Abstract
Aim
To assess the relative efficacies of clozapine plus Electroconvulsive Therapy (ECT) compared against non-clozapine typical and atypical antipsychotics plus ECT for the treatment of “Treatment Resistant Schizophrenia” (TRS). Primarily to assess if clozapine delivers a significant improvement over other antipsychotics when combined with ECT.
Design
Major electronic databases were searched between 1990 and March 2017 for trials measuring the effects of either clozapine augmented ECT, other antipsychotic-augmented ECT, or both. After the systematic review of the data, a random-effects meta-analysis was conducted measuring the relative effect sizes of the different treatment regimens.
Subjects
1179 patients in 23 studies reporting the usage of ECT augmentation with antipsychotics. A total of 95 patients were tested with clozapine, and ECT (9 studies) and 1084 patients were tested with non-clozapine antipsychotics (14 studies) such as flupenthixol, chlorpromazine, risperidone, sulpiride, olanzapine, and loxapine with concurrent ECT treatment considered for systematic review. Of these, 13 studies reported pre and post-treatment scores were included in the meta-analysis.
Main outcome measures
The main outcome measure was the presence and degree of both positive and negative psychotic symptoms, as measured by either of two standardized clinician administered tests, the Brief Psychiatric Rating Scale (BPRS), and the Positive and Negative Symptom Scale (PANSS).
Results
The comparison of the different antipsychotics established the supremacy of ECT-augmented clozapine treatment against other typical and atypical antipsychotics. The Forest Plot revealed that the overall standard mean difference was 0.891 for non-clozapine studies and 1.504 for clozapine studies, at a 95% interval. Furthermore, the heterogeneity plots showed that while clozapine studies showed no significant heterogeneity, non-clozapine studies showed an I2 statistic value at 42.19%, suggesting moderate heterogeneity. Lastly, publication bias showed asymmetrical plots and significant values of Kendal's tau and Egger's rank test.
Conclusion
ECT augmentation technique was found to be effective in the reduction of psychometric scale scores, and the resulting improvement was significant. Clozapine maintained its stance as the most effective treatment for Treatment-Resistant Schizophrenia, followed by flupenthixol.
Keywords: Evidence-based medicine, Psychiatry
1. Introduction
Schizophrenia is a complex mental disorder in which the individual suffers from an altered perception of his/her surroundings, characterized by severe impairment of one’s thoughts, feelings, and actions (National Institute of Mental Health). The underlying cause of the disease has not yet been fully established; hence the treatment focuses more on the abatement of the disabling symptoms (National Institute of Mental Health). The treatment follows an intensive course of pharmacological and non-pharmacological therapies, which are used in combination to design the desired course of treatment for the patient [1]. Clozapine is the gold standard and most effective pharmacological drug prescribed for the treatment of schizophrenia [2]. Besides clozapine, other typical and atypical antipsychotics are also prescribed to the patients, depending upon the side effects and target symptom manifestations.
Clozapine is the favored choice of treatment due to its numerous advantages, such as its superior efficacy for treating both positive symptoms and negative symptoms, relatively high compliance rate compared to other treatment options, advantage in reversing tardive dyskinesia, cost-effectiveness over other agents. Most importantly, clozapine also has the added advantage of mediating addictive behaviors in patients addicted to drugs and the potential to reduce rates of suicide in this population [3]. The studies have shown that 10%–60% of the patients show insufficient improvement following antipsychotic therapy, thereby exhibiting resistance to the drugs even to clozapine [4, 5, 6, 7]. Due to the high Quality of Life burden of schizophrenia, and the low rates of treatment success with antipsychotics alone, augmentation techniques may be pursued to improve therapeutic outcomes [8]. Augmentation techniques are employed in the most serious manifestations of the disease, and as a last line of treatment when antipsychotic treatment alone has failed [9]. Augmentation strategies are applied such as combining more than one antipsychotic agent, adding antidepressants, mood stabilizers, other agents such as glycine, d-cycloserine, d-serine and electroconvulsive therapy (ECT). Some antipsychotics are used for augmentation in various combinations with or without clozapine such as risperidone, olanzapine, amisulpride, quetiapine, aripiprazole [10]. The mood stabilizers such as lithium lamotrigine and valproic acid are frequently used, under the category of antidepressants some are commonly used such as citalopram, duloxetine, and venlafaxine to alleviate symptoms in schizophrenia. However, Electroconvulsive therapy (ECT), has been agreed upon as the most robust and effective augmentation strategy producing valuable results [10].
Many studies have shown that the augmentation of the drug responses by employing electroconvulsive therapies often rapidly improves patient’s symptoms in treatment-refractory schizophrenia [9, 11, 12, 13]. Augmentation with ECT may be used with any antipsychotics, but the best results have been found in conjunction with clozapine. Clozapine is the most effective course of treatment for schizophrenia, especially in severe stages along with ECT augmentation, but the clozapine is a highly underutilized drug because of weekly blood monitoring to detect the presence of agranulocytosis. However the risk of developing agranulocytosis is very low [2, 6]. Therefore, this study is aimed to compare the relative efficacies of ECT augmented antipsychotic therapies with that of clozapine. Such comparative meta-analysis between clozapine and other drugs will help to determine the stark differences between the capabilities of these drugs combined with ECT augmentation strategies. The relative side effects of both clozapine and other antipsychotic drugs were also reported in the systematic review.
Existing literature on the treatment of schizophrenia offers ample insights into the efficacy of augmentation therapies [11, 14] but the relative analysis between the efficacies of different ECT-drug combinations have not been investigated into in much detail. While the current practice is to use clozapine unless contraindicated due to its gold standard status for psycho-active treatment alone, augmented therapy may differ in results. Thus, the present study aimed to compare the relative efficacies of clozapine-ECT therapy over other ECT- augmented psychoactive drugs. A meta-analysis comparing clozapine + ECT against other anti-psychotic drugs in patients with TRS will provide important guidance for clinicians attempting to treat patients with this highly debilitating, chronic condition. The present study improves upon previous studies of [15, 16, 17] by widening the body of included research and comparing them statistically on the basis of pre and post scores.
However, the meta-analysis suffered from a lack of standardization with respect to the research designs adopted across the included studies, specifically the inclusion of both clinical trials and case series. Due to the infrequent use of ECT and the high symptom burden of TRS, individual case reports and case series are more common than clinical trials and it was anticipated that this would create a bias in the literature.
Clinical psychology studies utilize a number of different scales to evaluate the patient’s mental health in terms of psychosis, cognitive abilities, and other global symptoms. The types of scales utilized in the present study were the Brief Psychiatric Rating Scale (BPRS), Positive and Negative Syndrome Scale (PANSS), Clinical Global Impressions (CGI), Mini-Mental State Examination (MMSE), and Global Assessment of Functioning (GAF). These scales are frequently used in clinical studies and assessments of a patient's condition [18, 19, 20, 21]. However, the present study is centered on the BPRS and PANSS scales of psychosis, because these scales presented psychotic improvement in schizophrenia patients and helped to overcome the challenges associated with the efficient comparison of treatment outcomes in meta-analysis. A majority of the studies also adopted the PANSS and BPRS assessment scales. The PANSS scale is superior to BPRS with respect to its predictive power and greater ability to explain the variance. Hence in the present study, the BPRS scores were converted to PANSS as specified in the methodology section below [19].
2. Hypothesis
The study aimed to evaluate and compare the effectiveness of combination therapy involving ECT combined with clozapine, against ECT combined with other anti-psychotics, for patients with TRS. The objectives of the study are as follows:
-
1.
To determine the efficacy of ECT plus clozapine therapy in relieving symptoms of Schizophrenia.
-
2.
To determine the efficiency of ECT and other antipsychotics (non-clozapine) in relieving symptoms of Schizophrenia.
-
3.
To assess whether clozapine + ECT was significantly more effective in the treatment of TRS than other antipsychotics.
3. Methodology
A literature review was conducted in order to identify all published literature of augmented anti-psychotic treatment of TRS, including RCT's, open-label trials, and case series. The search was conducted across the time period of 1990 to March 2017. Analysis of data was conducted using Comprehensive-Meta Analysis V3 Software (Biostat, Inc.).
3.1. Research design
The present systematic review followed the PRISMA guidelines [22]. The first task was defining relevant and appropriate objectives that clearly addressed the research topic. The definition of objectives was followed by the identification of the relevant studies, sourced from public databases like Cochrane, PubMed, and EMBASE, for journals, reports, thesis, and other relevant files.
The collected studies were assessed for their quality by subjecting them to critical review by multiple authors as per the defined inclusion and exclusion criteria that satisfied the study design. The evidence that has been collected by following the research guidelines and questions and quality assessment was then properly summarized.
3.2. Inclusion criteria
In order to be included in the current systematic review, studies meet the following criteria:
-
•
Studies must have been published in English.
-
•
Studies have been published during the period of 1990–March 2017.
-
•
The design of the reported studies either double-blind, controlled trials or case series.
-
•
All participants in included studies must be diagnosed with schizophrenia as per the standardized operational criteria included in the studies.
-
•
Study protocols must have included ECT augmentation with the antipsychotic drug within the treatment plan.
3.3. Exclusion criteria
Exclusion criteria were instituted both to ensure the quality of data that would be included and to ensure only relevant trials would be identified in the literature search. The exclusion criteria were defined as follows:
-
•
Studies reporting either exclusively only ECT treatment or antipsychotics drug treatment alone.
-
•
Any study which included adjunct therapies for augmentation of clozapine such as antidepressants, mood stabilizers etc.
-
•
ECT augmentation, whether clozapine or any other antipsychotic drugs, used in the treatment of any other mental disorders
-
•
Antipsychotics not used concurrently with ECT procedure during treatment.
-
•
Studies including subjects with the history of substance abuse.
-
•
Case reports, systemic reviews, or previously published metanalyses.
3.4. Information sources and searches
The literature collected in the present research involved the findings of open trials, case series, retrospective studies, randomized clinical trials and open-label trials and studies. English language databases were searched, including PubMed, EMBASE, Cochrane Review Library, and PubMed Clinical inquiries, for the period of 1990–March 2017. The keywords employed for performing the research were those mentioned in Table 1.
Table 1.
Keywords used for searching the databases for relevant literature.
| Individual Keywords |
Combination of Keywords |
||
|---|---|---|---|
| Antipsychotics | In combination with “ECT." | In combination with “ECT + Schizophrenia” | |
| Electroconvulsive therapy (ECT) | Clozapine | Antipsychotics | |
| Risperidone | Schizophrenia | ||
| Schizophrenia | Olanzapine | Clozapine | |
| Quetiapine | Risperidone | ||
| Ziprasidone | Olanzapine | ||
| Chlorpromazine | Quetiapine | ||
| Flupenthixol | Ziprasidone | ||
| Risperdal | Chlorpromazine | ||
| Lurasidone | Flupenthixol | ||
| Zyprexa | Risperdal | ||
| Aripiprazole | Lurasidone | ||
| Haloperidol | Zyprexa | ||
| Fluphenazine | Aripiprazole | ||
| Thiothixene | Haloperidol | ||
| Trifluoperazine | Fluphenazine | ||
| Loxapine | Thiothixene | ||
| Sulpiride | Trifluoperazine | ||
| Loxapine | |||
| Sulpiride |
3.5. Study selection and exclusion
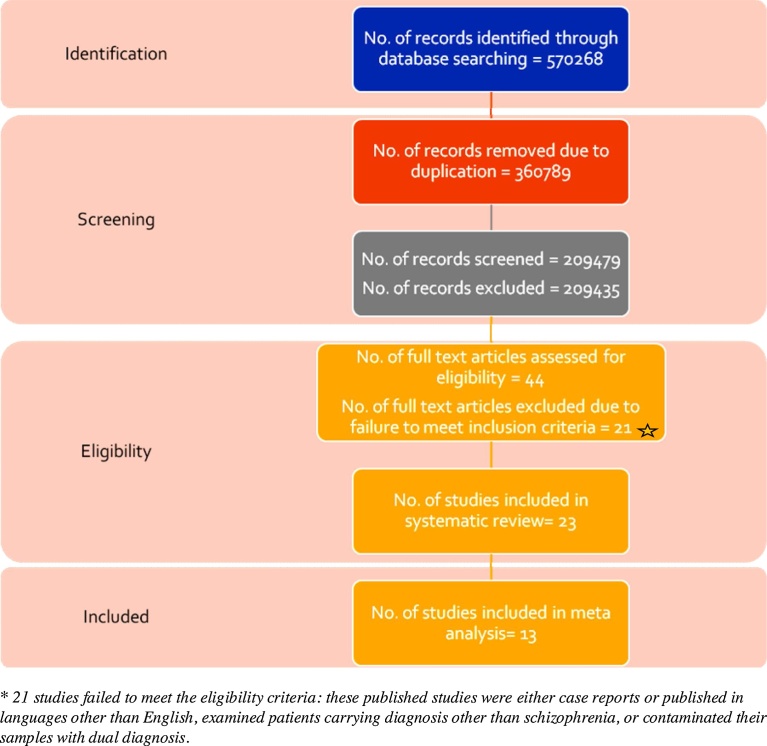
Different electronic databases were searched using single keywords or combinations of different keywords as mentioned in Table 1. The search yielded a total of 570,268 results. During the screening of the abstracts of these articles; only 44 articles were found to have fulfilled the inclusion criteria which was defined for the current study. Moreover; duplicates and studies not meeting the basic requirements of the research were eliminated. Only 23 of the 44 studies were found to be eligible for systematic review; dedicated to the specific course of treatment and patient sub groups. Out of these; 13 studies relating to the use of ECT-clozapine treatment and ECT-nonclozapine treatment (all the antipsychotics other than clozapine) were differentiated and reviewed separately during metanalysis. This was done essentially to evaluate and compare the relative efficacies of the two therapeutic methods. The overall layout of the selection process is depicted in Fig. 1.
Fig. 1.
Literature search and selection flow chart of studies included.
3.6. Primary outcome
The refined results of literature review resulted in 23 studies, as shown in Fig. 1, which were further classified into two groups, clozapine (9 studies) and non-clozapine (14 studies) antipsychotic drug treatments, augmented by ECT. All trials involved concomitant administering of ECT and antipsychotic medications, although duration and dosage varied. Some of the studies were randomized and controlled using ECT and placebo ECT treatments, whereas others were open clinical trials, case series, or retrospective chart reviews of the patients already admitted for schizophrenia treatment (see Table 2).
Table 2.
Study design and demographic profile of patients included in the selected studies.
| Details | Clozapine (N = 9) | Non-Clozapine (N = 14) |
|---|---|---|
| Study Design | ||
| Randomized Control Trials | 2 | 4 |
| Case Series | 1 | 0 |
| Open-Label Trials | 6 | 10 |
| Demographic Details | ||
| Target Population | 98 | 1084 |
| Age | 35.7 | 32.85 |
| Gender*(female/male) | 39/56 | 529/456 |
| Duration of Illness**(yr) | 15.36 | 11.11 |
| Dosage of drug***(mg/day) | 392.18 | Chlorpromazine = 235.99 |
| Flupenthixol = 21.64 | ||
| Risperidone = 6.26 | ||
| Loxapine = 36.7 | ||
| No. of ECT treatments**** | 13.44 | 13.2 |
3.7. Data extraction
Data-extraction was performed by three authors (S.A., A.K., and H.M.) independently and using a pre-devised format. In the event of any discrepancies in data-extraction, one of the authors independently analyzed the study (S.A.) and the combined results were discussed. Data extracted included: author, year, patient demographic information (gender, age, and country), study design, number of participants, treatment plans (number of ECT treatments, types of antipsychotics, and dosage), clinical interview type and results.
4. Analysis
Post-systematic reviews of the 23 selected studies were re-examined for inclusion, on the basis of the type of psychiatric scales used for evaluation of mental health and relative availability of the pre- and post-treatment scores in the meta-analysis. Thus, the studies which failed to report the results on either BPRS or PANSS scales, with respect to both the pre and post treatment scores, could not be analyzed. The meta-analysis, according to the random-effects model, was then conducted on the included studies, using comprehensive meta-analysis software (CMA v3, Englewood, NJ, USA). The random-effects model was chosen as a meta-analytic strategy due to the non-negligible differences in its methodology and design. An overall effect within each treatment group was calculated based on the clinician-administered interviews (BPRS and PANSS), and on differences in mean values with 95% confidence intervals. The data from the BPRS and PANSS scales for both the clozapine and non-clozapine group of studies was used, and the studies were analyzed by utilizing the linked score database as generated [23], according to the EQUIPERCENTILE method. Heterogeneity was calculated using the I2 test statistic. Finally, publication bias was estimated using funnel plots, Begg and Mazumdar correlation rank tests and Egger’s linear regression intercepts [24, 25]. Sensitivity analysis was done by removing the studies which had effect sizes of SMD > 2.0, which represented an outlying effect size. Correlation values between the pre and post scores were imputed since they were not available for any of the studies [26].
5. Results
A total of 23 studies were included in the systematic review, with 9 assessing ECT and clozapine treatment, and 14 studies assessing augmented ECT treatment with other antipsychotics. Demographic details, disease characteristics, and average dosage were included and represented in Table 2. Information regarding non-clozapine-ECT treatment is mentioned in Table 3 and clozapine-ECT treatment in Table 4, respectively. The 23 studies included 1179 patients, wherein 95 patients were treated with concurrent administration of clozapine and ECT, and 1084 patients received ECT treatments with simultaneous administration of drugs belonging to the non-clozapine group. The main drugs belonging to the non-clozapine class, encountered in the present studies were flupenthixol, chlorpromazine, risperidone, olanzapine, sulpiride, and loxapine, with the majority being administrations of flupenthixol.
Table 3.
Systematic review of studies administering non-clozapine augmented with ECT.
| Author (Year, Place) | Age (mean± SD) | Design | Number of patients | Setting | General anesthesia, electrode placement, seizure threshold | Duration of Illness (mean± SD in years) | Dosage of Drug (mg/day) | Mean number of treatments | Scales used for Evaluation | Result |
|---|---|---|---|---|---|---|---|---|---|---|
| Chanpattana et al. (2000, Thailand) | 32.2 ± 7.2 | Open-label trial | Total = 21 | Inpatient and Outpatient | General anesthesia, bilateral electrodes, seizure threshold: NA | 10.8 ± 6.2 | Flupenthixol = 12- 24 | 11.4 ± 5 | Psychotic: BPRS | Overall improvement was seen as BPRS score were found to be reduced from 50.5 ± 9.1 to 14.2 ± 7.8 at the end of Phase 2 |
| Chanpattana et al. (2000, Thailand) | 1ST* = 35.1 ± 8.3; 2ST = 35.2 ± 8.2; 4ST = 33.5 ± 7.4 | Randomized, double-blind | Total = 62; 1ST-Remitter = 11;Non-remitters = 10 2ST-Remitter = 11;Non-remitters = 10 4ST- Remitter = 11;Non-remitters = 9 | NA | General anesthesia, bilateral electrodes, seizure threshold: Baseline: 1ST = 75.4 ± 30; 2ST = 79.5 ± 23.4; 4ST = 81.2 ± 28.7 Tenth: 1ST = 184.3 ± 95.2; 2ST = 188.9 ± 105.5; 4ST = 229.5 ± 75.3 Increment: 1ST = 107.5 ± 84.8; 2ST = 108.8 ± 111; 4ST = 147.8 ± 86, empirical titration technique | 1ST = 15.7 ± 7.9; 2ST = 14.0 ± 7.3; 4ST = 12.9 ± 5.5 | Flupenthixol 1ST = 22.9 ± 2.4; 2S = 23.1 ± 2.2; 4ST = 23.1 ± 2.2 | At first improvement: 1ST = 13.6 ± 5.0; 2ST = 7.5 ± 3.8; 4ST = 4.2 ± 1.5 At the end of the study: 1ST = 18.6 ± 5; 2ST = 12.5 ± 3.8; 4ST = 9.2 ± 1.5 | Psychotic: BPRS | Overall improvement was seen as BPRS scores for the three groups 1ST, 2ST, & 4ST were found to be reduced by 62.6%, 60.8% & 65.6% from baseline scores of 51.8 ± 10.7, 48.7 ± 7.2 & 47.9 ± 6.1 respectively. |
| Chanpattana & Chakrabhand et al. (2001, Thailand) | Responders = 31.9; Non-responders = 37.1 | Clinical Trial | Total = 293; Responder = 160; Non-responders = 133 | Inpatient and outpatient | General anesthesia, bilateral electrodes, Initial seizure threshold: women: 93.6 ± 32.3; men: 94.2 ± 35.3; Responders: Women: 89.1 ± 35.8; men: 92.2 ± 37.0; non-responders: women: 97.6 ± 27.3; men: 90.6 ± 33.0; empirical titration technique | Responders = 11; Non-responders = 16.6 | Flupenthixol 12 mg/day during the first week then increased to 24 mg/day. | Responders = 12.5; Non-responders = 20.2 | Psychotic: BPRS | Significant reduction was observed in the values of different parameters of BPRS profile. There was a marked improvement in positive symptoms, but the negative symptoms showed limited improvement. |
| Chanpattana & Sackeim et al. (2010, Thailand) | Responders = 31.9; Non-responders = 36.7 | Clinical Trials | Total = 253; Responders = 138; Non-responders = 115 | Inpatient and outpatient | General anesthesia, bilateral electrode, initial seizure threshold: responders: 94.5; non-responders: 94.9, empirical titration technique. | Responders = 10.9; Non-Responders = 16.2 | Flupenthixol Responders = 21.5; Non-responders = 22.1 | Responders = 12.5; Non-responders = 20.2 | Psychotic: BPRS | Improvement in BPRS scores was observed with females showing greater extent of improvement than males. |
| Goswami et al. (2003, India) | ECT = 29.8 ± 8.54; Sham-ECT = 29.1 ± 5.7 | Randomized, Double blind, Controlled | Total = 25; ECT + Chlorpromazine = 15; Sham-ECT + Chlorpromazine = 10 | NA | General anesthesia, Bilateral electrode, seizure threshold- 50 to 200% | ECT = 7.6; Sham-ECT = 6.9 | Chlorpromazine = 500 mg/day | Psychotic: BPRS | Both the groups showed a reduction in the BPRS scores with ECT group showing the slightly higher level of score reduction, 44 ± 7.6 from 55 ± 7.2.CGI scores improved only for the ECT group from baseline 4.9 to 4.13 in week 4. ECT + drug treatment is better | |
| Hirose et al. (2001, Japan) | 29.5 ± 8.959(calculated) | Open trials | Total = 10 (males only) | Inpatient | Anesthesia: NA, bilateral electrode placement, seizure threshold: NA | 9.85 ± 8.794 (calculated) | Risperidone = 6.2 ± 2.097 (calculated) | 6.6 ± 1.712 (calculated) | Psychotic: BPRS | A significant reduction in the HS from the baseline value of 6.6 ± 0.16 to 1.1 ± 0.1. PS score reduced from 13.2 ± 2.6 to 4.1 ± 0.11. |
| Ravanic et al. (2009, Serbia) | Sulpiride = 38.52; Risperidone = 33.30; Olanzapine = 33.6 | Open labeled Active control | Total = 70; Sulpiride = 17; Risperidone = 26; Olanzapine = 27 | Outpatient | Anesthesia: NA, unilateral electrode, seizure threshold: NA | Sulpiride group = 10.43; Risperidone group = 7.1; Olanzapine group = 9.08 (calculated) | Sulpiride = 294.52; Risperidone = 6.32; Olanzapine = 6.82 | Sulpiride = 2.5; Risperidone = 6.32; Olanzapine = 6.82 | Psychotic: PANSS | The PANSS scores for general psychopathology reduced from 55.88 ± 14.28 to 25.13 ± 6.03 for Sulpiride, 55.78 ± 15.62 to 20.81 ± 5.85 for Risperidone, and 56.11 ± 16.27 to 19.23 ± 5.19 for Olanzapine. As observed from the score change, Olanzapine is the most effective of all the drugs. |
| Sarita et al. (1998, India) | 18-45 | Double-blind randomized Controlled study | Total = 36; ECT + Drug = 24; Sham ECT + Drug = 12 | NA | NA | > 2 | Group 1: unilateral ECT + haloperidol = 14.2 mg; Group2: bilateral ECT+ haloperidol = 14.6 mg; Sham ECT + Drug = 18.3 | 3 | Psychotic: BPRS | There was a reduction in BPRS scores of the ECT and Sham-ECT groups. However, not very significant differences in the treatment result were observed. |
| Chanpattana & Kramer et al. (2003, Thailand) | 32 ± 6.4 | Open trials | PHASE 1 Total = 59; Responders = 52; Non-responders = 7 | NA | Anesthesia: NA, bilateral electrode, seizure threshold: NA, titration | NA | Phase 1 − Flupenthixol 12 to 24 mg |
12.3 ± 4.5 | Psychotic: BPRS | Significant results were obtained regarding the improvement of condition. Reduction in BPRS scores from Baseline = 48.5 ±7.3 to Endpoint = 17.1 ± 9.9; |
| 32 ± 6.4 | Open trial | PHASE 2 Total = 52; Responders = 46; Dropouts = 6 | NA | Anesthesia: NA, bilateral electrode, seizure threshold: NA, titration | 9.9 ± 5.1 | Phase 2 − Flupenthixol 23.1 ± 2.2 |
24.6 ± 2.4 | |||
| Chanpattana et al. (1999, Thailand) | PHASE 1 Responders = 33.2 ± 8.0; Non-responders = 38.6 ± 7.2 | Open trials | PHASE 1 Total = 101; Responders = 58; Non-responders = 43 | Inpatient and outpatient | General anesthesia, Bilateral electrode seizure threshold: NA | PHASE 1 Responders = 12.4 ± 6.7; Non- responders = 18.1 ± 7.7 | PHASE 1- Flupenthixol Responders = 21.0 ± 4.2; Non-responders = 23.6 ± 3.8 | PHASE 1 Responders = 13.9 ± 4.8; Non-responders = 20.4 ± 0.8 | Psychotic: BPRS | PHASE 1 Reduction in the BPRS scores of responders from baseline score of 49.1 ± 9.6 to 18.7 ± 7.2 at the end of phase I of the treatment was observed. |
| ECT = 32.7 ± 8.4; ECT + drug = 36.7 ± 8.2; Drug = 33 ± 6.8 | PHASE2; Total = 45; ECT(I) = 15; ECT(II) + Flupenthixol = 15; Flupenthixol (III) = 15 |
Inpatient and outpatient | General anesthesia, Bilateral electrode seizure threshold: NA | ECT = 11.9 ± 6.8 ECT + drug = 13.7 ± 5.5; Drug = 14.2 ± 6.4 | Phase 2 − ECT = 0; ECT + Flupenthixol = 22.0 ± 3.7; Flupenthixol = 22.4 ± 2.7 | Significant reduction in the BPRS scores was observed in patients receiving the combination of drug and ECT, from 44.9 ± 8.2 at entry to 16.7 ± 5.9 at the end for treatment I, 51.4 ± 9.0 to 14.1 ± 7.9 for treatment II and 49.0 ± 8.6 to 18.1 ± 4.2 for treatment III. | ||||
| Sajatovic et al. (1993, USA) | 28.9 ± 7.6 | Open trial | Total = 9; ECT + Loxapine Responders = 5; Non-responders = 4 | Inpatient | Anesthesia: NA, Unilateral and bilateral electrode placement, seizure threshold: NA | 10.4 ± 3.8 (calculated) | Loxapine = 36.7 ± 36.1 | 7.9 ± 2.3 | Psychotic: BPRS | Change in BPRS score from baseline = 51.6 ± 12.7 to endpoint = 35.8 ± 9.7 in 5 patients. 4 proved to be Non-responders |
| Chanpattana et al. (2000, Thailand) | Responders = 32.4 ± 7.9; Non-Responders = 38.4 ± 7.2 | Open trial | Total = 93; Responders = 56; Non-responders = 37 | NA | General anesthesia, bilateral electrode placement, seizure threshold − based on Thymatron and MECTA default settings | Responders = 12.00 ± 6.4; Non- responders = 18.0 ±7.9 | Flupenthixol; Responders = 22.8 ± 2.7; Non-responders = 23.1 ±3.0 | Responders = 13.1 ± 4.3; Non-responders = 20.3 ± 0.9 | Psychotic: BPRS | BPRS scores reduced by 62.4 ± 16.7% from an initial score of 48.7 ± 9.1. |
| Ukpong et al. (2002, Nigeria) | ECT = 27.7 ± 10.3 Sham-ECT = 24.3 ± 5.5 | Double-blind randomized control study | Total = 20; ECT+ Chlorpromazine = 11 (2); Sham-ECT+ Chlorpromazine = 9 (2) | Inpatient and outpatient | General anesthesia, bilateral electrode placement, seizure threshold: NA | ECT = 8.4 ± 9.19; Sham-ECT = 5.0± 6.0 | Chlorpromazine; ECT + Drug = 306.5; Sham ECT + drug = 285 | 12 for both groups | Psychotic: BPRS | Reduction in BPRS scores occurred for both the groups- ECT + Drug − Baseline = 22.33 ± 7.83, Endpoint = 1 ± 3; Sham ECT + Drug– Baseline = 19.43 ±7.28, Endpoint = 1.29 ±3.42 |
| Chanpattana & Chakrabhand et al. (2001, Thailand) | Weekly = 30.1 ± 7.5; Biweekly = 33 ± 6.2; Triweekly = 28.7 ± 7.3 | Open trial | Total = 32; Weekly ECT = 8; Biweekly ECT = 17; Triweekly ECT = 7 | NA | Anesthesia: NA bilateral electrode, seizure threshold: NA, empiric titration method | Weekly = 13.6 ± 6.0; Biweekly + 11.7 ± 5.5; Triweekly = 7.7 ± 3.6 | Flupenthixol Weekly = 21 ± 5.6; Biweekly = 22.2 ± 3.5; Triweekly = 18.9 ± 4.1 |
Weekly = 15 ± 4.5; Biweekly = 11 ± 3.7; Triweekly = 13.4 ± 7.2 | Psychotic: BPRS | Reduction in BPRS scores of selected items of the rating scale, besides the positive and negative symptoms. |
*Seizure Threshold.
*NA: not available.
Table 4.
Systematic review of studies administering clozapine augmented with ECT.
| Author (Year, Country) | Age (mean years) | Design | Number of patients | Setting | General anesthesia, electrode placement, seizure threshold | Duration of Illness (mean years) | Dosage of Drug (mg/day) | Mean number of treatments | Scales Used For Evaluation | Inference |
|---|---|---|---|---|---|---|---|---|---|---|
| Petrides et al. (2015, USA) | ECT+ Clozapine = 35.7 ± 2.27; Clozapine = 42.78 ± 1.82 | Randomized, Controlled, Single-blind, Crossover trial | Total = 39; ECT+ clozapine = 20; clozapine = 19 | Inpatient | General anesthesia, bilateral electrodes, seizure threshold: 5 to 60% | > 2 | ECT+ Clozapine = 525; clozapine = 511.1 | Randomized phase = 15.8 ± 4.2; Crossover phase = 14.3 ± 5.3 | Psychotic: BPRS | 50% response rate was recorded, 40% reduction of the symptoms, & 60% response rate for the congenial response criteria. |
| Gray & James et al. (1999, UK) | 30 | Open-Label Trial | Total = 6 | Inpatient | General anesthesia, bilateral electrodes, seizure threshold: NA | 14.33 | 525 | 12 | Psychotic: BPRS | Improvement in BPRS from Baseline = 71 to Endpoint = 48 |
| Kho et al. (2004, Netherlands) | 43 | Open-label trial | Total = 11 | Inpatient, one patient was outpatient | General anesthesia, Unilateral, later bilateral electrodes, seizure threshold: based on age | 16.16 | Nil | 8.1 | Psychotic: PANSS | The PANSS scores reduced from 74.54 to 49.181 |
| Masoudzadeh et al. (2007, Iran) | Clozapine = 31; ECT = 33; combined therapy = 30 | Randomized control trial (Unblinded study) | Total = 18; ECT = 6; clozapine = 6; Clozapine + ECT = 6 | Inpatient | Drug-induced sedation without causing seizure, Unilateral electrode | Nil | 200 | 12 | Psychotic: PANSS | 46% reduction in the PANSS score of sham ECT group, 40% reduction in sham clozapine group, and 71% reduction was seen in the ECT + Clozapine group. PANSS scores for the combination therapy group reduced from 99 to 29, with p-value 0.001. |
| Kim et al. (2017, Korea) | Female = 44.4 ± 14.2; Male = 34.0 ± 15.6 | Retrospective Case series study | Total = 7; Female = 5; Male = 2 | NA | General anesthesia, bilateral electrodes, seizure threshold: NA | Female = 16.0 ± 8.9; Male = 12.5 ± 10.6 | Before ECT = 350 ± 146.5 After ECT = 260.7 ± 95.6 During ECT >350 | 13.4 ± 4.6 | Psychotic: PANSS | Significant reduction in the mean PANSS scores from 70.1 ± 17.9 to 52.3 ± 17.9; One patient did not respond to ECT. |
| Benatov et al. (1996, Israel) | 42.33 (calculated) | Case series | Total = 3 | Inpatient | General anesthesia NA, Bilateral electrode placement, Seizure threshold: NA |
23.33 (calculated) | 416.66 (calculated) | 14.33(calculated) | Psychotic: BPRS & PANSS | All the patients showed an overall improvement in BPRS, PANSS as follows: Patient 1: BPRS − 68 to 39 PANSS − 122 to 78 Patient 2: BPRS- reduced by 40% from 75 PANSS- 125 to 72 Patient 3: Positive effect on symptoms |
| Frankenburg et al. (1993, USA) | 37.66 ± 8.731 (calculated) | Open trial | Total = 12 Responders = 10; Non-responders = 2 | Inpatient | General anesthesia, unilateral electrode, stimulus to cause seizure for 30 −60 secs. | 14.916 ± 5.017 (calculated) | 322.91(calculated) | 10.08 ± 4.813 (calculated) | No scale was reported. | Improvement was reported in terms of the extent of clinical response. 3 patients showed high response, 1 showed moderate response, 4 a minimal response, 2 minimal to no response, and 2 no response. |
| Cardwell et al. (1995, USA) | 41.25 | Retrospective chart review | Total = 7; Male = 3; Female = 4 | NA | NA | 1 year | 580 | 21.6 | Psychotic: BPRS | BPRS scores improved by 26.9% |
| Grover et al. (2015, India) | 32.7 ± 6.4 | Total = 11 | Outpatient | Anesthesia: NA, Bilateral electrodes, seizure threshold: NA | 123.4 ± 60.5 months | 339.8 ± 120.8 | 12.81 ± 6.6 | Psychotic: PANSS | PANSS scores reduced from baseline 77.1 ± 15.1 to Endpoint = 52.8 ± 9.6 |
5.1. BPRS: Brief Psychiatric Rating Scale; PANSS: Positive and Negative Syndrome Scale; MMSE: Mini-Mental State Examination
All the studies reported improvement in the BPRS or PANSS scores of the patients after they underwent ECT augmentation with antipsychotics. However, in two studies [36, 37], exacerbation of negative symptoms of schizophrenia, post-ECT augmentation with flupenthixol, was observed in some patients. Other studies [27, 31, 38, 39] reported exhibition of mild extrapyramidal symptoms in some patients. Also in the study conducted by Chanpattana and Sackeim et al., 2010 [36] improvement of the BPRS scores was observed to a greater extent in females than males. Another unique study [40] recorded higher improvement in the scores of female subjects across all three of its study groups. The study groups differed on the basis of the intensity of the ECT treatment administered to the patients.
Two studies researched to ascertain the efficacies of ECT augmentation by grouping the patients into ECT and placebo-ECT groups [35, 27]. These studies found that ECT-group patients demonstrated higher improvement in the psychometric analysis. However, the resulting differences were not statistically significant. In a comparative study Ravanić DB et al., 2009 [31] involving three drugs, sulpiride, risperidone, and olanzapine, olanzapine augmentation showed the highest improvement in outcome measures, with the highest reduction in PANSS scores observed from 56.11 ± 16.27 to 19.23 ±5.19, post-treatment. The highest degree of success of ECT augmentation therapy was reported by Chanpattana et al., 1999 in open trials carried out in two phases, using ECT augmentation with risperidone, with BPRS scores improving from 49.1 ±9.6 at entry to 18.7 ± 7.2 for the responders, and 51.4 ±9.4 to 21.7 ± 17.7 for non-responder groups [38].
5.2. ECT augmentation of clozapine
All studies testing clozapine-augmented ECT-therapy found an improvement in BPRS and PANSS scores, post-ECT augmentation treatment, and showed promising results in stabilization of disabling symptom even in aggressive patients [41]. It was also seen that clozapine administration produces only mild extra-pyramidal symptoms, contrary to the belief that clozapine is a harmful drug with significant side effects, resulting in its underutilization [6]. Similar insufficiency of life-threatening side effects as a reason for rejection of clozapine have been demonstrated by epidemiological studies carried out in the USA and Finland [42, 43].
5.3. Meta-analysis
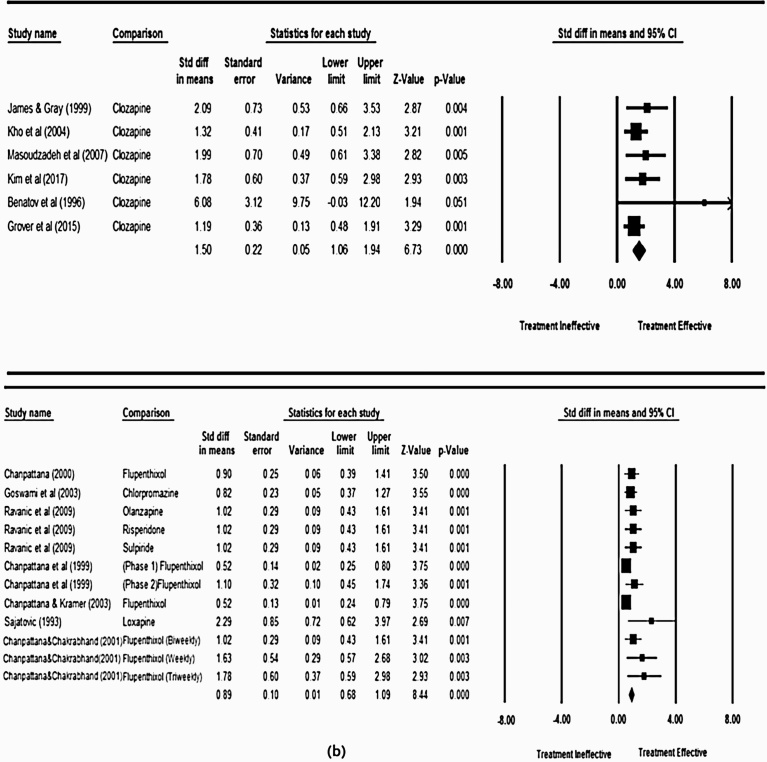
The present meta-analysis drew its conclusions from pre- and post-treatment scores of the psychometric scales, which were provided in only 13 studies out of the 23 studies. In these studies, the treatment was assessed using either BPRS or PANSS scales. The analyses were conducted separately for clozapine and non-clozapine studies, to compare treatment efficacy. Effect sizes were calculated in order to determine whether clozapine and non-clozapine treatment regimens lead to different results, with efficacy and sensitivity of the treatment calculated according to Durlak et al., 2009 [44]. The larger the value of effect size, the larger the difference in outcome, and the greater the efficacy of the superior regime. The overall effect size (standard difference in means) for non-clozapine and clozapine groups was 0.89 and 1.50, at 95% confidence interval respectively, as shown in Fig. 2. This indicated the higher efficacy of combined clozapine and ECT procedure in the treatment of schizophrenia, as compared to other antipsychotics.
Fig. 2.
Forest plot for (a) Non Clozapine plus ECT and (b) Clozapine plus ECT treatment efficacies respectively.
Within-group analysis allowed the assessment of best possible methodology for the treatment of treatment resistant schizophrenia. This was done by comparing the within-group effect sizes, such that a large effect size indicated a larger improvement from pre- to post-intervention test scores. In the non-clozapine group, the tri-weekly ECT treatment accompanied with flupenthixol drug as administered by Chanpattan et al., 2000 [40] was found to be the most effective with an effect size of 1.78, p = 0.003 as shown in Fig. 2 (a). The study by Satajovic and Meltzer et al., 1993 [32] in the clozapine group and Benatov et al., 1996 [45] in non-clozapine group had higher values of effect size, 2.29 and 6.08, but a wide confidence interval. Hence, these studies were concluded to be less precise [46]. Similarly, within the clozapine group of studies, the results from the study [41] presented by the effect size of 2.09, p = 0.004, were found to represent the largest effect size. The current metaanalysis included the administration of an average of 12 ECT treatments in participants, with a mean clozapine dosage of 525 mg/day.
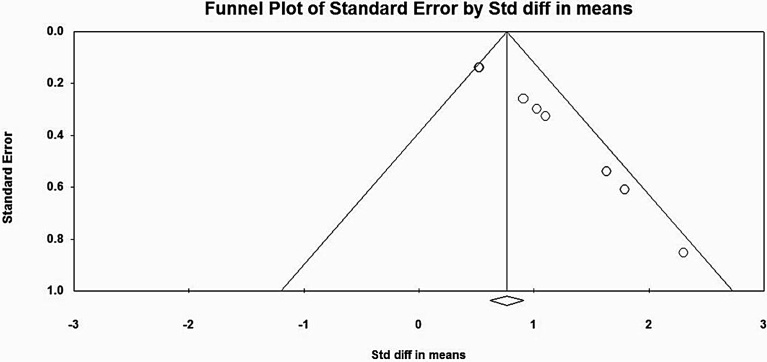
Concerning heterogeneity studies, only the non-clozapine group of studies showed evidence of heterogeneity. The value of I2 statistic was 42.19, indicating moderate heterogeneity among the studies [47]. Publication bias was tested, and the funnel plot was obtained as shown in Fig. 3 and Fig. 4. The plot for the non-clozapine group was asymmetrical as shown in Fig. 3, and the Begg Mazumdar (Kendall’s tau = 0.98, p = 0.00) and Egger’s rank test (intercept = 2.80, p = 0.00) indicated the presence of bias.
Fig. 3.
Funnel plot for Publication bias in Non-Clozapine group of studies.
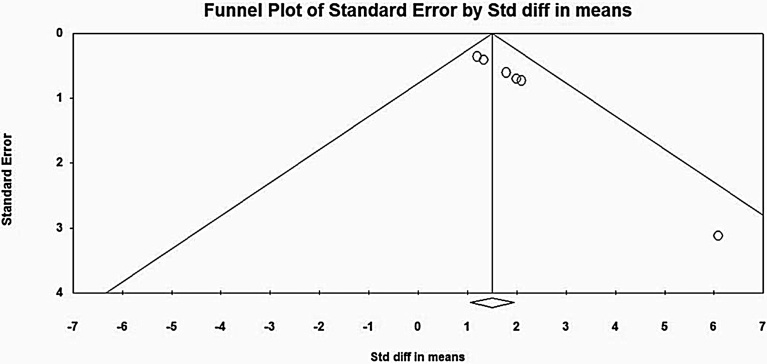
Fig. 4.
Funnel Plot for Publication Bias in Clozapine group of studies.
However, the clozapine group of studies did not demonstrate heterogeneity (I2 = 0), indicating that the studies showed similar intervention effects. Following similar methodology, publication bias was assessed with a funnel plot as shown in Fig. 4. The plot was asymmetrical, and again, the Begg-Mazumdar (Kendall's tau = 0.93, p = 0.004) and Egger's rank test (intercept = 1.97, p = 0.00) indicated the presence of bias. Sensitivity analysis was carried out for both groups of studies separately, and no significant change in the overall effect size was observed. Hence the results could be regarded with high certainty [47].
5.4. Side effects of ECT
ECT can be very effective in treating many psychiatric conditions, but there are potential concerns regarding its more adverse effects, ranging from cognitive effects, prolonged seizures, alterations in blood pressure, and cardiovascular complications. Earlier in its history, ECT was administered without anesthesia; later anesthesia was added to it in order to reduce its side effects, such as muscle damage and bone fractures. Also, back in the beginning, sine-wave electrical current ECT devices were the most commonly used ones. However, because of their cognitive side effects, for example, memory impairment, the American Psychiatric Association eventually considered this type of ECT unjustified [48], and now it is recommended to use brief-pulse wave as the standard ECT treatment [48, 49]. Despite concerns regarding the safety of ECT, the majority of studies from this meta-analysis didn't observe the occurrence of any significant adverse effects with combination treatment [11, 13, 16, 30, 31, 32, 34, 50]. Conversely, a few studies did report potential side-effects from ECT [22]. Grover et al., 2015 reported that two of its study participants developed prolonged seizures, and one patient developed a transient rise in blood pressure [29]. Another study also reported that patients with ECT treatment developed seizures [33]. Still another study reported a higher incidence of a headache and memory impairment in subjects who received ECT [14].
6. Discussion and conclusion
Treatment Resistant Schizophrenia (TRS) is defined as schizophrenia cases where there have been at least two failed treatment trials with antipsychotic medications administered with a minimum duration and dosage [51]. A patient is found to have treatment-resistant schizophrenia when (1) there are at least three periods of treatment in the preceding 5 years with neuroleptic agents (belonging to two different chemical classes) at dosages equivalent to or greater than 1000 mg/day of chlorpromazine for a period of 6 weeks, each without significant symptomatic relief, and (2) no period of good functioning within the preceding 5 years. The present meta-analysis aimed to determine whether clozapine in conjunction with ECT was more effective than other typical and atypical antipsychotics in conjunction with ECT in the TRS population. The results of the meta-analysis demonstrate that clozapine is significantly more effective than chlorpromazine, although there are concerns about the heterogeneity of the literature, and evidence of publication bias. This analysis is unique in that it has compared different groups of antipsychotic drugs used for schizophrenia treatment by utilizing pre- and post-treatment self-report scores of function and not just the proportion of patients showing improvement as a study done by Lally et al., 2016 [15]. The analysis of scores produced significant information with respect to the degree of relief provided by the treatment design during the course of procedure. It also allowed for intra- and inter-class comparison of the non-clozapine and clozapine drugs respectively. This inter-drug comparison found clozapine to be more effective in treating schizophrenia symptoms. This is in-line with previous research [15, 52, 53]. It was also seen that, among the non-clozapine groups, flupenthixol was most frequently used, and was found to be the most efficient in relieving treatment-resistant schizophrenia, when augmented with ECT. Previous analyses and studies [14, 54] have focused on the comparison of treatment and control groups, with respect to a specific drug class, and this has led to the ignorance and exclusion of other important information generated by the open trials. Hence, the comparison of the results produced by these other studies, directed towards relieving the symptoms of treatment-resistant schizophrenia as researched in this present study, not only identified the superior drug for treatment, but also allowed identification of the most effective drug within the different classes.
6.1. Limitations
The current meta-analysis has benefited from access to a large sample size derived from studies of acceptable quality. However, there are still limitations to the scope of this work which would benefit from being expanded in future research. The first limitation was the methods used in measuring efficacy of the treatment regimes. The decision to use pre/post survey data led to some of the studies necessarily being excluded, as this data did not match their design. Future research would benefit from devising scoring systems which include these studies. The systematic review also revealed that there was a lack of RCTs conducted using pharmaceutically augmented ECT regimes. The present metaanalysis included published literature up to March 2017. The latest published retrospective case series study by Kim et al., 2017 [11], was found eligible for the study. However, the dearth of RCTs studies, which led to reliance on open-label trials, subsequently introduces the possibility of bias due to lack of randomization blinding. Heterogeneity in the literature also limited the findings of this current study. Particularly within the non-clozapine trials, methodological differences were commonplace. This makes drawing inferences difficult, but no less important. Future assessments would benefit in further expanding the literature in non-clozapine-augmented ECT studies, even expanding upon the findings of Chanpattana et al., 2010 [36], which found gender differences in the efficacy of flupenthixol. Furthermore, only studies published in English were considered for analysis. Research conducted in other languages would not doubt contribute significantly to the knowledge base around this subject. The authors encourage any researchers with findings in another language to present or publish them. Translation was considered for the current study, but the risk of introducing errors into the data was considered too great.
6.2. Implications of the research
The conclusions and limitations of the present research provide fertile ground for future research. The absence of a large field of RCT’s on this subject is a significant limitation to informed clinical practice, and considering the efficacy demonstrated in the current meta-analysis, future work in this area would seem worthwhile. ECT has a controversial history. But in light of its potential in treatment-resistant conditions of many kinds, including schizophrenia, coupled with the current lack of understanding of its various mechanisms of action and outcomes, further, more in-depth research is likely to provide patients with significant benefits.
It is recommended that clozapine be utilized as the “gold-standard” antipsychotic medication in conjunction with ECT. Any future trials should seek to test ECT-augmented antipsychotic therapy both against a placebo control, and a gold-standard therapy. Both superiority and non-inferiority trials would be of value because clozapine has a significant side-effect profile, and is, despite its benefits, still not prescribed by many psychiatrists [55].
Identification of the most efficacious combination therapy and assessment of how well these therapies are tolerated could lead to improvements in both the quality of life and compliance for patients currently experiencing poor outcomes. Although this metaanalysis has provided significant new information suggesting a new first-line treatment, the body of research in this field continues and should continue to grow, with further analyses being conducted to update its findings. Assessment of long-term studies focusing on compliance, long-term side-effect profiles, and symptom-management will inform treatment recommendations in a way that was, unfortunately, not possible in the present analysis.
Future trials could improve on the current literature by increasing duration of follow-up assessment. Concerns of consent rates and patient attitudes towards ECT are also of concern. Specific programs designed to better explain and address concerns about this procedure would likely improve consent, compliance, and retention.
Declarations
Author contribution statement
Saeed Ahmed: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ali Mahmood Khan: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Hema Madhuri Mekala: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hema Venigalla: Performed the experiments.
Rizwan Ahmed, Amira Etman: Contributed reagents, materials, analysis tools or data.
Michael Esang: Conceived and designed the experiments; Performed the experiments, Wrote the paper.
Mustafa Qureshi: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Patel K.R., Cherian J., Gohil K., Atkinson D. Schizophrenia overview and treatment options. Pharmacy and Therapeutics. 2014;39(9):638. [PMC free article] [PubMed] [Google Scholar]
- 2.Meltzer H.Y. Clozapine balancing safety with superior antipsychotic efficacy. Clin. Schizophr. Relat. Psychoses. 2012;6(3):134–144. doi: 10.3371/CSRP.6.3.5. [DOI] [PubMed] [Google Scholar]
- 3.Joober R., Boksa P. Clozapine a distinct, poorly understood and under-used molecule. J. Psychiatry Neurosci. 2010;35(3):147. doi: 10.1503/jpn.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkis H. Therapy-Resistant Schizophrenia. Karger Publishers; 2010. History and current definitions of treatment-resistant schizophrenia; pp. 1–8. Vol. 26. [Google Scholar]
- 5.Englisch S., Zink M. Treatment-resistant schizophrenia: evidence-based strategies. Mens Sana Monogr. 2012;10(1):20. doi: 10.4103/0973-1229.91588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cetin M. Taylor and Francis; 2014. Clozaphobia fear of prescribers of clozapine for treatment of schizophrenia. [Google Scholar]
- 7.Kaneda Y., Jayathilak K., Meltzer H.Y. Determinants of work outcome in schizophrenia and schizoaffective disorder: role of cognitive function. Psychiatry Res. 2009;169(2):178–179. doi: 10.1016/j.psychres.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Lehman A.F., Lieberman J.A., Dixon L.B. Practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiatry. 2004:161. (2 SUPPL) [PubMed] [Google Scholar]
- 9.Seida J.C., Schouten J.R., Mousavi S.S. 2012. First-and second-generation antipsychotics for children and young adults. [PubMed] [Google Scholar]
- 10.Lambert M., Naber D. Current Schizophrenia. 2012. Phase-specific treatments for schizophrenia; p. 72. [Google Scholar]
- 11.Kim H.S., Kim S.H., Lee N.Y. Effectiveness of Electroconvulsive Therapy Augmentation on Clozapine-Resistant Schizophrenia. Psychiatry Investig. 2017;14(1):58–62. doi: 10.4306/pi.2017.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suehs B., Argo P.T.R., Bendele B.S.D., Crismon M.L., Trivedi M.H., Kurian B. Texas medication algorithm project procedural manual. Major depressive disorder algorithms. July 2008 [Google Scholar]
- 13.Petrides G., Malur C., Braga R.J. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. Am. J. Psychiatry. 2014;172(1):52–58. doi: 10.1176/appi.ajp.2014.13060787. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W., Cao X.-L., Ungvari G.S. Electroconvulsive therapy added to non-clozapine antipsychotic medication for treatment resistant schizophrenia: meta-analysis of randomized controlled trials. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0156510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lally J., Tully J., Robertson D., Stubbs B., Gaughran F., MacCabe J.H. Augmentation of clozapine with electroconvulsive therapy in treatment resistant schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2016;171(1):215–224. doi: 10.1016/j.schres.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Braga R.J., Petrides G. The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. J. ECT. 2005;21(2):75–83. doi: 10.1097/01.yct.0000165500.60784.05. [DOI] [PubMed] [Google Scholar]
- 17.Painuly N., Chakrabarti S. Combined use of electroconvulsive therapy and antipsychotics in schizophrenia: the Indian evidence. A review and a meta-analysis. J. ECT. 2006;22(1):59–66. doi: 10.1097/00124509-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Aas I.M. Guidelines for rating global assessment of functioning (GAF) Ann. Gen. Psychiatry. 2011;10(1):2. doi: 10.1186/1744-859X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell M., Milstein R., Beam-Goulet J., Lysaker P., Cicchetti D. The Positive and Negative Syndrome Scale and the Brief Psychiatric Rating Scale: reliability, comparability, and predictive validity. J. Nerv. Ment. Dis. 1992;180(11):723–728. doi: 10.1097/00005053-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Busner J., Targum S.D. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28. [PMC free article] [PubMed] [Google Scholar]
- 21.Kurlowicz L., Wallace M. The mini-mental state examination (MMSE) J. Gerontol. Nurs. 1999;25(5):8–9. doi: 10.3928/0098-9134-19990501-08. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leucht S., Rothe P., Davis J., Engel R. Equipercentile linking of the BPRS and the PANSS. Eur. Neuropsychopharmacol. 2013;23(8):956–959. doi: 10.1016/j.euroneuro.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Komossa K., Rummel-Kluge C., Schmid F. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2010:1. doi: 10.1002/14651858.CD006625.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothstein H.R., Sutton A.J., Borenstein M. John Wiley and Sons; 2006. Publication bias in meta-analysis: Prevention, assessment and adjustments. [Google Scholar]
- 26.Fu R., Vandermeer B.W., Shamliyan T.A. 2013. Handling continuous outcomes in quantitative synthesis. [PubMed] [Google Scholar]
- 27.Sarita E., Janakiramaiah N., Gangadhar B., Subbakrishna D., Rao K.J. Efficacy of combined ECT after two weeks of neuroleptics in schizophrenia: a double blind controlled study. Nimhans Journal. 1998;16(4):243–252. [Google Scholar]
- 28.Chanpattana W., Kramer B.A. Acute and maintenance ECT with flupenthixol in refractory schizophrenia: sustained improvements in psychopathology, quality of life, and social outcomes. Schizophr. Res. 2003;63(1):189–193. doi: 10.1016/s0920-9964(02)00330-4. [DOI] [PubMed] [Google Scholar]
- 29.Grover S., Hazari N., Chakrabarti S., Avasthi A. Augmentation of clozapine with ECT: observations from India. Am. J. Psychiatry. 2015;172(5):487. doi: 10.1176/appi.ajp.2014.14091170. [DOI] [PubMed] [Google Scholar]
- 30.Masoudzadeh A., Khalilian Z. Comparative Study of Clozapine, Electroshock and the Combination of ЕСТ with Clozapine in Treatment-Resistant Schizophrenic Patients. Pak. J. Biol. Sci. 2007;10(23):4287–4290. doi: 10.3923/pjbs.2007.4287.4290. [DOI] [PubMed] [Google Scholar]
- 31.Ravanić D.B., Pantović M.M., Milovanović D.R. Long-term efficacy of electroconvulsive therapy combined with different antipsychotic drugs in previously resistant schizophrenia. Psychiatr. Danub. 2009;21(2):179–186. [PubMed] [Google Scholar]
- 32.Sajatovic M., Meltzer H. The effect of short-term electroconvulsive treatment plus neuroleptics in treatment-resistant schizophrenia and schizoaffective disorder. J. ECT. 1993;9(3):167–175. [PubMed] [Google Scholar]
- 33.Cardwell B., Nakai B. Seizure activity in combined clozapine and ECT: A retrospective view. J. ECT. 1995;11(2):110–113. [PubMed] [Google Scholar]
- 34.Kho K., Blansjaar B., De Vries S., Babuskova D., Zwinderman A., Linszen D. Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254(6):372–379. doi: 10.1007/s00406-004-0517-y. [DOI] [PubMed] [Google Scholar]
- 35.Goswami U., Kumar U., Singh B. Efficacy of electroconvulsive therapy in treatment resistant schizophreinia: A double-blind study. Indian J. Psychiatry. 2003;45(1):26. [PMC free article] [PubMed] [Google Scholar]
- 36.Chanpattana W., Sackeim H.A. Electroconvulsive therapy in treatment-resistant schizophrenia: prediction of response and the nature of symptomatic improvement. J. ECT. 2010;26(4):289–298. doi: 10.1097/YCT.0b013e3181cb5e0f. [DOI] [PubMed] [Google Scholar]
- 37.Chanpattana W., Chakrabhand M.S. Combined ECT and neuroleptic therapy in treatment-refractory schizophrenia: prediction of outcome. Psychiatry Res. 2001;105(1):107–115. doi: 10.1016/s0165-1781(01)00321-3. [DOI] [PubMed] [Google Scholar]
- 38.Chanpattana W., Chakrabhand M.S., Sackeim H.A. Continuation ECT in treatment-resistant schizophrenia: a controlled study. J. ECT. 1999;15(3):178–192. [PubMed] [Google Scholar]
- 39.Hirose S., Ashby C.R., Jr., Mills M.J. Effectiveness of ECT combined with risperidone against aggression in schizophrenia. J. ECT. 2001;17(1):22–26. doi: 10.1097/00124509-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Chanpattana W., Chakrabhand M.S., Buppanharun W., Sackeim H.A. Effects of stimulus intensity on the efficacy of bilateral ECT in schizophrenia: a preliminary study. Biol. Psychiatry. 2000;48(3):222–228. doi: 10.1016/s0006-3223(00)00830-1. [DOI] [PubMed] [Google Scholar]
- 41.James D.V., Gray N. Elective combined electroconvulsive and clozapine therapy. Int. Clin. Psychopharmacol. 1999;14(2):69–72. doi: 10.1097/00004850-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Tiihonen J., Lönnqvist J., Wahlbeck K. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) The Lancet. 2009;374(9690):620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 43.Tiihonen J., Walhbeck K., Lönnqvist J. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224. doi: 10.1136/bmj.38881.382755.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durlak J.A. How to select, calculate, and interpret effect sizes. J. Pediatr. Psychol. 2009;34(9):917–928. doi: 10.1093/jpepsy/jsp004. [DOI] [PubMed] [Google Scholar]
- 45.Benatov R., Sirota P., Megged S. Neuroleptic-resistant schizophrenia treated with clozapine and ECT. J. ECT. 1996;12(2):117–121. [PubMed] [Google Scholar]
- 46.Ried K. 2006. Interpreting and understanding meta-analysis graphs: a practical guide. [PubMed] [Google Scholar]
- 47.Higgins J.P., Green S. John Wiley and Sons; 2011. Cochrane handbook for systematic reviews of interventions. Vol 4. [Google Scholar]
- 48.Scott A.I. RCPsych Publications; 2005. The ECT handbook: the third report of the Royal College of Psychiatrists' Special Committee of Ect. Vol 128. [Google Scholar]
- 49.Association A.P. Association American Psychiatric Pub.; 2008. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging (A task force report of the American Psychiatric Association) [Google Scholar]
- 50.Ukpong D., Makanjuola R., Morakinyo O. A controlled trial of modified electroconvulsive therapy in Schizophrenia in a Nigeria Teaching Hospital. West Afr. J. Med. 2002;21(3):237–240. doi: 10.4314/wajm.v21i3.28039. [DOI] [PubMed] [Google Scholar]
- 51.Kane J., Honigfeld G., Singer J., Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 52.Havaki-Kontaxaki B.J., Ferentinos P.P., Kontaxakis V.P., Paplos K.G., Soldatos C.R. Concurrent administration of clozapine and electroconvulsive therapy in clozapine-resistant schizophrenia. Clin. Neuropharmacol. 2006;29(1):52–56. doi: 10.1097/00002826-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Kupchik M., Spivak B., Mester R. Combined electroconvulsive-clozapine therapy. Clin. Neuropharmacol. 2000;23(1):14–16. doi: 10.1097/00002826-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Wahlbeck K., Cheine M., Essali A., Adams C. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am. J. Psychiatry. 1999;156(7):990–999. doi: 10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- 55.Kane J.M., Malhotra A. The future of pharmacotherapy for schizophrenia. World Psychiatry. 2003;2(2):81. [PMC free article] [PubMed] [Google Scholar]