Abstract
Because of the requirement of oxygen (O2) to produce energy, aerobic organisms developed mechanisms to protect themselves against a shortage of oxygen in both acute status and chronic status. To date, how organisms tolerate acute hypoxia and the underlying mechanisms remain largely unknown. Here, we identify that Tet1, one member of the ten-eleven translocation (TET) family of methylcytosine dioxygenases, is required for hypoxia tolerance in zebrafish and mice. Tet1-null zebrafish and mice are more sensitive to hypoxic conditions compared with their wild-type siblings. We demonstrate that Tet1 stabilizes hypoxia-inducible factor α (HIF-α) and enhances HIF-α transcription activity independent of its enzymatic activity. In addition, we show that Tet1 modulates HIF-2α and HIF-1α through different mechanisms. Tet1 competes with prolyl hydroxylase protein 2 (PHD2) to bind to HIF-2α, resulting in a reduction of HIF-2α hydroxylation by PHD2. For HIF-1α, however, Tet1 has no effect on HIF-1α hydroxylation, but rather it appears to stabilize the C-terminus of HIF-1α by affecting lysine site modification. Furthermore, we found that Tet1 enhances rather than prevents poly-ubiquitination on HIF-α. Our results reveal a previously unrecognized function of Tet1 independent of its methylcytosine dioxygenase activity in hypoxia signaling.
INTRODUCTION
In the Precambrian, the single-celled organisms (cynobacteria) converted solar energy, carbon dioxide, and water into the chemical energy of carbon bonds and produced oxygen (O2) as a side product through photosynthesis, leading to an increase of atmospheric O2 in the Earth (1–3). Amongst the essential factors required for speciation and evolution of aerobic organisms, particularly of complex multicellular organisms, O2 is indispensable because of its crucial role in energy generation. Through the oxidative phosphorylation that transfers chemical energy stored in carbon bonds of organic molecules to the high-energy phosphate bond in adenosine triphosphate (ATP), organisms produce energy for fundamental cellular functions, such as growth, proliferation, metabolism and synthesis of cellular constituents. In this process, O2 serves as the final electron receptor (1,3,4). During speciation, organisms not only have evolved sophisticated cellular sensors responding to O2 gradients, but also have developed elaborate systematic physiological systems that adapt to environments with variant O2 levels (5,6).
The heterodimeric transcription hypoxia-inducible factors 1α and 2α (HIF-1α and HIF-2α) are master regulators of the cellular response to O2 (2,3,5). HIF-1α and HIF-2α orchestrate this cellular response to hypoxia by regulating the expression of a wide set of genes involved in multiple biological processes, including growth, proliferation, apoptosis, and metabolism (4). Under well-oxygenated conditions (normoxia), the prolyl hydroxylases (PHD1, PHD2 and PHD3) use O2 and 2-oxoglutarate as substrates to hydroxylate HIF-1α and HIF-2α at specific proline residues. The hydroxylated HIF-1α and HIF-2α are bound by the von Hippel-Lindau (VHL) protein. VHL recruits an ubiquitin ligase complex that targets HIF-1α and HIF-2α for proteasomal degradation. In response to low O2 levels (hypoxia), because of the lack of O2 as a substrate, the enzymatic activity of PHDs is inhibited, and they lose their function to hydroxylate of HIF-1α and HIF-2α. This in turn leads to HIF-α protein stabilization and the induction of transcriptional activity, which provides a molecular mechanism for the transduction of changes in the availability of O2 in response to changes in gene expression (7,8). Because of the fine regulation of HIF-mediated hypoxia signaling by PHDs, the attenuation of PHDs function results in an up-regulation of hypoxia-inducible genes and leads to changes in cellular response to variant O2 levels (9).
Global variations of O2 levels have been correlated with adaptive evolutionary changes in organism physiology (6). Even though it is evident that the structure and function of some cells, tissues, or organs in the organisms have evolved to adapt to these variant O2 levels, accumulating evidence indicates that the hypoxia adaptation of aerobic organisms meets the HIF pathway at the molecular level (5). In high-altitude adaption, including Tibetans, Tibetan dogs and Tibetan grey wolf, genetic signatures have been identified in the HIF-2α (EPAS1) gene and PHD2 (EGLN1) gene (10–17). In addition to these two genes, other factors also have been shown to correlate with hypoxia adaption (10,18).
The ten-eleven translocation proteins (Tet1, Tet2 and Tet3), as well as PHD1, PHD2 and PHD3, belong to a large family of nonheme Fe2+/2-oxoglutarate-dependent dioxygenases (2-OGDO) (19). The TET proteins contain a conserved double-stranded β-helix (DSBH) domain, a cysteine-rich domain, and binding sites for the cofactors Fe2+ and 2-oxoglutarate that together form the core catalytic region in the C-terminus (20). The TET proteins convert 5mC to 5-hydroxymethylcytosine (5hmC) and its oxidative derivatives 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC), providing a first step toward DNA demethylation (21). The TET proteins are presented in all jawed vertebrates that have roles in diverse biological processes, including epigenetic regulation of gene expression, embryogenesis, stem cell function and diseases (22).
Some evidence suggests a relationship exists between hypoxia and TET function (23,24). Given the O2 dependence of TET enzymes, a hypoxic environment may reduce TET activity (23). It is evident that the activity of TET proteins is reduced by tumor hypoxia in human and mouse cells, independent of hypoxia-associated alternations of Tet expression (25). It is also observed that Tet1 is induced by hypoxia, resulting in an increase of globe 5-hmC, with an accumulation of 5hmC density at the canonical hypoxia response genes (26). In addition, Tet1 is found to be induced by hypoxia and serves as a coactivator of HIF-1α to regulate hypoxia-induced epithelial-mesenchymal transition, independent of its enzymatic activity (27). Even though these observations are somewhat controversial, the role of TET proteins in hypoxia signaling appears to be suggested. But how TET proteins act in their function in hypoxia signaling and whether TET proteins have any impact on hypoxia adaption (chronic hypoxia) or hypoxia tolerance (acute hypoxia) remain largely unknown.
In this study, we find that knockout of Tet1 in both zebrafish and mice causes Tet1-null zebrafish and mice to be more sensitive to hypoxia compared with their wild-type siblings. Moreover, Tet1 stabilizes the HIF-α protein, which is independent of its enzymatic activity. Therefore, we reveal a previously unrecognized function of Tet1 in hypoxia signaling.
MATERIALS AND METHODS
Generation of tet1-null, tet2-null and tet3-null zebrafish
Disruption of tet1, tet2 and tet3 in zebrafish was accomplished via TAL effector nucleases (TALENs) or CRISPR/Cas9 technologies. The target sequences of TALENs on tet1, tet2 and tet3 were designed using the tools provided on the website (https://tale-nt.cac.cornell.edu). For tet1, the left target sequence is 5′-ACAAATGACCATCTAGGT-3′; the right target sequence is 5′-AGGCATGAATGTGAACAA-3′. For tet2, the left target sequence is 5′-CTCACCAAGCACCAAAAT-3′; the right target sequence is 5′- AGTCTTGTCTCAGCAG-3′. For tet3, the left target sequence is 5′- TATTAAACCAGGAACTCT-3′; the right target sequence is 5′-ATGCATCTAATGAAGA-3′. The TALENs of tet1, tet2 and tet3 were constructed by FastTALE TALEN Assembly kit (Sidansai Biotechnology, Shanghai, China). The mRNA of tet1, tet2 and tet3 TALENs were synthesized by Amplicap SP6 High Message Maker Kit (Cell Script).
Tet1 sgRNA was designed using the tools provided on the website (http://crispr.mit.edu). The zebrafish Codon Optimized Cas9 plasmid was digested with XbaI, and purified and transcribed using the T7 mMessage mMachine Kit (Ambion). PUC9 gRNA vector was used for amplifying the sgRNA template. The primers for amplifying tet1 gRNA are: 5′-GTAATACGACTCACTATAGGACTGTCGTCTGGGCTGTAGTTTTAGAGCTAGAAATAGC-3′ and 5′-AAAAGCACCGACTCGGTGCC-3′. SgRNA was synthesized using the Transcript Aid T7 High Yield Transcription Kit (Fermentas).
Zebrafish (Danio rerio) strain AB was raised, maintained, reproduced, and staged according to standard protocol. DNA (Cas9) and mRNA were injected into embryos at one-cell stage. mRNA of the TALENs of tet1, tet2 or tet3 was injected at 0.075 ng/per embryo. Cas9 RNA and sgRNA were injected at 0.75–1.25 ng/per embryo and 0.075 ng/per embryo, respectively. The mutations were initially detected by HMA (heteroduplex mobility assay) as previously described (28). If the results were positive, the remainder embryos were raised up to adulthood as the F0, which were back-crossed with wildtype zebrafish (AB line) to generate the F1, which were genotyped by HMA initially and confirmed by sequencing of targeting sites. The heterozygous F1 were back-crossed to wildtype zebrafish (AB line; none of their own parents) to obtain F2. The F2 adult zebrafish carrying the same mutation were inter-crossed to generate the F3 offspring, which should contain wildtype (+/+), heterozygote (+/–) and homozygote (–/–). The primers used for mutant identification are listed in Supplementary Table S1.
The guidelines for zebrafish nomenclature (http://wiki.zfin.org/display/general/ZFIN+Zebrafish+Nomenclature+Guidelines) were followed for naming the four mutants, tet1 inb2017/ihb2017(http://zfin.org/ZDB-ALT-170421-5), tet1 inb2018/ihb2018 (http://zfin.org/ZDB-ALT-170421-8), tet2 inb2019/ihb2019(http://zfin.org/ZDB-ALT-170421-9) and tet3 inb2020/ihb2020 (https://zfin.org/ZDB-ALT-170424-10).
Zebrafish were maintained in a re-circulating water system according to standard protocol. All experiments with zebrafish were approved by the Institutional Animal Care and Use Committee of Institute of Hydrobiology, Chinese Academy of Sciences under protocol number 2014-001.
Tet1-null mice
Tet1 −/− mice have been described previously (29). Tet1+/− mice used in this study were originally obtained from Jackson Laboratory and we mated them with C57BL/6 mice to generate heterozygote mice (tet1+/−) for further experiments. The wildtype (+/+), heterozygote (+/−) and homozygote (−/−) mice were obtained by intercrossing of heterozygote (+/−) mice. All the mice were housed in the SPF animal facility and the animal experiments were performed at the age of 5–7 weeks with the use of protocols approved by the Institutional Animal Care and Use Committee of Institute of Hydrobiology, Chinese Academy of Sciences under protocol number 2014-002.
Cell culture and transfection
HEK293T and N2a (Neuro-2a) cell lines were originally obtained from ATCC and maintained in Dulbecco's modified Eagle's medium (Hyclone) supplemented with 10% fetal bovine serum (FBS; Hyclone) at 37°C in a humidified atmosphere incubator containing 5% CO2. All cell lines were verified to be free of Mycoplama contamination before use. Vigofect (Vigorous Biotechnology, Beijing, China) was used for cell transfection.
Mouse Tet1−/− and Tet1+/+ MEF cells was established as described previously (30).
Luciferase reporter assay
N2a cells were seeded in 24-well plates and transfected with the indicated plasmids together with Hypoxia Response Element luciferase reporter (HRE-Luc.) (kindly provided by Navdeep Chandel) and pTK-Renilla as an internal control. Luciferase activity was measured 20–24 h after transfection using the Dual-luciferase Reporter Assay System (Promega). Data were normalized to Renilla luciferase.
Hypoxia treatment
The Ruskinn INVIVO2 I-400 workstation was used for hypoxia treatment on cells and animals (zebrafish and mice). Before use, the O2 concentration was adjusted to the indicated values ahead of time.
In the pilot experiments, we noticed that the animal body weight (zebrafish and mice) could affect hypoxia tolerance significantly. Thus, before hypoxia treatments, we weighed animal body weight individually and tried to choose animals with similar body weight, then, to test their hypoxia tolerance.
For hypoxia treatment on adult zebrafish, tet1-null zebrafish were put into one 1000-ml flask filled with 750 ml water and their sibling controls were also put into another 1000-ml flasks filled with 750 ml water. In addition, before putting zebrafish, the oxygen concentration in water of these flaks was measured by a LDO101 probe (HQ30d, HACH) respectively (in all experiment, the oxygen concentration in water of the flasks for holding tet1-null zebrafish was 7.70 ± 0.07 mg/l and the oxygen concentration in water of the flasks for holding wildtype siblings was 7.74 ± 0.05 mg/l). Subsequently, the flasks were set in the Ruskinn INVIVO2 I-400 workstation simultaneously, in which the oxygen concentration was adjusted to 5% ahead of time (5.06kPa). Then, the behavior of zebrafish was closely monitored, recorded, photographed or videotaped.
For obtaining the survival curve of zebrafish under hypoxia, tet1-null zebrafish fries (45 dpf, 0.16 ± 0.02 g, n = 15) and their wild-type siblings (45 dpf, 0.16 ± 0.02 g, n = 15) were placed in the hypoxia workstation simultaneously. The numbers of dead zebrafish were counted once an hour. These experiments were repeated three times.
For hypoxia treatment on adult mice, Tet1-null mice as well as their wildtype and heterozygous siblings were weighed individually and the mice with similar weight were chosen (17.5 ± 0.5 g) for further assays. Each mouse was put into one flask respectively. The flasks were sealed with gauze and were then put into the hypoxia workstation. The oxygen concentration in the hypoxia workstation was adjusted to 10% (10.1 kPa) ahead of time. After treatment for 2 h (10% O2), the oxygen concentration in the hypoxia workstation adjusted to 8% (8.1 kPa). The behavior of mice in the flasks was closely monitored, recorded, photographed or videotaped. Because it was difficult to obtain mice with different genetic background (+/+, +/−, −/−, respectively) but similar body weight at the same time, we used mice from different litters. The hypoxia treatment experiments were conducted at the different stages of this study. The experiments were repeated five times (n = 5/each group (+/+, +/−, −/−)). The total number of mice used in hypoxia treatment experiments was 15 (n = 15).
Oxygen consumption rate measurement
Oxygen consumption rate measurement was conducted in 500-ml flasks with a same amount of water (500 ml) (n = 12). Before putting zebrafish, the oxygen concentration of water in the flasks was measured by a LDO101 probe (HQ30d, HACH) (7.70 ± 0.07 mg/l). Tet1-null zebrafish (n = 6, 0.16 ± 0.02 g) as well as their wild-type siblings (n = 6, 0.16 ± 0.02 g) were simultaneously put into those 12 flasks respectively. Then the flasks were sealed tightly. After 4 h, the oxygen concentration in three flasks with tet1-null zebrafish and three flasks with wildtype siblings was measured with a LDO101 probe individually. After another 4 h, the oxygen concentration in the remaining three flasks with tet1-null zebrafish and the remaining three flasks with wild-type siblings was measured with a LDO101 probe individually. The data were recorded and calculated.
Immunohistochemistry staining and TUNEL assay
Immunohistochemistry staining was performed for detecting 5-hmC using anti-5hmC antibody (cat#39769, Active Motif) in zebrafish brain by a standard protocol.
The numbers of apoptotic cell numbers in both zebrafish and mouse brain were detected by TUNEL assay using the Apoptag Peroxidase In Situ Apoptosis Detection Kit (Millipore) according to manufacturer's instructions.
The staining was analyzed using an Olympus IX-81 microscope fitted with a SPOT camera and software.
Antibodies and Western blot
The antibodies used were as follows: anti-c-Myc antibody (9E10, 1:1000 for IB analysis, Santa Cruz), anti-Flag antibody (F1804, 1:1000 for IB analysis, Sigma), anti-HA antibody (1:5000 for IB analysis, Covance), anti-GAPDH antibody (SC-47724, 1:1000 for IB analysis, Santa Cruz), anti-α-tubulin antibody (EPR1333, 1:10 000 for IB analysis, Epitomics), anti-HIF-2α antibody (AB199, 1:1000 for IB analysis, Abcam), anti-HIF-1α antibody (A6265, 1:1000 for IB analysis, Abclone), anti-HIF-OH antibody (3434S, 1:1000 for IB analysis, Cell Signaling).
Western blot was performed as described previously (31). The Fuji Film LAS4000 mini luminescent image analyzer was used for photographing the blots. Multi Gauge V3.0 was used for quantifying the protein levels based on the band density obtained in western blot analysis.
Plasmid constructs and mutants
The original wildtype mouse Tet1 was kindly provided by Guoliang Xu and its truncated mutants were constructed by PCR and cloned into indicated expression vectors. PHD2 construct was ordered from Addgene (Cat# 18963). HIF-1α, HIF-2α and their mutants have been described previously or were constructed by PCR (31).
Co-immunoprecipitation assay and hydroxylation assay
The experimental procedure of co-immunoprecipitation has been described previously (31).
For hydroxylation assays, because overexpression of Tet1 could enhance HIF-2α or HIF-1α protein level dramatically, in order to get a clear picture about the real effect of Tet1 on HIF-2α or HIF-1α hydroxylation, based on the ratio of HIF protein levels obtained from the pilot experiments, we adjusted the loading amount of protein and tried to let Flag-HIF-2α or Flag-1α loaded at the same level between the samples with and without Tet1 overexpression after co-immunoprecipitation using anti-flag conjugated agarose beads (Figures 5B and 7B).
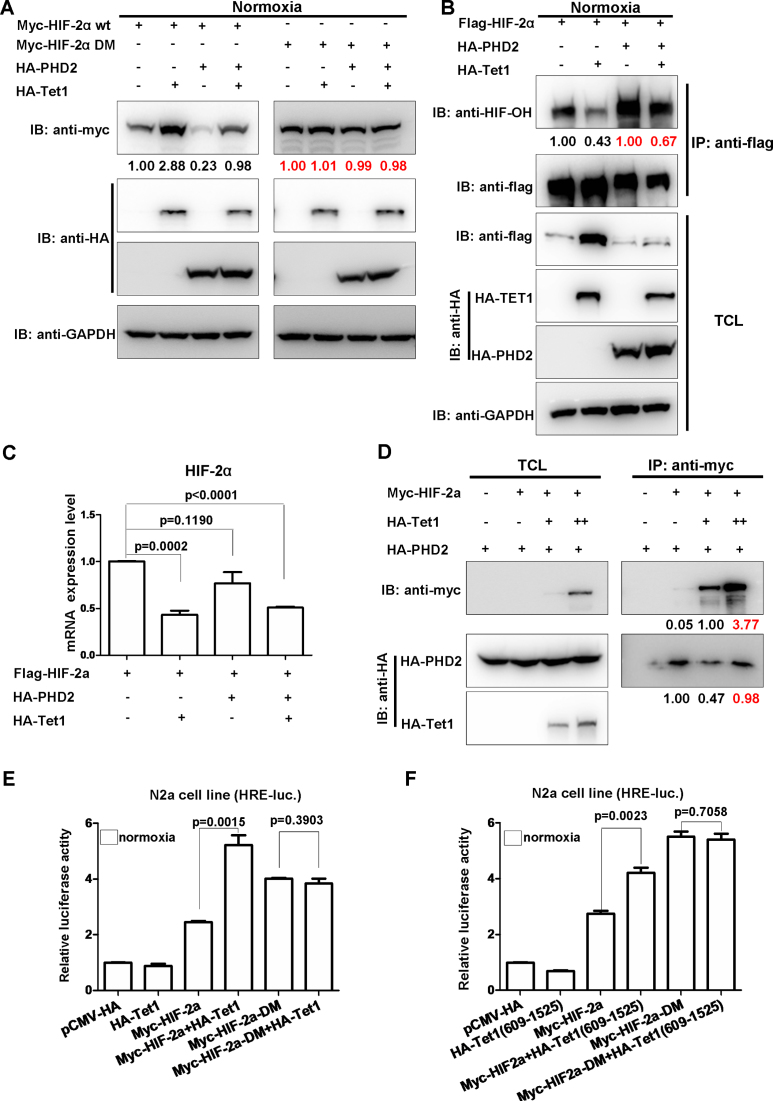
Figure 5.
Tet1 reduces hydroxylation of HIF-2α by disturbing PHD2 function on HIF-2α. (A) Tet1 stabilized wild-type HIF-2α, but not the hydroxylated site mutated HIF-2α (HIF-2α DM) under normoxia. (B) Overexpression of Tet1 diminished HIF-2α hydroxylation under normoxia. HEK293T cells were transfected with the indicated plasmids. After co-immunoprecipitation using anti-flag conjugated agarose beads, the loading amount of protein was adjusted to similar levels between the samples with and without Tet1 overexpression based on the pilot experiments. (C) Overexpression of Tet1 did not cause HIF-2α mRNA to increase. HEK293T cells were transfected with the indicated plasmids. After 16–20 h, the cells were harvested for RT-PCR assays. (D) Co-expression of Tet1 diminished PHD2 binding to HIF-2α. (E) Overexpression of Tet1 enhanced wildtype HIF-2α activity on the hypoxia response element luciferase (HRE-luc.) reporter in the N2a cell line under normoxia, but not that of the hydroxylated site-mutated HIF-2α (HIF-2α DM). (F) Overexpression of the Tet1 domain covering 609–1525aa enhanced the activity of wild-type HIF-2α on the HRE-luc. reporter in the N2a cell line under normoxia, but not that of the hydroxylated site-mutated HIF-2α (HIF-2α DM).
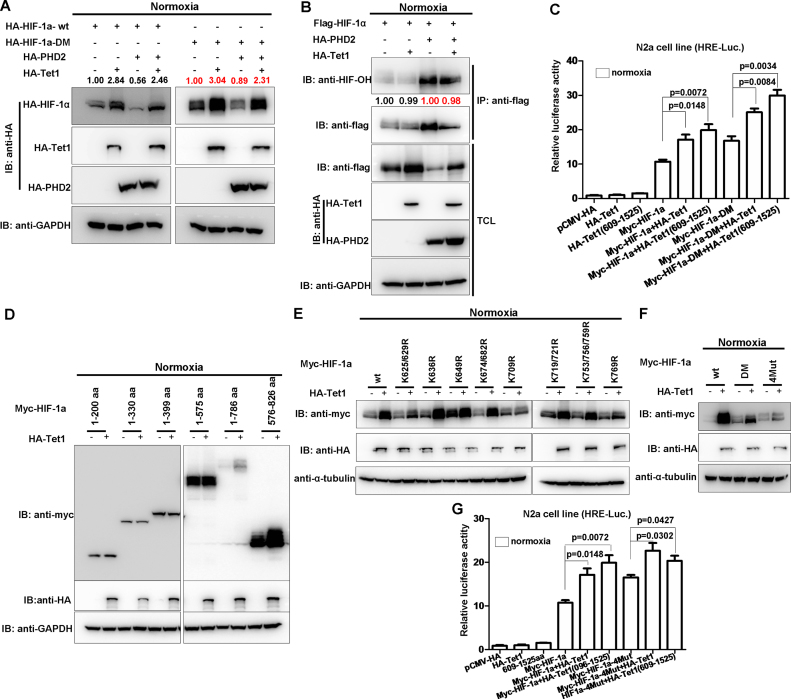
Figure 7.
Tet1 does not affect PHD2 function on HIF-1α and stabilizes the C-terminal of HIF-1α. (A) The hydroxylated site-mutated HIF-1α (HIF-1α DM) still was stabilized by Tet1. (B) Tet1 had no effect on hydroxylation of HIF-1α. HEK293T cells were transfected with the indicated plasmids. After co-immunoprecipitation using anti-flag conjugated agarose beads, the loading amount of protein was adjusted to the similar level between the samples with and without Tet1 overexpression based on the pilot experiments. (C) Overexpression of mouse Tet1 (tet1-wt) and its domain covering 609-1525aa enhanced the activity of the hydroxylated site mutated HIF-1α (HIF-1α DM) on the hypoxia response element luciferase (HRE-luc.) reporter in the N2a cell line under normoxia. (D) Tet1 stabilized the C-terminal of HIF-1α (576-826 aa). (E) Tet1 stabilized the different lysine mutants of HIF-1α. (F) The four-lysine site mutated HIF-1α (HIF-1α-4Mut) (K625/629/709/769R) still was stabilized by Tet1, but it was not as dramatic as that of wild-type HIF-1α or the hydroxylated site mutated HIF-1α (DM). (G) Overexpression of mouse Tet1 (tet1-wt) and its domain covering 609-1525aa could still enhance the activity of HIF-1α-4Mut on the HRE-luc. reporter in the N2a cell line under normoxia.
Ubiquitination assay
HEK293T cells were co-transfected with Flag-HIF-2α or Myc-HIF-1α, HA-Tet1, His-ubiquitin or His-ubiquitin- mutants (K6, K11, K27, K29, K33, K48 or K63 only). Ubiquitination assays with His-ubiquitin or His-ubiquitin- mutants were performed by affinity purification on Ni2+-NTA resin (Novagen) as described previously (32). An anti-HIF-2α or anti-Myc antibody was used for detecting polyubiquitination of HIF-2α or HIF-1α respectively.
Semi-quantitative real-time PCR analysis
Total RNA was extracted from cells using the Trizol reagent (Invitrogen), and cDNA was synthesized using a first strand cDNA synthesis kit (Fermentas). The primers used for RT-PCR analysis are listed in Supplementary Table S1.
Statistical analysis
Luciferase assay, semi-quantitative RT-PCR and oxygen consumption rate measurement data are reported as mean ± S.E.M. of three independent experiments performed in triplicate. The statistic analysis was performed using GraphPad Prism 5 (unpaired t -test) (GraphPad Software Inc.).
RESULTS
Tet1-null zebrafish exhibit less tolerant to hypoxia
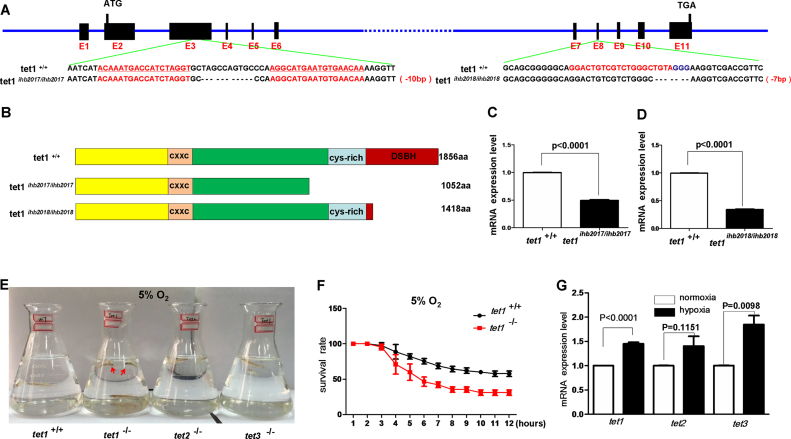
Growing evidence indicates the importance of TET proteins in DNA hydroxymethylation, accounting for their functions in development, differentiation, pluripotency, immunity, and tumorigenesis (20–22). Similar to mammalians, zebrafish have three orthologs of Tet1, Tet2 and Tet3, which contain main functional domains as those in mammalians, suggesting that the TET protein is conserved evolutionarily between mammalians and zebrafish (33,34). To systematically define the roles of TET proteins in vivo by zebrafish model, we initially introduced mutations into zebrafish tet1, tet2 and tet3 using TAL effector nucleases (TALENs) and obtained three mutants respectively, tet1ihb2017/ihb2017, tet2ihb2019/ihb2019 and tet3ihb2020/ihb2020 (Figure 1A; Supplementary Figure S1A and D). The predicted truncated proteins of tet1, tet2 and tet3 in the mutants indicate that the enzymatic domain was completely deleted in all three proteins (Figure 1B; Supplementary Figure S1B and E). Semi-quantitative real time polymerase chain reaction (RT-PCR) analysis indicted that tet1 mRNA, tet2 mRNA and tet3 mRNA were reduced in tet1ihb2017/ihb2017, tet2ihb2019/ihb2019 and tet3ihb2020/ihb2020 mutants, respectively, which suggested that tet1, tet2 and tet3 were disrupted effectively (Figure 1C; Supplementary Figure S1C and F). From the embryonic stage to adulthood, the heterozygote (tet1+/−, tet2+/− and tet3+/−) and homozygote (tet1ihb2017/ihb2017, tet2ihb2019/ihb2019 and tet3ihb2020/ihb2010) of tet1, tet2 and tet3 were indistinguishable from their wild-type siblings, similar to what was reported previously (34). All of these mutants and their wild-type siblings were raised in the tanks of a re-circulating water system separated by their genetic background. To enrich water O2 in the tanks, water was dropped slowly on the water surface of each tank. Interestingly, we noticed that the tet1−/− zebrafish often swam near to the water surface in the tanks (Supplementary Video S1), but the others, including wild-type, tet2−/− and tet3−/− zebrafish swam across the whole tanks (Supplementary Videos S2, S3 and S4). After we measured the O2 concentration of the water surface and water bottom in the tanks using an LDO101 probe, we found that the O2 concentration of the water surface of tanks was always higher than that in the water bottom of tanks. The behaviors of tet1−/− zebrafish preferring to swim near to the water surface of tanks might suggest that tet1−/− could not tolerate low O2 existed in the water bottom of tanks. To further test this hypothesis, we chose three each of wild-type, tet1−/−, tet2−/− and tet3−/−zebrafish with similar body weight and put them into four flasks. Subsequently, we put the flasks into a hypoxia workstation with 5% O2 adjusted ahead of time and closely observed their behaviors. After 4 h, tet1−/− zebrafish started to die. When all three tet1−/− zebrafish died completely, the other zebrafish, including wildtype, tet2−/− and tet3−/− zebrafish were still alive (Figure 1E; Supplementary Videos S5 and S6). To document the data quantitatively, we made a survival rate curve that indicated that more tet1−/− mutant zebrafish died than their wildtype siblings under the same hypoxic conditions. The mRNA levels of tet1, tet2 and tet3 was induced under hypoxia (Figure 1G). In addition, to determine whether the different phenotypes exhibited between wild-type (tet1+/+) and tet1−/− zebrafish under hypoxia were caused by O2 consumption rate, we measured the O2 consumption rate and found no significant difference between wild-type (tet1+/+) and tet1−/− zebrafish (Supplementary Figure S2A). Intriguingly, modest reduction of 5hmC was observed in the brain of tet1−/− mutant compared with that of their wild-type siblings (Supplementary Figure S2B), similar to what has been reported previously (34). These data suggest that tet1 might facilitate zebrafish hypoxia tolerance.
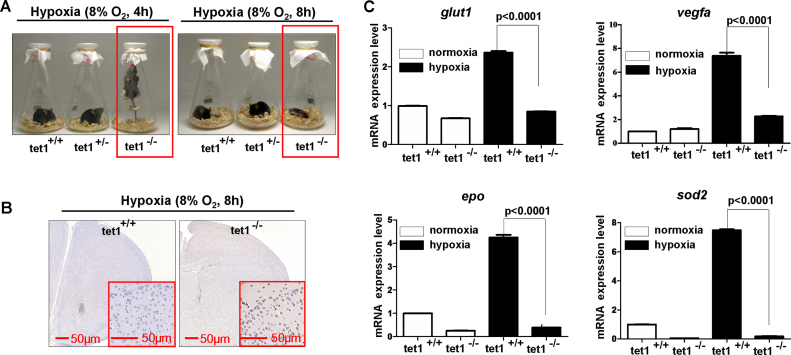
Figure 1.
Zebrafish tet1 facilitates hypoxia tolerance. (A) Targeting strategy for generating zebrafish tet1 mutants by TALEN and CRISPR/Cas9 technology. The targeting sequences by TALEN or CRISPR/Cas9 are underlined and marked with red font; the sequence differences between the mutants and their wild-type siblings are indicated by dashes. (B) The predicted protein products of tet1 in the mutants and their wild-type siblings. The major domains of Tet1 including CXXC, cys-rich and DSBH are marked. In the mutant line tet1ihb2017/ihb2017, cys-rich domain and DSBH domain were deleted; in the mutant line tet1ihb2018/ihb2018 DSBH domain was deleted. (C) Expression of tet1 in tet1-null (tet1ihb2017/ihb2017) zebrafish embryos was down-regulated significantly compared with that in wild-type (tet1+/+) (P < 0.0001). (D) Expression of tet1 in tet1-null (tet1ihb2018/ihb2018) zebrafish embryos was down-regulated significantly compared with that wild-type (tet1+/+) (P < 0.0001). (E) Tet1-null zebrafish exhibit more sensitive to hypoxia (5%) compared with their wild-type siblings, as well as tet2-null zebrafish and tet3-null zebrafish. (F) The survival rate curve of tet1-null zebrafish and their wild-type sibling. Three groups of tet1-null zebrafish fries (45 dpf, n = 15 each group) and three groups of their wild-type siblings (45 dpf, n = 15 each group) were placed in the hypoxia workstation (Ruskinn INVIVO2 400) simultaneously. The oxygen concentration of the hypoxia workstation was adjusted to 5% ahead of time. The numbers of dead zebrafish were counted once an hour. (G) The mRNA levels of tet1 as well as tet2 and tet3 were induced under hypoxia.
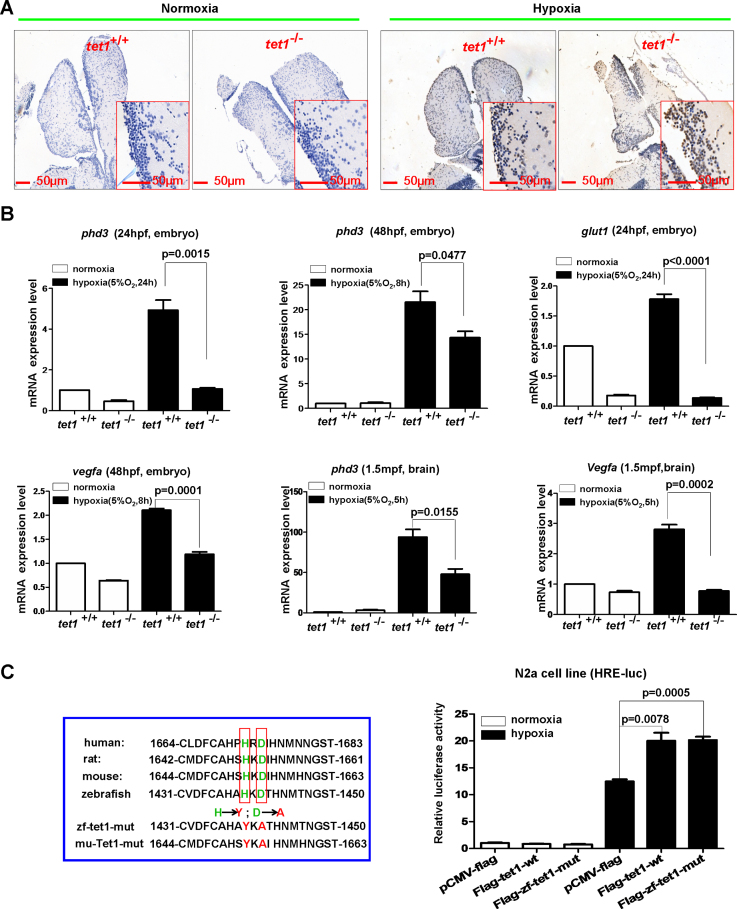
To uncover the reason why tet1−/− zebrafish were more sensitive to hypoxia compared with their wild-type siblings, we examined apoptosis in the brain cells by TUNEL assays. Under normoxia, no obvious apoptotic cells were detected in the brains of both tet1−/− zebrafish and their wild-type siblings (Figure 2A). Under hypoxia, however, more apoptotic cells were counted in tet1−/− zebrafish compared with their wild-type siblings (Figure 2A). The fact that more cells went through apoptosis in the brain of tet1−/− zebrafish compared with their wild-type siblings under hypoxia might account for the sensitivity of tet1−/− to hypoxia.
Figure 2.
Knockout of tet1 in zebrafish causes more apoptotic cells in the brain and reduction of hypoxia-inducible gene expression under hypoxia. (A) Compared with those in the brain of wild-type siblings, more apoptotic cells were detected in the brain of tet1-null zebrafish by TUNEL assays under hypoxia (5% O2, 5 h). (B) Expressions of hypoxia-inducible genes including phd3, glut1, vegfa were reduced significantly in the brain of tet1-null zebrafish embryos and adults compared with those of their wild-type siblings under hypoxia. (C) Overexpression of both zebrafish tet1 and its enzymatic inactive form (zf-tet1-mut) could activate the hypoxia response element luciferase (HRE-luc.) reporter in the N2a cell line under hypoxia. N2a cells were transfected with the indicated plasmids and luciferase activity was measured 20–24 h after transfection. The left red box indicates the alignment of partial Tet1 sequences surrounding Tet1 enzymatic activity region and the enzymatic inactive mutant (mut) with histidine mutated to tyrosine and aspartic acid mutated to alanine. zf-tet1-mut, zebrafish tet1 mutant; mu-Tet1-mut, mouse Tet1 mutant.
Subsequently, we examined the expression of well-defined hypoxia-inducible genes, including phd3, glut1, and vegfa in the embryos and adult brains. As shown in Figure 2B, under hypoxia, the expression of phd3, glut1 and vegfa were reduced dramatically in the embryos and the adult brains of tet1−/− zebrafish compared with their wild-type siblings (Figure 2B). These observations suggest that tet1 might enhance hypoxia-inducible gene expression. Furthermore, to determine whether the enhancement of hypoxia-inducible gene expression depended on the methylcytosine dioxygenase activity of tet1, we constructed a tet1 mutant in which the two enzymatic activity sites were mutated (Flag-zf-tet1-mut) and examined its effect on the hypoxia response element luciferase (HRE-Luc.) reporter. Surprisingly, this tet1 mutant still activated the HRE reporter, similar to the effect of wild-type tet1, suggesting that the effect of tet1 on hypoxia-inducible gene expression might not depend on tet1 enzymatic activity (Figure 2C). Considering that a modest reduction of 5hmC was detected in the brain cells of the tet1−/− mutant, the hypoxia-sensitive phenotype exhibited in tet1−/− zebrafish might not be due to the loss of methylcytosine dioxygenase activity in tet1, suggesting that an enzymatic-independent function of tet1 might be responsible for its role on hypoxia tolerance.
To further validate the phenotypes displayed in tet1ihb2017/ihb2017 zebrafish, we made another tet1 mutant (tet1ihb2018/ihb2018) using CRISPR/Cas9 technology. Similar to what we detected in tet1ihb2017/ihb2017, tet1ihb2018/ihb2018 zebrafish died more quickly than their wild-type siblings under hypoxia and expression of phd3 or vegfa also was reduced in tet1ihb2018/ihb2018 zebrafish, ruling out the possibility that the phenotypes exhibited in tet1−/− zebrafish resulted from an off-targeting effect of the tet1 TALEN or gRNA (Supplementary Figure S3).
Tet1-null mice exhibit less tolerant to hypoxia
Zebrafish tet1 contains the main function domains identified in mammalian TET1 protein (Supplementary Figure S4). To determine whether the effect of tet1 on hypoxia tolerance was conserved evolutionarily, we took advantage of Tet1-null mice. Similar to what we treated for zebrafish, we put the wild-type, tet1+/− and tet1−/− mice in three flasks and sealed the bottleneck with the gauze. Then, the flasks were put into the hypoxia workstation with 10% O2 adjusted ahead of time. After 2 h (10% O2), Tet1-null mice (Tet1−/−) and Tet1 heterozygous mice (Tet1+/−) appeared to be very uncomfortable and started to scratch themselves constantly, but the wild-type mice seemed to be relaxing relatively (Supplementary Video S7). After the mice stayed in the hypoxia workstation for 6 h (10% for 2 h, 8% for 4 h), Tet1−/− mice first started to jump and tried to reach the gauze that sealed the bottleneck. Subsequently, Tet1 heterozygous mice (Tet1+/−) started to jump (Supplementary Videos S8 and S9; Figure 3A). However, the wild-type mice (Tet1+/+) still seemed to be relaxing at that time point (Supplementary Videos S8 and S9; Figure 3A, left panel). After the mice were treated under hypoxia for 10 h (10% for 2 h, 8% for 8 h), Tet1−/- mice died, but heterozygous (Tet1+/−) and the wildtype mice (Tet1+/+) were still alive (Supplementary Videos S10 and S11; Figure 3A, right panel). These data suggest that knockout of Tet1 in mice impairs hypoxia tolerance, similar to what was displayed in tet1-null zebrafish. Consistently, more apoptotic cells were detected in the brain of Tet1−/− mice compared with their wild-type siblings(Tet1+/+) under hypoxia (Figure 3B). Semi-quantitative RT-PCR analysis revealed that expression of hypoxia-inducible genes was reduced in the brains of Tet1−/− mice compared with their wild-type siblings under hypoxia (Figure 3C).
Figure 3.
Tet1-null mice exhibit more sensitive to hypoxia. (A) Compared with their wild-type siblings and Tet1 heterozygous mice, Tet1-null mice exhibited less tolerant to hypoxia after they were put into the hypoxia workstation. The O2 concentration in the hypoxia chamber was adjusted to 10% ahead of time; 2 h later, it was adjusted to 8%. (B) Compared with those in the brain of wild-type sibling, more apoptotic cells were detected in the brain of tet1-null mice by TUNEL assays under hypoxia (10% O2 for 2 h plus 8% O2 for 8h). (C) Expressions of hypoxia-inducible genes including Glut1, Vegfa, Epo and Sod2 were reduced significantly in the brain of Tet1-null mice compared with those of their wildtype siblings under hypoxia (10% O2 for 2 h plus 8% O2 for 8 h).
Taken together, these data suggest that Tet1 plays an important role in hypoxia tolerance, which is conserved evolutionarily between fish and mammalians; knockout of Tet1 causes more apoptotic cells in the brain and reduction of hypoxia-inducible gene expression under hypoxia.
Tet1 stabilizes HIF-2α protein and enhances HIF-2α transcriptional activity independent of its enzymatic activity
Similar to zebrafish tet1, mouse Tet1 also activated the HRE reporter independent of its enzymatic activity (Figure 4A).
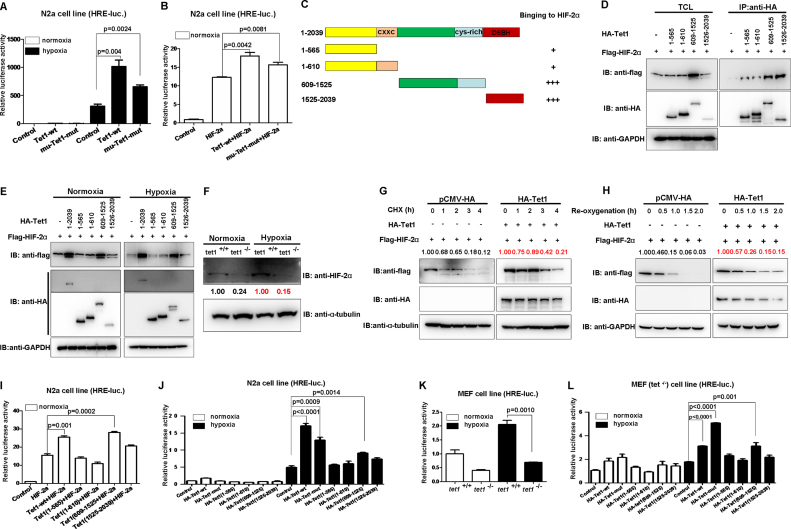
Figure 4.
Tet1 stabilizes HIF-2α protein and enhances HIF-2α transcriptional activity independent of its enzymatic activity. (A) Overexpression of both mouse Tet1 (WT) and its enzymatic inactive form (mu-tet1-mut) could activate hypoxia response element luciferase (HRE-luc.) reporter in the N2a cell line under hypoxia. (B) Overexpression of both mouse Tet1 (WT) and its enzymatic inactive form (mu-tet1-mut) together with HIF-2α could activate the hypoxia response element luciferase (HRE-luc.) reporter induced by HIF-2α in the N2a cell line under normoxia. (C) Schematic of the Tet1 domains. The extent of the interaction between HIF-2α and the Tet1 domains is indicated the number of plus sign (+). (D) Domain mapping indicates that the domain covering 609-1525aa and the domain covering 1525-2039aa of Tet1 interact with HIF-2α. (E) Tet1 enhanced HIF-2α stability under normoxia and hypoxia. HEK293T cells were transfected with indicated plasmids. After 16–20 h under normoxia (21% O2) or hypoxia (5% O2), cells were harvested and detected by Western blot analysis. (F) The protein level of HIF-2α was reduced in the MEF cells of Tet1-null mice compared with that in wild-type siblings. (G) Overexpression of Tet1 enhanced stabilization of HIF-2α in the presence of cycloheximide (CHX, 50 μg/ml) under normoxia. HEK293T cells were transfected with the indicated plasmids for 18–20 h, then, cycloheximide was added to culture medium with the final concentration 50 μg/ml. At different time points, the cells were harvested for western blot analysis. (H) Overexpression Tet1 enhanced stabilization of HIF-2α during re-oxygenation. HEK293T cells were transfected with the indicated plasmids for 16–18 h. Subsequently, the cells were placed in a hypoxia incubator (NBS Galaxy 48R) for 8 h (5% O2). Then, the cells were transferred into a regular incubator (21% O2). At different time points, the cells were harvested for Western blot analysis. (I) Overexpression of mouse Tet1 (WT) and its domain covering 609-1525 aa together with HIF-2α could activate the HRE-luc. reporter induced by HIF-2α in the N2a cell line under normoxia. (J) Overexpression of mouse Tet1 (tet1-wt), its enzymatic inactive form (tet1-mut) and its domain covering 609-1525aa could activate the HRE-luc. reporter in the N2a cell line under hypoxia. (K) The activity of the HRE-luc. reporter was lower in Tet1-null MEF cells compared with that in wild-type MEF cells under hypoxia. (L) In Tet1-null MEF cells (Tet1−/−), overexpression of mouse Tet1 (tet1-wt), its enzymatic inactive form (tet1-mut) and its domain covering 609-1525aa could activate the HRE-luc. reporter under hypoxia.
In light of the well-defined function of HIF-2α on hypoxia signaling and hypoxia adaptation, to gain insights into the mechanisms by which pathways are linked to the role of Tet1 on hypoxia tolerance (5), we initially examined whether Tet1 could affect HIF-2α transactivity. As shown in Figure 4B, overexpression of HIF-2α activated the HRE reporter dramatically. Co-expression of wild-type Tet1 as well as its enzymatic dead mutant (mu-tet1-mut) enhanced HIF-2α transactivity significantly (Figure 4B). Again, these data suggest that Tet1 enhances HIF-2α transactivity independent of its enzymatic activity.
Subsequently, we examined whether Tet1 interacts with HIF-2α by co-immunoprecipitation assays. It appeared that the whole Tet1 could interact with HIF-2α, and its domain (609-1525aa) had stronger binding ability, which also stabilized HIF-2α dramatically (Figure 4C and D). Then, we examined the stabilization of HIF-2α by Tet1 under both normoxia and hypoxia. The full-length Tet1 and the domain (609-1525aa) had stronger capability to stabilize HIF-2α (Figure 4E). To validate the stabilizing effect of Tet1 on HIF-2α endogenously, we checked HIF-2α protein levels in Tet1-null MEF cells and wild-type MEF cells. Consistently, HIF-2α in Tet1−/− MEF cells was much lower than that in Tet1+/+ MEF cells (Figure 4F). When the protein synthesis inhibitor, cycloheximide (CHX), was added, the degradation rate of HIF-2α was slower in co-expression of Tet1 compared with that in co-expression empty vector control, suggesting new protein synthesis was not required in this process (Figure 4G). Moreover, during re-oxygenation, the degradation rate of HIF-2α was slower in co-expression of Tet1 compared with that in the co-expression empty vector control (Figure 4H). These data suggest that Tet1 stabilizes HIF-2α.
We further confirmed that the full-length Tet1 and the domain (609-1525aa) which could stabilize HIF-2α dramatically and also enhanced HIF-2α transactivity (Figure 4I). In addition, under hypoxia, the full-length Tet1, as well as the enzymatic-deficient mutant and the domain (609–1525aa), activated the HRE reporter significantly (Figure 4J). In Tet1−/− MEF cells, the HRE reporter activity was lower than that in Tet1+/+ MEF cells under both normoxia and hypoxia (Figure 4K), consistent with Tet1 protein levels (Figure 4F). Moreover, we validated the enhancement effect of the full-length Tet1, the enzymatic-deficient mutant and the domain (609–1525aa) on the HRE reporter in Tet1−/− MEF cells (Figure 4L).
Taken together, these data suggest that Tet1 enhances HIF-2α activity by stabilizing HIF-2α protein independent of its enzymatic activity.
Tet1 reduces hydroxylation of HIF-2α by disturbing PHD2 function on HIF-2α
TET proteins and PHD proteins belong to an ancient family of nonheme Fe2+/2-OGDO) (19). PHD2, other than PHD1 and PHD3, has long been recognized as the major regulator of hypoxia signaling pathway (35). In addition, the genetic changes in both PHD2 and HIF-2α have indicated a strong correlation with high-altitude adaptation (5). We therefore speculated that Tet1 might affect PHD2 function on HIF-2α to act its function on hypoxia tolerance. To test this hypothesis, we subsequently conducted a series of experiments to address it. As shown in Figure 5A, when PHD2 was co-expressed with wild-type HIF-2α, as expected, the HIF-2α protein level was reduced. When Tet1 was co-expressed, the HIF-2α protein level was increased (Figure 5A). When PHD2 was co-expressed with the two proline-site mutated HIF-2α (HIF-2α-DM, P405/531A), however, the HIF-2α protein level was not changed. Interestingly, co-expression of Tet1 also could not cause HIF-2α-DM protein to change. This phenomenon suggests that Tet1 might affect HIF-2α stability by disturbing PHD2’s function on HIF-2α. In agreement with this notion, overexpression of Tet1 indeed could diminish HIF-2α hydroxylation by PHD2 (Figure 5B). Of note, overexpression of Tet1 did not cause HIF-2α mRNA to increase, further suggesting that Tet1 enhanced HIF-2α at the protein level but not at the mRNA level (Figure 5C). In fact, Tet1 could compete with PHD2 for binding to HIF-2α (Figure 5D). As shown in Figure 5D, HIF-2α was increased from 0.05 fold to 1.00 fold, but the binding of PHD2 to HIF-2α dropped from 1.00 fold to 0.47 fold when Tet1 was co-expressed. Consistently, Tet1 enhanced the transactivity of wild-type HIF-2α, but not the transactivity of HIF-2α-DM (Figure 5E). Moreover, the domain (609-1525aa) of Tet1 enhanced wild-type HIF-2α transactivity, but it had no effect on HIF-2α-DM transactivity (Figure 5F).
It is well defined that hydroxylated HIF-2α recruits VHL E3 complex for ubiquitin-dependent proteasomal degradation (36–38). To determine whether Tet1 could affect proteasomal degradation of HIF-2α, we used a proteasome inhibitor, MG132, for validation. When MG132 was added, the enhancement of HIF-2α by Tet1 disappeared (Supplementary Figure S5A). Subsequently, we sought to know whether Tet1 also affect ubiquitination of HIF-2α. We examined ubiquitination of wild-type HIF-2α and HIF-2α-DM in the presence or absence of Tet1. To our surprise, Tet1 enhanced poly-ubiquitination of wild-type HIF-2α instead of preventing poly-ubiquitination of wild-type HIF-2α, but for HIF-2α-DM, Tet1 had no effect on its poly-ubiquitination, which was consistent with the effect of Tet1 on stabilization of HIF-2α and HIF-2α-DM, respectively (Supplementary Figure S5B). This unexpected phenomenon prompted us to hypothesize that Tet1 might enhance poly-ubiquitination linked through other site lysines instead of linking through K48 poly-ubiquitination, which mainly mediates protein degradation (39). After we used different ubiquitin for poly-ubiquitination assays, including wild-type, K6, K11, K27, K29, K33, K48 and K63-only ubiquitins, we found that Tet1 enhanced poly-ubiquitination of HIF-2α linked through K29 and K48, obviously, in contrast to the consequence of protein poly-ubiquitination linked through K48 (Supplementary Figure S5C).
It is well defined that pVHL typically binds to hydroxylated HIF-α (36–38). To further ensure that Tet1 could diminish HIF-2α hydroxylation, we performed co-immunoprecipitation between pVHL and HIF-2α with or without Tet1 over-expression. As shown in Supplementary Figure S5D, when Tet1 was over-expressed, the protein level of HIF-2α was increased from 1.33-fold to 1.00-fold as revealed by co-immunoprecipitation assays (Supplementary Figure S5D, left panel). However, the binding amount of pVHL to HIF-2α was dropped from 1.00-fold to 0.70-fold (Supplementary Figure S5D, left panel). When the amount of overexpressed Tet1 was further increased, the protein level of HIF-2α was increased from 1.00-fold to 2.14-fold in co-immunoprecipitation assays, but, the binding amount of pVHL to HIF-2α was only increased from 1.00-fold to 1.16-fold (Supplementary Figure S5D, left panel). Therefore, Tet1 diminished pVHL binding to HIF-2α, further supporting that Tet1 could indeed cause reduction of HIF-2α hydroxylation.
Taken together, these data suggest that Tet1 competes with PHD2 for binding to HIF-2α, resulted in the attenuation of HIF-2α hydroxylation. In addition, Tet1 inhibits proteasome-mediated degradation of HIF-2α, but it enhances poly-ubiquitination of HIF-2α.
Tet1 stabilizes HIF-1α protein and enhances HIF-1α transcriptional activity independent of its enzymatic activity
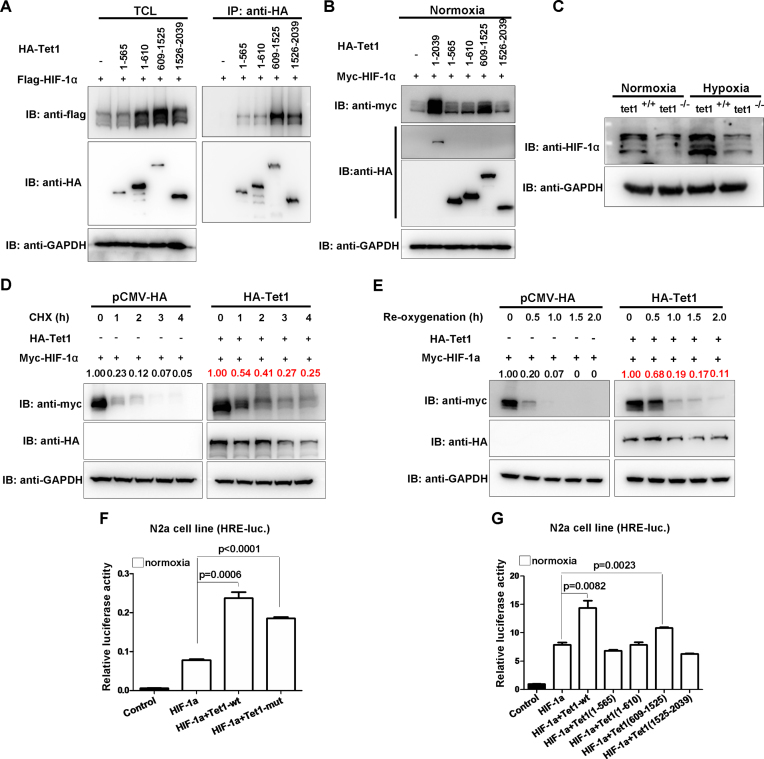
To understand the role of Tet1 in hypoxia tolerance and the underlying mechanisms more thoroughly, we further examined the effect of Tet1 on HIF-1α. Consistent with what was reported previously (27), Tet1 interacted with HIF-1α and its C-terminus had stronger binding ability to HIF-1α (Figure 6A). Similar to that on HIF-2α, Tet1 and its domain (609–1525aa) also stabilized HIF-1α dramatically (Figure 6B). In Tet1−/− MEF cells, the protein level of HIF-1α was much lower than that in Tet1+/+ MEF cells under normoxia and hypoxia (Figure 6C). When cycloheximide (CHX) was added, overexpression of Tet1 prevented HIF-1α degradation compared with those in the overexpression of the empty vector control (Figure 6D). Moreover, during re-oxygenation, the degradation rate of HIF-1α was slower in overexpression of Tet1 compared with that in the overexpression of empty vector control (Figure 6E). In addition, overexpression of wild-type (Tet1-WT), the enzymatic-dead mutant (Tet1-mut) and the domain (609-1525) of Tet1 also enhanced HIF-1α transactivity on the HRE reporter (Figure 6F and G).
Figure 6.
Tet1 stabilizes HIF-1α protein and enhances HIF-1α transcriptional activity independent of its enzymatic activity. (A) Domain mapping of Tet1 protein binding to HIF-1α. (B) Tet1 and its different domains stabilized HIF-1α protein. (C) The protein level of HIF-1α was reduced in Tet1-null MEF cells compared with that in wild-type MEF cells under normoxia and hypoxia. (D) Overexpression of Tet1 enhanced stabilization of HIF-1α in the presence of cycloheximide (CHX, 50μg/mL) under normoxia. HEK293T cells were transfected with the indicated plasmids for 18-20 h, then, cycloheximide was added to culture medium with the final concentration 50μg/mL. At different time points, the cells were harvested for Western blot analysis. (E) Overexpression Tet1 enhanced stabilization of HIF-1α during re-oxygenation. HEK293T cells were transfected with the indicated plasmids for 16-18 h. Subsequently, the cells were placed in a hypoxia incubator (NBS Galaxy 48R) for 8 h (5% O2). Then, the cells were transferred into a regular incubator (21% O2). At different time points, the cells were harvested for Western blot analysis. (F) Overexpression of mouse Tet1 (Tet1-wt) and its enzymatic inactive form (Tet1-mut) together with HIF-1α could activate the hypoxia response element luciferase (HRE-luc.) reporter induced by HIF-1α in the N2a cell line under normoxia. (G) Overexpression of mouse Tet1 (Tet1-wt) and its domain covering 609-1525aa together with HIF-1α could activate the HRE-luc. reporter induced by HIF-1α in the N2a cell line under normoxia.
Taken together, these data suggest that Tet1 stabilizes HIF-1α and enhances HIF-1α transcriptional activity independent of its enzymatic activity.
Tet1 does not affect PHD2 function on HIF-1α and stabilizes the C-terminal of HIF-1α
To determine whether Tet1 also affects hydroxylation of HIF-1α by disturbing PHD2 function, we examined the effect of Tet1 on the two-proline site mutated HIF-1α (HIF-1α-DM, P403/564A). In contrast to overexpression on HIF-2α, overexpression of Tet1 still stabilized the protein level of HIF-1α-DM (Figure 7A). Consistently, Tet1 has no effect on HIF-1α hydroxylation (Figure 7B). Moreover, overexpression of wild-type (Tet1-WT), the enzymatic-dead mutant (Tet1-mut) and the domain (609-1525) of Tet1 enhanced HIF-1α-DM transactivity on the HRE reporter (Figure 7C).
To further clarify the mechanistic aspects of HIF-1α stabilized by Tet1, we conducted experiments to map which parts of HIF-1α were stabilized by Tet1. As shown in Figure 7D, Tet1 stabilized the C-terminus of HIF-1α (576–826aa). To define which lysine sites located in the C-terminus were responsible for Tet1-mediated HIF-1α stabilization, we mutated all lysine sites in the C-terminus of HIF-1α to arginine and examined the effect of Tet1 on these mutants. Compared with the stabilizing effect of Tet1 on wild-type HIF-1α, Tet1 had relative weak effect on the mutants, K625/629R, K709R and K769R (Figure 7E)F. In light of the different effect of Tet1 on HIF-1α and HIF-2α, and the C-terminus of HIF-1α mainly were stabilized by Tet1, we compared the sequence of C-terminus between HIF-1α and HIF-2α and found that K629, K709 and K769 were different (Supplementary Figure S6). Thus, we made a mutant with four-site mutations (4Mut; K625/629/709/769R). Overexpression of Tet1 still stabilized the mutant 4Mut, even though the stabilization of 4Mut by Tet1 was not as dramatic as that of wild-type HIF-1α, suggesting that an unknown mechanism other than disturbing PHD2 function and lysine site modification accounted for the Tet1 function on HIF-1α stabilization (Figure 7F). Consistent with these observations, overexpression of Tet1 and its domain (609-1525) also enhanced HIF-1α and HIF-1α-4Mut transactivity on the HRE reporter (Figure 7G).
Similar to that on HIF-2α, Tet1 stabilized HIF-1α by preventing proteasome-mediated degradation of HIF-1α because MG132 also could block the enhancement of HIF-1α by Tet1 (Supplementary Figure S7A). In addition, Tet1 promoted poly-ubiquitination of wild-type HIF-1α and HIF-1α-DM, but had a relatively weak effect on the poly-ubiquitination of HIF-1α-4Mut (Supplementary Figure S7B).
In contrast to the effect of Tet1 on interaction between pVHL and HIF-2α, overexpression of Tet1 did not diminish pVHL binding to HIF-1α because the increasing tendency of HIF-1α protein resulted from overexpression of Tet1 was the same as the tendency of pVHL binding to HIF-1α (Supplementary Figure S7C), further reinforcing that Tet1 has no effect on HIF-1α hydroxylation.
Taken together, these data suggest that Tet1 stabilizes HIF-1α through an unknown mechanism rather than by disturbing of PHD2 function and lysine site modification.
DISCUSSION
Since the initial finding of the enzymatic activity in modifying methylcytosine and erasing DNA methylation, Tet1 and its family members Tet2 and Tet3 have gained much more attention (20–22). It has yet to be demonstrated whether the enzymatic-independent functions are owned by Tet1. As reported, Tet1 could regulate hypoxia-induced epitheliar-mesenchymal transition by acting as a coactivator of HIF-1α, independent of its enzymatic activity (27). In the current study, we found a subtle change of 5hmC in the brain of tet1-null zebrafish, indicating a mild effect of tet1 on the demethylation of zebrafish DNA, which is consistent with the observations reported previously (34). Furthermore, the enzymatic activity of Tet1 is not required for the enhancement of protein stability and transactivity of HIF-α. Knockout of Tet1 in both zebrafish and mice, however, caused Tet1-deficient animals to be more sensitive to hypoxic conditions. This finding suggests an enzymatic-independent function of Tet1 in hypoxia signaling.
Given the crucial role of O2 in species evolution, investigations on hypoxia adaptation (chronic hypoxia), particularly for high-altitude adaptation, have attracted more attention. Multiple lines of evidence support that the genetic changes in the HIF-2α (EPAS1) gene and the PHD2 (EGLN1) gene account for this kind of adaptation, which also indicate that the HIF pathway is mainly responsible for high-altitude adaptation (5,10,11,13–18). Basically, the high-altitude adaptation may represent a kind of adaptation for chronic hypoxic conditions, which supposes to go through a long historical geological period (5). How animals adapt to acute hypoxic conditions (hypoxia tolerance) and the underlying mechanisms remain unclear, probably because of a lack of feasible tools for exploration.
In this study, we took advantage of a hypoxia workstation and set up a practical procedure to evaluate hypoxia tolerance of zebrafish and mice. We demonstrated that Tet1 facilitates hypoxia tolerance by stabilizing HIF-α proteins and enhancing their transcriptional activity, demonstrating that the acute hypoxia adaptation (hypoxia tolerance) also meets the HIF signaling. Furthermore, we revealed that Tet1 disturbs PHD2 function on HIF-2α, reinforcing the critical role of PHD2 and HIF-2α on both chronic hypoxia adaptation and acute hypoxia adaptation (hypoxia tolerance). On the basis of this study, however, we still do not know whether Tet1 also contributes to chronic hypoxia adaptation (such as high-altitude adaptation). Further elucidation on the role of Tet1 in chronic hypoxia adaptation might provide a new clue for understanding aerobic organisms in high-altitude adaptation. Another possibility is that animals adapt to chronic hypoxia and acute hypoxia through different mechanisms. For chronic hypoxia adaptation, the genetic changes in major factors of hypoxia signaling might represent a main way because of a long term adaptation. However, for acute hypoxia adaptation, the regulation of gene expression related to hypoxia signaling might be a main cause because of quick response. In order to adapt to hypoxia, animals might develop multiple mechanisms to modulate HIF pathway.
It appears that the regulating mechanism of Tet1 on HIF-1α is more complicated than that on HIF-2α. Uncovering the underlying mechanism of Tet1 on HIF-1α probably will open a new window for understanding the mystery of hypoxia tolerance. In spite of the evidence we provided here demonstrating that Tet1 might facilitate hypoxia tolerance by affecting the HIF pathway, we cannot rule out that other pathways may be involved in mediating Tet1 function in hypoxia tolerance. Multiple genes probably act in concert to mediate Tet1 function in hypoxia tolerance.
Intriguingly, we noticed that double proline mutated HIF-2α and HIF-1α are also ubiquitinated. These mutants presumably would not be binding pVHL. Therefore, in addition to pVHL, other E3 ligases or E3 ligase complexes might also involve in HIF-α ubiquitination. To further address this phenomenon will help us to understand the regulation of HIF-α protein stability more thoroughly.
To date, multiple lines of evidence support that proteasome-mediated degradation is the major cause for degradation of HIF-1α and HIF-2α under either normoxia or hypoxia (4,40–46). Here, we also confirmed that Tet1 stabilizes HIF-α by disturbing the proteasome-mediated degradation of HIF-α. Unexpectedly, however, Tet1 enhances poly-ubiquitination of HIF-α instead of preventing the poly-ubiquitination of HIF-α. Further assays showed that Tet1 mainly enhances poly-ubiquitination of HIF-2α linked through K29 and K48, but not through K63 or other lysine sites. These phenomena are inconsistent with the classic protein degradation pathway that is mediated mainly by the ubiquitin-proteasome system (47). In fact, in addition to several types of ubiquitin modifications that target proteins for degradation, certain proteins appears to degraded by the proteasome in an ubiquitin-independent manner (48–51). Therefore, it seems that HIF-α could be mediated by ubiquitin-independent proteasomal degradation, but Tet1 disturbs this pathway, resulting in HIF-α stabilization. Given that Tet1 stabilizes HIF-2α by interfering with PHD2 function, it indicates that PHD2 not only might mediate HIF-2α degradation in this ubiquitin-dependent manner but also might mediate HIF-2α degradation in an ubiquitin-independent manner. The ability to further define the ubiquitin-independent proteasomal degradation of HIF-α and the role of Tet1 in this process will delineate a clear picture for HIF-α regulation, and in turn, also may reveal the mechanisms of Tet1 in hypoxia tolerance.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Drs. Peter Ratcliffe, William Kaelin, Frank Lee, Navdeep Chandel, Guoliang Xu, Chen Dong, Bo Zhang and Jingwei Xiong for the generous gift of reagents.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR online.
FUNDING
Strategic Priority Research Program of the Chinese Academy of Sciences [XDA08010208 to W.X.]; NSFC [31461163003, 91631102, 31671315 and 31721005]; NSFC [31601051 to J.W.]. Funding for open access charge: Charge for publication.
Conflict of interest statement. None declared.
REFERENCES
- 1. Aragones J., Fraisl P., Baes M., Carmeliet P.. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009; 9:11–22. [DOI] [PubMed] [Google Scholar]
- 2. Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012; 148:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Majmundar A.J., Wong W.J., Simon M.C.. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010; 40:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014; 9:47–71. [DOI] [PubMed] [Google Scholar]
- 5. Bigham A.W., Lee F.S.. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev. 2014; 28:2189–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prabhakar N.R., Semenza G.L.. Regulation of carotid body oxygen sensing by hypoxia-inducible factors. Pflugers Arch. 2016; 468:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prabhakar N.R., Semenza G.L.. Oxygen sensing and homeostasis. Physiology (Bethesda). 2015; 30:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bishop T., Ratcliffe P.J.. HIF hydroxylase pathways in cardiovascular physiology and medicine. Circ. Res. 2015; 117:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pugh C.W., Ratcliffe P.J.. New horizons in hypoxia signaling pathways. Exp. Cell Res. 2017; 356:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bigham A.W. Genetics of human origin and evolution: high-altitude adaptations. Curr. Opin. Genet. Dev. 2016; 41:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gou X., Wang Z., Li N., Qiu F., Xu Z., Yan D., Yang S., Jia J., Kong X., Wei Z. et al. . Whole-genome sequencing of six dog breeds from continuous altitudes reveals adaptation to high-altitude hypoxia. Genome Res. 2014; 24:1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang W., Fan Z., Han E., Hou R., Zhang L., Galaverni M., Huang J., Liu H., Silva P., Li P. et al. . Hypoxia adaptations in the grey wolf (Canis lupus chanco) from Qinghai-Tibet Plateau. PLoS Genet. 2014; 10:e1004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song D., Li L.S., Arsenault P.R., Tan Q., Bigham A.W., Heaton-Johnson K.J., Master S.R., Lee F.S.. Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J. Biol. Chem. 2014; 289:14656–14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tashi T., Scott Reading N., Wuren T., Zhang X., Moore L.G., Hu H., Tang F., Shestakova A., Lorenzo F., Burjanivova T. et al. . Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2alpha) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J. Mol. Med. (Berl.). 2017; 95:665–670. [DOI] [PubMed] [Google Scholar]
- 15. Huerta-Sanchez E., Jin X., Asan Bianba Z., Peter B.M., Vinckenbosch N., Liang Y., Yi X., He M., Somel M. et al. . Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature. 2014; 512:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeong C., Alkorta-Aranburu G., Basnyat B., Neupane M., Witonsky D.B., Pritchard J.K., Beall C.M., Di Rienzo A.. Admixture facilitates genetic adaptations to high altitude in Tibet. Nat. Commun. 2014; 5:3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorenzo F.R., Huff C., Myllymaki M., Olenchock B., Swierczek S., Tashi T., Gordeuk V., Wuren T., Ri-Li G., McClain D.A. et al. . A genetic mechanism for Tibetan high-altitude adaptation. Nat. Genet. 2014; 46:951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilbert-Kawai E.T., Milledge J.S., Grocott M.P., Martin D.S.. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology (Bethesda). 2014; 29:388–402. [DOI] [PubMed] [Google Scholar]
- 19. Salminen A., Kauppinen A., Kaarniranta K.. 2-Oxoglutarate-dependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: potential role in the regulation of aging process. Cell. Mol. Life Sci. 2015; 72:3897–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pastor W.A., Aravind L., Rao A.. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013; 14:341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu H., Zhang Y.. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011; 25:2436–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rasmussen K.D., Helin K.. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016; 30:733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thienpont B., Galle E., Lambrechts D.. TET enzymes as oxygen-dependent tumor suppressors: exciting new avenues for cancer management. Epigenomics. 2016; 8:1445–1448. [DOI] [PubMed] [Google Scholar]
- 24. Kao S.H., Wu K.J., Lee W.H.. Hypoxia, epithelial-mesenchymal transition, and TET-mediated epigenetic changes. J. Clin. Med. 2016; 5:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thienpont B., Steinbacher J., Zhao H., D’Anna F., Kuchnio A., Ploumakis A., Ghesquiere B., Van Dyck L., Boeckx B., Schoonjans L. et al. . Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature. 2016; 537:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mariani C.J., Vasanthakumar A., Madzo J., Yesilkanal A., Bhagat T., Yu Y., Bhattacharyya S., Wenger R.H., Cohn S.L., Nanduri J. et al. . TET1-mediated hydroxymethylation facilitates hypoxic gene induction in neuroblastoma. Cell Rep. 2014; 7:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai Y.P., Chen H.F., Chen S.Y., Cheng W.C., Wang H.W., Shen Z.J., Song C., Teng S.C., He C., Wu K.J.. TET1 regulates hypoxia-induced epithelial-mesenchymal transition by acting as a co-activator. Genome Biol. 2014; 15:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ota S., Hisano Y., Muraki M., Hoshijima K., Dahlem T.J., Grunwald D.J., Okada Y., Kawahara A.. Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells. 2013; 18:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dawlaty M.M., Ganz K., Powell B.E., Hu Y.C., Markoulaki S., Cheng A.W., Gao Q., Kim J., Choi S.W., Page D.C. et al. . Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011; 9:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao W., Ai J., Habermacher G., Volpert O., Yang X., Zhang A.Y., Hahn J., Cai X., Wang Z.. U19/Eaf2 binds to and stabilizes von hippel-lindau protein. Cancer Res. 2009; 69:2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X., Chen Z., Xu C., Leng X., Cao H., Ouyang G., Xiao W.. Repression of hypoxia-inducible factor alpha signaling by Set7-mediated methylation. Nucleic Acids Res. 2015; 43:5081–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J., Zhang W., Ji W., Liu X., Ouyang G., Xiao W.. The von hippel-lindau protein suppresses androgen receptor activity. Mol. Endocrinol. 2014; 28:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogdanovic O., Smits A.H., de la Calle Mustienes E., Tena J.J., Ford E., Williams R., Senanayake U., Schultz M.D., Hontelez S., van Kruijsbergen I. et al. . Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 2016; 48:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li C., Lan Y., Schwartz-Orbach L., Korol E., Tahiliani M., Evans T., Goll M.G.. Overlapping requirements for Tet2 and Tet3 in normal development and hematopoietic stem cell emergence. Cell Rep. 2015; 12:1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meneses A.M., Wielockx B.. PHD2: from hypoxia regulation to disease progression. Hypoxia (Auckl.). 2016; 4:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E., Wykoff C.C., Pugh C.W., Maher E.R., Ratcliffe P.J.. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999; 399:271–275. [DOI] [PubMed] [Google Scholar]
- 37. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J.M., Lane W.S., Kaelin W.G. Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001; 292:464–468. [DOI] [PubMed] [Google Scholar]
- 38. Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., von Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J. et al. . Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001; 292:468–472. [DOI] [PubMed] [Google Scholar]
- 39. McDowell G.S., Philpott A.. Ubiquitin-mediated proteolysis in Xenopus extract. Int. J. Dev. Biol. 2016; 60:263–270. [DOI] [PubMed] [Google Scholar]
- 40. Kaelin W.G., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer. 2008; 8:865–873. [DOI] [PubMed] [Google Scholar]
- 41. Liu Y.V., Baek J.H., Zhang H., Diez R., Cole R.N., Semenza G.L.. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cell. 2007; 25:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Montagner M., Enzo E., Forcato M., Zanconato F., Parenti A., Rampazzo E., Basso G., Leo G., Rosato A., Bicciato S. et al. . SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors. Nature. 2012; 487:380–384. [DOI] [PubMed] [Google Scholar]
- 43. Oh E.T., Kim J.W., Kim J.M., Kim S.J., Lee J.S., Hong S.S., Goodwin J., Ruthenborg R.J., Jung M.G., Lee H.J. et al. . NQO1 inhibits proteasome-mediated degradation of HIF-1alpha. Nat. Commun. 2016; 7:13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu H.T., Kuo Y.C., Hung J.J., Huang C.H., Chen W.Y., Chou T.Y., Chen Y., Chen Y.J., Chen Y.J., Cheng W.C. et al. . K63-polyubiquitinated HAUSP deubiquitinates HIF-1alpha and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat. Commun. 2016; 7:13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le Moan N., Houslay D.M., Christian F., Houslay M.D., Akassoglou K.. Oxygen-dependent cleavage of the p75 neurotrophin receptor triggers stabilization of HIF-1alpha. Mol. Cell. 2011; 44:476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang S.J., Park Y.S., Cho J.H., Moon B., An H.J., Lee J.Y., Xie Z., Wang Y., Pocalyko D., Lee D.C. et al. . Regulation of hypoxia responses by flavin adenine dinucleotide-dependent modulation of HIF-1alpha protein stability. EMBO J. 2017; 36:1011–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dikic I. Proteasomal and autophagy degradation systems. Annu. Rev. Biochem. 2017; 86:193–224. [DOI] [PubMed] [Google Scholar]
- 48. Erales J., Coffino P.. Ubiquitin-independent proteasomal degradation. Biochim. Biophys. Acta. 2014; 1843:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu J., Zhou L., Ji L., Chen F., Fortmann K., Zhang K., Liu Q., Li K., Wang W., Wang H. et al. . The REGgamma-proteasome forms a regulatory circuit with IkappaBvarepsilon and NFkappaB in experimental colitis. Nat. Commun. 2016; 7:10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stewart D.P., Koss B., Bathina M., Perciavalle R.M., Bisanz K., Opferman J.T.. Ubiquitin-independent degradation of antiapoptotic MCL-1. Mol. Cell. Biol. 2010; 30:3099–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li X., Amazit L., Long W., Lonard D.M., Monaco J.J., O’Malley B.W.. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol. Cell. 2007; 26:831–842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.