Abstract
Methotrexate (MTX) is the most used drug in rheumatoid arthritis (RA) treatment. However, it shows variability in clinical response, which is explained by an association with genetic polymorphisms. This study aimed to elucidate the role of the two gene polymorphism C677T and A1298C of the methylenetetrahydrofolate reductase (MTHFR) in response to MTX in Algerian RA patients. Study included 54 early RA patient treated with MTX for one year. MTX efficiency and toxicity were evaluated at 6 and 12 months respectively and the two gene polymorphisms were genotyped. No association was found between A1298C polymorphism and MTX toxicity. However, T allele of the C677T polymorphism was associated with the occurrence of MTX adverse effects (p = 0,019, OR: 3,63, 95% CI [1,12 - 12,80]). No association was found between C677T polymorphism and MTX efficiency, while A allele of the A1298C polymorphism was associated with good and moderate response (p = 0,02, OR = 3,28, 95% CI: [1,11– 9,42]). The study of RA biological markers kinetics showed that MTX did not affect antibodies rate unlike inflammatory markers. Our study suggests that MTHFR C677T and A1298C genotyping are associated to MTX toxicity and efficiency, respectively, in RA patients. This offers new perspectives in the personalization of RA treatment in Algeria.
Keywords: Genetics, Clinical genetics, Internal medicine, Health sciences
1. Introduction
The current objective in the management of rheumatoid arthritis (RA) is to control the pain, prevent and control the articular damages and avoid, in the long term, a functional handicap. Disease-modifiying anti rheumatic drugs (DMARDs) are the main treatment of RA, allowing the control of the symptoms as well as the long-term articular progress. In spite of the big diversity inside this family of molecules, the Methotrexate (MTX), which is an analogue of the folic acid, is the most widely used since its discovery in 1980, making it the first-line treatment for this pathology [1, 2, 3, 4].
Despite its superior efficacy in comparison to other DMARDs, considerable intra- and inter-individual variation has been reported, limiting the use of this drug. Indeed, a therapeutic failure (toxicity or low efficiency) was described in one third of patients [5, 6, 7].
Several factors influence the response to MTX such as personal factors, disease related factors or genetic ones [8, 9, 10, 11].
Since the absence of clinical and molecular markers for predicting the response to MTX, the pharmacogenetics provides many answers, by allowing the identification of patients who are susceptible to be good or poor responders, as well as those who will develop side effects. Thus, it will improve the progress of long-term RA [12].
Many studies were reported on the genetic polymorphism of the enzymes interfering in the mechanisms of action of MTX. The most informative results concern the genetic polymorphisms of the methylene tetrahydrofolate reductase (MTHFR), which activates the folic acid and indirectly inhibits the MTX actions. Because of the key-role of this enzyme in the DNA synthesis, repair and methylation, the polymorphisms of this gene, localized on the chromosome 1 (1p36.3), were the object of numerous works. Two missence mutations were mostly studied: the first is the C677T substitution (rs1801133) and the second is the A1298C one (rs1801131), which were described as associated to the efficiency and/or the toxicity of MTX in RA. However, results of these studies are controversial and inconclusive [13, 14].
The main objective of our study is finding an association between both polymorphisms C677T and A1298C of the MTHFR enzyme, with the toxicity and/or the efficiency of MTX in the RA in Algeria. In another hand, we studied the kinetics of the biological markers of this autoimmune disease during one year of treatment by MTX.
2. Materials and methods
2.1. Patients
Our prospective study included 54 patients (47 women and 7 men, average age: 44,26 ± 14,41 years) diagnosed with early RA (disease duration <24 months) according to the ACR/EULAR 2010 criteria (American college of rheumatology/European League Against Rheumatism) [15]. They were recruited in our Immunology department of the Beni Messous University Hospital (Algiers, Algeria), from the Rheumatology department of Ben Aknoun Hospital (Algiers, Algeria) during the period from January 2014 to June 2015. All patients were treated with the association: MTX and low dose corticosteroid (Table 1). No biological DMARDs were associated to MTX. A one year follow up with three check points was conducted for all patients: at diagnosis (T0) and after 6 (T6) and 12 months (T12) of treatment. Laboratory tests were performed at each time, including: erythrocyte sedimentation rate measure (ESR), C-reactive protein (CRP), uremia, creatinemia and transaminases dosage, IgM isotype rheumatoid factor (IgM-RF) and anti-citrullinated protein/peptid antibodies (ACPA) determination, anti-nuclear antibodies (ANA) detection, complete blood count (CBC) and serum proteins electrophoresis (SPE). Similarly, Disease activity score 28 (DAS28) was calculated and MTX side effects were noted. Finally, the response to MTX was evaluated using the DAS28-based EULAR response criteria [16]. Finally, whole blood samples (EDTA) was used for the genetic study. Our study was approved by the ethics committee of CHU Béni-Messous. Informed consent of each patient was given at the time of study.
Table 1.
Demographic, clinical and biological characteristics of patients at diagnosis.
| Characteristics | n = 54 (Frequency %) |
|---|---|
| 1. Demographic | |
| Age in years (M ± SD) | 44,26 ± 14,41 |
| Men | 7 (12,96%) |
| Women | 47 (87,04%) |
| Sex ratio (W/M) | 6,7 |
| 2. Clinical: | |
| Arthritis | 54 (100%) |
| Diagnosis delay in months (M ± SD) | 10,24 ± 7,28 |
| Radiological abnormalities | 13 (24,04%) |
| DAS28-CRP (M ± SD) | 4,45 ± 1,75 |
| Disease activity: | |
| Low DAS28 ≤ 3,2 | 13 (24,07%) |
| Moderate 3,2<DAS28≤5,1 | 22 (40,74%) |
| High DAS28>5,1 | 19 (35,19%) |
| Treatment: | |
| MTX + Methylprednisolone (4 mg/day) | 54 (100%) |
| MTX average dose/week (M ± SD) | 15,05 mg ± 2,40 |
| Folic acid supplementation (5 mg/week) | 54 (100%) |
| 3. Biological | |
| ESR mm/1ère h (M ± SD) | 53,40 ± 30,57 |
| CRP mg/l (M ± SD) | 24,21 ± 25,31 |
| Anemia | 15 (27,78%) |
| Abnormal transaminases | 0 (0%) |
| Abnormal uremia/creatinemia | 0 (0%) |
| RF-IgM positive | 30 (55,55%) |
| Anti-CCP3 IgG antibodies positive | 34 (62,96%) |
Demographic, clinical and biological characteristics of patients at diagnosis.
2.2. Methods
DNA was extracted from leukocytes via salting-out method and the two genetic polymorphisms of the MTHFR enzyme: A1298C (Rs1801131) and C677T (Rs1801133) were analyzed by real time PCR (TaqMan®, ThermoFisher Scientific).
2.3. Statistical analysis
Statistical analysis was performed using the Graph Pad prism version 6.0 and the compare 2 softwares. The Pearson Chi-square test or the Fisher’s exact test were used for comparing genotypic and allelic frequencies. Means comparison was done using the Student t-test or the one way ANOVA test. The Friedman test and the Wilcoxon signed-rank test were used for the kinetic analysis of the biological markers. Correlations studies were done using the Pearson or Spearman tests. Two-sided P-value less than 0.05 was considered statistically significant.
3. Results
3.1. Association between MTHFR polymorphisms and MTX toxicity
MTX side effects were reported in nine patients (16,67%) after one year of treatment (Table 2). The comparison of Genotypic and allelic frequencies of the A1298C polymorphism according to MTX toxicity did not show any significant difference. Similarly, no significant difference was found by analyzing the C677T SNP allelic frequencies according to MTX toxicity. However, T allele was significantly associated with MTX adverse effects (p = 0,019, OR: 3,63, 95% CI [1,12–12,80]), while C allele seemed to have a protective effect (p = 0,019, OR: 0,28, 95% CI [0,08 –0,90]) (Table 3).
Table 2.
MTX side effects after one year treatment.
| Side effects (n = 54) | n = 9 (16,67%) |
|---|---|
| Digestive intolerance (Nausea/Vomiting) | 4 (44,44%) |
| Liver intolerance | 2 (22,22%) |
| Alopecia | 1 (11,11%) |
| Haematologic toxicity: | 2 (22,22%) |
| Normocytic-normochromic anemia | 1 (50%) |
| Thrombopenia | 1 (50%) |
Table 3.
A1298C and C677T gene polymorphisms and MTX toxicity.
| SNP | Side effects+ | Side effects- | OR (95% CI) | p |
|---|---|---|---|---|
| SNP A1298C | n = 9 | n = 45 | ||
| Genotype | ||||
| AA | 5 (55,56%) | 25 (55,56%) | / | 1,00 |
| AC | 4 (44,44%) | 18 (40%) | / | 1,00 |
| CC | 0 (0%) | 2 (4,44%) | / | 1,00 |
| Allele | ||||
| A | 14 (77,78%) | 68 (75,56%) | / | 1,00 |
| C | 4 (22,22%) | 22 (24,44%) | / | 1,00 |
| SNP C677T | n = 9 | n = 45 | ||
| Genotype | ||||
| CC | 1 (11,11%) | 20 (44,44%) | / | 0,07 |
| CT | 4 (44,44%) | 18 (40%) | / | 1,00 |
| TT | 4 (44,44%) | 7 (15,56%) | / | 0,07 |
| Allele | ||||
| C | 6 (33,33%) | 58 (64,44%) | 0,28 (0,08–0,90) | 0,019 |
| T | 12 (66,67%) | 32 (35,56%) | 3,63 (1,12 − 12,80) | 0,019 |
3.2. Association between MTHFR polymorphisms and MTX efficiency
After six months of MTX treatment, 57,41% of the patients were good responders, 18,52% were moderate ones and 24,07% were non responders, according to EULAR response criteria [16]. Genotypic frequencies analysis of the A1298C SNP did not show any significant difference. However, A allele was more frequent in good (82,26%) and moderate (80%) responders. A significant difference was found by comparing the A1298C SNP allelic frequencies and EULAR response levels (p = 0,04) (Table 4). Moreover, A allele was significantly associated with good and/or moderate EULAR response to MTX (p = 0,02, OR = 3,28, 95% CI: [1,11–9,42]). At the opposite, C allele was significantly associated with a poor EULAR response (Table 4).
Table 4.
A1298C and C677T gene polymorphisms and MTX efficiency according to EULAR response criteria [16].
| SNP | Good response n = 31 |
Moderate response n = 10 |
Non response n = 13 |
p | Good or Moderate response n = 41 |
No response n = 13 |
p | OR (CI 95%) |
|---|---|---|---|---|---|---|---|---|
| A1298C | ||||||||
| Genotype | ||||||||
| AA | 20 (64,52%) | 6 (60%) | 4 (30,77%) | 0,11 | 26 (63,41%) | 4 (30,77%) | 0,05 | / |
| AC | 11 (35,48%) | 4 (40%) | 7 (53,85%) | 15 (36,59%) | 7 (53,85%) | 0,34 | / | |
| CC | 0 (0%) | 0 (0%) | 2 (15,38%) | 0 (0%) | 2 (15,38%) | 0,05 | / | |
| Allele | ||||||||
| A | 51 (82,26%) | 16 (80%) | 15 (57,69%) | 0,04 | 67 (81,71%) | 15 (57,69%) | 0,02 | 3,28 (1,11–9,42) |
| C | 11 (17,74%) | 4 (20%) | 11 (42,31%) | 15 (18,29%) | 11 (42,31%) | 0,02 | 0,31 (0,11 − 0,90) | |
| C677T | ||||||||
| Genotype | ||||||||
| CC | 9 (29,03%) | 5 (50%) | 7 (53,84%) | 0,22 | 14 (34,15%) | 7 (53,84%) | 0,33 | / |
| CT | 17 (54,84%) | 2 (20%) | 3 (23,08%) | 19 (46,34%) | 3 (23,08%) | 0,20 | / | |
| TT | 5 (16,13%) | 3 (30%) | 3 (23,08%) | 8 (19,51%) | 3 (23,08%) | 1,00 | / | |
| Allele | ||||||||
| C | 35 (56,45%) | 12 (60%) | 17 (65,39%) | 0,74 | 47 (57,32%) | 17 (65,39%) | 0,50 | / |
| T | 27 (43,55%) | 8 (40%) | 9 (34,61%) | 35 (42,68%) | 9 (34,61%) | 0,50 | / | |
In addition, no significant difference was found by comparing genotypic and allelic frequencies and EULAR response levers, for the second MTHFR polymorphism (Table 4).
3.3. Kinetics of RA biological markers under MTX treatment
3.3.1. CRP and ESR
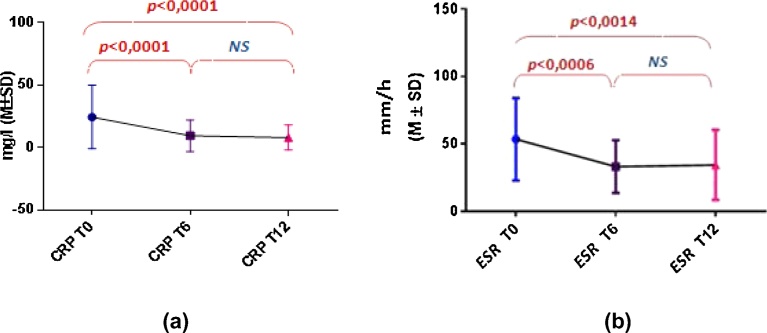
CRP and ESR parameters significantly decreased between T0 and T6, but stabilized in the following six months (Fig. 1).
Fig. 1.
CRP and ESR kinetics after 6 and 12 months of MTX treatment. Both CRP (a) and ESR (b) levels decreased significantly during the first six months of MTX treatment, but did not change beyond.
3.3.2. Autoantibodies
No significant difference was observed by comparing IgM-RF and ACPA average levels, at the three check points T0, T6 and T12. However, level changes were noted in few patients between baseline and one year therapy. Indeed, a RF seroreversion to positive was observed in 11 patients (36,67% of RF+ patients) while 3 patients RF seronegative at diagnosis, produce these autoantibodies after one year (12,5% of RF- patients). Moreover, 13 patients experienced a ≥50% modification in RF level (43,33% of RF+ patients). Concerning ACPA antibodies level changes, a ≥50% level modification was observed in 17 patients (50% of ACPA+ patients). However, only two patients ACPA+ at diagnosis became seronegative (5,88% of ACPA+ patients) and only one seroconversion was observed (5% of ACPA- patients) after one year MTX therapy.
3.3.3. Correlation between RF and ACPA
A positive correlation between these two immunological markers of RA was observed in 45 patients, at T0 (p = 0,02, r = 0,35), T6 (p = 0,0015, r = 0,47), and T12 (p = 0,0059, r = 0,37).
4. Discussion
MTHFR genetic polymorphisms A1298C and C677T were widely studied in RA patients all over the world. Most reported data concern the C677T polymorphism.
Association studies between the two MTHFR gene polymorphisms and MTX toxicity showed a considerable heterogeneity from country to country, and even in the same one.
In our study, the comparison between the genotypic and allelic frequencies of the A1298C SNP, and MTX toxicity did not show any significant difference. The same results were obtained from most studies [17, 18, 19, 20, 21, 22, 23, 24, 25] such as the English meta-analysis of 2013, which included 17 reports about the MTHFR gene polymorphisms’ influence on MTX toxicity [20]. However, few studies reported an association between this polymorphism and MTX toxicity, with sometimes conflicting results. Indeed, while few studies reported an association between the AC and CC genotypes as well as the C allele with MTX side effects’ development in RA [26, 27, 28, 29, 30], others described a protective effect of the CC genotype [31, 32]. Moreover, Hughes et al. related the A allele to MTX toxicity, in Caucasians [33].
On another hand, the C677T polymorphism analysis revealed an association between the T allele and MTX side effects’ development, with a result at the limit of statistical significance for the TT genotype, probably due to our small sample size. Contrarily, the C allele had a protective effect on MTX toxicity in RA patients. Many studies reported the association of the T allele with MTX toxicity [17, 19, 23, 25, 34, 35]. Ranganathan et al. reported an association of this allele with alopecia, in African-American RA patients [36]. Other studies described an association between the TT and/or CT genotypes with MTX toxicity [17, 19, 24, 27, 37], as Weisman et al. who reported an association of the TT genotype with a central nervous system toxicity [38].
Unlike our results, many studies did not found any significant association between The C677T polymorphism and MTX side effects’ development in RA [17, 20, 21, 22, 27, 28, 29, 33, 39, 40].
Various approaches were reported in order to study MTX efficiency, as: the ACR or EULAR response criteria, DAS or DAS28 improvement from baseline, RA disease activity, mean DAS or DAS28. Moreover, Hodkinson et al. reported that patients who did not respond after six months of treatment by conventional DMARDs, have a very poor chance to improve their response after one year [41]. Thus, in our study, we evaluated the response to MTX using the EULAR response criteria [16], after six months of MTX therapy.
Many studies showed a lack of association between the two MTHFR gene polymorphism and MTX efficacy [20, 21, 22, 23, 33, 40, 42], unlike our study. In fact, the A allele of the A1298C polymorphism was significantly associated with a good or moderate EULAR response. The AA genotype was also associated with MTX response at the limit of statistical significance, due to our small sample size. However, no significant association was found by comparing genotypic and allelic frequencies of the C677T gene polymorphism and the EULAR response levels.
One study published by Dervieux et al. in 2006, studied the MTX response in RA using the EULAR criteria. It included 48 RA patients treated by MTX for six months [29]. Unlike our results, the TT genotype of the C677T polymorphism was associated with a poor response to MTX [29]. On the other hand, Wessels et al. studied the association between the two MTHFR polymorphisms and DAS improvement, in 205 patients. A good DAS improvement (> 1,2) was reported in carriers of the CC and AA genotypes of the C677T and A1298C polymorphisms respectively [30]. Furthermore, no association was found between a moderate DAS improvement (> 0,6) and the two gene polymorphism [30]. Moreover, Kato et al. found that AA genotype of the A1298C polymorphism carriers had a lower DAS28 mean than the AC and CC genotypes’ carriers, after six months of MTX therapy [18].
Lastly, in a polish study of 2007, T and C alleles of the C677T and A1298C gene polymorphisms respectively, were associated with a high remission rate [43].
Additionally, a number of studies on the polymorphisms of genes involved in the MTX metabolism have focused on the simultaneous evaluation of multiple polymorphisms in an attempt to determine a genetic profile that would be characterized by the highest efficacy and lowest incidence of adverse effects, in addition to the MTHFR ones, such as: RFC-1 (reduced folate carrier −1), TYMS (thymidylate synthase), GGH (gammaglutamyl hydrolase), and TC (transcobalamin),…. [24, 25, 30, 34, 36, 38, 40].
Concerning the kinetics of RA biological markers, CRP and ESR significantly decrease during the first six months of treatment in our study, and stabilize beyond (from T6 to T12).
These results corroborate those of Sraub et al. who followed up CRP and ESR and some cytokines evolution in 20 RA patients, for three years. A significant decrease from baseline was observed for the two inflammatory markers as well as interleukins 6 and 2, after 12, 24 and 36 months of treatment by DMARDs [44].
On another hand, RF-IgM and ACPA rates did not significantly change after one year of MTX treatment in our study. However, rate changes were observed in few patients. Several research works studied the RF-IgM and ACPA kinetics in RA but the most of them interested in the biotherapies influence, especially anti-Tumor Necrosis Factor alpha (TNFα) antibodies, with controversial results [45, 46, 47]. Only few studies reported the conventional DMARDs effect on RA autoantibodies.
Our results were supported by those of a Swedish study of 2004, which include 242 patients with an early RA, followed up for three years [48]. ACPA rate stability was also reported by Burr et al. after five years of treatment by conventional DMARDs as MTX [49].
Furthermore, Mikulis et al. reported a >25% autoantibodies rate decrease after one year of treatment by conventional DMARDs, in half of the RA patients [70]. Interestingly, this diminution was observed in patients with a diagnosis delay less than twelve months [50].
Similarly, a German study of 2009 observed a 50% rate decrease of RF-IgM and ACPA antibodies, after 40 weeks of treatment by a DMARDs combination [51].
Unlike our study results, Ally et al. reported a significantly decrease of ACPA rate, after six months of treatment by MTX [52].
5. Conclusion
In our study, we found an association between the two genetic polymorphisms of the MTHFR enzyme, and the efficiency and/or the toxicity of MTX, after 6 months and 12 months of treatment, respectively. First of all, the T allele of the C677T SNP was significantly associated with the development of MTX side effects. As regards the efficacy, an EULAR response to the treatment (good or moderate) was obtained with the carriers of the A allele of the A1298C SNP. Unfortunately, these results still lacking significance and without clinical applications at present, due to the big heterogeneity of the studies’ results reported in the literature, especially concerning the A1298C polymorphism.
Therefore, it is likely that the individual’s susceptibility to a treatment is under the dependence of numerous factors among which some are genetic, epigenetic or related to the co-morbidities and environment. The influence of a particular gene is very low.
It is thus desirable to confirm these results on a larger sample and study the association of other genes involved in the pharmacokinetics of MTX, in order to find one or several haplotypes of susceptibility to the treatment, according to which new therapeutic strategies can be envisaged.
On the other hand, we studied the kinetics of the inflammatory markers and auto-antibodies after one year of treatment by MTX. Concerning the IgM-RF and ACPA, their average levels did not vary significantly after 6 and 12 months of treatment. Consequently, it is useless to repeat the evaluation of these autoantibodies after treatment by MTX.
In perspective, we intend to extend the follow-up of these patients and study other biological parameters of RA under treatment with MTX, as cytokines and chimiokines, as well as follow-up the clinical and radiological progress of the disease under treatment.
Declarations
Author contribution statement
Lilya Berkani: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fadia Rahal: Performed the experiments.
Ines Allam: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Soraya Mouaki Benani, Aicha Ladjouze: Conceived and designed the experiments.
Reda Djidjik: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Weinblatt M.E., Coblyn J.S., Fox D.A., Fraser P.A., Holdsworth D.E., Glass D.N. Efficacy of low-dose methotrexate in rheumatoid arthritis. N. Engl. J. Med. 1985;312(13):818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- 2.Williams H.J., Willkens R.F., Samuelson C.O., Jr., Alarcon G.S., Guttadauria M., Yarboro C. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis A controlled clinical trial. Arthritis Rheum. 1985;28(7):721–730. doi: 10.1002/art.1780280702. [DOI] [PubMed] [Google Scholar]
- 3.Le Loet X., Berthelot J.M., Cantagrel A., Combe B., De Bandt M., Fautrel B. Clinical practice decision tree for the choice of the first disease modifying antirheumatic drug for very early rheumatoid arthritis: a 2004 proposal of the French Society of Rheumatology. Ann. Rheum. Dis. 2006;65:45–50. doi: 10.1136/ard.2005.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokka T., Kautiainen H., Toloza S., Makinen H., Verstappen S.M., Lund Hetland M. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann. Rheum. Dis. 2007;66:1491–1496. doi: 10.1136/ard.2006.069252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alarcon G.S., Tracy I.C., Blackburn W.D., Jr. Methotrexate in rheumatoid arthritis: Toxic effects as the major factor in limiting long-term treatment. Arthritis Rheum. 1989;32:671–676. doi: 10.1002/anr.1780320603. [DOI] [PubMed] [Google Scholar]
- 6.Gispen J.G., Alarcon G.S., Johnson J.J., Acton R.T., Barger B.O., Koopman W.J. Toxicity of methotrexate in rheumatoid arthritis. J. Rheumatol. 1987;14:74–79. [PubMed] [Google Scholar]
- 7.Salliot C., van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann. Rheum. Dis. 2009;68:1100–1104. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoekstra M., van Ede A.E., Haagsma C.J., van de Laar M.A., Huizinga T.W., Kruijsen M.W. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003;62:423–426. doi: 10.1136/ard.62.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson J.J., Wells G., Verhoeven A.C., Felson D.T. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43:22–29. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Hider S.L., Silman A.J., Thomson W., Lunt M., Bunn D., Symmons D.P. Can clinical factors at presentation be used to predict outcome of treatment with methotrexate in patients with early inflammatory polyarthritis. Ann. Rheum. Dis. 2009;68:57–62. doi: 10.1136/ard.2008.088237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Ede A.E., Laan R.F., Rood M.J., Huizinga T.W., van de Laar M.A., van Denderen C.J. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001;44:1515–1524. doi: 10.1002/1529-0131(200107)44:7<1515::AID-ART273>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Finckh A., Liang M.H., van Herckenrode C.M., de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006;55:864–872. doi: 10.1002/art.22353. [DOI] [PubMed] [Google Scholar]
- 13.Frosst P., Blom H.J., Milos R., Goyette P., Sheppard C.A., Matthews R.G. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 14.van der Put N.M., Gabreels F., Stevens E.M., Smeitink J.A., Trijbels F.J., Eskes T.K. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects. Am. J. Hum. Genet. 1998;62:1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. January 2010;69(9):1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 16.Van Gestel A.M., Anderson J.J., van Riel P.L., Boers M., Haagsma C.J., Rich B. ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Associations for Rheumatology. J. Rheumatol. March 1999;26(3):705–711. [PubMed] [Google Scholar]
- 17.Xiao H., Xu J., Zhou X., Stankovich J., Pan F., Zhang Z. Associations between the genetic polymorphisms of MTHFR and outcomes of methotrexate treatment in rheumatoid arthritis. Clin. Exp. Rheumatol. October 2010;28(5):728–733. [PubMed] [Google Scholar]
- 18.Kato T., Hamada A., Mori S., Saito H. Genetic polymorphisms in metabolic and cellular transport pathway of methotrexate impact clinical outcome of methotrexate monotherapy in Japanese patients with rheumatoid arthritis. Drug Metab. Pharmacokinet. 2012;27(2):192–199. doi: 10.2133/dmpk.dmpk-11-rg-066. [DOI] [PubMed] [Google Scholar]
- 19.Cáliz R., del Amo J., Balsa A., Blanco F., Silva L., Sanmarti R. The C677T polymorphism in the MTHFR gene is associated with the toxicity of methotrexate in a Spanish rheumatoid arthritis population. Scand. J. Rheumatol. February 2012;41(1):10–14. doi: 10.3109/03009742.2011.617312. [DOI] [PubMed] [Google Scholar]
- 20.Owen S.A., Lunt M., Bowes J., Hider S.L., Bruce I.N., Thomson W. MTHFR gene polymorphisms and outcome of methotrexate treatment in patients with rheumatoid arthritis: analysis of key polymorphisms and meta-analysis of C677T and A1298C polymorphisms. Pharmacogenomics J. April 2013;13(2):137–147. doi: 10.1038/tpj.2011.42. [DOI] [PubMed] [Google Scholar]
- 21.Salazar J., Moya P., Altés A., Díaz-Torné C., Casademont J., Cerdà-Gabaroi D. Polymorphisms in genes involved in the mechanism of action of methotrexate: are they associated with outcome in rheumatoid arthritis patients? Pharmacogenomics. 2014;15(8):1079–1090. doi: 10.2217/pgs.14.67. [DOI] [PubMed] [Google Scholar]
- 22.Morgan M.D., Al-Shaarawy N., Martin S., Robinson J.I., Twigg S. MTHFR functional genetic variation and methotrexate treatment response in rheumatoid arthritis: a meta-analysis. Pharmacogenomics. March 2014;15(4):467–475. doi: 10.2217/pgs.13.235. [DOI] [PubMed] [Google Scholar]
- 23.Saleh M.M., Irshaid Y.M., Mustafa K.N. Methylene tetrahydrofolate reductase genotypes frequencies: association with toxicity and response to methotrexate in rheumatoid arthritis patients. Int. J. Clin. Pharmacol. Ther. February 2015;53(2):154–162. doi: 10.5414/CP202242. [DOI] [PubMed] [Google Scholar]
- 24.Chaabane S., Marzouk S., Akrout R., Ben Hamad M., Achour Y., Rebai A. Genetic Determinants of Methotrexate Toxicity in Tunisian Patients with Rheumatoid Arthritis: A Study of Polymorphisms Involved in the MTX Metabolic Pathway. Eur. J. Drug Metab. Pharmacokinet. August 2016;41(4):385–393. doi: 10.1007/s13318-015-0288-z. [DOI] [PubMed] [Google Scholar]
- 25.Świerkot J., Ślęzak R., Karpiński P., Pawłowska J., Noga L., Szechiński J., Wiland P. Associations between single-nucleotide polymorphisms of RFC-1, GGH, MTHFR, TYMS, and TCII genes and the efficacy and toxicity of methotrexate treatment in patients with rheumatoid arthritis. Pol. Arch. Med. Wewn. 2015;125(3):152–161. doi: 10.20452/pamw.2707. [DOI] [PubMed] [Google Scholar]
- 26.Davis L.A., Polk B., Mann A., Wolff R.K., Kerr G.S., Reimold A.M. Folic acid pathway single nucleotide polymorphisms associated with methotrexate significant adverse events in United States veterans with rheumatoid arthritis. Clin. Exp. Rheumatol. June 2014;32(3):324–332. [PMC free article] [PubMed] [Google Scholar]
- 27.Song G.G., Bae S.-C., Lee Y.H. Association of the MTHFR C677T and A1298C polymorphisms with methotrexate toxicity in rheumatoid arthritis: a meta-analysis. Clin. Rheumatol. December 2014;33(12):1715–1724. doi: 10.1007/s10067-014-2645-8. [DOI] [PubMed] [Google Scholar]
- 28.Mena J.P., Salazar-Páramo M., González-López L., Gámez-Nava J.I., Sandoval-Ramirez L., Sánchez J.D. Polymorphisms C677T and A1298C in the MTHFR gene in Mexican patients with rheumatoid arthritis treated with methotrexate: implication with elevation of transaminases. Pharmacogenomics J. August 2011;11(4):287–291. doi: 10.1038/tpj.2010.32. [DOI] [PubMed] [Google Scholar]
- 29.Dervieux T., Greenstein N., Kremer J. Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis Rheum. October 2006;54(10):3095–3103. doi: 10.1002/art.22129. [DOI] [PubMed] [Google Scholar]
- 30.Wessels J.A.M., de Vries-Bouwstra J.K., Heijmans B.T., Slagboom P.E., Goekoop-Ruiterman Y.P.M., Allaart C.F. Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single-nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis Rheum. April 2006;54(4):1087–1095. doi: 10.1002/art.21726. [DOI] [PubMed] [Google Scholar]
- 31.van Schouwenburg P.A., Rispens T., Wolbink G.J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. March 2013;9(3):164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 32.Berkun Y., Levartovsky D., Rubinow A., Orbach H., Aamar S., Grenader T. Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Ann. Rheum. Dis. October 2004;63(10):1227–1231. doi: 10.1136/ard.2003.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes L.B., Beasley T.M., Patel H., Tiwari H.K., Morgan S.L., Baggott J.E. Racial or ethnic differences in allele frequencies of single-nucleotide polymorphisms in the methylenetetrahydrofolate reductase gene and their influence on response to methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. September 2006;65(9):1213–1218. doi: 10.1136/ard.2005.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi A., Urano W., Tanaka E., Furihata S., Kamitsuji S., Inoue E. Validation of the associations between single nucleotide polymorphisms or haplotypes and responses to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a proposal for prospective pharmacogenomic study in clinical practice. Pharmacogenet. Genomics. June 2007;17(6):383–390. doi: 10.1097/01.fpc.0000236326.80809.b1. [DOI] [PubMed] [Google Scholar]
- 35.Urano W., Taniguchi A., Yamanaka H., Tanaka E., Nakajima H., Matsuda Y. Polymorphisms in the methylenetetrahydrofolate reductase gene were associated with both the efficacy and the toxicity of methotrexate used for the treatment of rheumatoid arthritis, as evidenced by single locus and haplotype analyses. Pharmacogenetics. April 2002;12(3):183–190. doi: 10.1097/00008571-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ranganathan P., Culverhouse R., Marsh S., Mody A., Scott-Horton T.J., Brasington R. Methotrexate (MTX) pathway gene polymorphisms and their effects on MTX toxicity in Caucasian and African American patients with rheumatoid arthritis. J. Rheumatol. April 2008;35(4):572–579. [PubMed] [Google Scholar]
- 37.van Ede A.E., Laan R.F., Blom H.J., Huizinga T.W., Haagsma C.J., Giesendorf B.A. The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis Rheum. November 2001;44(11):2525–2530. doi: 10.1002/1529-0131(200111)44:11<2525::aid-art432>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Weisman M.H., Furst D.E., Park G.S., Kremer J.M., Smith K.M., Wallace D.J. Risk genotypes in folate-dependent enzymes and their association with methotrexate-related side effects in rheumatoid arthritis. Arthritis Rheum. February 2006;54(2):607–612. doi: 10.1002/art.21573. [DOI] [PubMed] [Google Scholar]
- 39.Zeng Q.-Y., Wang Y.-K., Xiao Z.-Y., Chen S.-B. Pharmacogenetic study of 5, 10-methylenetetrahydrofolate reductase C677T and thymidylate synthase 3R/2R gene polymorphisms and methotrexate-related toxicity in Chinese Han patients with inflammatory arthritis. Ann Rheum Dis. August 2008;67(8):1193–1194. doi: 10.1136/ard.2007.085266. [DOI] [PubMed] [Google Scholar]
- 40.Ghodke-Puranik Y., Puranik A.S., Shintre P., Joshi K., Patwardhan B., Lamba J., Niewold T.B., Chopra A. Folate metabolic pathway single nucleotide polymorphisms: a predictive pharmacogenetic marker of methotrexate response in Indian (Asian) patients with rheumatoid arthritis. Pharmacogenomics. December 2015;16(18):2019–2034. doi: 10.2217/pgs.15.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodkinson B., Musenge E., Ally M., Meyer P.W.A., Anderson R., Tikly M. Response to traditional disease-modifying anti-rheumatic drugs in indigent South Africans with early rheumatoid arthritis. Clin. Rheumatol. April 2012;31(4):613–619. doi: 10.1007/s10067-011-1900-5. [DOI] [PubMed] [Google Scholar]
- 42.Hashiguchi M., Tsuru T., Miyawaki K., Suzaki M., Hakamata J., Shimizu M., Irie S., Mochizuki M. Preliminary study for predicting better methotrexate efficacy in Japanese patients with rheumatoid arthritis. J. Pharm. Health Care Sci. June 2016;7(2):13. doi: 10.1186/s40780-016-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurzawski M., Pawlik A., Safranow K., Herczynska M., Drozdzik M. 677C > T and 1298A > C MTHFR polymorphisms affect methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics. November 2007;8(11):1551–1559. doi: 10.2217/14622416.8.11.1551. [DOI] [PubMed] [Google Scholar]
- 44.Straub R.H., Müller-Ladner U., Lichtinger T., Schölmerich J., Menninger H., Lang B. Decrease of interleukin 6 during the first 12 months is a prognostic marker for clinical outcome during 36 months treatment with disease-modifying anti-rheumatic drugs. Br. J. Rheumatol. December 1997;36(12):1298–1303. doi: 10.1093/rheumatology/36.12.1298. [DOI] [PubMed] [Google Scholar]
- 45.Modi S., Soejima M., Levesque M.C. The effect of targeted rheumatoid arthritis therapies on anti-citrullinated protein autoantibody levels and B cell responses. Clin. Exp. Immunol. July 2013;173(1):8–17. doi: 10.1111/cei.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alessandri C., Bombardieri M., Papa N., Cinquini M., Magrini L., Tincani A. Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann. Rheum. Dis. October 2004;63(10):1218–1221. doi: 10.1136/ard.2003.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H.A., Lin K.C., Chen C.H., Liao H.T., Wang H.P., Chang H.N. The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann. Rheum. Dis. January 2006;65(1):35–39. doi: 10.1136/ard.2005.038851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kastbom A., Strandberg G., Lindroos A., Skogh T. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project) Ann. Rheum. Dis. September 2004;63(9):1085–1089. doi: 10.1136/ard.2003.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burr M.L., Viatte S., Bukhari M., Plant D., Symmons D.P., Thomson W. Long-term stability of anti-cyclic citrullinated peptide antibody status in patients with early inflammatory polyarthritis. Arthritis Res. Ther. 2012;14(3):R109. doi: 10.1186/ar3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikuls T.R., O’Dell J.R., Stoner J.A., Parrish L.A., Arend W.P., Norris J.M. Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti-cyclic citrullinated peptide antibody. Arthritis Rheum. December 2004;50(12):3776–3782. doi: 10.1002/art.20659. [DOI] [PubMed] [Google Scholar]
- 51.Van Tuyl L.H.D., Lems W.F., PJSM Kerstens, Voskuyl A.E., Dijkmans B a. C, Boers M. IgM-rheumatoid factor and anti-cyclic citrullinated peptide decrease by 50% during intensive treatment in early rheumatoid arthritis. Ann. Rheum. Dis. October 2009;68(10):1652–1653. doi: 10.1136/ard.2008.103184. [DOI] [PubMed] [Google Scholar]
- 52.Ally M.M.T.M., Hodkinson B., Meyer P.W.A., Musenge E., Tintinger G.R., Tikly M. Circulating anti-citrullinated peptide antibodies, cytokines and genotype as biomarkers of response to disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. BMC Musculoskelet. Disord. 2015;16:130. doi: 10.1186/s12891-015-0587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]