐propeller (βP) domains, and the Dishevelled EGL‐10 and pleckstrin (DEP) and Dishevelled and Axin (DIX) domains (Dann et al., 2001; Gammons et al., 2016b). In the absence of Wnt, inactive complexes of FZD, LRP5/6 and DVL pose as static sensors for Wnt binding (Chen et al., 2014) (Figure 1A). When Wnt binds FZD receptors and LRP5/6, the FZD receptor and LRP5/6 complexes are activated and oligomerize to create an extensive scaffold for Dishevelled stabilization and interaction with LRP5/6 (Bilic et al., 2007) (Figure 1B). Dishevelled then co‐polymerizes with Axin through shared DIX domains to form a trap which sequesters the β‐catenin destruction complex (Schwarz‐Romond et al., 2007; Fiedler et al., 2011). Consequentially, β‐catenin translocates to the nucleus where it works in concert with Wnt transcription factors to turn on Wnt target genes (van de Wetering et al., 1991).
Figure 1.
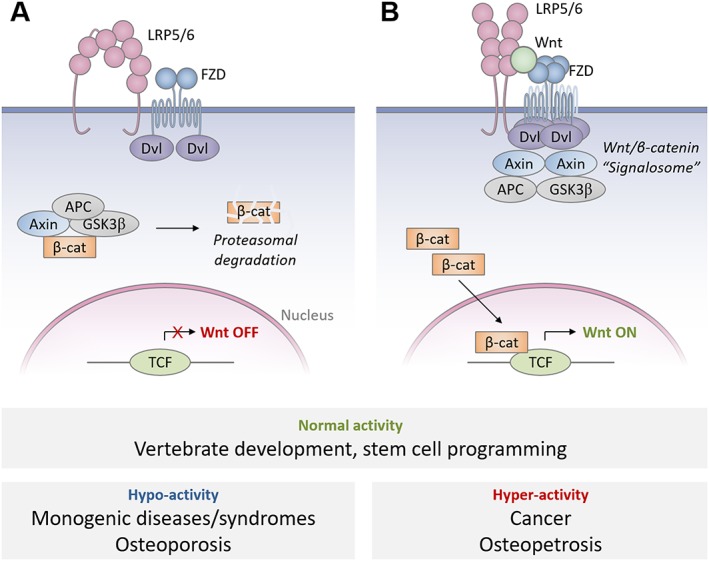
Schematic illustration of Wnt/β‐catenin signalosome assembly. (A) Emerging model of Wnt ‘off’ state in which FZD receptor dimers and inactive LRP5/6 dimers form inactive complexes, which bind monomeric Dishevelled (Dvl). (B) Emerging model of Wnt ‘on’ state in which Wnts drive FZD oligomerization by stabilizing FZD CRD interactions, inducing conformational changes in LRP5/6 dimers and thus increasing local concentrations of Dishevelled, which ultimately permits efficient Dishevelled/Axin co‐polymerization. These events are responsible for intracellular Wnt signalosome assembly and sequestration of the β‐catenin destruction complex. Normal levels of Wnt signalling determine proper vertebrate development and stem cell programming, among many other functions, while excessively high or low levels of signalling have pathogenic outcomes.
Wnt family ligands are FZD‐specific, highly conserved and often determine developmental patterning and cell fate (van de Wetering et al., 1997). Many of these physiological consequences are the result of Wnt/β‐catenin signalling which involves the assembly of an intracellular Dishevelled/Axin signalosome (Cong et al., 2004; Schwarz‐Romond et al., 2007). Unlike norrin, an atypical FZD4/LRP5 agonist, all 19 human Wnts share a highly conserved two‐domain structure which enables it to attach the FZD receptor CRD and binding to the LRP5/6 β‐propellers (Bazan et al., 2012). In this review, we focus on the FZD receptor and LRP5/6 interfaces involved in Wnt binding and discuss the mechanisms by which Wnts may alter the assemblies of FZD receptors and LRP5/6.
Class FZD GPCRs comprise a family of 10 closely related FZD receptors in humans and an ancestral member, smoothened (SMO). FZD receptors share similar structures, possess common mechanisms of activation and achieve similar downstream activities (Schulte and Bryja, 2007). All FZD receptors share a highly conserved CRD that consists of five helices cross‐braced by five disulfide bonds, forming a rigid, globular domain (Dann et al., 2001). A flexible, mostly un‐conserved linker connects the CRD to the transmembrane domain (TMD) (Byrne et al., 2016). The seven‐transmembrane domain of smoothened resembles that of GPCRs, most closely that of Class B GPCRs (Wang et al., 2013). The intracellular loops and C‐terminal tails of FZD receptors are likely to be unstructured, diverse and sometimes very short, yet they all include several conserved Dishevelled‐binding motifs (Wong et al., 2003; Tauriello et al., 2012; Gammons et al., 2016b). The role of FZD receptors in Wnt signalosome assembly is just beginning to be appreciated.
Wnt coreceptors such as LRP5/6 function in tandem with FZD receptor to orchestrate pathway specificity, facilitate Wnt binding and drive signalosome assembly. LRP5 and LRP6 are coreceptors for the Wnt/β‐catenin pathway and are composed of four globular β‐propeller domains, three LDL‐like domains, a single‐pass transmembrane domain and an approximately 200‐residue cytoplasmic tail containing multiple stability and effector elements (Cheng et al., 2011). The β‐propeller domains of LRP5/6 can bind Wnts or Wnt pathway inhibitors such as Dickkopf 1 (DKK1) and sclerostin (Cheng et al., 2011). The conformations of the β‐propeller domains, together with the proteins to which they are bound (including Wnt, norrin, FZD, DKK1 and sclerostin), may regulate the intracellular proximity of LRP5/6 tails to one another and their corresponding activities (Matoba et al., 2017). The existence of LRP5/6 oligomers are well established based on a series of immunocytochemistry and Western blotting experiments focusing on phosphorylated LRP6 after stimulation with Wnt (Bilic et al., 2007), but recently published data using light microscopy to study LRP6 dynamics provide a mechanism for LRP6 oligomerization, which we discuss in the last section of this article.
In this review, we begin with a discussion of the structure and dimerization of the FZD receptors, then review the structures and requirements of Wnt pathway agonists and use this information to better interpret emerging data on Wnt‐CRD recognition, stoichiometry and mechanisms of FZD receptor oligomerization. We integrate this emerging model of FZD receptor oligomerization into the existing understanding of LRP5/6 oligomer assembly, discuss the inter‐dependence between the assembly of the FZD/LRP complex and the Dishevelled/Axin signalosome and suggest novel mechanisms in Wnt signalosome assembly, which can be therapeutically targeted.
FZD receptor structure and dynamics
FZD receptors, like many of their canonical GPCR counterparts, are regulated by ligand binding, oligomerization status and extracellular domain conformations which cooperate to trigger formation of intracellular GPCR effector complexes. The full‐length structure of FZD receptors is currently only known from smoothened, the most divergent family member. The extracellular and transmembrane domains (TMDs) of smoothened have been crystallized (Figure 2A), revealing a structured CRD very similar to that of the FZD4 and FZD8 receptors a flexible and largely unstructured CRD‐to‐TMD linker, a TMD bundle very reminiscent of class B GPCRs and a long extracellular loop 3 (ECL3) which coordinates with the CRD and linker to encase the CRD sterol‐binding pocket (Byrne et al., 2016; Zhang et al., 2017). Since FZD receptor TMDs and CRDs are very similar with regard to sequence to smoothened (Figure 2C), it is probable that the most substantial structural differences will be due to the linker and ECL3 length and minor variations in CRD structure (Figure 2D). Indeed, while smoothened has a long ECL3 and a comparatively short CRD‐to‐TMD linker, FZD receptors have much longer linkers and shorter ECL3 loops. This suggests greater independence between the TMD and the CRD in FZD receptors compared to smoothened, permitting CRD oligomerization or the binding of Wnts. It is still unclear how the signal initiated by Wnt binding to the CRD of the FZD receptor is transduced across the membrane.
Figure 2.
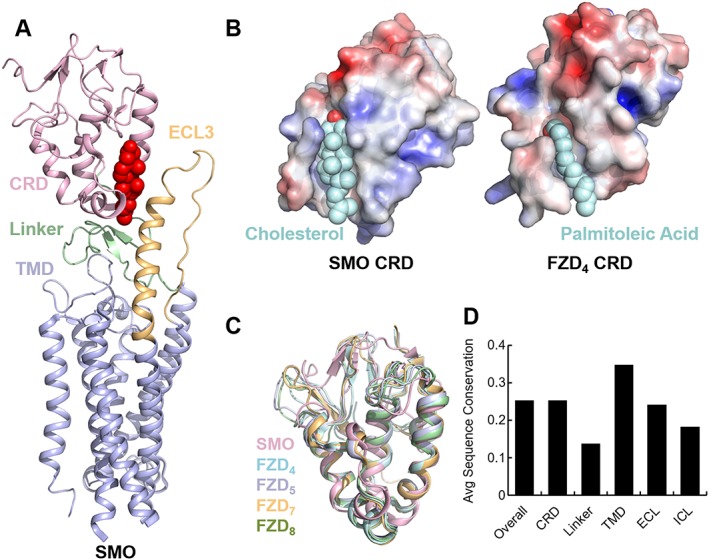
Smoothened (SMO) as a model for FZD receptor structure. (A) Structure of smoothened extracellular and transmembrane domains (TMDs), showing an extracellular extension of transmembrane helix 5 and a partially structured CRD‐to‐TMD linker which closes off the sterol‐binding pocket with bound cholesterol (shown in red spheres). (B) Comparison of palmitoyl coordination in FZD4 receptor CRD and cholesterol coordination in smoothened. (C) Available crystal structures of FZD receptor CRDs aligned to show structural similarity. (D) Graph of overall sequence proportion in each smoothened domain shared with FZD family members. Total conservation was calculated from 50% gap‐trimmed multiple sequence alignment of hSMO with all hFZDs and averaged for all residues in a given topology or domain.
Cholesterol has been crystallized with the smoothened CRD and identified as its native ligand (Byrne et al., 2016), and it is reasonable to ask whether FZD receptors may also bind cholesterol. We noticed that the smoothened CRD coordinates the hydroxyl group of cholesterol at a glutamic acid highly conserved across species (D95 in humans), while FZD receptors 1–10 have no polar residues near that location in any higher species. In general, the CRDs of FZD receptors are characterized by a tighter lipid‐binding groove than smoothened (Dann et al., 2001; Janda et al., 2012) (Figure 2B). We also note that smoothened is not known to bind palmitoleic acid, while palmitoleoylated Wnts bind FZD receptors and FZD receptors have not been reported to bind cholesterol in the sterol‐binding pocket. It is not obvious how FZD receptors would bind cholesterol at this site in the CRD, although cholesterol certainly may play a role in allosteric FZD receptor regulation elsewhere, and the importance of membrane lipids in GPCR structure and function cannot be overstated.
FZD receptor dimerization has long been thought to contribute to Wnt signal propagation (Carron et al., 2003; Ke et al., 2013; Wang et al., 2013), yet evidence has only recently emerged for Wnt‐induced FZD receptor oligomerization based on BRET data for the FZD4 receptor and emerging evidence for other FZD receptors (DeBruine et al., 2017). FZD receptors appear to have two different dimerization interfaces, one in the TMD and one in the CRD, that together allow formation of FZD receptor homo‐oligomers. It may be possible to infer the structural basis of TMD dimerization from the crystal structure of parallel smoothened TMD dimers which interact through an interface at helices IV and V (Wang et al., 2013). This is analogous to what has been observed for class B GPCRs (Harikumar et al., 2007). Recently, the dimerization interface of the TMD of the FZD6 receptor has indeed been shown to be formed by these two helices (Petersen et al., 2017). While smoothened dimerization is thought to be important for receptor activation, FZD receptors can weakly auto‐dimerize, and ligand binding further stabilizes dimerization and stimulates oligomerization as recently demonstrated for the FZD4 receptor and Wnt‐5a (DeBruine et al., 2017). Surprisingly, however, a recent study which used fluorescence cross‐correlation spectroscopy (FCCS) to monitor FZD6 receptor dimerization status observed a transient dissociation of dimers in response to Wnt dissociation, followed by re‐association after a few minutes (Petersen et al., 2017). It is unclear how Wnt would accomplish this mechanistically but might perhaps permit conformational switching of the FZD/LRP complex.
Phylogenetic clustering of human FZD receptors reveals four major subfamilies: FZD 1/2/7, 3/6, 4/9/10 and 5/8 (Schenkelaars et al., 2015), although the CRD within each of these subfamilies is remarkably conserved. Each family shares a hallmark characteristic: FZD5 and FZD8 receptors have exceptionally long CRD‐to‐TMD linkers; FZD3 and FZD6 receptors have long cytoplasmic tails with many regulatory elements; FZD4, FZD9 and FZD10 have very short C‐terminal tails and the shortest CRD‐to‐TMD linkers; and FZD1, FZD2 and FZD7 receptors seem to possess a CRD most different in sequence and structure from other FZD receptors. Few structural differences are readily apparent within these subfamilies, especially within the CRDs, and the diversification of tissue‐specific expression patterns seems to have driven divergent evolution most dramatically (Schenkelaars et al., 2015). Further structural studies of individual FZD subfamilies may indicate whether these differences are therapeutically tractable and can be used to increase specificity of FZD inhibitors. Notably, the transmembrane domains of these FZD receptors are highly conserved and may be capable of hetero‐dimerization with other FZD family members. However, the CRDs may differ enough between subfamilies to restrict hetero‐oligomerization and thus provide limited selectivity for therapeutic targeting.
Structures and function of Wnt pathway agonists
The structure of Xenopus Wnt‐8 (XWnt‐8) in complex with the mouse FZD8 receptor's CRD revealed a two‐domain architecture for Wnts that has been described as a grasping hand, consisting of a ‘palm’ (saposin domain) and an ‘index finger’ (cystine knot‐like domain) (Janda et al., 2012) (Figure 3B). The saposin domain is modified by a 16‐carbon palmitoleic acid that stabilizes the FZD receptor‐CRD interaction. The cystine‐knot‐like domain is similar in structure, but not in sequence, to TGF‐β, Noggin and bone morphogenic protein. Both the saposin and cystine‐knot‐like domains are known to interact with the FZD receptor's CRD at unique sites opposite one another, rendering a unique horseshoe‐shaped structure. This structure appears to have the potential for opening and closing about an unstructured central hinge, providing flexibility for grasping pre‐assembled FZD receptor complexes (Bazan et al., 2012). The sequences of all 19 ‘classical’ human Wnts are highly conserved and the structures are almost certainly super‐imposable.
Figure 3.
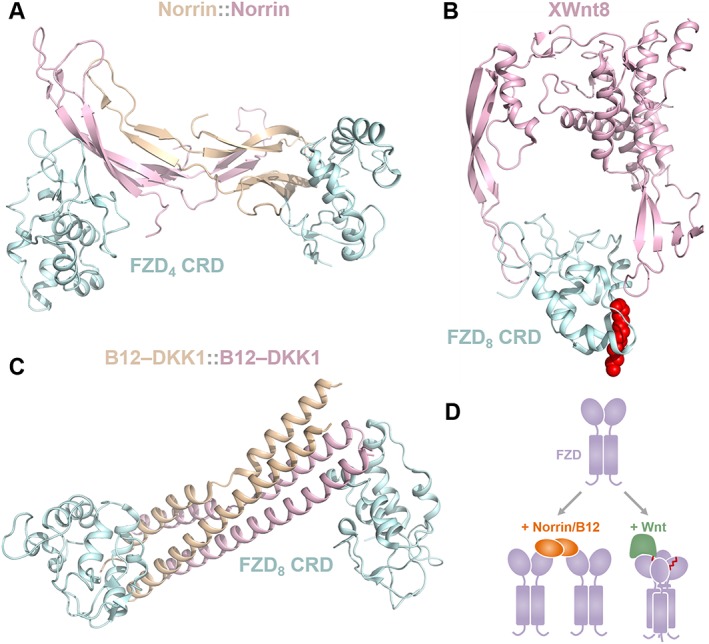
Architecture of Wnt pathway agonists. (A) Norrin homodimers bind two non‐adjacent FZD4 receptor CRDs in an orientation completely unlike any other CRD dimer structures crystallized (PDB 5CL1). (B) Structure of XWnt‐8 and the mFZD8 receptor CRD reveals two interaction interfaces, one of which is strengthened by insertion of the Wnt cis‐C16:Δ9 palmitoleoyl moiety (shown in red spheres) into a conserved hydrophobic groove in the FZD8 CRD (PDB 4F0A). (C) The Wnt pathway B12‐DKK1 surrogate (B12 homodimers shown here) binds FZD8 CRDs in a manner that is strikingly similar to norrin, showing nearly identical FZD CRD geometry and CRD‐CRD orientations, only reflected about a single axis (PDB 5UN5, 5UN6). (D) A hypothetical cartoon model contrasting the mechanism of Wnt‐mediated versus norrin/B12‐mediated FZD signalosome assembly.
Norrin is an atypical Wnt morphogen and cystine‐knot‐like protein which is active only as a covalent homodimer (Ke et al., 2013) and surprisingly bears no sequence or structural similarity to ‘classical’ Wnts (Figure 3A), thus providing an instructive case of convergent evolution towards Wnt signal activation through an alternative mechanism. Norrin specifically activates Wnt/β‐catenin signalling through only the FZD4 receptor and LRP5/6 (Xu et al., 2004). The basis of this specificity is not completely clear as FZD9 and FZD10 receptors are close homologues to the FZD4 receptor and seem to share the norrin binding surface in the FZD4 receptor. Perhaps the basis of norrin–FZD4 specificity is dependent on TSPAN12, an FZD4‐specific co‐receptor (Lai et al., 2017). It is also possible that the basis of norrin–FZD4 is mechanistic and depends on the remarkably short ECL3 of the FZD4 receptor. Functional domains that are required for signal activation have been identified by mapping genetic mutations in patients with familial exudative vitreoretinopathy, a norrin‐dependent monogenic disease (Robitaille et al., 2002). Specifically, mutations in the FZD4‐ and LRP5‐binding sites on norrin abolish signalling, as do corresponding mutations on FZD4 and LRP5 at norrin‐binding sites (Toomes et al., 2004). Interestingly, mutations in the FZD4 linker domain are also associated with strong disease phenotypes, suggesting a key role for the linker in CRD‐to‐TMD signal transmission. Finally, mutations disrupting norrin homodimerization prevent signalling, suggesting that norrin homodimerization is a requirement and confirming the functional significance of structural observations that the binding two FZD4 and two LRP5 receptors is essential (Ke et al., 2013; Chang et al., 2015; Shen et al., 2015). If norrin activates signalling by recruiting more than a single FZD4 receptor, it is reasonable to consider that Wnts may also do the same.
Recently, Wnt surrogates have been engineered, which are able to activate Wnt/β‐catenin signalling with an efficiency comparable to natural Wnts (Janda et al., 2017). These surrogates were designed by fusing an LRP5/6‐binding module (DKK1, a Wnt pathway inhibitor) to a FZD receptor CRD‐binding module (either an scFv of the therapeutic anti‐FZD receptor antibody vantictumab or B12, a synthetic helical protein). The crystal structure of this synthetic four‐helix bundle domain protein (named B12) binds only FZD5 and FZD8 receptors with nanomolar affinity. The crystal structure of B12 in complex with FZD8 CRD revealed a dimer geometry very similar to that of norrin (Figure 3C), showing similar distances between CRDs and similar angles of the CRD relative to each other, despite different binding surfaces. However, it should be noted that size exclusion chromatography indicates monomeric B12 in solution. Strikingly, the optimal linker length between B12 and DKK1 for Wnt activity was found to be between 10 and 30 bp, consistent with the approximately 10 base pairs between the FZD4 receptor and LRP5 binding surfaces mapped on norrin (Ke et al., 2013). The structures of B12::FZD8, norrin::FZD4 and XWnt‐8::FZD8 provide valuable insights into Wnt pathway activation, and based on more recent work with Wnt‐mediated CRD interactions, a model arises where FZD dimers are brought together either by homodimerized FZD‐binding ligands or by ligands such as Wnt, which have been proposed to stabilize CRD interactions through their lipid modification (Figure 3D).
Wnt‐CRD recognition and selectivity
Classical Wnts are known to bind FZD receptors at two sites in the CRD, although no biochemical studies have directly considered the possibility of Wnt binding FZDs beyond the CRD. A ‘Site 1’ CRD interaction is prominently characterized by a palmitoleoyl moiety covalently attached to a conserved serine – S187 in the case of XWnt‐8 – which inserts into a FZD CRD lipid‐binding groove and is stabilized by a conserved network of hydrophobic interactions. This groove in FZD receptors is similar to the cholesterol‐binding groove found in smoothened but is slightly narrower and does not possess a hydrophilic tyrosine to coordinate oxysterol binding. A ‘Site 2’ Wnt–FZD interaction is located opposite the ‘Site 1’ interaction on the FZD CRD where the Wnt cystine‐knot‐like domain is in contact with a highly conserved surface on the CRD. This second interaction seems to stabilize the stronger ‘Site 1’ interaction but might also fulfil other roles in the FZD/LRP complex assembly. While these two interaction sites have been confirmed by mutational mapping (Dann et al., 2001; Janda et al., 2012), additional interaction sites may exist. For example, it is surprising that the horseshoe‐shaped Wnt is in contact with the FZD receptor only at its tips in the crystal structure of XWnt‐8 bound to the FZD8 receptor, leaving a very large solvent‐exposed space between the CRD and the hydrophobic interior surface of the Wnt. Another surprise is that the most highly conserved regions on a Wnt bind the most highly conserved domain in FZD receptors and yet Wnts demonstrate intricate selectivity for FZD receptors. A possible explanation for many of these observations is that a less conserved Wnt–FZD interaction surface might exist.
All Wnts demonstrate selectivity for subfamilies of FZD receptors, an observation which has been partially explored in the context of the CRD (Dijksterhuis et al., 2015). However, it seems that this selectivity is not fully accounted for by the CRD, as suggested by a systematic Topflash assay of Wnt/β‐catenin signalling stimulated by every possible Wnt‐FZD pairing (Yu et al., 2012). In this assay, Yu et al. demonstrate that Wnt–FZD selectivity tends to mirror phylogenetic clustering: distinct subfamilies of Wnts signal most efficiently through distinct subfamilies of FZD receptors. The extent of Wnt–FZD selectivity in this assay and the limited amount of Wnt–CRD selectivity in biochemical assays suggest a possible non‐CRD effect on determining selectivity.
Classical Wnt‐mediated FZD receptor activation involves Wnt binding to the FZD receptor and LRP5/6 (Cong et al., 2004). However, the mechanism by which Wnt activates FZD receptors remains largely unknown. Crystallography studies of FZD4, FZD5 and FZD7 receptor CRDs in the presence of unsaturated fatty acids have shown that FZD CRD dimers can adopt orientations with lipid‐binding grooves aligned back‐to‐back, thus forming a continuous hydrophobic pocket in which the lipid stabilizes the CRD in the dimeric conformation (DeBruine et al., 2017; Nile et al., 2017). In the case of the FZD4 receptor, these CRDs were crystallized with cis‐C16:Δ9 palmitoleic acid (the predominant lipid modification of Wnts; ref.) and formed a tetrameric assembly consisting of two unstable lipid‐binding dimers, which cross‐braced to form a stable tetrameric assembly and thus completely protect the lipid from solvent. A similar tetrameric form of FZD4 CRDs has been previously crystallized (PDB 5BPB) (Chang et al., 2015), as have two dimer forms which are present in this tetrameric form (PDB 5CM4, 5BPQ) (Chang et al., 2015; Shen et al., 2015). In the case of FZD5 and FZD7 receptors, which were crystallized with C16:Δ9 palmitoleic acid and trans‐C24:Δ15 tetracos‐15‐enoic acid, respectively, the lipid‐bound dimers formed apparently stable complexes with a robust dimerization interface that completely protected the lipid from solvent. Alignment of the Wnt structure to these complexes (and the smoothened structures) reveals that both of these complexes are sterically able to bind Wnts (Figure 4A, B). The role of Wnt in altering FZD CRD oligomerization, however, is less well understood. It is possible that FZD CRDs exist as monomers, which dimerize upon Wnt binding, or it is possible that FZD CRDs exist as dimers, which bind Wnts as dimers, or finally, it is possible that Wnts mediate FZD CRD tetramer assembly and that lipid‐bound dimer forms merely represent an intermediate state (Figure 4C). Studies of purified FZD5 and FZD7 receptor CRDs in solution with varying lengths of unsaturated lipids have shown that lipid binding can drive CRD dimerization (Nile et al., 2017), and BRET data of full‐length FZD4 receptors suggest that Wnt‐5a binding mediates FZD4 receptor oligomerization through the CRD (DeBruine et al., 2017). The ability of the CRD to form dimers has been previously studied, as dimeric forms of FZD8 receptor CRDs have previously been described (Dann et al., 2001), and artificial stimulation of FZD7 receptor CRD dimerization has been shown to promote Wnt signalling (Carron et al., 2003), but the significance of these dimers is only now becoming clear due to recent structural studies. In conclusion, signalosome assembly probably depends on Wnt‐mediated FZD–FZD interactions upon binding. Future studies should explore the stoichiometry at each step in this mechanism.
Figure 4.
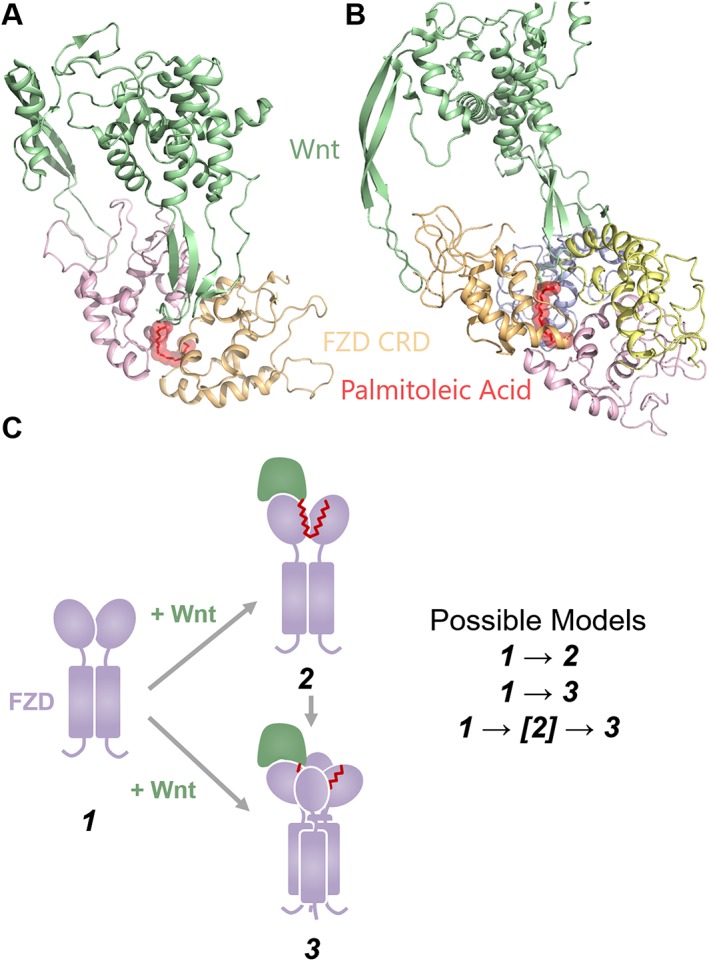
Models of Wnt‐CRD recognition. (A) Crystal structure of FZD5 receptor CRDs (5URY) in complex with C16:Δ9 aligned to XWnt‐8 (4F0A). (B) Crystal structure of FZD4 receptor CRDs (5UWG) in complex with C16:Δ9 aligned to XWnt‐8 (4F0A). FZD linkers (modelled based on SMO structures) do not impede Wnt binding in either (A) or (B). (C) Possible models of Wnt‐CRD recognition, in which Wnts may bind either CRD dimers, CRD tetramers, or utilize a CRD dimer‐bound state as an intermediate towards a CRD tetramer‐bound state.
Evidence for non‐CRD binding sites is provided by observations of Wnt‐dependent signalling through FZD receptor constructs lacking a CRD. Experiments in Drosophila lacking a FZD receptor CRD showed normal patterns of development, concluding that the CRD is dispensable for transducing Wnt signalling in vivo (Chen et al., 2004). A biochemical study also supported this observation, but demonstrated that high levels of overexpression are necessary to achieve a signalling response and that FZD activity can be restored when the CRD is replaced by Wnt‐binding proteins such as Wnt inhibitory factor (Povelones and Nusse, 2005). These studies must be interpreted with caution until more definite evidence can be obtained, but they seem to point towards additional Wnt‐binding sites on FZD receptors. These findings further underscore the role of the CRD as a regulatory domain and not as a domain fundamental to FZD receptor activation.
In summary, while the one‐to‐one interaction between Wnt thumb and index finger and CRD lipid‐binding groove and C‐terminal loops, respectively, is well established, additional sites of Wnt–FZD contact may exist both within the CRD (due to higher‐order CRD interactions induced or stabilized by Wnt) and beyond the CRD (which may confer Wnt–FZD selectivity and capacity for Wnt‐dependent CRD‐independent signalling). The basis of Wnt–FZD selectivity remains obscure, especially because known Wnt–FZD interaction surfaces are highly conserved. Finally, the effect of Wnt on higher‐order FZD interactions merits further study. It is unlikely that a single Wnt binds to only a single FZD receptor, and yet, our current structural knowledge does not extend beyond this simplistic model.
LRP5/6 dynamics and signalosome assembly
While nearly a decade has passed since LRP6 signalosomes were first described (Bilic et al., 2007), the structural mechanism of LRP5/6 oligomerization had remained unclear until very recently. LRP5 and LRP6 are similar proteins, which are structurally understood only from LRP6 β‐propellers and small fragments of the LDL repeats (Cheng et al., 2011; Matoba et al., 2017). These β‐propellers serve as binding scaffolds for agonists of the Wnt pathway such as Wnts and norrin (Janda et al., 2012; Ke et al., 2013), as well as antagonists such as DKK1 and sclerostin (Veverka et al., 2009; Cheng et al., 2011).
Prior to Wnt binding, inactive complexes of LRP5/6 and the FZD receptor may form (Chen et al., 2014). It is unclear whether this is dependent on experimental conditions, overexpression, or is physiologically relevant. If this interaction is physiological, it would run contrary to the idea that Wnts recruit LRP5/6 and FZD receptors to form an active signalling complex. Further studies need to address the significance of these interactions, as an interface between the FZD receptor and LRP would be novel and potentially able to be targeted. Specifically, negatively charged surfaces on FZD receptors should be investigated as candidates for binding the LRP5/6 β‐propellers, although transmembrane and C‐terminal tail interactions may also be possible.
Dimerization of LRP5/6 has been associated with both inactive and active receptor states (Liu et al., 2003; Chen et al., 2014), and both DKK1 and Wnts have been found to mediate LRP5/6 dimerization (Liu et al., 2003; Matoba et al., 2017). Since DKK1 and Wnt have opposite effects on Wnt signal activation, these findings were perplexing until a recent study, which used negative stain electron microscopy and 2D classification and showed that the conformations of the LRP6 β‐propellers in dimer complexes can control receptor activity (Matoba et al., 2017). The hinge between the rigid N‐terminal (βP1/2) and C‐terminal (βP3/4) pairs of β‐propeller domains can permit more than 180o of rotation, resulting in the sampling of a wide array of conformations in an inactive state, but the restriction of these conformations when bound to a ligand. Furthermore, conformational flexibility of the β‐propellers was also limited upon N‐glycosylation of a residue in the immediate vicinity of the βP12/βP34 hinge. Active conformations of LRP5/6 β‐propellers would, in theory, permit transmembrane and intracellular domain dimerization. DKK1 ligand binding to the β‐propeller scaffolds reduced the conformational freedom of LRP5/6 and therefore prohibited the assembly of active dimers. Indeed, biochemical approaches using bispecific antibodies which mediate LRP6 β‐propeller interactions have been shown to increase Wnt signalling activity (Gong et al., 2010).
Wnts manifest various requirements for LRP5 and LRP6 and selectivity for either βP1/2 or βP3/4. Despite many uncertainties, it is evident that Wnt‐1, Wnt‐9b and Wnt‐10b require both LRP5 and LRP6 for Wnt/β‐catenin signalling (Goel et al., 2012) and that Wnt‐3 and Wnt‐3a bind βP1/2 while other Wnts seem to bind βP3/4 (Ettenberg et al., 2010). These findings may reveal surfaces within Wnt subfamilies that mediate LRP5/6 selectivity and could be a therapeutic target. They also raise the possibility of synergy between Wnts 3/3a and other Wnts by binding alternate LRP β‐propellers, thus mediating dimerization and activation more effectively.
LRP5/6 are reported to form large signalosomes, yet no evidence for LRP oligomerization exists in the absence of the FZD receptor. In the context of the signalosome induced by classical Wnts, it is possible that repeating units of LRP dimers form complexes with FZD receptor dimers or tetramers each bound to a single Wnt and that these complexes then oligomerize in response to multiple Wnt binding events or other co‐receptor recruitment. Norrin may act through different mechanisms, as it homodimerizes to bind two FZD4 CRDs whereas classical Wnts have different FZD receptor‐binding modes (Ke et al., 2013). Thus, a norrin complex may perhaps even bring the FZD CRD and LRP5/6 β‐propeller in contact, while classical Wnts will mediate CRD interactions through the palmitoleoyl modification while binding LRP5/6 through a negatively charged surface relatively distant from the site of CRD contact. The anticipated result would be a tunable and diverse mechanism for the complex activation of the FZD receptor and LRP5/6, which may have significant implications for the design of effective Wnt pathway surrogates and inhibitors (Moraga et al., 2015).
The mechanisms of LRP5/6 intracellular activation and interactions with Dishevelled have been excellently described and reviewed elsewhere (Bilic et al., 2007; Bienz, 2014; Feng and Gao, 2015). The role of LRP5/6 clustering to trigger CK1γ phosphorylation and Axin recruitment, the role of Dishevelled stabilization at the LRP/FZD scaffold and the significance of Dishevelled/Axin co‐polymerization are not the focus of this review, yet they are all requirements for sequestration of the β‐catenin destruction complex and activation of nuclear β‐catenin signalling.
Role of Dishevelled in intracellular signalosome assembly
The role of intracellular loops in FZD receptor function is understood largely through several studies of Dishevelled. Dishevelled consists of globular PSD‐95/Dlg1/ZO‐1 (PDZ), DEP and DIX domains separated by unstructured linkers, giving the impression of ‘balls on a string’. The PDZ domain anchors Dishevelled to the FZD receptor at two non‐contiguous motifs in the FZD C‐terminal tail, serving as a scaffold for recruiting Dishevelled to the FZD receptor (Zhang et al., 2009). The DEP domain binds the intracellular core of the FZD TMD upon receptor activation and undergoes a highly thermostable domain swap (formation of an intermolecular β‐sheet) to form dimers (Gammons et al., 2016a). It is possible that coordination of DEP domains at FZD dimers is a requirement for this domain‐swapping event (Gammons et al., 2016b). We also note that domain‐swapped DEP dimers formed tetrameric assemblies in two different crystal forms (Gammons et al., 2016a). While this tetrameric interaction is weak and there is no biochemical evidence to support this observation, it coincides with structural data for tetrameric assemblies of FZD4 receptor CRDs (Chang et al., 2015). Finally, the DIX domain seems independent of the FZD receptor and becomes capable of co‐polymerizing with Dishevelled or Axin DIX domains upon DEP domain swapping (Schwarz‐Romond et al., 2007; Fiedler et al., 2011). Ultimately, FZD receptor oligomerization will form an intracellular scaffold capable of stabilizing high local concentrations of Dishevelled and promoting Dishevelled/Axin co‐polymerization.
Emerging models and therapeutic opportunities
The recent discovery of Wnt‐dependent FZD oligomerization and the hypothesis of tunable LRP5/6 dimerization suggest a dynamic receptor signalosome which functions as a Dishevelled‐activating scaffold. While Dishevelled may be pre‐associated with FZDs at the membrane and FZDs and LRP5/6 may have already formed inactive complexes, the stimulus of Wnt binding the FZD CRD and inducing FZD oligomerization is what is required for Dishevelled DEP domain swapping and DIX domain co‐polymerization with Axin.
A better understanding of this signalosome will reveal many novel protein–protein interfaces for therapeutic inhibition. For instance, peptide inhibitors or antibodies blocking CRD–CRD interactions may be designed with specificity for subfamilies of FZD receptors. FZD CRD–TMD contacts may be required for propagating conformational changes intracellularly and permitting Dishevelled DEP domain swapping. Perhaps small molecule inhibitors or peptides will be discovered, which would disrupt these feedback mechanisms and prevent regulation of the TMD by the CRD in a manner similar to smoothened inhibition. Despite the current success in targeting Wnt‐FZD receptor interfaces at the CRD, perhaps non‐CRD Wnt‐binding sites exist, which would be less conserved and present an opportunity for more specific targeting of Wnt–FZD receptor pairs. Alhough little is known about FZD–LRP interactions, perhaps the disruption of these interactions would destabilize the membrane signalosome and prevent the effect of Wnt on driving a viable Dishevelled‐binding scaffold. Finally, manipulating the LRP5/6 β‐propeller domains in a manner that prevents the assembly of active dimers (by affecting flexibility of the hinge or restricting β‐propeller movement) is also a promising strategy, although the structural effect of agonists and several antagonists is not yet understood in the context of LRP5/6 β‐propeller flexibility.
Inhibiting Wnt signalling through FZD receptors has so far met with limited clinical success. The monoclonal antibody OMP18R5 (trade name vantictumab) targets FZD receptors 1/2/7 and 5/8, presumably interfering with Wnt ligand binding, but also perhaps preventing higher‐order CRD interactions (Gurney et al., 2012). Small peptide inhibitors represent a promising new approach for preventing FZD CRD oligomerization, as they can be diversely synthesized and screened efficiently. Ongoing discovery efforts are certain to yield promising results for therapies targeted against subfamilies of FZD receptors such as 5/8 and 1/2/7 which have been implicated in various cancers (Fernandez et al., 2014; Steinhart et al., 2017). These peptides may be developed against surfaces, which prevent Wnt binding, obstruct the CRD–CRD interactions that are thought to be required for the assembly of FZD receptor signalosomes or interfere with CRD‐TMD contacts and auto‐regulation.
The development of surrogates and agonists for the Wnt/β‐catenin pathway will also benefit greatly from the emerging model of the FZD receptor and LRP5/6 oligomeric scaffold. An effective Wnt surrogate must restrict the LRP5/6 β‐propeller domain flexibility to active states in which the transmembrane domains are in close proximity and must also mediate FZD receptor CRD dimerization and do so without tuning FZD/LRP orientations to a conformation unable to facilitate Dishevelled activation. Based on these observations, it is possible that fusions of norrin FZD‐binding peptides and DKK1 or sclerostin LRP5/6‐binding domains might mimic norrin or that bi‐specific LRP5/6 antibodies coupled with palmitoleoyl mimics may mimic classical Wnts.
In summary, the model of FZD/LRP oligomerization is intrinsically linked to Dishevelled/Axin signalosome assembly (Figure 5). When Wnts induce FZD receptor oligomerization and restrict LRP5/6 dimers to active conformations, an activation scaffold is created in which Dishevelled DEP domain swapping occurs and permits DIX‐dependent co‐polymerization with Axin. The role of FZD receptors in mediating these crucial steps in Wnt signalosome assembly is only beginning to be understood and promises to be a therapeutically attractive vantage point from which the Wnt pathway may be effectively and specifically targeted.
Figure 5.
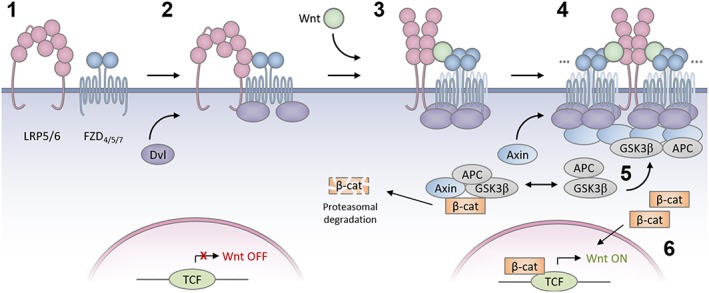
A hypothetical model of Wnt signalosome assembly in which (1) LRP5/6 and FZD receptors exist as inactive dimers; (2) LRP5/6, FZD receptor and Dishevelled form inactive complexes; (3) Wnt stabilizes re‐arrangement of LRP5/6 dimers to an active form and activates FZD receptor, permitting Dishevelled DEP domain dimerization. (4) Multiple Wnts may drive assembly of large receptor signalosomes. Dishevelled co‐polymerizes with Axin. (5) Large Dishevelled/Axin signalosomes sequester components of the β‐catenin destruction complex thus (6) permitting β‐catenin stabilization, nuclear translocation and activation of TCF family transcription factors and Wnt pathway target gene transcription.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Bart Williams and Gunnar Schulte for insightful discussions regarding this work, and we thank Michelle Martin for administrative support.
DeBruine, Z. J. , Xu, H. E. , and Melcher, K. (2017) Assembly and architecture of the Wnt/β‐catenin signalosome at the membrane. British Journal of Pharmacology, 174: 4564–4574. doi: 10.1111/bph.14048.
Contributor Information
Zachary J DeBruine, Email: zach.debruine@vai.org.
Karsten Melcher, Email: karsten.melcher@vai.org.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, Janda CY, Garcia KC (2012). Structural architecture and functional evolution of Wnts. Dev Cell 23: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M (2014). Signalosome assembly by domains undergoing dynamic head‐to‐tail polymerization. Trends Biochem Sci 39: 487–495. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M et al (2007). Wnt induces LRP6 signalosomes and promotes dishevelled‐dependent LRP6 phosphorylation. Science 316: 1619–1622. [DOI] [PubMed] [Google Scholar]
- Byrne EF, Sircar R, Miller PS, Hedger G, Luchetti G, Nachtergaele S et al (2016). Structural basis of Smoothened regulation by its extracellular domains. Nature 535: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carron C, Pascal A, Djiane A, Boucaut JC, Shi DL, Umbhauer M (2003). Frizzled receptor dimerization is sufficient to activate the Wnt/beta‐catenin pathway. J Cell Sci 116: 2541–2550. [DOI] [PubMed] [Google Scholar]
- Chang TH, Hsieh FL, Zebisch M, Harlos K, Elegheert J, Jones EY (2015). Structure and functional properties of Norrin mimic Wnt for signalling with Frizzled4, Lrp5/6, and proteoglycan. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Strapps W, Tomlinson A, Struhl G (2004). Evidence that the cysteine‐rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc Natl Acad Sci U S A 101: 15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yan H, Ren DN, Yin Y, Li Z, He Q et al (2014). LRP6 dimerization through its LDLR domain is required for robust canonical Wnt pathway activation. Cell Signal 26: 1068–1074. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L et al (2011). Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol 18: 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H (2004). Wnt signals across the plasma membrane to activate the beta‐catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115. [DOI] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ (2001). Insights into Wnt binding and signalling from the structures of two Frizzled cysteine‐rich domains. Nature 412: 86–90. [DOI] [PubMed] [Google Scholar]
- DeBruine ZJ, Ke J, Harikumar KG, Gu X, Borowsky P, Williams BO et al (2017). Wnt5a promotes Frizzled‐4 signalosome assembly by stabilizing cysteine‐rich domain dimerization. Genes Dev 31: 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis JP, Baljinnyam B, Stanger K, Sercan HO, Ji Y, Andres O et al (2015). Systematic mapping of WNT‐FZD protein interactions reveals functional selectivity by distinct WNT‐FZD pairs. J Biol Chem 290: 6789–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD et al (2010). Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand‐binding regions by LRP6 antibodies. Proc Natl Acad Sci U S A 107: 15473–15478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Gao N (2015). Keeping Wnt signalosome in check by vesicular traffic. J Cell Physiol 230: 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao S et al (2014). The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proc Natl Acad Sci U S A 111: 1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M, Mendoza‐Topaz C, Rutherford TJ, Mieszczanek J, Bienz M (2011). Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down‐regulating beta‐catenin. Proc Natl Acad Sci U S A 108: 1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammons MV, Renko M, Johnson CM, Rutherford TJ, Bienz M (2016a). Wnt signalosome assembly by DEP domain swapping of Dishevelled. Mol Cell 64: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammons MV, Rutherford TJ, Steinhart Z, Angers S, Bienz M (2016b). Essential role of the Dishevelled DEP domain in a Wnt‐dependent human‐cell‐based complementation assay. J Cell Sci 129: 3892–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Chin EN, Fakhraldeen SA, Berry SM, Beebe DJ, Alexander CM (2012). Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J Biol Chem 287: 16454–16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY et al (2010). Wnt isoform‐specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One 5: e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L et al (2012). Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A 109: 11717–11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Pinon DI, Miller LJ (2007). Transmembrane segment IV contributes a functionally important interface for oligomerization of the Class II G protein‐coupled secretin receptor. J Biol Chem 282: 30363–30372. [DOI] [PubMed] [Google Scholar]
- Janda CY, Dang LT, You C, Chang J, de Lau W, Zhong ZA et al (2017). Surrogate Wnt agonists that phenocopy canonical Wnt and beta‐catenin signalling. Nature 545: 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC (2012). Structural basis of Wnt recognition by Frizzled. Science 337: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykas A, Yang‐Snyder J, Heroux M, Shah KV, Bouvier M, Moon RT (2004). Mutant Frizzled 4 associated with vitreoretinopathy traps wild‐type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol 6: 52–58. [DOI] [PubMed] [Google Scholar]
- Ke J, Harikumar KG, Erice C, Chen C, Gu X, Wang L et al (2013). Structure and function of Norrin in assembly and activation of a Frizzled 4‐Lrp5/6 complex. Genes Dev 27: 2305–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MB, Zhang C, Shi J, Johnson V, Khandan L, McVey J et al (2017). TSPAN12 is a norrin co‐receptor that amplifies Frizzled4 ligand selectivity and signaling. Cell Rep 19: 2809–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Bafico A, Harris VK, Aaronson SA (2003). A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol 23: 5825–5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba K, Mihara E, Tamura‐Kawakami K, Miyazaki N, Maeda S, Hirai H et al (2017). Conformational freedom of the LRP6 ectodomain is regulated by N‐glycosylation and the binding of the Wnt antagonist Dkk1. Cell Rep 18: 32–40. [DOI] [PubMed] [Google Scholar]
- Moraga I, Wernig G, Wilmes S, Gryshkova V, Richter CP, Hong WJ et al (2015). Tuning cytokine receptor signaling by re‐orienting dimer geometry with surrogate ligands. Cell 160: 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile AH, Mukund S, Stanger K, Wang W, Hannoush RN (2017). Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc Natl Acad Sci U S A 114: 4147–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Wright SC, Rodriguez D, Matricon P, Lahav N, Vromen A et al (2017). Agonist‐induced dimer dissociation as a macromolecular step in G protein‐coupled receptor signaling. Nat Commun 8: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Nusse R (2005). The role of the cysteine‐rich domain of Frizzled in Wingless‐Armadillo signaling. EMBO J 24: 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP et al (2002). Mutant frizzled‐4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32: 326–330. [DOI] [PubMed] [Google Scholar]
- Schenkelaars Q, Fierro‐Constain L, Renard E, Hill AL, Borchiellini C (2015). Insights into Frizzled evolution and new perspectives. Evol Dev 17: 160–169. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V (2007). The Frizzled family of unconventional G‐protein‐coupled receptors. Trends Pharmacol Sci 28: 518–525. [DOI] [PubMed] [Google Scholar]
- Schwarz‐Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y et al (2007). The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol 14: 484–492. [DOI] [PubMed] [Google Scholar]
- Shen G, Ke J, Wang Z, Cheng Z, Gu X, Wei Y et al (2015). Structural basis of the Norrin‐Frizzled 4 interaction. Cell Res 25: 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z, Pavlovic Z, Chandrashekhar M, Hart T, Wang X, Zhang X et al (2017). Genome‐wide CRISPR screens reveal a Wnt‐FZD5 signaling circuit as a druggable vulnerability of RNF43‐mutant pancreatic tumors. Nat Med 23: 60–68. [DOI] [PubMed] [Google Scholar]
- Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA et al (2012). Wnt/beta‐catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci U S A 109: E812–E820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA et al (2004). Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet 74: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veverka V, Henry AJ, Slocombe PM, Ventom A, Mulloy B, Muskett FW et al (2009). Characterization of the structural features and interactions of sclerostin: molecular insight into a key regulator of Wnt‐mediated bone formation. J Biol Chem 284: 10890–10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W et al (2013). Structure of the human smoothened receptor bound to an antitumour agent. Nature 497: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J et al (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Oosterwegel M, Dooijes D, Clevers H (1991). Identification and cloning of TCF‐1, a T lymphocyte‐specific transcription factor containing a sequence‐specific HMG box. EMBO J 10: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D et al (2003). Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C‐terminal region of Frizzled. Mol Cell 12: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C et al (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled‐4, a high‐affinity ligand‐receptor pair. Cell 116: 883–895. [DOI] [PubMed] [Google Scholar]
- Yu H, Ye X, Guo N, Nathans J (2012). Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development 139: 4383–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao F, Wu Y, Yang J, Han GW, Zhao S et al (2017). Crystal structure of a multi‐domain human smoothened receptor in complex with a super stabilizing ligand. Nat Commun 8: 15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Appleton BA, Wiesmann C, Lau T, Costa M, Hannoush RN et al (2009). Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nat Chem Biol 5: 217–219. [DOI] [PubMed] [Google Scholar]


