Abstract
Trypanosoma cruzi induces serious cardiac alterations during the chronic infection. Intense inflammatory response observed from the beginning of infection, is critical for the control of parasite proliferation and evolution of Chagas disease. Peroxisome proliferator-activated receptors (PPAR)-α, are known to modulate inflammation.
In this study we investigated whether a PPAR-α agonist, Fenofibrate, improves cardiac function and inflammatory parameters in a murine model of T. cruzi infection. BALB/c mice were sequentially infected with two T. cruzi strains of different genetic background. Benznidazole, commonly used as trypanocidal drug, cleared parasites but did not preclude cardiac pathology, resembling what is found in human chronic chagasic cardiomyopathy. Fenofibrate treatment restored to normal values the ejection and shortening fractions, left ventricular end-diastolic, left ventricular end-systolic diameter, and isovolumic relaxation time. Moreover, it reduced cardiac inflammation and fibrosis, decreased the expression of pro-inflammatory (IL-6, TNF-α and NOS2) and heart remodeling mediators (MMP-9 and CTGF), and reduced serum creatine kinase activity. The fact that Fenofibrate partially inhibited NOS2 expression and NO release in the presence of a PPAR-α non-competitive inhibitor, suggested it also acted through PPAR-α-independent pathways. Since IκBα cytosolic degradation was inhibited by Fenofibrate, it can be concluded that the NFκB pathway has a role in its effects. Thus, we demonstrate that Fenofibrate acts through PPAR-α-dependent and -independent pathways.
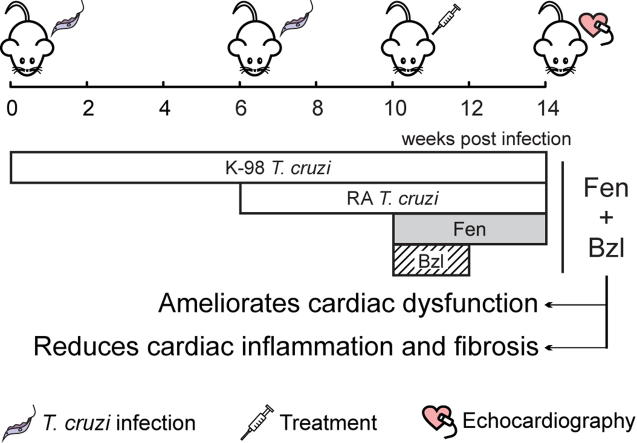
Our study shows that combined treatment with Fenofibrate plus Benznidazole is able both to reverse the cardiac dysfunction associated with the ongoing inflammatory response and fibrosis and to attain parasite clearance in an experimental model of Chagas disease.
Keywords: Trypanosoma cruzi, Heart dysfunction, PPAR-α, Fenofibrate treatment, Inflammatory mediators
Graphical abstract
Highlights
-
•
A murine model resembling some aspects of human chagasic cardiomyopathy was developed.
-
•
Fenofibrate is able to ameliorate cardiac dysfunction in mice infected with T. cruzi.
-
•
Cardiac lesions are reduced in T. cruzi infected mice treated with Fenofibrate.
-
•
Benznidazole reduces parasite load but does not preclude cardiac pathology.
1. Introduction
Almost 7 million people worldwide are estimated to be infected with Trypanosoma cruzi, the etiological agent of Chagas disease (WHO - World Health Organization, 2016). This disease is endemic throughout Central and South America, representing a major public health problem. The acute phase of infection is characterized by the presence of parasites in the host bloodstream and other tissues, promoting a severe inflammatory response (Teixeira et al., 2002). After the acute phase, generally asymptomatic, the infection evolves to a silent chronic phase. However, after a variable period of time (10–30 years after the onset of infection), 30–40% of the patients develop symptomatic cardiac alterations, including heart failure, arrhythmias, and thromboembolism, which cause major disabilities with high economic and social impact (Benziger et al., 2017). Diverse factors contribute to the development of chagasic dilated cardiomyopathy. Disruptions of the capillary network, due to the inflammatory infiltrate, induce focal myocytolysis, generating microvascular injury and myocardial remodeling. Substantial evidence has shown that the cardiac tissue, an important target of T. cruzi infection, induces the production of marked amounts of pro-inflammatory cytokines, chemokines and enzymes, including inducible nitric oxide synthase (NOS2) and metalloproteinases (MMPs), resulting in inflammation and cardiac remodeling due to the parasite infection (Penas et al., 2013). In response, fibroblasts proliferate and the interstitial collagen matrix is increased (Mitelman and Argentinian Society of Cardiology, 2011).
Benznidazole (N-benzyl-2-nitroimidazole acetamide, Bzl) is the trypanocidal drug of choice in the majority of the endemic countries, including Argentina, since has less severe side effects than Nifurtimox (Maguire, 2015). Although this drug is effective reducing the parasite load during the acute and the chronic phases of the disease, treatment during the latter does not correlate with a better outcome, since both Bzl-treated and placebo-treated patients have similar clinical progression in terms of development of chronic chagasic cardiomyopathy (Morillo et al., 2015). Besides, unwanted side effects are common at the currently used doses (Pérez-Molina et al., 2009, Viotti et al., 2009, Miller et al., 2015, Noguerado-Mellado et al., 2016). These side effects force about 10% of patients to abandon the treatment being the main disadvantage of its use.
Peroxisome proliferator-activated receptors (PPARs), members of the steroid hormone receptor superfamily, are ligand-dependent nuclear transcription factors. Fenofibrate (Fen), a PPAR-α ligand, is a third-generation fibric acid derivative currently used clinically as a hypolipidemic agent to lessen the risk of atherosclerosis (Ling et al., 2013).
Growing evidence has demonstrated the efficacy of PPAR agonists, including Fen, as regulators of inflammation and extracellular matrix remodeling of the heart (Lockyer et al., 2010). Fen can prevent myocardial inflammation and fibrosis in diabetic mice (Zhang et al., 2016). Moreover, Fen has been shown to exert cardioprotective effects against various cardiac disorders in in vivo and in vitro rat models (Zou et al., 2013, Cheng et al., 2016) and in patients (Yin et al., 2013).
We have previously reported that PPAR-γ ligands reduce the inflammatory reaction using in vivo and in vitro models of T. cruzi infection (Penas et al., 2013) and LPS-stimulated (Hovsepian et al., 2010) or T. cruzi-stimulated cardiomyocytes (Hovsepian et al., 2011). Other authors have shown that PPAR-α can inhibit the cardiovascular inflammatory response by reducing NF-kB activity, and through regulation of cytokine-receptor and growth-factor receptor signaling (Lockyer et al., 2010). However, the role of PPAR-α ligands in Chagas disease is currently unknown.
Based on the premise of the Drugs for Neglected Diseases Initiative (DNDi), to develop new therapeutical strategies for the treatment of neglected tropical diseases, relying on drugs already available in the pharmaceutical market, we sought to determine whether the administration of Fen, as an anti-inflammatory drug, improves the cardiac outcome in an experimental model of T. cruzi infection in the context of low-dose Bzl treatment.
Our results show, for the first time, that Fen treatment restores echocardiographic parameters to normal, and reduces tissue inflammation and fibrosis in a murine model of mixed T. cruzi infection, while a low dose of Bzl is capable to clear blood parasitaemia and heart parasite load.
2. Methods
2.1. Ethics statement
Mice used in this study were bred and maintained in the animal facility at the Instituto de Investigaciones en Microbiología y Parasitología Médica, Universidad de Buenos Aires – CONICET. All procedures carried out with mice were approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL, Facultad de Medicina de la Universidad de Buenos Aires, CD No 2271/2014) and are in accordance with guidelines of the Argentinean National Administration of Medicines, Food and Medical Technology (ANMAT), Argentinean National Service of Sanity and Agrifoods Quality (SENASA) and also based on the US NIH Guide for the Care and Use of Laboratory Animals.
2.2. Mice and infection
Eight-week-old BALB/c mice (7 per group) were infected by intraperitoneal route with 1x105 bloodstream trypomastigotes of the non-lethal K-98 clone of T. cruzi (TcI) for 6 weeks, followed by re-infection with 100 bloodstream trypomastigotes of the lethal RA (pantropic/reticulotropic) strain of T. cruzi (TcVI) for 4 weeks (Celentano and González Cappa, 1993, Zingales et al., 2009).
2.3. In vitro model: neonatal mouse primary cardiomyocytes culture and infection
One-to 3-day old neonatal outbred CF-1 strain mice were euthanized by decapitation after CO2 exposure, and cardiomyocytes were obtained as previously described (Hovsepian et al., 2013). Cardiomyocytes were cultured in FBS 10%-DMEM-M199 medium at 37 °C under 5% CO2 atmosphere, and infected at a 5:1 (parasite: cell) ratio in six well polystyrene plates. After 3 h, the infected cultures were washed five times with fresh 1% FBS-DMEM: M-199 medium to remove free parasites. Each experiment was carried out 3 times with 5 replicates per group.
2.4. Treatments
2.4.1. In vivo treatments
Mice were treated by oral gavage with Benznidazole (Abarax®, ELEA, Argentina. PubChem Compound Database CID = 31593, Bzl) and/or Fenofibrate (Daunlip®, Montpellier S.A, Argentina. PubChem Compound Database CID = 3339, Fen) suspended in corn oil. Fen dose optimization was carried out using 50, 100, 200 or 300 mg/kg/day for 30 consecutive days. The chosen doses were 25 mg/kg/day for Bzl (Cevey et al., 2016) for 15 consecutive days and 100 mg/kg/day for Fen treatment, for 30 consecutive days. Each experiment was carried out three times.
2.4.2. In vitro treatments
Fen was suspended in PBS. According to the experiment, cells were treated with 50, 100 or 150 μM Fen for 30 min before T. cruzi infection. In some experiments cells were pre-treated for 30 min with the non-competitive antagonist of PPAR-α (MK886, 10 μM) before the addition of Fen.
2.5. Parasitaemia and survival
Parasitaemia was evaluated by microhematocrit (Feilij et al., 1983) or the method of Pizzi modified by Brener (1962) every three to seven days. Survival was registered daily until the end of the experiment.
2.6. Doppler echocardiography
Transthoracic echocardiography was performed using an Acuson Sequoia C 512 ultrasound system with a 14-MHz linear transducer. Echocardiographic experiments were performed under light anesthesia (287.5 mg/kg of 2.5% filtered 2,2,2-Tribromoethanol; Sigma-Aldrich). The two-dimensional parasternal short-axis imaging plane was used to obtain M-mode tracings at the level of the papillary muscles. Left ventricular (LV) internal dimensions and LV wall thickness (LVWT) were determined at systole and diastole using leading-edge methods and guidelines of the American Society of Echocardiography (Sahn et al., 1978). End-diastolic measurements were taken at the maximal LV diastolic dimension, and end systole was defined as the time of the most anterior systolic excursion of the posterior wall. Measurements were taken from three consecutive beats for each mouse. Ejection fraction (EF) and shortening fraction (SF) were calculated and used as ejective indexes of systolic function. EF was estimated from LV dimensions by the cubed method as follows: EF (%) = [(LVEDD3 − LVESD3)/LVEDD3] × 100, where LVEDD is LV end-diastolic diameter and LVESD is LV end-systolic diameter. The isovolumic relaxation time (IVRT) was measured from the Doppler-echo study.
2.7. Histopathological studies
Mouse hearts were fixed in PBS-buffered 4% formaldehyde and included in paraffin. Six non-contiguous sections (5 μm) were stained with hematoxylin-eosin or picrosirius red. Images from thirty random microscopic fields (400x) were acquired using an Eclipse E600 microscope (Nikon Inc.) equipped with a Spot RT digital camera. Image analysis was performed using the Image J software (NIH, USA).
2.8. Creatine kinase (CK) activity
Serum CK activity was determined using photometric NADP reduction assay according to manufacturer's instructions (Wiener Lab, Rosario, Argentina). Absorbance was measured at 340 nm.
2.9. mRNA and gDNA purification
Total RNA was obtained from heart tissue homogenates using Quickzol reagent (Kalium), treated with DNAse (Life Technologies). Total RNA was reverse transcribed using Expand Reverse Transcriptase (Promega Corporation), according to manufacturer's instructions. gDNA was obtained from heart tissue using phenol-chloroform extraction (Laird et al., 1991).
2.10. Quantitative real-time polymerase chain reaction (qPCR) and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Both qPCR and RT-qPCR were performed using 5X HOT FIREPOL EVAGREEN qPCR (Solis BioDyne) in an Applied Biosystems 7500 sequence detector. mRNA expression was analysed by RT-qPCR. The parameters were: 52 °C for 2 min, 95 °C for 15 min, and 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 1min. Normalization was carried out using 18S rRNA. Parasite load was assessed by qPCR with the TCZ primers (Cummings and Tarleton, 2003, Duffy et al., 2009). These amplify a 146-bp sequence of the highly repetitive satellite genomic DNA. The sensitivity of the technique allows the detection of less than 1 parasite/ml, which is defined as parasite equivalents. Quantification was performed using the comparative Ct method (Schmittgen and Livak, 2008).
2.11. Primer sequences
TCZ:Fw 5′ TCCCTCTCATCAGTTCTATGGCCC 3′;
Rv 5′ CAGCAAGCATCTATGZCACTTAGACCCC.
18S:Fw 5′ AACACGGGAAACCTCACCC 3′;
Rv 5′ CCACCAACTAAGAACGGCCA 3′.
IL-6: Fw 5′ TGATGCACTTGCAGAAAACAA 3′;
Rv 5′ GGTCTTGGTCCTTAGCCACTC 3′.
TNF-α:Fw 5′ CGGGCAGGTCTACTTTGGAG 3′;
Rv 5′ ACCCTGAGCCATAATCCCCT 3′.
NOS2: Fw 5′ CACAGCAATATAGGCTCATCCA 3′;
Rv 5′GGATTTCAGCCTCATGGTAAAC 3′.
CTGF: Fw 5′ CCTAAAATCGCCAAGCCTGT 3′;
Rv 5′ CACCCCGCAGAACTTAGCC 3′.
PPAR-α: Fw 5′ GCTGGTGTACGACAAGTG3′;
Rv 5′ GTGTGACATCCCGACAGAC3′.
2.12. Cytokine ELISA
IL-6 and TNF-α serum concentrations were measured using ELISA kits according to the manufacturer's instructions (BD Biosciences OptEIA). The reaction was detected by peroxidase-conjugated Streptavidin, followed by incubation with hydrogen peroxide as a substrate and ABTS (Sigma Aldrich Co., St. Louis, USA) as a chromogen. Sample cytokine concentrations were interpolated from standard curves of recombinant IL-6 and TNF-α. Absorbance readings were made at 405 nm.
2.13. Protein extraction and Western blot analysis
Heart total and cytosolic protein extracts were prepared as previously described by our group (Cevey et al., 2016). Protein concentration was determined by the method of Bradford using a commercial protein assay (Bio-Rad, USA) and bovine serum albumin (BSA) (Sigma-Aldrich Co.) as a standard (Kruger, 1994).
Fifty μg of protein extracts separated by 8–12% SDS-PAGE gels were blotted onto a Hybond-P membrane (GE Health-care, Spain) to detect NOS2, MMP-9, PPAR-α and IkBα (Santa Cruz Biotechnology, USA), and α-actin (Sigma-Aldrich Co), using specific antibodies. Blots were revealed by enhanced chemoluminiscence in an Image Quant 300 cabinet (GE Healthcare Biosciences, USA). Band intensity was analysed using the Image J software.
2.14. NO measurement
To determine the amount of NO released into the culture medium, nitrate was reduced to nitrite and measured spectrophotometrically by the Griess reaction (Díaz-Guerra et al., 1996, Bryan and Grisham, 2007). The absorbance at 540 nm was compared with a standard curve of NaNO2.
2.15. Statistical analysis
Data are expressed as the mean of 3 independent experiments ± SEM for each treatment (Seven mice or five culture replicates/group). One-way ANOVA was used to analyse the statistical significance of the differences observed between the infected, treated or untreated groups. The Tukey post-hoc test was performed to compare every mean with every other mean. Differences were considered statistically significant when P < 0.05. All analyses were performed using the Prism 7.00 Software (GraphPad, USA).
3. Results
3.1. Fenofibrate reverses cardiac functional failure in mice infected with Trypanosoma cruzi
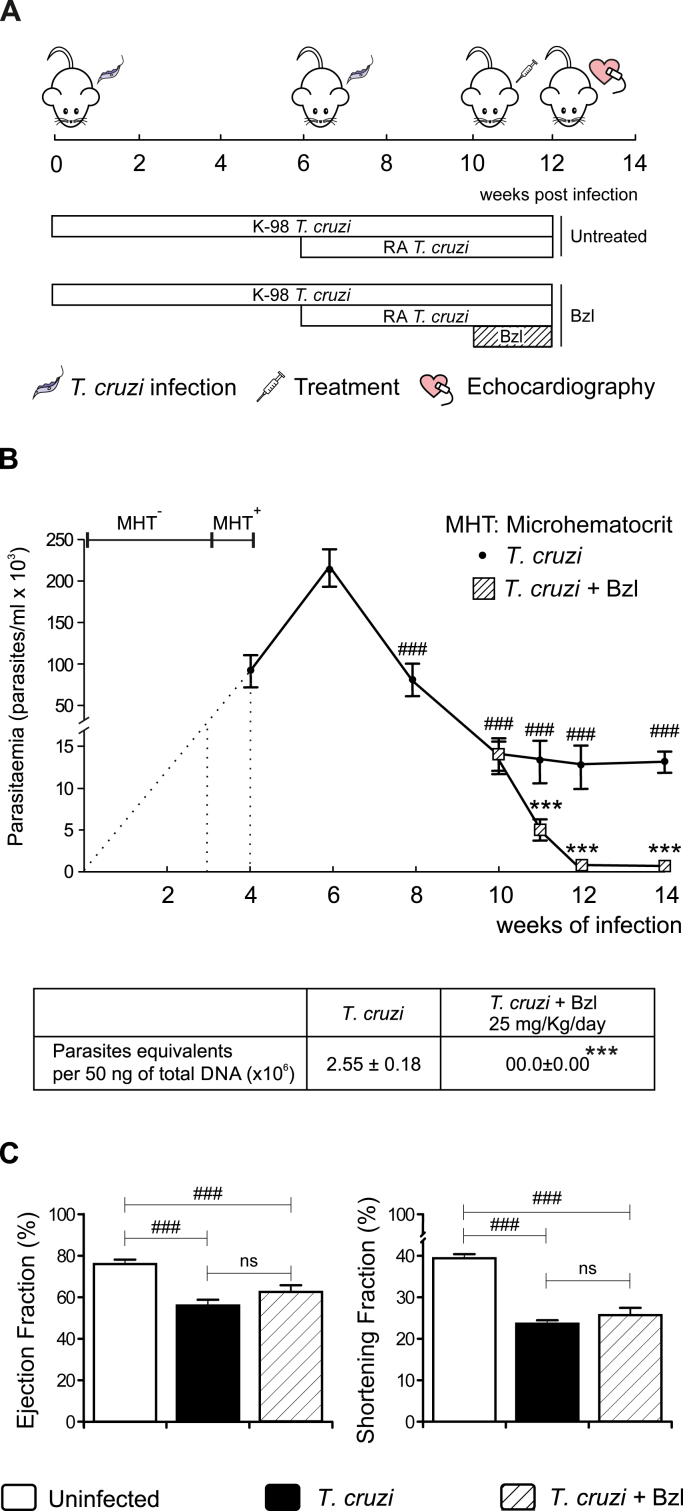
Human populations in endemic areas are exposed to repeated infections by T. cruzi. Since reinfection with strains differing in their genetic background may have a significant effect in the development of pathology, we designed an experimental model that took this fact into account. BALB/c mice were first infected with 1x105 bloodstream trypomastigotes of the K-98 clone (CA-I strain) for six weeks, and re-infected with 100 bloodstream trypomastigotes of the RA strain for four additional weeks (Fig. 1A). Parasitaemia was detectable by microhematocrit method from 3 weeks post-infection (w.p.i) and could be measured by the method of Pizzi since 4 w.p.i, increasing up to 6 w.p.i. Thereafter, parasitaemia decreased up to 10 w.p.i, remaining steady until the end of the experiment (14 w.p.i). Besides, heart parasitism was detected by qPCR at 14 w.p.i. (Fig. 1B).
Fig. 1.
Benznidazole reduces parasitaemia but does not ameliorate cardiac dysfunction in Trypanosoma cruzi-infected mice. BALB/c mice were infected by intraperitoneal route with 1 x 105 bloodstream trypomastigotes of the non-lethal K-98 clone of T. cruzi for 6 weeks, followed by intraperitoneal re-infection with 100 bloodstream trypomastigotes of the lethal RA strain of T. cruzi for 4 weeks. Infected mice were treated with 25 mg/kg/day Benznidazole (Bzl) for 15 consecutive days since 10 w.p.i. (A). Parasitaemia (parasites/mL x 103) and heart parasite load were analysed in infected or infected-treated mice (B). The ejection fraction and shortening fraction were evaluated by echocardiography (C). Results are expressed as the mean of three independent experiments (seven mice/group) ± SEM. White bar: Uninfected control mice. Black bar: T. cruzi-infected mice. Hatched white bar: T. cruzi and Bzl treated mice. ***P < 0.0001, T. cruzi-infected and Bzl treated mice vs T. cruzi-infected mice. ###P < 0.0001, T. cruzi-infected mice vs. uninfected mice.
We have previously shown that treatment with 25 mg/kg/day of Bzl clears parasitaemia and tissue parasitism in an acute model of Chagas disease (Cevey et al., 2016). To assess whether this treatment was effective in the mixed infection model, mice were treated with Bzl from week 10 p.i, as soon as the left ventricular systolic dysfunction (evaluated by echocardiography) was detected. Parasites were undetectable in mouse blood and heart after 2 weeks of treatment, time at which it was suspended (Fig. 1B).
Infection with T. cruzi induced a significant decrease in both indexes of systolic function, Ejection fraction (EF) and Shortening Fraction (SF), in comparison with uninfected mice. Interestingly, Bzl treatment did not modify EF and SF in comparison with infected untreated mice. In this regard, our model mimics what was found in the BENEFIT trial (Morillo et al., 2015) (Fig. 1C).
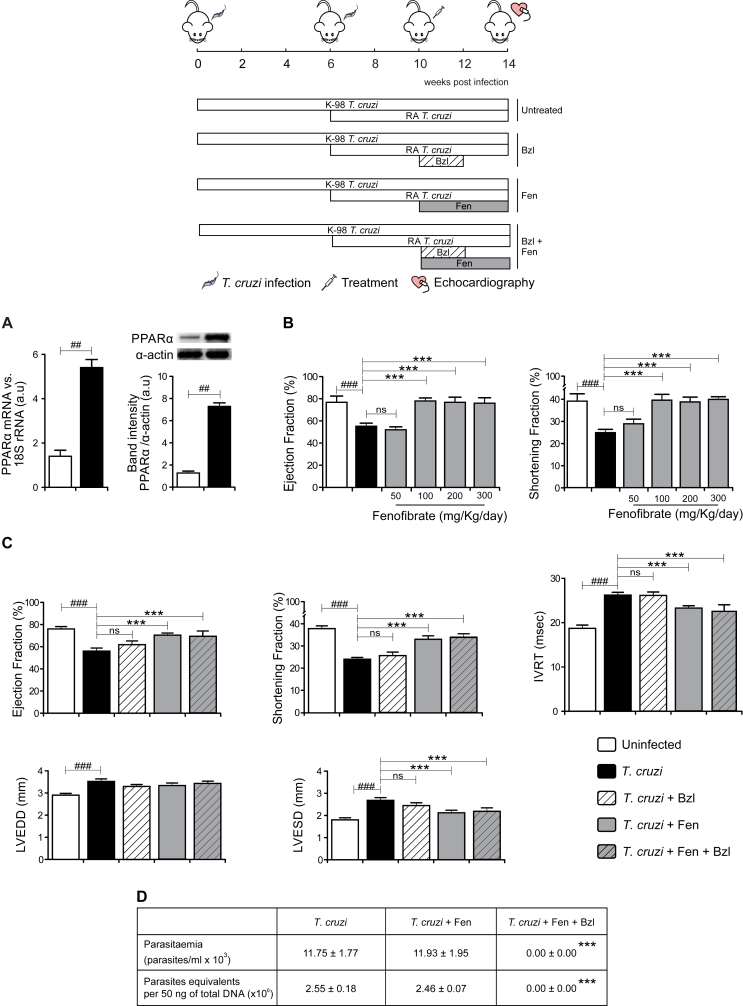
Since PPAR ligands have emerged as key regulators of inflammation and cardiac function we sought to determine whether Fen treatment might be a rational approach to ameliorate the cardiac alterations in our experimental model. First, we analysed the expression of the PPAR-α receptor in T. cruzi-infected mice. PPARα mRNA level was more than 5-fold higher in infected than in uninfected mice, as measured by RT-qPCR. Accordingly, WB showed increased PPAR-α protein expression in infected mice (Fig. 2A). Thus, PPARα seems a feasible target to improve cardiac function in T. cruzi-infected mice. To test the effectiveness of the treatment of infected mice with Fenofibrate (Fen), a synthetic PPAR-α agonist, we performed a dose-response study. We found that treatment with Fen 100 mg/kg/day for 30 days, was the lower dose capable to restore EF and SF (Fig. 2B) when treatment was initiated at the 10 weeks p.i., time at which the cardiac dysfunction was already observed. Thus, Fen improves the ventricular function in T. cruzi-infected mice. These changes occurred without significant differences in the heart rate (data not shown). The LVEDD and LVESD were significantly increased in T. cruzi-infected mice, evidencing LV dilatation. Interestingly, treatment with Fen attenuated LV dilatation and restored LVESD. Left ventricular diastolic function was evaluated through the IVRT. The IVRT was also prolonged in untreated T. cruzi-infected mice and Fen restored its behavior. Treatment with Bzl alone was unable to restore any of the cardiac parameters to normal values (Fig. 2C).
Fig. 2.
Fenofibrate reverses cardiac functional failure in mice infected with Trypanosoma cruzi. BALB/c mice were infected by intraperitoneal route with 1 x 105 bloodstream trypomastigotes of the non-lethal K-98 clone of T. cruzi for 6 weeks, followed by intraperitoneal re-infection with 100 bloodstream trypomastigotes of the lethal RA strain of T. cruzi for 4 weeks. PPAR-α mRNA levels and PPAR-α expression were determined by RT-qPCR and Western blot with specific primers and antibodies, respectively. The RT-qPCR results were normalized against 18S rRNA. Protein levels were normalized against α-actin (A). Infected mice were treated with different doses of Fenofibrate (Fen). Ejection fraction and Shortening fraction were evaluated by echocardiography (B). Infected mice were treated with 25 mg/kg/day Bzl for 15 consecutive days. At the same time treatment with 100 mg/kg/day Fen was initiated and continued for 30 consecutive days. The ejection fraction, shortening fraction, left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD) and isovolumic relaxation time (IVRT) were evaluated by echocardiography (C). Parasitaemia (parasites/mL x 103) and heart parasite load were analysed in infected or infected-treated mice (D). Results are expressed as the mean of three independent experiments (seven mice/group) ± SEM. White bar: Uninfected control mice. Black bar: T. cruzi-infected mice. Hatched white bar: T. cruzi and Bzl treated mice. Grey bar: T. cruzi and Fen treated mice. Hatched grey bar: T. cruzi and Fen-Bzl treated mice. **P < 0.001, ***P < 0.0001, T. cruzi-infected and treated mice (Fen or Fen plus Bzl) vs. T. cruzi-infected mice; ##P < 0.001, ###P < 0.0001, T. cruzi-infected mice vs. uninfected mice.
Noteworthy, combined Fen plus Bzl treatment was as effective as Fen alone to restore cardiac parameters. Besides, Fen neither modified the course of parasitaemia nor heart parasite load of T. cruzi-infected and Bzl-treated or Bzl-untreated mice (Fig. 2D).
3.2. Fenofibrate reduces the inflammatory response and cardiac injury
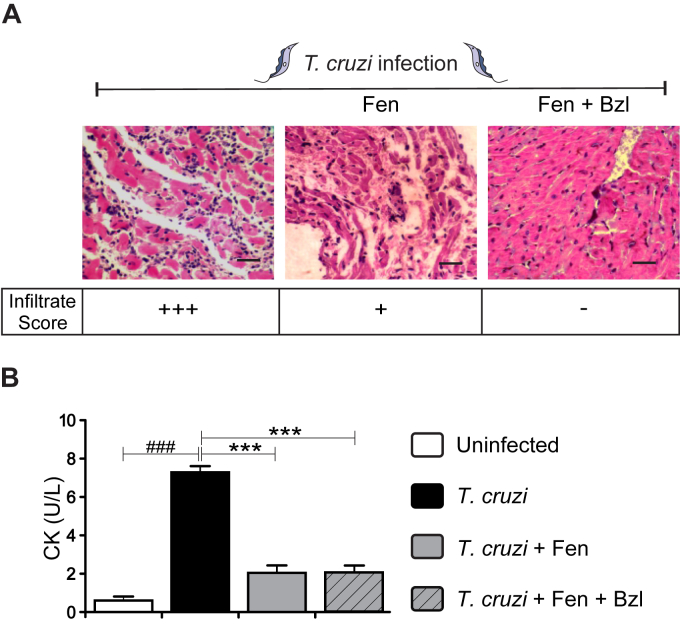
Mixed infection resulted in intense mononuclear cell infiltration in the heart at 14 w.p.i. Treatment with Fen significantly reduced the inflammatory infiltrates in comparison with infected untreated mice (Fig. 3A). Moreover, the combined treatment with Bzl did not modify this effect. Creatine kinase (CK) activity increased in the sera of infected mice, as expected. Interestingly, Fen reduced CK serum activity irrespectively of Bzl treatment. Therefore, it can be concluded that Fen is able to ameliorate cardiac damage associated with T. cruzi infection (Fig. 3B).
Fig. 3.
Fenofibrate reduces the inflammatory response and cardiac injury in mice infected with Trypanosoma cruzi. Histological sections of the hearts of T. cruzi-infected untreated mice (left panel), T. cruzi-infected and Fen-treated mice (center panel) and T. cruzi-infected and Fen plus Bzl-treated mice (right panel). The inflammatory reaction was evaluated by Hematoxylin-Eosin stain. Infiltrate score: (−) No infiltrates, (+) Scarce focal infiltrates compromising less than 10% ventricle area, (++) Focal or scattered infiltrates compromising 10–25% ventricle area, (+++) Diffuse myocardial inflammation including coalescent infiltrates. Magnification: 400×. Bar: 100 μm. (A). Creatine kinase (CK) activity in sera was determined as a marker of heart injury (B). Results are expressed as the mean of three independent experiments (seven mice/group) ± SEM. White bar: Uninfected control mice. Black bar: T. cruzi-infected mice. Grey bar: T. cruzi-infected and Fen treated mice. Hatched grey bar: T. cruzi-infected and Fen plus Bzl treated mice. ***P < 0.0001 T. cruzi-infected and treated mice (Fen or Fen plus Bzl) vs. T. cruzi-infected mice; ###P < 0.0001, T. cruzi-infected mice vs. uninfected mice.
3.3. Fenofibrate reduces pro-inflammatory mediators in Trypanosoma cruzi-infected mice
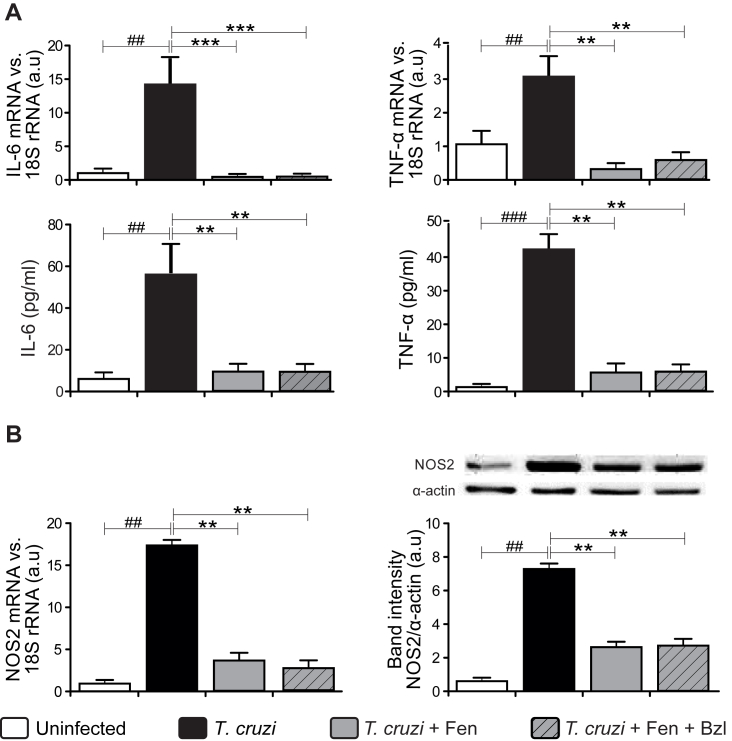
The expression of TNF-α and IL-6 increased significantly in the heart and sera of T. cruzi-infected mice. Interestingly, Fen inhibited the expression of pro-inflammatory cytokines in T. cruzi-infected mice (Fig. 4A). Also, increased NOS2 mRNA and protein levels were observed in the mixed infection model, while Fen treatment reduced their expression (Fig. 4B). Noteworthy, these effects were unchanged when Fen was combined with Bzl.
Fig. 4.
Fenofibrate reduces pro-inflammatory mediators in Trypanosoma cruzi-infected mice. IL-6 and TNF-α mRNA levels were analysed by RT-qPCR in total RNA heart extracts of T. cruzi-infected mice or T. cruzi-infected and treated mice (upper panels). The concentration of these cytokines in sera was measured by ELISA (lower panels) (A). NOS2 mRNA and protein levels were determined by RT-qPCR and Western blot with specific primers and antibodies, respectively (B). The RT-qPCR results were normalized against 18S rRNA. Protein levels were normalized against α-actin. Results are expressed as the mean of three independent experiments (seven mice/group) ± SEM. White bar: Uninfected control mice. Black bar: T. cruzi-infected mice. Grey bar: T. cruzi-infected and Fen treated mice. Hatched grey bar: T. cruzi-infected and Fen plus Bzl treated mice **P < 0.001, T. cruzi-infected and treated mice (Fen or Fen plus Bzl) vs. T. cruzi-infected mice; ##P < 0.001, T. cruzi-infected mice vs. uninfected mice.
3.4. Fenofibrate attenuates heart fibrosis and remodeling in Trypanosoma cruzi-infected mice
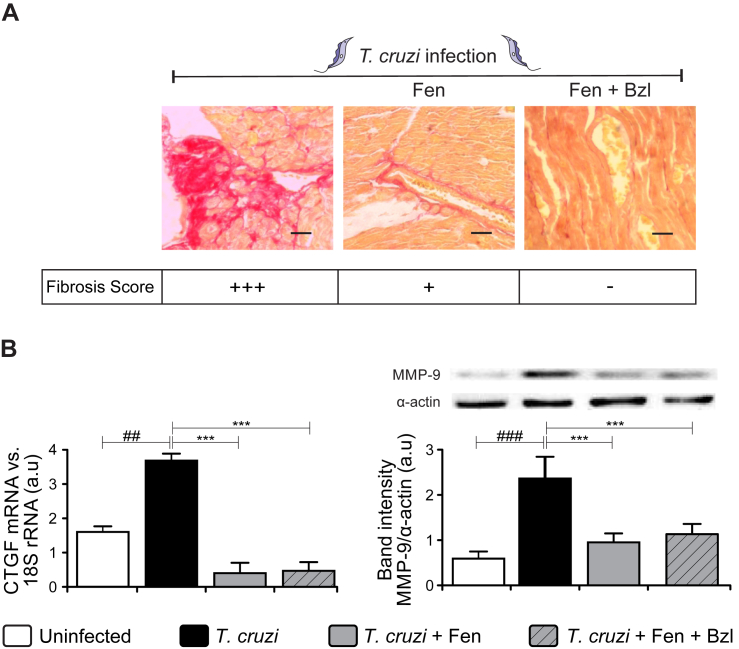
Fibrosis was observed in heart sections of T. cruzi-infected mice, as shown by picrosirius red staining. Increased interstitial and perivascular collagen deposits were detected in untreated animals (Fig. 5A). In agreement with this, the expression of CTGF and MMP-9 were higher in the heart of infected mice (Fig. 5B). Also, Fen treatment significantly reduced fibrosis (Fig. 5A) and mediators of connective tissue remodeling in infected mice (Fig. 5B).
Fig. 5.
Fenofibrate attenuates heart fibrosis and remodeling in Trypanosoma cruzi-infected mice.
Fibrosis was analysed in histological sections of the hearts of T. cruzi-infected untreated mice (left panel), T. cruzi-infected Fen-treated mice (center panel) and T. cruzi-infected Fen plus Bzl-treated mice (right panel), at the end of treatment. Collagen deposits were evaluated by Picrosirius red stain. Fibrosis score (% fibrosis area): (−) <1%, (+) 1–5%, (++) 5–10%, (+++) >10%. Magnification: 400×. Bar: 100 μm (A). Connective Tissue Growth Factor (CTGF) mRNA levels were analysed by RT-qPCR in total RNA heart extracts of T. cruzi-infected mice or T. cruzi-infected and treated mice. MMP-9 expression was determined by Western blot with specific antibodies (B). The RT-qPCR results were normalized against 18S rRNA. Protein levels were normalized against α-actin. Results are expressed as the mean of three independent experiments (seven mice/group) ± SEM. White bar: Uninfected control mice. Black bar: T. cruzi-infected mice. Grey bar: T. cruzi-infected and Fen treated mice. Hatched grey bar: T. cruzi-infected and Fen plus Bzl treated mice. ***P < 0.0001 T. cruzi-infected and treated mice (Fen or Fen plus Bzl) vs. T. cruzi-infected mice; ##P < 0.01, ###P < 0.0001 T. cruzi-infected mice vs. uninfected mice.
3.5. Participation of PPARα-independent pathways in the effects of Fenofibrate on the cardiomyocyte inflammatory response
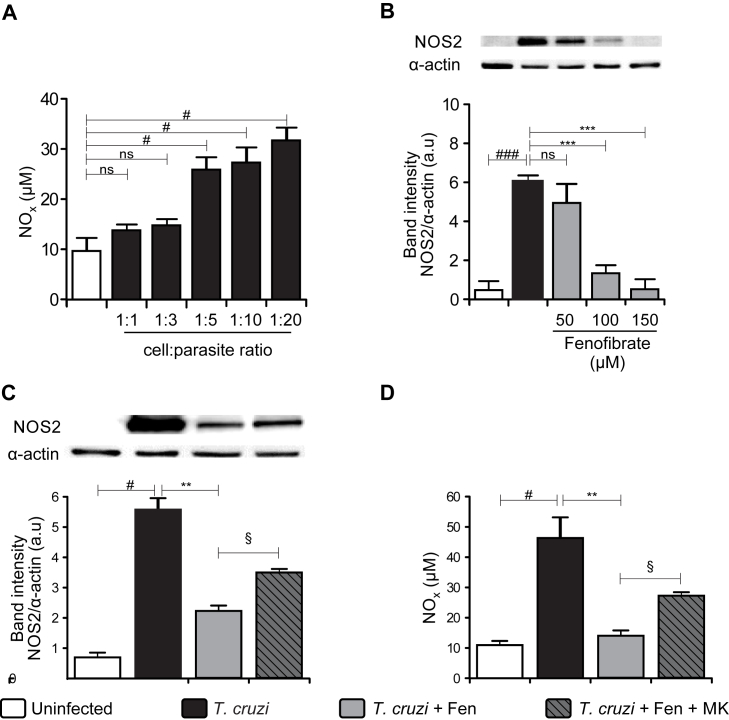
We have previously reported that PPAR-γ agonists potently inhibit MAPK and NF-κB pathways by additional PPAR-γ-independent mechanisms in isolated cardiomyocytes (Hovsepian et al., 2010, Hovsepian et al., 2011). To determine whether Fen acts through PPARα-independent mechanisms, primary culture cardiomyocytes were infected with the RA strain of T. cruzi. Then, activation of signaling pathways such as NF-κB were analysed. To test T. cruzi ability to induce a typical inflammatory response, NOS2 expression was evaluated by WB. To this end, first we analysed the optimal cardiomyocyte:parasite ratio to induce nitric oxide (NO) production. We found that 1:5 was the minimum ratio necessary to induce a significant NO level, which could be detected in supernatants of infected cardiac cells (Fig. 6A). Also, we determined that 100 μM Fen was the optimal concentration in vitro able to inhibit NOS2 expression after infection (Fig. 6B). A significant increase in NOS2 expression was observed in infected cardiomyocytes in comparison with uninfected cells (Fig. 6C). In agreement with this, high levels of NO were detected in supernatants of infected cells (Fig. 6D). The pre-treatment with Fen inhibited NOS2 expression and NO release (Fig. 6C and D). As it is known, Fen is a high-affinity PPAR-α ligand. To evaluate PPAR-α participation in the effects of Fen, infected cells were pre-treated with MK886, a potent non-competitive PPAR-α inhibitor. In the presence of MK886, Fen partially inhibited both NOS2 expression and NO release (Fig. 6C and D), suggesting the possible participation of PPAR-α-independent pathways of Fen.
Fig. 6.
Fenofibrate inhibits NOS2 expression and NO release in primary cultures of cardiomyocytes infected with Trypanosoma cruzi. Cardiomyocytes were obtained and cultured at 37 °C under a 5% CO2 atmosphere. Cardiomyocytes were infected at different parasite: cell ratios in six well polystyrene plates for 48 h. NO release was quantified by the Griess reaction in culture supernatants (A). T. cruzi-infected cardiomyocytes were treated with different doses of Fenofibrate. NOS2 expression was determined by Western blot with a specific antibody and normalized against α-actin (B). Cardiomyocytes were treated with 10 μM MK886, an antagonist of PPAR-α, 30 min before the addition of 100 μM Fen. 30 min after Fen treatment cells were infected with T. cruzi. After 48 h, NO levels were quantified by the Griess reaction in culture supernatants (C) and NOS2 expression was determined by Western blot with specific antibodies (D). Cardiomyocytes were treated with 100 μM Fen 30 min before infection with T. cruzi. After 15 or 30 min, IκBα cytosolic expression was determined by Western blot with specific antibodies (E). Protein levels were normalized against α-actin. Results are expressed as the mean of three independent experiments (three replicates/treatment) ± SEM. White bar: Uninfected control cells. Black bar: T. cruzi-infected cells. Grey bar: T. cruzi and Fen treated cells. Hatched dark-grey bar: MK pretreated, T. cruzi infected and Fen treated cells. **P < 0.001, ***P < 0.0001, T. cruzi-infected and Fen treated cells vs. infected cells; #P < 0.05, T. cruzi-infected vs. uninfected cells; §P < 0.05, T. cruzi-infected MK pre-treated and Fen treated cells vs. T. cruzi infected-Fen treated cells.
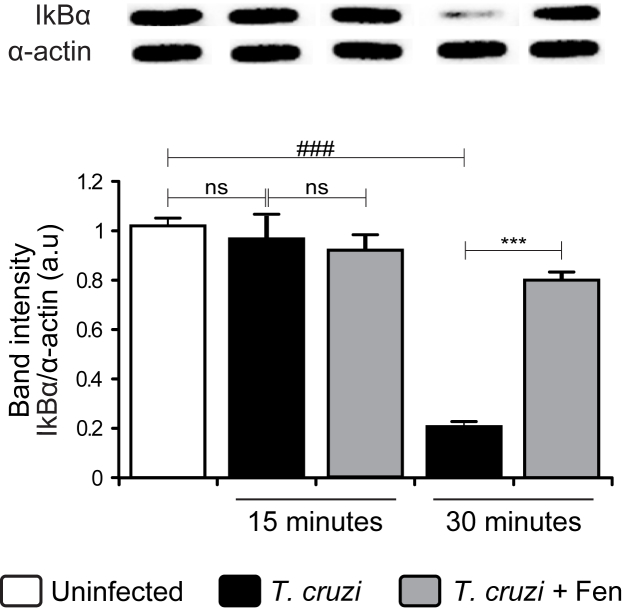
It is widely reported that Fen inhibits NF-κB signaling in different experimental models (Shen et al., 2014, Zuo et al., 2015, Garcia-Ramírez et al., 2016). In light of our results showing the ability of Fen to inhibit inflammatory parameters, we sought to determine whether Fen effects were also exerted through the NF-κB pathway. Results shown in Fig. 7 confirm NF-κB activation, since IκBα inhibitor was reduced after 30 min of infection. Furthermore, Fen treatment inhibited IκBα degradation, confirming its role in the regulation of inflammation not only through PPAR-α but also through the NF-κB pathway.
Fig. 7.
Fenofibrate impairs NF-κB activation in primary cultures of Trypanosoma cruzi-infected cardiomyocytes. Cardiomyocytes were treated with 100 μM Fen 30 min before infection with T. cruzi. After 15 or 30 min post-infection, IκBα cytosolic expression was determined by Western blot with specific antibodies. Results are expressed as the mean of three independent experiments (three replicates/treatment) ± SEM. White bar: Uninfected control cells. Black bar: T. cruzi-infected cells. Grey bar: T. cruzi-infected and Fen treated cells. ***P < 0.0001, T. cruzi-infected and Fen treated cells vs. T. cruzi-infected cells; ###P < 0.0001, T. cruzi-infected cells vs. uninfected cells.
4. Discussion
The use of a mouse model involving mixed T. cruzi strain infection, that reveals systolic and diastolic dysfunction resembling human chagasic chronic cardiomyopathy, allowed us to test whether Fen is able to ameliorate the cardiac dysfunction. Most experimental murine models of infection with T. cruzi that study the immunological and/or pathophysiological consequences of chronic Chagas disease, address these issues using single parasite strain infection. While this fact may be of benefit in terms of model simplicity, this does not represent what usually happens in nature. Furthermore, we couldn't reproduce the cardiac dysfunction characteristic of human Chagas disease, using single population infections. Besides, in the context of active vector-driven transmission in rural and some suburban areas, an individual may be exposed to reinfection by several T. cruzi populations (Tomasini et al., 2017), some of which may differ in their virulence, tropism, immunogenicity and genetics. This, of course, may be conditioned by the diversity of genotypes present in a specific geographic area. Thus, the model described herein, includes this variable, representing a situation that is closer to what happens in the actual world. The biological (tissue tropism, lethality, etc) (González Cappa et al., 1980, González Cappa et al., 1981a, González Cappa et al., 1981b, Mirkin et al., 1994, Mirkin et al., 1997), immunological (development of humoral and cellular response) (Muller et al., 1986, Vogt et al., 2008) and genetic (DTU) (Zingales et al., 2009) characteristics of the parasite populations used in this study have already been characterized. This allowed us to design a mixed infection model that, both in terms of cardiac dysfunction as well as in its pathophysiological consequences, resembles what happens in chronic Chagas disease patients. In support of this, previous studies have shown that multiple infections lead to worsening of the inflammatory reaction (Bustamante et al., 2002, Bustamante et al., 2003, Bustamante et al., 2004, Andrade et al., 2006) and immunopathological (Guerreiro et al., 2015) consequences.
The main finding of our study is the ability of Fen to reverse left ventricular dysfunction. It is known that Bzl is highly effective in the acute stage of the disease, especially in childhood. In contrast, data on the effectiveness of treatment of chronic disease have been controversial (Garcia et al., 2005, Viotti et al., 2006). It has been demonstrated that, although Bzl is effective in parasite clearance in the chronic phase it is unable to ameliorate or preclude the progression of cardiac pathology (Morillo et al., 2015). Additionally, treatment with Bzl has serious limitations, such as the side effects exhibited by a significant percentage of patients (Pérez-Molina et al., 2009, Viotti et al., 2009, Miller et al., 2015, Noguerado-Mellado et al., 2016). Therefore, it is very desirable to find antiparasitic doses lower than those currently used, since its known that the adverse effects are dose-dependent. In a previous work we showed that optimal effects of Bzl, in terms of parasite clearance from blood and heart tissue, can be achieved at a dose significantly lower than those usually used for the treatment in an experimental murine model, using a highly virulent Bzl-susceptible T. cruzi strain (Cevey et al., 2016). In the present study, we show that such low dose of Bzl completely eliminates blood parasites, but is unable to restore the EF and SF in infected mice. These results are in agreement with those of Zaidenberg et al., who showed that high doses of Bzl reduce the PR interval prolongation but failed to sustain an improvement of cardiac function at the end of the study (Zaidenberg et al., 2006). Moreover, a recent study carried out in a mouse model of experimental Chagas heart disease with a Colombian strain showed that Bzl does not restore to normal the heart rate-corrected QT (QTc) intervals (Vilar-Pereira et al., 2016b).
Our work shows, for the first time, that treatment with Fen, a potent hypolipidemic drug with additional anti-inflammatory properties, improves heart dysfunction in experimental Chagas disease. We demonstrate that Fen improves LV function, decreases the IVRT and attenuates ventricular remodeling. From our point of view, these effects are closely linked to the anti-inflammatory effects of Fen. These anti-inflammatory and protective effects have been previously evaluated in models of autoimmune myocarditis (Cheng et al., 2016), skeletal muscle inflammation (Dai et al., 2016), and cardiac ischemia/reperfusion (Sugga et al., 2012). Moreover, we have previously demonstrated that a PPAR-γ agonist modulates the exacerbated heart inflammatory response using in vivo models of early lethal and non-lethal T. cruzi infection, as well as using in vitro models with a highly virulent T. cruzi strain (Hovsepian et al., 2011, Penas et al., 2013).
Here we show that mixed infection with T. cruzi for 14 weeks resulted in intense inflammatory reaction with high TNF-α and IL-6 heart expression, consistent with increased serum levels of these pro-inflammatory cytokines. Noteworthy, we demonstrate that after 4 weeks of treatment with Fen, the inflammatory infiltrates were significantly reduced and that pro-inflammatory markers like NOS2, TNF-α and IL-6 were inhibited and restored to control levels. A similar response was observed in terms of the effects of Fen on the reduction of cardiac fibrosis and tissue-remodeling mediators such as MMP-9 and CTGF. As a whole, these results reveal that Fen treatment prevents myocardial damage. In this context, fibrates have been regarded as potent therapeutic agents. Clofibrate and Fen exert cardioprotection in I/R-induced myocardial injury in rats, as assessed in terms of reduction in myocardial infarct size, LDH and CK activity in coronary effluent, along with reduction in I/R-induced oxidative stress (Sugga et al., 2012). In agreement with these results, we observed a significant reduction in serum CK activity following the Fen treatment, further suggesting reduction of cardiac injury.
In this study, we also analysed the ability of in vitro treatment with Fen to down-regulate inflammatory mechanisms of cardiomyocytes in response to T. cruzi infection. We determined that treatment with Fen exerted an anti-inflammatory effect, reducing NOS2 protein expression and NO levels. Previously, our group demonstrated that PPAR-γ agonists inhibit the induction of inflammatory genes in both lipopolysaccharide-stimulated and in in vitro T. cruzi-infected cardiomyocytes through PPAR-γ-dependent or -independent pathways, like NF-κB (Hovsepian et al., 2010, Hovsepian et al., 2011). Moreover, other authors have demonstrated that the natural PPAR-γ ligand 15-deoxy-Δ12,14 prostaglandin J2 exerts a strong anti-inflammatory effect by attenuating the expression of pro-inflammatory mediators in activated monocytes/macrophages, an effect mainly exerted through the inhibition of NF-κB-dependent transcription of inflammatory genes (Straus et al., 2000). In this sense, the inhibition of the NF-κB signaling pathway by Fen has been reported in different experimental models (Shen et al., 2014). Jen et al. suggested that activated PPAR-α can decrease the activation of adiponectin and NF-κB and inhibit Endothelin-1-induced cardiomyocyte hypertrophy in human cardiomyocyte cultures (Jen et al., 2016). Other findings suggest that the anti-inflammatory effects of Fen through inhibition of NF-κB activity are beneficial for treating diabetic macular edema (Garcia-Ramírez et al., 2016). Because NF-κB has a pivotal role in the regulation of inflammation and cell survival (Papa et al., 2009), a fine tuned targeting to this factor may be needed to properly treat diseases in which its dysregulation is involved. It must be noted, however, that the treatment with Fen precludes its activation, resulting in a beneficial outcome in terms of tissue inflammation and functional restoration.
Finally, this work shows that Fen reverses relevant aspects of Chagas cardiac dysfunction like EF, SF, LVESD and IVRT. It is known that the alteration of these parameters are involved in the high morbidity of Chagas disease patients (Ribeiro et al., 2012, Vilar-Pereira et al., 2016a). Fen effects add up to the complete reduction of the parasite burden by Bzl in low doses, reducing the inflammatory response and attenuating heart fibrosis and pro-fibrotic cytokines in T. cruzi-infected mice. Lastly, this study is in line with the WHO/DNDi proposal that promotes new therapeutic approaches for neglected tropical diseases, using drugs already available for the treatment of other diseases. Taking into account all the above, we believe that the combined therapy of Fen plus Bzl has potential for clinical trials in patients with chronic Chagas disease.
Conflict of interest
There are none.
Funding source
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas [Grant number PIP 0672]; Universidad de Buenos Aires [Grant number 20020130100774BA] and Agencia Nacional de Promoción Científica y Tecnológica [Grant number 2014-1049].
Acknowledgments
We are grateful to Mr. Eduardo Alejandro Giménez and Mr. Ricardo Chung for their excellent technical assistance. We would also like to thank Ms. Victoria González Eusevi for her assistance in English grammar and spelling corrections.
References
- Andrade S.G., Campos R.F., Castro Sobral K.S., Magalhães J.B., Pereira Guedes R.S., Guerreiro M.L. Reinfections with strains of Trypanosoma cruzi, of different biodemes as a factor of aggravation of myocarditis and myositis in mice. Rev. Soc. Bras. Med. Trop. 2006;39:1–8. doi: 10.1590/s0037-86822006000100001. [DOI] [PubMed] [Google Scholar]
- Benziger C.P., do Carmo G.A.L., Ribeiro A.L.P. Chagas cardiomyopathy. Cardiol. Clin. 2017;35:31–47. doi: 10.1016/j.ccl.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Brener Z. Observations on immunity to superinfections in mice experimentally inoculated with Trypanosoma cruzi and subjected to treatment. Rev. Inst. Med. Trop. Sao Paulo. 1962;4:119–123. [PubMed] [Google Scholar]
- Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J.M., Rivarola H.W., Fernández A.R., Enders J.E., Fretes R., Palma J.A., Paglini-Oliva P.A. Indeterminate Chagas' disease: Trypanosoma cruzi strain and re-infection are factors involved in the progression of cardiopathy. Clin. Sci. (Lond) 2003;104:415–420. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Bustamante J.M., Rivarola H.W., Fernández A.R., Enders J.E., Fretes R., Palma J.A., Paglini-Oliva P.A. Trypanosoma cruzi reinfections in mice determine the severity of cardiac damage. Int. J. Parasitol. 2002;32:889–896. doi: 10.1016/s0020-7519(02)00023-1. [DOI] [PubMed] [Google Scholar]
- Bustamante J.M., Rivarola H.W., Palma J.A., Paglini-Oliva P.A. Electrocardiographic characterization in Trypanosoma cruzi reinfected mice. Parasitology. 2004;128:415–419. doi: 10.1017/s0031182003004591. [DOI] [PubMed] [Google Scholar]
- Celentano A.M., González Cappa S.M. In vivo macrophage function in experimental infection with Trypanosoma cruzi subpopulations. Acta Trop. 1993;55:171–180. doi: 10.1016/0001-706x(93)90075-m. [DOI] [PubMed] [Google Scholar]
- Cevey Á.C., Mirkin G.A., Penas F.N., Goren N.B. Low-dose benznidazole treatment results in parasite clearance and attenuates heart inflammatory reaction in an experimental model of infection with a highly virulent Trypanosoma cruzi strain. Int. J. Parasitol. Drugs Drug Resist. 2016;6:12–22. doi: 10.1016/j.ijpddr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Xi Y., Chi X., Wu Y., Liu G. Fenofibrate treatment of rats with experimental autoimmune myocarditis by alleviating Treg/Th17 disorder. Cent. Eur. J. Immunol. 2016;1:64–70. doi: 10.5114/ceji.2016.58817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings K.L., Tarleton R.L. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 2003;129:53–59. doi: 10.1016/s0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- Dai F., Jiang T., Bao Y., Chen G., Chen L., Zhang Q., Lu Y. Fenofibrate improves high-fat diet-induced and palmitate-induced endoplasmic reticulum stress and inflammation in skeletal muscle. Life Sci. 2016;157:158–167. doi: 10.1016/j.lfs.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Díaz-Guerra M.J., Velasco M., Martín-Sanz P., Boscá L. Evidence for common mechanisms in the transcriptional control of type II nitric oxide synthase in isolated hepatocytes. Requirement of NF-kappaB activation after stimulation with bacterial cell wall products and phorbol esters. J. Biol. Chem. 1996;271:30114–30120. doi: 10.1074/jbc.271.47.30114. [DOI] [PubMed] [Google Scholar]
- Duffy T., Bisio M., Altcheh J., Burgos J.M., Diez M., Levin M.J., Favaloro R.R., Freilij H., Schijman A.G. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in chagas disease patients. PLoS Negl. Trop. Dis. 2009;3:e419. doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilij H., Muller L., Gonzalez Cappa S.M. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J. Clin. Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramírez M., Hernández C., Palomer X., Vázquez-Carrera M., Simó R. Fenofibrate prevents the disruption of the outer blood retinal barrier through downregulation of NF-κB activity. Acta Diabetol. 2016;53:109–118. doi: 10.1007/s00592-015-0759-3. [DOI] [PubMed] [Google Scholar]
- Garcia S., Ramos C.O., Senra J.F.V., Vilas-Boas F., Rodrigues M.M., Campos-de-Carvalho A.C., Ribeiro-Dos-Santos R., Soares M.B.P. Treatment with benznidazole during the chronic phase of experimental Chagas' disease decreases cardiac alterations. Antimicrob. Agents Chemother. 2005;49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Cappa S.M., Bijovsky A.T., Freilij H., Muller L., Katzin A.M. Isolation of a Trypanosoma cruzi strain of predominantly slender form in Argentina. Med. (B. Aires) 1981;41:119–120. [PubMed] [Google Scholar]
- González Cappa S.M., Chiale P., del Prado G.E., Katzin A.M., de Martini G.W., de Isola E.D., Abramo Orrego L., Segura E.L. Isolation of a strain of Trypanosoma cruzi from a patient with chronic Chagas cardiomyopathy and its biological characterization. Med. (B. Aires) 1980;40(Suppl. 1):63–68. [PubMed] [Google Scholar]
- González Cappa S.M., Katzin A.M., Añasco N., Lajmanovich S. Comparative studies on infectivity and surface carbohydrates of several strains of Trypanosoma cruzi. Med. (B. Aires) 1981;41:549–555. [PubMed] [Google Scholar]
- Guerreiro M.L. da S., Morais I.R.B., Andrade S.G. Immunological response to re-infections with clones of the Colombian strain of Trypanosoma cruzi with different degrees of virulence: influence on pathological features during chronic infection in mice. Mem. Inst. Oswaldo Cruz. 2015;110:500–506. doi: 10.1590/0074-02760140286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovsepian E., Mirkin G.A., Penas F., Manzano A., Bartrons R., Goren N.B. Modulation of inflammatory response and parasitism by 15-Deoxy-Δ(12,14) prostaglandin J(2) in Trypanosoma cruzi-infected cardiomyocytes. Int. J. Parasitol. 2011;41:553–562. doi: 10.1016/j.ijpara.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hovsepian E., Penas F., Goren N.B. 15-deoxy-Delta12,14 prostaglandin GJ2 but not rosiglitazone regulates metalloproteinase 9, NOS-2, and cyclooxygenase 2 expression and functions by peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms in cardiac cells. Shock. 2010;34:60–67. doi: 10.1097/SHK.0b013e3181cdc398. [DOI] [PubMed] [Google Scholar]
- Hovsepian E., Penas F., Siffo S., Mirkin G.A., Goren N.B. IL-10 inhibits the NF-κB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes. PLoS One. 2013;8:e79445. doi: 10.1371/journal.pone.0079445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen H.-L., Liu P.-L., Chen Y.-H., Yin W.-H., Chen J.-W., Lin S.-J. Peroxisome proliferator-activated receptor α reduces endothelin-1-caused cardiomyocyte hypertrophy by inhibiting nuclear factor- κ B and adiponectin. Mediat. Inflamm. 2016;2016:1–11. doi: 10.1155/2016/5609121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- Laird P.W., Zijderveld A., Linders K., Rudnicki M.A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H., Luoma J.T., Hilleman D. A review of currently available fenofibrate and fenofibric acid formulations. Rev. Cardiol. Res. @BULLET. 2013;4:47–55. doi: 10.4021/cr270w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer P., Schisler J.C., Patterson C., Willis M.S. Minireview: won't get fooled again: the nonmetabolic roles of peroxisome proliferator-activated receptors (PPARs) in the heart. Mol. Endocrinol. 2010;24:1111–1119. doi: 10.1210/me.2009-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J.H. Treatment of chagas' disease — time is running out. N. Engl. J. Med. 2015;373:1369–1370. doi: 10.1056/NEJMe1510170. [DOI] [PubMed] [Google Scholar]
- Miller D.A., Hernandez S., Rodriguez De Armas L., Eells S.J., Traina M.M., Miller L.G., Meymandi S.K. Tolerance of benznidazole in a United States chagas disease clinic. Clin. Infect. Dis. 2015;60:1237–1240. doi: 10.1093/cid/civ005. [DOI] [PubMed] [Google Scholar]
- Mirkin G.A., Celentano A.M., Malchiodi E.L., Jones M., González Cappa S.M. Different Trypanosoma cruzi strains promote neuromyopathic damage mediated by distinct T lymphocyte subsets. Clin. Exp. Immunol. 1997;107:328–334. doi: 10.1111/j.1365-2249.1997.267-ce1166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin G.A., Jones M., Sanz O.P., Rey R., Sica R.E., González Cappa S.M. Experimental Chagas' disease: electrophysiology and cell composition of the neuromyopathic inflammatory lesions in mice infected with a myotropic and a pantropic strain of Trypanosoma cruzi. Clin. Immunol. Immunopathol. 1994;73:69–79. doi: 10.1006/clin.1994.1171. [DOI] [PubMed] [Google Scholar]
- Mitelman J., Argentinian Society of Cardiology Consensus statement on Chagas-Mazza disease. Argent. J. Cardiol. 2011;79:544–564. [Google Scholar]
- Morillo C.A., Marin-Neto J.A., Avezum A., Sosa-Estani S., Rassi A., Rosas F., Villena E., Quiroz R., Bonilla R., Britto C., Guhl F., Velazquez E., Bonilla L., Meeks B., Rao-Melacini P., Pogue J., Mattos A., Lazdins J., Rassi A., Connolly S.J., Yusuf S. Randomized trial of benznidazole for chronic chagas' cardiomyopathy. N. Engl. J. Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- Muller L.A., Añasco N., González Cappa S.M. Trypanosoma cruzi: isolate dependence in the induction of lytic antibodies in the mouse and rabbit. Exp. Parasitol. 1986;61:284–293. doi: 10.1016/0014-4894(86)90183-9. [DOI] [PubMed] [Google Scholar]
- Noguerado-Mellado B., Rojas-Pérez-Ezquerra P., Calderón-Moreno M., Morales-Cabeza C., Tornero-Molina P. Allergy to benznidazole: cross-reactivity with other nitroimidazoles. J. Allergy Clin. Immunol. Pract. 2016 doi: 10.1016/j.jaip.2016.09.047. doi: 10.10, 827–828. [DOI] [PubMed] [Google Scholar]
- Papa S., Bubici C., Zazzeroni F., Franzoso G. Mechanisms of liver disease: cross-talk between the NF-kappaB and JNK pathways. Biol. Chem. 2009;390:965–976. doi: 10.1515/BC.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penas F., Mirkin G.A., Hovsepian E., Cevey Á., Caccuri R., Sales M.E., Goren N.B. PPARγ ligand treatment inhibits cardiac inflammatory mediators induced by infection with different lethality strains of Trypanosoma cruzi. Biochim. Biophys. Acta - Mol. Basis Dis. 2013;1832:239–248. doi: 10.1016/j.bbadis.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Pérez-Ayala A., Moreno S., Fernández-González M.C., Zamora J., López-Velez R. Use of benznidazole to treat chronic Chagas' disease: a systematic review with a meta-analysis. J. Antimicrob. Chemother. 2009;64:1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.L., Nunes M.P., Teixeira M.M., Rocha M.O.C. Diagnosis and management of Chagas disease and cardiomyopathy. Nat. Rev. Cardiol. 2012;9:576–589. doi: 10.1038/nrcardio.2012.109. [DOI] [PubMed] [Google Scholar]
- Sahn D.J., DeMaria A., Kisslo J., Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shen W., Gao Y., Lu B., Zhang Q., Hu Y., Chen Y. Negatively regulating TLR4/NF-κB signaling via PPARα in endotoxin-induced uveitis. Biochim. Biophys. Acta - Mol. Basis Dis. 2014;1842:1109–1120. doi: 10.1016/j.bbadis.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Straus D.S., Pascual G., Li M., Welch J.S., Ricote M., Hsiang C.H., Sengchanthalangsy L.L., Ghosh G., Glass C.K. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugga G.S., Khanam R., Khan M.U., Khanam R. Protective role of fibrates in cardiac ischemia/reperfusion. J. Adv. Pharm. Technol. Res. 2012;3:188. doi: 10.4103/2231-4040.101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M.M., Gazzinelli R.T., Silva J.S. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- Tomasini N., Ragone P.G., Gourbière S., Aparicio J.P., Diosque P., Cohen J. Epidemiological modeling of Trypanosoma cruzi: low stercorarian transmission and failure of host adaptive immunity explain the frequency of mixed infections in humans. PLoS Comput. Biol. 2017;13:e1005532. doi: 10.1371/journal.pcbi.1005532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar-Pereira G., Carneiro V.C., Mata-Santos H., Vicentino A.R.R., Ramos I.P., Giarola N.L.L., Feijó D.F., Meyer-Fernandes J.R., Paula-Neto H.A., Medei E., Bozza M.T., Lannes-Vieira J., Paiva C.N. Resveratrol reverses functional chagas heart disease in mice. PLoS Pathog. 2016;12:e1005947. doi: 10.1371/journal.ppat.1005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar-Pereira G., Resende Pereira I., de Souza Ruivo L.A., Cruz Moreira O., da Silva A.A., Britto C., Lannes-Vieira J. Combination chemotherapy with suboptimal doses of benznidazole and pentoxifylline sustains partial reversion of experimental chagas' heart disease. Antimicrob. Agents Chemother. 2016;60:4297–4309. doi: 10.1128/AAC.02123-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R., Vigliano C., Lococo B., Alvarez M.G., Petti M., Bertocchi G., Armenti A. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev. anti. Infect. Ther. 2009;7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- Viotti R., Vigliano C., Lococo B., Bertocchi G., Petti M., Alvarez M.G., Postan M., Armenti A. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann. Intern. Med. 2006;144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- Vogt J., Alba Soto C.D., Mincz M.P., Mirkin G.A. Impaired Trypanosoma cruzi-specific IFN-γ secretion by T cells bearing the BV9 T-cell receptor is associated with local IL-10 production in non-lymphoid tissues of chronically infected mice. Microbes Infect. 2008;10:781–790. doi: 10.1016/j.micinf.2008.04.013. [DOI] [PubMed] [Google Scholar]
- WHO - World Health Organization . WHO; 2016. Chagas Disease (American Trypanosomiasis) [PMC free article] [PubMed] [Google Scholar]
- Yin W.-H., Chen J.-W., Chen Y.-H., Lin S.-J. Fenofibrate modulates HO-1 and ameliorates endothelial expression of cell adhesion molecules in systolic heart failure. Acta Cardiol. Sin. 2013;29:251–260. [PMC free article] [PubMed] [Google Scholar]
- Zaidenberg A., Luong T., Lirussi D., Bleiz J., Del Buono M.B., Quijano G., Drut R., Kozubsky L., Marron A., Buschiazzo H. Treatment of experimental chronic chagas disease with trifluralin. Basic Clin. Pharmacol. Toxicol. 2006;98:351–356. doi: 10.1111/j.1742-7843.2006.pto_253.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cheng Y., Gu J., Wang S., Zhou S., Wang Y., Tan Y., Feng W., Fu Y., Mellen N., Cheng R., Ma J., Zhang C., Li Z., Cai L. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016;130:625–641. doi: 10.1042/CS20150623. [DOI] [PubMed] [Google Scholar]
- Zingales B., Andrade S.G., Briones M.R.S., Campbell D.A., Chiari E., Fernandes O., Guhl F., Lages-Silva E., Macedo A.M., Machado C.R., Miles M.A., Romanha A.J., Sturm N.R., Tibayrenc M., Schijman A.G. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zou J., Le K., Xu S., Chen J., Liu Z., Chao X., Geng B., Luo J., Zeng S., Ye J., Liu P. Fenofibrate ameliorates cardiac hypertrophy by activation of peroxisome proliferator-activated receptor-α partly via preventing p65-NFκB binding to NFATc4. Mol. Cell. Endocrinol. 2013;370:103–112. doi: 10.1016/j.mce.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Zuo N., Zheng X., Liu H., Ma X. Fenofibrate, a PPARα agonist, protect proximal tubular cells from albumin-bound fatty acids induced apoptosis via the activation of NF-kB. Int. J. Clin. Exp. Pathol. 2015;8:10653–10661. [PMC free article] [PubMed] [Google Scholar]