Highlights
-
•
An innovative design that allows fully automated, fully integrated clot lysis potency determination for lot release and stability testing.
-
•
A comprehensive validation design to ensure accurate estimate of recovery (accuracy) and precision profile.
-
•
A comparability strategy that allows justification of specification based on statistical comparison between assays.
-
•
Evidence of significant improvement in assay performance after being transferred to QC laboratories.
Keywords: Potency assay, Clot lysis, Comparability, Automation
Abstract
This report describes the design, development, validation and long-term performance of tPA clot lysis activity assay using Advanced Chemistry Line Total Operational Performance (ACL TOP)™ Homeostasis Testing System. The results of the study demonstrated robust and stable performance of the analytical method. The accuracy of the assay, expressed by percent recovery is 98–99%. The intermediate precision and repeatability precision, expressed as Relative Standard Deviation (RSD), was 3% and less than 2% respectively. The validated range is from 70% to 130% of the target potency of 5.8 × 105 IU/mg. The linearity of this range, expressed in correlation coefficient, is 0.997. After the assay is transferred to a QC laboratory, the assay retained high accuracy and precision with a success rate of >99%.
1. Introduction
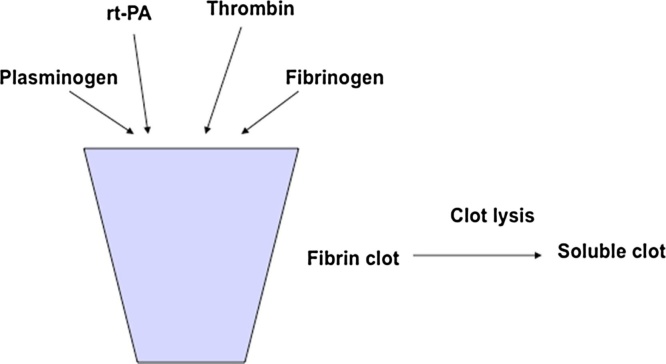
Recombinant human tissue Plasminogen Activator (rt-PA) is a serine protease with a molecular weight of about 60,000–110,000 Da [1], [2]. It proteolytically converts plasminogen to active plasmin that in turn degrades insoluble fibrin to soluble by products [3], [4]. This property has been shown to provide both clinical and cost effectiveness for the treatement of stroke [5]. Since the registration of rt-PA, many variants that confer the clot lysis therapeutic activity have been reported [6]. The activity of rt-PA can be determined by measuring the lysis of a synthetic fibrin clot over time [7], [8]. The in vitro potency measurement relies on simultaneous clot formation and clot lysis reactions triggered by mixing fibrinogen, plasminogen, thrombin and tPA together at once (Fig. 1). In practice, this is achieved by sequential addition of rtPA, plasminogen, thrombin and finally, fibrinogen, which triggered the clot formation and the following clot lysis cascade. The change in turbidity of the clot is monitored and the time needed to achieve a predetermined level of reduction in turbidity is used as a measurement of potency. Different forms of potency assays using the kinetic method have been developed; including a microcentrifugal analyzer (MCA, also referred to as the Monarch, manufactured by Instrumentation Laboratory) based semi-automated method [9], and plate-based methods [10]. The accuracy and precision of the protein activity determination is crucial to ensure the efficacy and safety of the therapeutic product, because the thrombolytic drugs can cause serious bleeding in the brain, which can be fatal (for a recent review: [11]).
Fig. 1.
Clot lysis assay design. Potency of rt-PA was determined by simultaneous clot formation and clot lysis. In this reaction, rt-PA (sample or standard) was mixed with plasminogen, thrombin and fibrinogen together to allow the formation of fibrin clot by thrombin and fibrinogen and subsequent lysis by plasmin converted from plasminogen by rtPA. The change in turbidity was used to determine the kinetics of enzyme reaction.
A new type of homeostasis analyzer with automation capability was introduced by Instrumentation Laboratory. The ACL TOP system is a fully-automated stand-alone random-access multiparameter coagulation analyzer [12] The optical reading unit allows 16 simultaneous reaction readings at two currently available wavelengths i.e. 405 and 671 nm. The cuvette loading area, located on the left side of the instrument, can be filled, even while running, with up to 20 clips of 10 cuvette-strips each for a total of 800 cuvettes (4 cuvettes per strip). A conveyor belt moves the cuvette-strips to a cuvette shuttle that places them in position to be used by the analyzer for sample handling. Up to 120 samples can be loaded at once using the rack system (12 racks of 10 tubes each). Technical evaluation has indicated the reliability of ACL TOP analyzer for clinical homeostasis testing [13].
A potency assay intended for lot release must meet pre-defined validation acceptance criteria of specificity, accuracy, precision, linearity, and range in accordance with ICH guidelines. For potency assays supporting marketed products, method comparability to the current assay must be demonstrated. Lastly, to reduce human error and ergonomic risk, new analytical methods should be automated if possible. This report describes the development and validation of an automated clot lysis activity assay using ACL TOP analyzer, with characterization of critical parameters and assessment of robustness of assay. Since the development, validation and transfer of the assay, it was accepted by multiple health authorities and proven to be highly consistent and reliable in supporting GMP activities. Although the method was developed for enzymatic clot lysis, the principle and setups can be applied to establish other functional assays as well.
2. Materials and methods
2.1. Critical reagents
Recombinant human fibrinogen, thrombin, and plasminogen were purchased from Calbiochem. The recombinant human tissue plasmin activator samples and reference standard were manufactured by Genentech. Instrument specific proprietary cleaning reagents (labeled as cleaning A and B reagents and rinse solutions) used for the ACL TOP instrument were purchased from Instrumentation Laboratory (Bedford, MA).
2.2. Final format
The automated clot lysis method utilizes the pre-dilution function of ACL TOP and each concentration is programmed into each test. Briefly, in house reference standard for the active pharmaceutical ingredient (API), control and samples are first diluted to 40 μg/mL with assay buffer, loaded into the ACL TOP sample chamber and recorded by the ACL TOP software as “patients”. Standards are generated by 5 tests, each of which specifies a dilution level during the pre-dilution step with assay buffer. Control, samples and blank are tested at the three middle dilution levels (corresponding to 3333.3 ng/mL, 2222.2 ng/mL, 1111.1 ng/mL of the reference standard). Twenty μL of diluted standards, samples and control are then loaded into individual cuvettes and mixed with 20 μL of 33 Units/mL thrombin. The clot formation/lysis reaction starts as soon as 200 μL of a cocktail of plasminogen (36 μg/mL)/fibrinogen (2.2 mg/mL) is added into the cuvettes. Absorbance at 405 nm is monitored from 10 s to 700 s after initiation of the reaction. Lysis time is determined using the 50% threshold of the absorbance reading. Standard curve is generated by linear regression of log(lysis time) against log(tPA concentration). The potency of control and samples is then calculated by interpolating lysis time readout on the standard curve.
2.3. Potency calculation and statistics
The clot lysis activities of control and samples using standard curve was calculated by parallel line analysis described in USP Chapter 1032, Design and Development of Biological Assays [14]. The potency values for samples and control were calculated by multiplying the potency estimate from PLA by the specific activity of the reference standard (5.8 × 105 IU/mg, reference [17]) used in the standard curve.
2.4. DOE for range finding and robustness confirmation
All Design of Experiments (DOE) for range finding and robustness studies were created using JMP version 7.0 or 8.01 with the Customer Design function. Statistical significance was determined with α = 0.05.
2.5. Comparability study
Forty-four samples were tested using the licensed procedure and the ACL TOP automated method. Samples included drug substance as well as various configurations of drug products under normal storage as well as stressed conditions, including heat, light exposure, low and high pH, and oxidation (with 2,2′-Azobis(2-amidinopropane) dihydrochloride) treatments., as described in ICH Harmonized Tripartite Guideline, Stability Testing of New Drug Substance and Products Q1A(R2), current Step 4 version, dated 6 February 2003. The mean of the paired difference of the results as well as the 95% confidence interval of the mean difference was calculated and compared to a pre-determined maximum allowable difference by Two One sided t Test (TOST). The two methods were considered comparable if the 95% confidence interval falls within the maximum allowable difference [15].
2.6. Validation of the method
Method validation was performed in accordance with the International Conference on Harmonisation (ICH) Guideline on the validation of analytical procedures (ICH Q2[R1]). Briefly, accuracy, intermediate precision, linearity and range were determined using samples of 70%, 85%, 100%, 115% and 130% of the target concentrations from results of 24 assays performed by three analysts on two instruments. Robustness evaluation was based on ANOVA with 95% confidence interval. Results are compared to pre-determined acceptance criteria in% recovery for accuracy, % relative standard deviation for precision, coefficient of determinations (R2) for linearity. Variant component analysis was performed to determine the contribution of analyst, day, and instrument to variability.
3. Results and discussion
3.1. Design of the clot lysis assay
The purpose of establishing a potency assay was to assess quality attribute of the therapeutic protein, not demonstrating the efficacy. While the efficacy of tPA has been demonstrated by the resolution of preformed clot, the potency assay design was based on simultaneous clot formation and clot lysis for the consistency and reproducibility of the reaction. A semi-automated potency method using the Monarch system and a plate based manual method have been described previously for lot release and stability testing for tPA (references [9] and [10]).
3.2. Adapting ACL TOP for in house clot lysis assay
3.2.1. Selection of wavelength
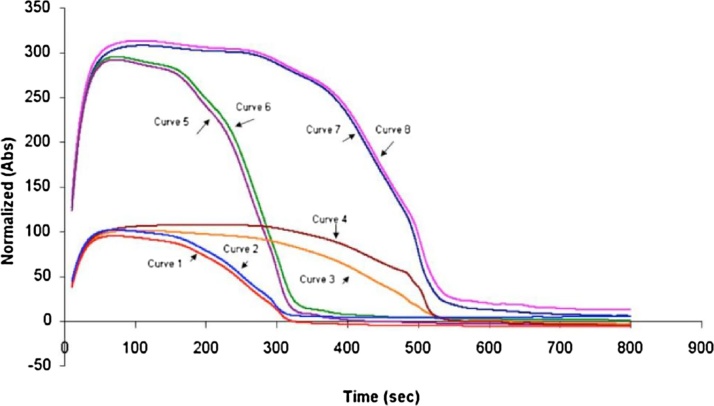
The ACL TOP instrument is capable of monitoring two wavelengths: 671 nm and 405 nm. The clot lysis curves were monitored using both wavelengths for two tPA concentrations. The results indicate much stronger absorption signals at 405 nm than at 671 nm (Fig. 2). Based on the result, the wavelength of 405 nm was selected for the analytical method.
Fig. 2.
Clot lysis curves detected by two different wavelengths. The absorbance at 405 nm was much higher than the absorbance at 671 nm. Curves 1–4: kinetic absorbance curves at 405 nm generated from 4 independent experiments. Curves 5–8: kinetic absorbance curves at 671 nm generated from 4 independent experiments. The experiments were conducted with a high level of rtPA (curves 1,2,5,6) and a low level of rtPA (curves 3,4,7, and 8) with two replicates for each condition.
3.2.2. Selection of rt-PA concentrations
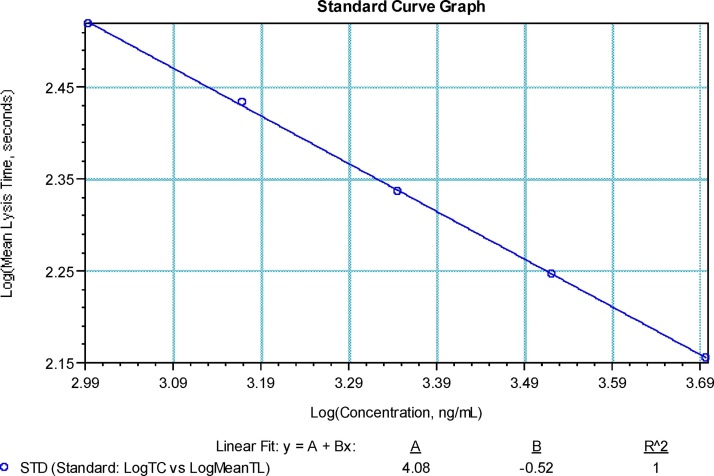
A large linear range of dose response, from 400 ng/mL to 10 μg/mL(corresponding to 30–830 ng/mL rt-PA in the final reaction buffer), was demonstrated for tPA (Data not shown). Several factors were considered to select the tPA concentrations for standard [1] Dilution capability of ACL TOP. The maximum capacity within one single dilution step is 148X by the ACL TOP instrument; [2] Even spacing between concentrations on the standard curve. Because the response curve is plotted on log scale, the doses should also be distributed on log scale to avoid bias toward any segment of the curve; and [3] Aberrant clot lysis profile for high tPA concentrations. In several occasions, aberrant clot lysis curves were observed with tPA concentration higher than 5 μg/mL. The final concentrations selected for standard were 5000.0 ng/mL, 3333.3 ng/mL, 2222.2 ng/mL, 1111.1 ng/mL and 987.7 ng/mL (Fig. 3). The coefficient of correlation ranged from −0.997 to −1.000 with the final format.
Fig. 3.
A typical standard curve for the automated clot lysis assay plotted in log scale for both standard concentration and lysis time.
To increase number of samples that can be analyzed within an assay, it is desirable to use minimal number of concentration points for each sample and control. The potency values calculated using 5 concentration points or 3 concentration points were compared using reference material as sample. The potency values from 5 points and 3 points were comparable. Based on the result, the use of minimal concentration points (3 points) was chosen for the sample and control.
3.2.3. Critical reagents
Early development work to optimize concentration of each critical reagent used in the assay shows a strong correlation of plasminogen and fibrinogen on lysis time as well as the profile of clot lysis curve, while thrombin has minimal impact on both [9], [16], [17]. A DOE was then created to verify the robustness of plasminogen and fibrinogen concentration. Two readouts were examined: lysis time and sample value, which was measured using assay control. For the concentration range of 0.5–3.0 mg/mL Fibrinogen and 4–150 μg/mL plasminogen, the interaction between the two reagents had a significant impact on lysis time; however, the impact is not statistically significant for the relative potency readout of a sample (p > 0.05; data not shown). The results were consistent with previous publication [9].
3.2.4. ACL TOP parameters
Parameters used in the automated dilution steps were evaluated for their impact on assay control results. The DOE analysis shows none of the factors has statistically significant impact on the sample readout (i.e. none of the factors had a p value <0.05)
Since the rinse and clean step after the loading of assay buffer is critical for removing residual tPA from dilution to dilution, the parameters used in this step is evaluated for its robustness. A DOE was designed to evaluate the hold time for the cleaning reagent (5–15 s), volume of the cleaning reagent (150–250 μL), air gap (10–20 μL) and agitation settings (off and on). None of the factors had a significant impact on the assay control value.
3.2.5. System suitability criteria
The system suitability criteria used throughout the development study were the same as the licensed manual assay. The criteria are [1] the correlation coefficient of the standard must be between −0.995 and −1.000 [2] the control value must fall within range of established mean ± 15% and [3] the CV of the control and sample replicates within the assay must be within 15%. Under these provisional criteria, the success rate of the ACL TOP method was 100%.
Because the automated method exhibited much better precision profile than the licensed manual method (data shown below) the precision acceptance criteria may be tightened to reflect the assay performance. The distributions of control values and%CV for samples and control were evaluated. The 95% prediction interval was approximately mean ± 8%. Based on the evaluation, the system suitability criteria for control range was proposed to be mean ± 8%. The correlation coefficient of the standard would remain the same. Since parallel line analysis (PLA) would be used to calculate potency results, a new set of system suitability criteria was to be implemented to replace the CV criteria for the replicates (described below)
As a prerequisite parallelism determination, a regression/linearity determination for the sample as well as reference standard was determined in order to verify the linearity of the dose responses. The correlation coefficients for standard in the development study were all between −0.995 and −1.000 with very few exceptions where aberrant clot lysis curves were observed. Therefore, the acceptance criterion of correlation coefficient for standard is set at between −0.995 and −1.000. The correlation coefficient for majority of samples in the development study were also between −0.995 and −1.000, with few of them falling between −0.990 and −0.995 without apparent causes. This is because samples contain only three concentrations whereas standard contains five concentrations; therefore, the likelihood for the correlation coefficient of samples to fall outside of the range of −0.995 to −1.000 is predicted to be higher than that of the reference standard. The slope ratio distributions were largely between 0.80 and 1.20. The 95/99 tolerance interval was 0.83–1.17. To produce geometrically symmetric criterion, a limit of 0.83–1.20 is proposed for the method.
3.3. Analytical method validation
The accuracy of the method was determined at 70–130% of the target potency by preparing 70–130% of the target concentration using reference material as a sample. Each sample was tested by three analysts who each performed eight assays using two instruments for a total of 24 assays. Standard, control and sample dilutions were prepared independently for each assay. Nominal potencies reported in Table 1 are expressed in IU/mg. The mean recovery at each concentration was calculated by dividing the mean measured potency by the expected potency. The mean recovery at 100% of the target potency was 98%, and the mean recoveries at 70, 85, 115, and 130% of the target potency ranged from 98 to 99%. These values are well within the accuracy acceptance criterion (95–105% recovery).
Table 1.
Recovery Study.
| Analyst | Assay | Instrument | Potency (×105 IU/mg) |
||||
|---|---|---|---|---|---|---|---|
| 130% | 115% | 100% | 85% | 70% | |||
| 1 | 1 | 1 | 7.7852 | 6.7764 | 5.5017 | 4.9011 | 4.0139 |
| 2 | 2 | 7.1465 | 6.2950 | 5.8276 | 4.7577 | 3.9402 | |
| 3 | 1 | 7.4216 | 6.5590 | 6.0203 | 4.9451 | 4.1253 | |
| 4 | 2 | 7.2003 | 6.4968 | 5.7958 | 4.7743 | 4.0228 | |
| 5 | 1 | 7.9173 | 6.5266 | 5.6373 | 4.7727 | 3.8350 | |
| 6 | 2 | 7.4679 | 6.5898 | 5.8274 | 4.9749 | 4.1294 | |
| 7 | 1 | 7.4625 | 6.6443 | 5.5970 | 4.9066 | 3.9890 | |
| 8 | 2 | 7.2515 | 6.4691 | 5.6157 | 4.7409 | 3.9496 | |
| 2 | 1 | 1 | 7.8943 | 6.7825 | 5.9011 | 4.9491 | 4.1017 |
| 2 | 2 | 7.4888 | 6.7419 | 5.7973 | 5.0416 | 4.2560 | |
| 3 | 1 | 7.5383 | 6.6131 | 5.7356 | 4.9065 | 4.2273 | |
| 4 | 2 | 7.2667 | 6.5035 | 5.6728 | 4.8413 | 3.9851 | |
| 5 | 1 | 7.3138 | 6.5463 | 5.5661 | 4.8144 | 3.8874 | |
| 6 | 2 | 7.3360 | 6.5947 | 5.7450 | 4.8699 | 4.1428 | |
| 7 | 1 | 7.3950 | 6.5971 | 5.5998 | 4.9234 | 4.0717 | |
| 8 | 2 | 7.3723 | 6.6256 | 5.4948 | 4.9459 | 4.0338 | |
| 3 | 1 | 1 | 7.8821 | 6.6256 | 5.6538 | 4.9285 | 3.9420 |
| 2 | 2 | 7.4389 | 6.4214 | 5.6608 | 4.8478 | 4.0973 | |
| 3 | 1 | 7.0796 | 6.3387 | 5.5095 | 4.7300 | 3.8839 | |
| 4 | 2 | 7.1578 | 6.2747 | 5.6356 | 4.8739 | 4.0319 | |
| 5 | 1 | 7.4217 | 6.6921 | 5.5307 | 4.7184 | 4.0109 | |
| 6 | 2 | 7.8395 | 6.9471 | 5.9129 | 5.0263 | 4.2174 | |
| 7 | 1 | 6.8343 | 6.4052 | 5.2345 | 4.4942 | 3.8723 | |
| 8 | 2 | 6.9902 | 6.4778 | 5.3741 | 4.6322 | 3.9826 | |
| Mean Potency | 7.41 | 6.56 | 5.66 | 4.85 | 4.03 | ||
| Expected Potency | 7.54 | 6.67 | 5.8 | 4.93 | 4.06 | ||
| Standard Deviation | 0.29 | 0.16 | 0.18 | 0.13 | 0.11 | ||
| Relative Standard Deviation (%) | 4 | 2 | 3 | 3 | 3 | ||
| Mean Recovery (%) | 98 | 98 | 98 | 98 | 99 | ||
The recovery study was performed by three analysts using two instruments over 8 independent assays. All results are reported from valid assays that passed all system suitability criteria.
Repeatability was determined using the assay control as a sample. Intra-assay RSD values between the 6 sample results from three independent assays were 1%, 1% and 2% (Table 2). All of these values are within the acceptance criterion of RSD ≤5% and demonstrate the repeatability of the assay.
Table 2.
Repeatability Precision.
| Sample ID | Potency (×105 IU/mg) |
||
|---|---|---|---|
| Assay 1 | Assay 2 | Assay 3 | |
| Sample Position 1 | 6.2103 | 6.0051 | 6.3268 |
| Sample Position 2 | 6.0590 | 5.8614 | 6.2202 |
| Sample Position 3 | 6.1222 | 5.9693 | 6.2176 |
| Sample Position 4 | 6.0477 | 5.8331 | 6.0152 |
| Sample Position 5 | 6.0008 | 5.9314 | 6.1535 |
| Sample Position 6 | 6.0861 | 5.8360 | 6.1851 |
| Mean | 6.09 | 5.91 | 6.19 |
| Standard Deviation | 0.07 | 0.07 | 0.10 |
| Relative Standard Deviation (%) | 1 | 1 | 2 |
Repeatability study was performed by one analyst using one instrument to evaluate variability within a single run (6 sample positions). The experiment was repeated in three different assays.
The intermediate precision of the assay was determined using data from the recovery study in the accuracy study by evaluation of overall assay-to-assay variation as well as the variances contributed by components such as analyst, day, and instrument. Since all assays in the recovery study were performed using independently prepared samples, the instrument-to-instrument variance contains the variance of sample preparation. As shown in Table 1, Table 3, the overall assay-to-assay, analyst-to-analyst, day-to-day within analyst and instrument-to-instrument RSD values for the target sample were 3%, 0%, 2% and 2%, respectively. The overall assay-to-assay RSD values for the samples at 70, 85, 115 and 130% of the target ranged from 2 to 4%. The analyst-to-analyst RSD values for the samples at 70, 85, 115 and 130% of the target sample were less than 1%. The day-to-day within analyst RSD values for the samples at 70, 85, 115 and 130% of the target sample ranged from 0 to 2%. The instrument-to-instrument RSD values for the samples at 70, 85, 115 and 130% of the target sample ranged from 2 to 3%. All these values are well within the acceptance criterion of RSD ≤5%.
Table 3.
Variance Component Estimate
| Sample | Source of Variance | Relative Standard Deviation (%) |
|---|---|---|
| Control | Analyst-to-analyst | 1 |
| Day-to-day within Analyst | 1 | |
| Instrument-to-instrument within daya | 2 | |
| Overall | 3 | |
| 70% of Target | Analyst-to-analyst | 0 |
| Day-to-day within Analyst | 0 | |
| Instrument-to-instrument within daya | 3 | |
| Overall | 3 | |
| 85% of Target | Analyst-to-analyst | 0 |
| Day-to-day within Analyst | 1 | |
| Instrument-to-instrument within daya | 2 | |
| Overall | 3 | |
| 100% of Target | Analyst-to-analyst | 0 |
| Day-to-day within Analyst | 2 | |
| Instrument-to-instrument within daya | 2 | |
| Overall | 3 | |
| 115% of Target | Analyst-to-analyst | 0 |
| Day-to-day within Analyst | 1 | |
| Instrument-to-instrument within daya | 2 | |
| Overall | 2 | |
| 130% of Target | Analyst-to-analyst | 0 |
| Day-to-day within Analyst | 2 | |
| Instrument-to-instrument within daya | 3 | |
| Overall | 4 |
The analysis was based on the data generated in the recovery study (Table 1) with three analysts, 2 instruments over 8 independent assays (one assay per day). Variance component analysis was performed to estimate the variance contributed by each factor.
Instrument-to-instrument variance contains the variance of sample preparation.
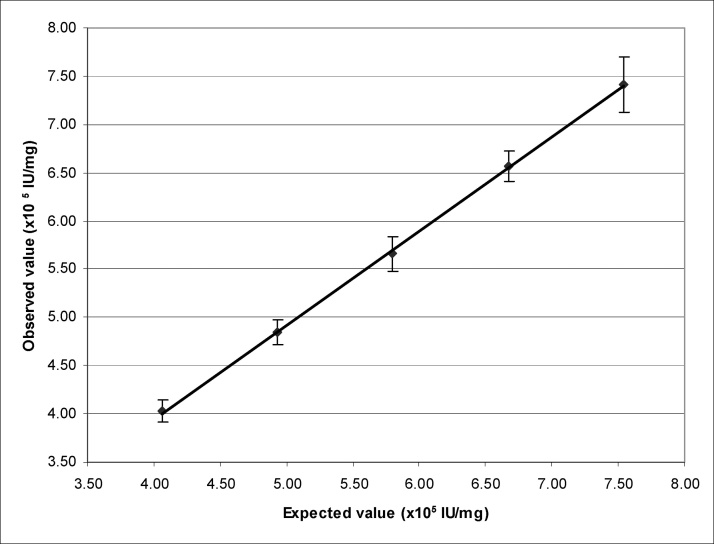
The data from the recovery study were analyzed to determine the linearity of the method. The mean measured potencies (n = 24) obtained for each of the samples were plotted against the expected potencies (Fig. 4 linearity). The correlation coefficient (r) obtained was 0.997, the slope is 0.97, the Y intercept was 0.05 × 105 IU/mg, and the residual sum of square was 0.003. The recovery at each concentration ranged from 98 to 99% (Table 1). This result meets the acceptance criterion of correlation coefficient (r) ≥0.99.
Fig. 4.
Linearity of the potency assay plotted for the expected potency values against the observed potency values. The potency values were generated using the ACL TOP method as described. The error bars indicated the standard deviation at each concentration.
The data from the recovery study were analyzed to determine the range of the method (Table 1). The validated range of the assay is determined to be 4.06–7.54 × 105 IU/mg based on the accuracy, precision, and linearity data from the recovery study.
The specificity of the method was evaluated in two ways: first by evaluating the activity of 10 Genentech commercial products (including enzymes, hormone and antibody products) in the assay; and second, by evaluating the effect of these materials on clot lysis activity. Products that did not induce clot lysis were considered to have no activity in the assay. Except for a related clot lysis enzyme (a modified rtPA), all products tested showed no activity in the assay. To evaluate the effects of other Genentech products on rt-PA potency, each product was spiked into the 40 μg/mL rt-PA sample to achieve a final concentration of 40 μg/mL for the non-rt-PA product. The potency of rt-PA ranged from 5.56 to 6.05 × 105 IU/mg when tested in the presence of the materials, with the exception of the related enzyme. This closely related enzyme was shown to have an additive effect on clot lysis assay. In conclusion, the potency method does not detect clot lysis activity with any other product manufactured in the same facility except for a closely related molecule. The assay meets acceptance criteria for specificity.
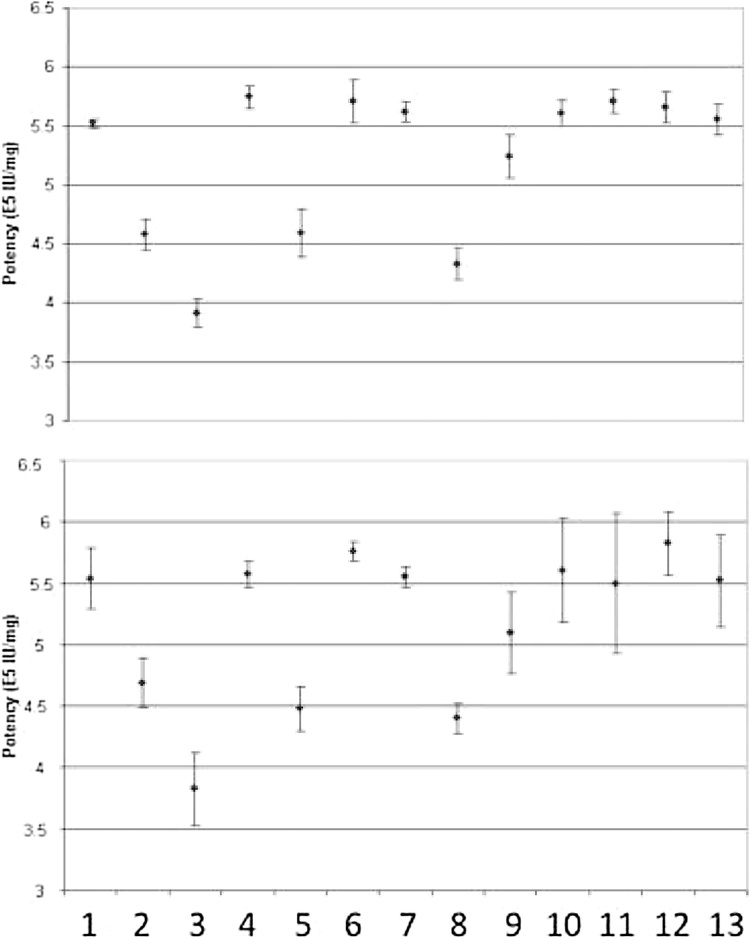
The ability of the method to detect changes in the activity of rt-PA subjected to various stress conditions was evaluated. The conditions include heat, light exposure, low and high pH, and oxidation (with 2,2′-Azobis(2-amidinopropane) dihydrochloride) treatments. The results are summarized in Fig. 5. Statistical analysis was performed based on ANOVA to determine if the potency of the stressed sample is significantly different from that of the control (P < 0.05). The results demonstrate that the potency method can detect changes in activity of rt-PA samples subjected to heat at 40 °C for 4 and 7 days, intense light (1.2 million lux hour) and acidic pH (pH4) conditions tested in the stability panel.
Fig. 5.
Stability indicating profiles for the automated assay (upper panel) and the manual method (lower panel). The conditions are 1. Control, 2. Thermal treatment 4 days at 40 °C, 3. Thermal treatment 7 days at 40 °C, 4. Control for light exposure, 5. Light exposure, 6. Control for agitation, 7. Agitation treatment, 8. pH4 treatment for 7 days at 30 °C, 9. pH10 treatment for 7 days at 5 °C, 10. Control for H2O2 treatment, 11. Oxidation with 1000 ppm H2O2, 12. Control for AAPH treatment, and 13. Oxidation with 10 mM AAPH.
3.4. Comparability strategy and result
Head-to-head comparison of the reportable results obtained using the licensed manual method and the automated methods were performed. Samples evaluated for comparability included bulk drug substance, all configurations of drug product, stressed stability samples (Fig. 5) as well as recovery samples containing 60–140% of target concentrations tested in the method validation study. Potency values ranged from 3.82 to 7.63 × 105 IU/mg (determined by the previously registered method), representing approximately 70% –130% of the potency value of the reference material. The absolute mean difference in the reportable results was determined as 0.03 × 105 IU/mg. The upper and lower limits of the 95% confidence interval of the mean difference are −0.07 × 105 IU/mg and 0.02 × 105 IU/mg respectively demonstrated the comparability of the method. There was no change in stability indicating profile. However, the loss of potency is more readily detected using the automated method due to enhanced precision profile (Fig. 5).
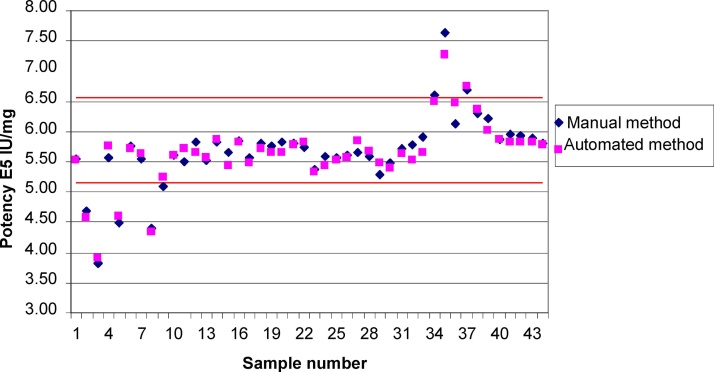
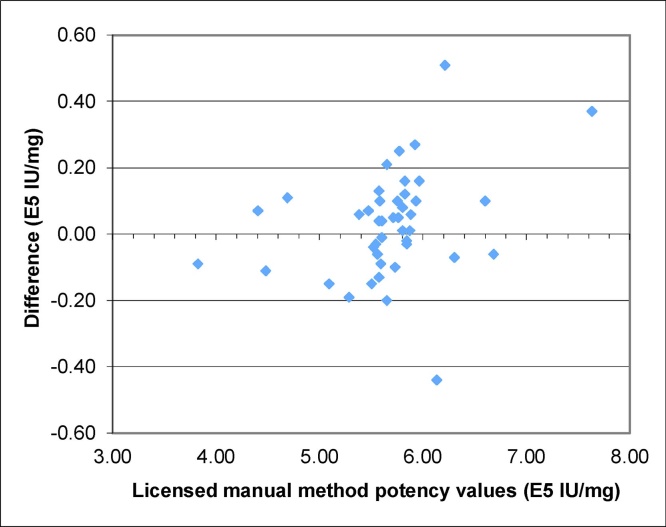
Distribution of reportable results obtained using automated method against those obtained using the licensed manual method is examined across the potency range of the 44 samples (Fig. 6). By visual examination the distribution of the differences is random across the entire range. No bias against the higher or lower end of the potency value was observed. The result further supports the comparability of the two methods (Fig. 7). As a result, there was no change in potency acceptance criteria proposed for any of the tPA DS or DP configurations.
Fig. 6.
Comparability between the manual and the automated methods for 44 samples (N = 44) including drug substance, drug product, stability samples stored at target temperature, accelerated conditions and stressed conditions as shown in Fig. 5. Samples simulating high potency samples (115% and 130% of target concentrations) were also included in the analysis.
Fig. 7.
Residual plot for method comparison. The samples are the same as in Fig. 6 (N = 44) of various conditions. For each sample, the potency value was determined using both manual method and the automated method. The difference between the methods is plotted against the potency value determined by the manual method.
3.5. Analytical method monitoring result
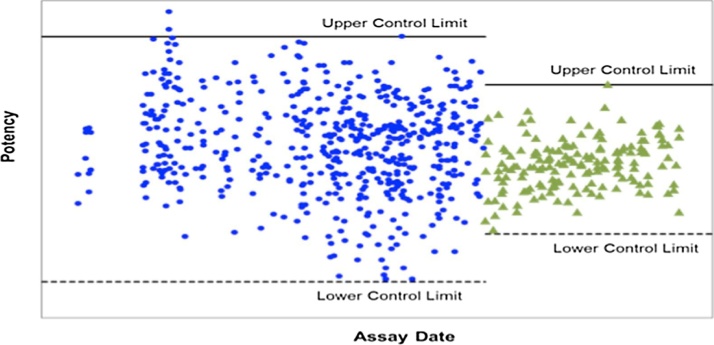
The assay performance was monitored after assay transfer into a routine testing laboratory. A product control whose potency value was determined in the originating laboratory was tested with samples in every assay as a system suitability acceptance criterion in the recipient laboratory. The long-term mean is within ±1% of the original mean, and the intermediate precision, expressed in RSD, was 3%, which is consistent with the results generated during assay validation (Fig. 8). The success rate of the assay is 99%. Taken together, these results indicate the reproducibility and robustness of the system.
Fig. 8.
Method monitoring profile for the manual method (blue circles) and the automated method (green triangles) using the same lot of assay control. The upper and lower control limits were used for the manual method (±15%) and the automated method (±9%) respectively.
4. Conclusion
An automated clot lysis method has been developed using ACL TOP for routine quality control purpose. The system significantly improves precision and robustness but is comparable in relative potency readout to the manual assay. The assay retains consistent performance two years after it was transferred from the development laboratory to a QC laboratory.
Conflict of interest
None.
Acknowledgements
The author would like to thank the technical support of Ariel Margulis, Ai Shih, and Helen Kumagai; information technology support of Faeq Manson, statistical design by Joseph Marhoul; and Genentech QC laboratory in Vacaville. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
References
- 1.Yim K.W. Fractionation of the human recombinant tissue plasminogen activator (rtPA) glycoforms by high-performance capillary zone electrophoresis and capillary isoelectric focusing. J. Chromatogr. 1991;559(1–2):401–410. doi: 10.1016/0021-9673(91)80089-y. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor J.V. rtPA is a well-characterized protein. In: Brown F., Lubiniecki A., Murano G., editors. vol. 96. Karger; Basel: 1998. pp. 113–121. (Characterization of Biotechnology Pharmaceutical Products. Develop. Biological Standardization). [Google Scholar]
- 3.Collen D. Molecular mechanisms of fibrinolysis and their application to fibrin-specific thrombolytic therapy. J. Cell. Biochem. 1987;33(2):77–86. doi: 10.1002/jcb.240330202. [DOI] [PubMed] [Google Scholar]
- 4.Gurman P., Miranda O.R., Nathan A., Washington C., Rosen Y., Elman N.M. Recombinant tissue plasminogen activators (rtPA): a review. Clin. Pharmacol. Ther. 2015;97(3):274–285. doi: 10.1002/cpt.33. [DOI] [PubMed] [Google Scholar]
- 5.Boudreau D.M., Guzauskas G., Villa K.F., Fagan S.C., Veenstra D.L.A. Model of cost-effectiveness of tissue plasminogen activator in patient subgroups 3 to 4.5 hours after onset of acute ischemic stroke. Ann. Emerg. Med. 2013;61(1):46–55. doi: 10.1016/j.annemergmed.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett W.F., Paoni N.F., Keyt B.A., Botstein D., Jones A.J.S., Presta L., Wurm F.M., Zoller M.J. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J. Biol. Chem. 1991;266(8):5191–5201. [PubMed] [Google Scholar]
- 7.Lisman T., de Groot P.G., Meijers J.C.M., Rosendaal F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thromobosis. Blood. 2005;105(3):1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 8.Cellai A.P., Lami D., Magi A., Liotta A.A., Rogolino A., Antonucci E., Bandinelli B., Abbate R., Prisco D. Assessment of fibrinolytic activity by measuring the lysis time of a tissue factor-induced clot: a feasibility evaluation. Clin. Appl. Thromb. Homeost. 2010;16(3):337–344. doi: 10.1177/1076029608325542. [DOI] [PubMed] [Google Scholar]
- 9.Carlson R.H., Garnick R.J., Jones A.J.S., Meunier A.M. The determination of recombinant human tissue-type plasminogen activator activity by turbidimetry using a microcentrifugal analyzer. Anal. Biochem. 1988;168(2):428–435. doi: 10.1016/0003-2697(88)90340-5. [DOI] [PubMed] [Google Scholar]
- 10.Jones A., Meunier A.M. A precise and rapid microtitre plate clot lysis assay: methodology, kinetic modeling and measurement of catalytic constants for plasminogen activation during fibrinolysis. Thromb. Haemost. 1990;64(3):455–463. [PubMed] [Google Scholar]
- 11.Wardlaw J.M., Murray V., Berge E., del Zoppo G.J. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014;(July) doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appert-Flory A., Fischer F., Jambou D., Toulon P. Evaluation and performance characteristics of the automated coagulation analyzer ACL TOP. Thromb. Res. 2007;120(5):733–743. doi: 10.1016/j.thromres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Lippi G., Ippolito L., Favaloro E.J. Technical evaluation of the Novel preanalytical module on Instrumentation Laboratory ACL TOP: advancing automation in homeostasis testing. J. Lab. Autom. 2013;18(5):382–390. doi: 10.1177/2211068213491747. [DOI] [PubMed] [Google Scholar]
- 14.United State Pharmacopoeia 〈1032〉 Design and Development of Biological Assays.
- 15.Schuirmann D.J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokinet. Biopharm. 1987;15(6):657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 16.Philo R.D., Gaffney P.J. Plasmin potency estimates. Influence of substrate used in assay. Thromb. Haemost. 1981;45(2):107–109. [PubMed] [Google Scholar]
- 17.Sands D., Whitton C.M., Merton R.E., Longstaff C.A. Collaborative Study to establish the 3rd International Standard for tissue plasminogen activator. Thromb. Haemost. 2002;88(2):294–297. [PubMed] [Google Scholar]